Abstract
The relationships between different dendritic cell (DC) populations are not clearly established. In particular, it is not known how DC generated in vitro relate to those identified in vivo. Here we have characterized rat bone marrow-derived DC (BMDC) and compared them with DC isolated from spleen (SDC) and pseudo-afferent lymph (LDC). BMDC express typical DC markers and are mostly OX41 positive and CD4 negative. In contrast to ex vivo DC, some BMDC express Fc receptors. FcR+ and FcR− BMDC express similar levels of major histocompatibility complex class II molecules (MHC) and are B7 positive, but some FcR− BMDC express high levels of B7. In contrast to freshly isolated or cultured ex vivo SDC and LDC, both BMDC subpopulations can express inducible nitric oxide synthase (iNOS) and can secrete nitric oxide (NO) in amounts similar to those secreted by peritoneal macrophages. Despite expressing MHC class II and B7, FcR+ BMDC stimulate only a very weak MLR and inhibit stimulation by FcR− BMDC and ex vivo DC. Inhibition is only partially NO dependent. FcR+ BMDC are not macrophages, as judged by adherence and phagocytosis. Both subpopulations are able to present antigen to primed T cells in vitro and are able to prime naïve CD4 T cells in vivo. However, unlike SDC, BMDC are unable to stimulate cytotoxic T-lymphocyte (CTL) responses to a minor histocompatibility antigen. Thus, BMDC show marked differences to ex vivo DC and their relationship to those of in vivo DC populations, to date, is unclear.
Introduction
Dendritic cells (DC) are professional antigen-presenting cells (APC), which play a pivotal role in the stimulation and regulation of immune responses.1 In animals, and to a lesser extent humans, DC can be prepared by digestion of peripheral tissues or central lymphoid tissues,2–4 or by cannulation of afferent or pseudo-afferent lymphatics.5–7 In mouse, rat and human, cells with phenotypic and functional DC characteristics can also be prepared by culture from different precursors, including bone-marrow stem cells, CD34+ blood cells and monocytes.8–12 It is clearly important, from both a straightforward biological viewpoint and because of the increasing use of DC in immunotherapy, to understand how these different DC populations relate to each other, and how DC generated in vitro relate to DC in vivo. It is now apparent that DC in vivo can arise from both myeloid and lymphoid precursors13,14 and there is evidence for distinct lineages in human DC generated from human blood mononuclear cells.11,15,16 It is also unclear how those DC migrating from tissues under steady-state conditions5,7,17,18 relate to those accumulating, or stimulated to migrate, by proinflammatory stimuli.19–21 The understanding of DC is further complicated because within a single lineage, DC exist at different stages of maturation or differentiation.
Relatively few studies have attempted to compare DC generated in vitro with those obtained ex vivo and, where this has been attempted, the comparisons have been limited.12,22 In the experiments described in the present report we have generated DC from rat bone marrow using granulocyte–macrophage colony-stimulating factor (GM–CSF) and interleukin (IL)-4 [bone marrow-derived DC (BMDC)], and have compared their phenotypic and functional properties with DC obtained ex vivo from spleen (SDC) and pseudo-afferent intestinal lymph (LDC).
We show that BMDC, although superficially similar to ex vivo DC, unlike ex vivo DC, can be stimulated to synthesize inducible nitric oxide synthase (iNOS) and to secrete large amounts of nitric oxide (NO). BMDC are much weaker stimulators of an allogeneic mixed lymphocyte reaction (MLR) than SDC or LDC, and their ability to stimulate an MLR is not increased by several factors associated with DC maturation; indeed, when large numbers of BMDC are used as stimulators, proliferation is inhibited. This inhibition is only partly the result of NO secretion. There are two populations of BMDC distinguishable by FcR expression. FcR+ BMDC are less stimulatory in an allogeneic MLR and inhibit the ability of other DC populations to stimulate an MLR. FcR− BMDC augment the MLR stimulation by LDC. Both BMDC and SDC can present antigen (Ag) to sensitized T cells and prime naïve CD4 T cells in vivo. In contrast to SDC, however, BMDC were unable to prime CD8+ CTL against a minor histocompatibility Ag. Therefore, BMDC differ functionally from ex vivo DC and it remains unclear how they fit into our current picture of DC in vivo.
Materials and methods
Animals
Rats were bred and maintained under specific pathogen-free (SPF) conditions at the MRC Cellular Immunology Unit (Oxford, UK). Strains used were PVG (RT1c), DA (RT1av1), LEW (RT1l) and F1 crosses of of DA/LEW. All experiments were carried out under the authority of a licence issued by the Home Office.
Antibodies and other reagents
Anti-iNOS monoclonal antibodies (mAbs) (ANOS5 and ANOS11)23 were used at 10 µg/ml for immunocytochemistry. Most mouse anti-rat antibodies used in this study were gifts from the MRC Cellular Immunology Unit. OX6 [mouse immunoglobulin G1 (IgG1)] recognizes rat major histocompatibility complex (MHC) class II; OX62 (mouse IgG1) recognizes an integrin-like molecule expressed solely on LDC and γδ T cells.24,25 The mouse anti-rat CD80 mAb (3H5) was a kind gift from Dr Okumura (Jutendo University, Tokyo, Japan).26 Anti-rat CD11c was purchased from Serotec (Oxford, UK). NG-monomethyl l-Arginine (NMMA), indomethacin and saponin (from Saponaria spp.) were purchased from Sigma (Poole, UK). GM–CSF (supernatant from an X63-Ag8 line transfected with murine GM–CSF) was a gift from Dr D. Gray (University of Edinburgh, Edinburgh, UK). Rat IL-4 [supernatant from a rat IL-4-transfected Chinese hamster ovary (CHO) line], recombinant rat interferon-γ (IFN-γ) and recombinant rat tumour necrosis factor-α (TNF-α) were gifts from Dr D. Mason (MRC Cellular Immunology Unit).
Cell culture
The media (R5) used was RPMI-1640, supplemented with 5% fetal calf serum (FCS), 2 mm l-glutamine, 1 mm sodium pyruvate, 10 U/ml penicillin and 50 µg/ml streptomycin (all from Gibco, Paisley, UK). Lipopolysaccharide (LPS) contamination of the FCS was < 0·4 ng/ml (as measured by the supplier). For MLRs, FCS was replaced by 5% DA rat serum. All cell cultures were performed at 37°, in an atmosphere of 5% CO2.
Generation and isolation of DC
Culture of bone marrow DC was based on a previously described procedure.8,27 Bone marrow was aspirated from femurs of 10–16-week-old male PVG (RT1c) or DA/l (RT1av11) rats. Red blood cells were removed by Geys lysis solution, and lymphocytes/MHC class II+ cells were removed by rosetting.28 Cells were cultured in 24-well plates at 106 cells/ml/well in complete media [R5 plus 1% vol/vol GM–CSF (30 ng/ml) and IL-4 (2000 U/ml)]. Medium was changed every 2–3 days and cells were harvested after 6 or 7 days. Semi- and non-adherent cells were harvested after 6 or 7 days. Cells used for fluorescence-activated cell sorter (FACS) analysis of iNOS were indirectly depleted of macrophages (Mφ) on day 6 by harvesting BMDC and replating in 24-well plates at 106 cells/ml.
Lymph DC were isolated from pseudo-afferent thoracic duct lymph, as described previously.5 Rats were not irradiated. PVG (RT1c) rats were used routinely, but in some experiments DA/L (RT1av11) rats were used; similar results were obtained.
Spleen DC were isolated using a modification of a previously described procedure,29,30 by digestion with 2 mg/ml collagenase D (Boehringer Mannheim, Mannheim, Germany) and 0·05% DNaseI (Boehringer Mannheim) followed by suspension in Ca2+-free Hanks' balanced salt solution (HBSS) (Gibco) containing 50 mm EDTA, at 4°, to separate clusters. Cells were then centrifuged over Nycoprep Animal 1·077 (Nycomed, Oslo, Norway), washed and incubated in Petri dishes in R5 for 2 hr at 37°. Non-adherent cells were washed away. The remaining cells were cultured overnight and non-adherent cells collected by pipetting. These cells were then rosetted with opsonized sheep red-blood cells (SRBC; TCS Biologicals, Botolph, Claydon, UK) to remove FcR+ cells. To obtain highly purified SDC, non-adherent cells were incubated with an antibody cocktail to remove lymphocytes, natural killer (NK) cells and Mφs. The resultant populations were judged 75–85% DC by morphology and immunocytochemistry. To obtain freshly isolated SDC, the overnight adherence was omitted and SDC were enriched using negative selection of lymphocytes, NK cells and Mφs (used for the antigen presentation in vivo, as shown in Fig. 9a).
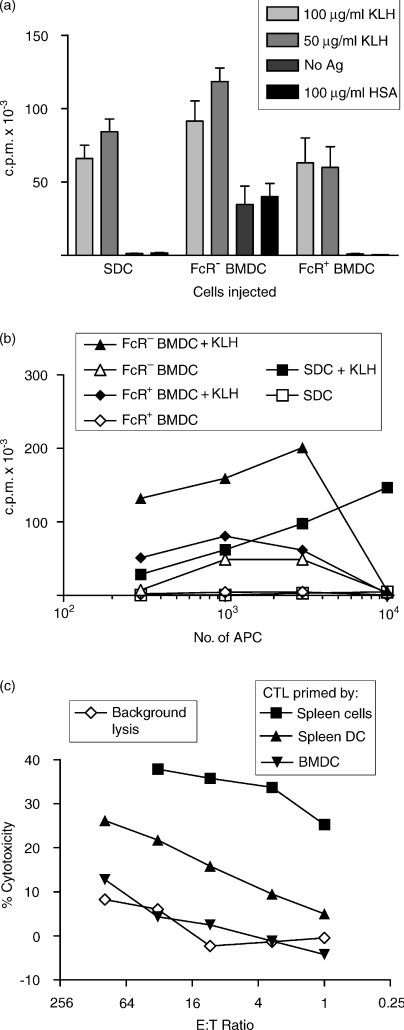
Figure 9.
Presentation of antigen (Ag) by bone marrow-derived dendritic cells (BMDC) in vivo and in vitro to CD4+ and CD8+ T cells. (a) Freshly isolated spleen DC (SDC) (no overnight culture), FcR− BMDC and FcR+ BMDC were pulsed with keyhole limpet haemocyanin (KLH) for 1 hr, washed and injected subcutaneously into naïve rats. Ten days later, rats were killed and 2 × 105 CLN cells were cultured with KLH, no antigen, or human serum albumin (HSA), for 5 days, with [3H]thymidine ([3H]TdR) added for the final 16 hr. [3H]TdR incorporation was measured by scintillation counting. This assay showed a non-specific background with FcR− BMDC that was not seen in subsequent experiments. (b) DC were pulsed with KLH and cultured with nylon wool-enriched T cells from spleens of rats primed with KLH 2 weeks previously, for 5 days with a pulse of [3H]TdR for the final 16 hr. The counts per minute (c.p.m.) were obtained by scintillation counting. (c) [Lewis (female) × DA (male)]F1 rats were primed with [DA (female) × Lewis (male)]F1 BMDC, SDC or spleen cells by subcutaneous injection. Three weeks later, lymph node cells were harvested and restimulated in vitro with [DA (female) × Lewis (male)] spleen cells. Cytotoxicity was assessed in a JAM assay.
Opsonization of SRBC and Fc rosetting of BMDC
SRBC (TCS Biologicals) were washed three times in phosphate-buffered saline (PBS), then resuspended in a 5% solution with 20 µg/ml rabbit anti-SRBC hyperimmune serum (Nordic, Tilburg, The Netherlands) in R5 and incubated for 1 hr at 37°, washed three times in R5 and made up to a 5% (vol/vol) solution. BMDC were separated into FcR+ and FcR− populations by incubation with opsonized SRBC (10 SRBC : 1 BMDC) for 30 min at 4° on a rotating wheel. Cells were then centrifuged on a Histopaque (Sigma) gradient, FcR− cells removed from the interface and FcR+ cells recovered from the pellet after lysis of SRBC.
Isolation of Mφs
Rats were killed by CO2 and ice-cold PBS injected into the peritoneal cavity. Resident peritoneal Mφs were collected by pipette after opening the peritoneal cavity. Thioglycollate-elicited Mφs were collected from the peritoneal cavity of freshly killed rats injected intraperitoneally (i.p.) with thioglycollate (Difco Brewers thioglycollate; Difco, West Molesey, UK) 3 days previously.
Phagocytosis and endocytosis assays
Phagocytosis of opsonized SRBC was measured using the following procedure. DC or Mφs were mixed with opsonized SRBC at ratios of 1 : 10 and 1 : 50 cells : SRBC and incubated for 1 hr at 37°. External SRBC were lysed using Geys solution, and cytospins were prepared and labelled with OX6 (anti-MHC class II) or OX42 (anti-CD11b/c). The numbers of SRBC internalized by 200 cells were counted. Endocytosis of fluorescein isothiocyanate (FITC)-conjugated beads was measured as follows. Various sizes of beads (Sigma, supplied as 5% beads, diluted 1 : 100 in the assay) were incubated with BMDC for 30 min at 37 or 4°. Cells were washed three times and the mean fluorescence intensity (MFI) measured using a fluorescence-activated cell sorter (FACscan; Becton-Dickinson, San Jose, CA). Specific uptake was assessed by subtracting the MFI of cells incubated at 4° from the value at 37°, to give the ΔMFI.
Immunocytochemistry and flow cytometry
Cells were resuspended at 5 × 105/ml in R5 media and mixed with an equal volume of 10% bovine serum albumin (BSA) (Sigma) and then spun onto glass slides in a cytospin for 5 min at 400 rpm. Slides were then fixed in ethanol for 10 min and washed in PBS. Slides were labelled with mouse anti-rat mAbs followed by rabbit anti-mouse IgG antibody conjugated to horseradish peroxidase (Dako, Glostrup, Denmark). Diaminodiamino-benzene (10 mm) (Kementec, Copenhagen, Denmark) and 0·005% H2O2 (Sigma) were then added to the slides for 10 min. Slides were then counterstained with Harris Haematoxylin and dehydrated through ethanol and Histoclear (National Diagnostics, Hessle, UK) and mounted with DePeX (BDH, Dorset, UK).
For flow cytometry, DC were harvested from plates by pipetting (leaving the adherent cells) and resuspended at 2 × 105 cells/200 µl in a 96-well round-bottom plate. Cells were permeabilized on ice for 1 hr with 0·3% saponin (Sigma) and 1% BSA in PBS (PBS/sap/BSA) and then stained for 30 min on ice with anti-iNOS, or with OX21 as a negative control or OX6 as a positive control. Cells were then washed with PBS/sap/BSA. Secondary FITC conjugated rabbit anti-mouse was then incubated with cells for 30 min. For double staining, 50 µl of OX6-biotin was used at 10 µg/ml for 30 min without saponin, then streptavidin Quantum red (Sigma) was used to detect MHC class II molecules. For surface labelling, cells were washed and stained in PBS containing 1% BSA and 10 mm sodium azide. Cells were extensively washed and analysed on a Becton-Dickinson FACscan connected to a BD FACSmate. Plots were analysed using Cell Quest and at least 10 000 events were collected for each sample.
Treatment with cytokines or endotoxin and measurement of NO
Cells were plated out in 24-well plates at 106 cells/well with IFN-γ (50 U/ml) and LPS (50 ng/ml), in R5 media. After 24–48 hr, cells were harvested, washed and analysed by FACS or immunocytochemistry.
Various populations of DC or Mφs were cultured at 5 × 105/150 µl in 96-well round-bottom plates. IFN-γ was added at 50 U/ml, TNF-α at 25 U/ml and LPS at 50 ng/well. After 48 hr of culture, NO release was assessed using the Greiss reagent [1% sulphanilamide (Sigma) in 5% phosphoric acid and 1%N-(1-naphtyl)ethylenediamine (NEDA) (Sigma) in H2O]. For each sample, a negative control was included that contained 250 µg/ml NMMA.
Allogeneic MLR
DC (generally RT1av1 xl, occasionally RT1c) were isolated as described above and graded numbers added to 2 × 105 allogeneic (generally RT1c occasionally RT1av1 xl) cervical lymph node cells in 96-well round-bottom plates in MLR medium. After 3 days, culture plates were pulsed with [3H]thymidine ([3H]TdR) (Amersham, Little Chalfont, UK), 0·5 µCi/well overnight, harvested onto betaplate filtermats and counted on a betaplate scintillation counter (Wallac, Milton Keynes, UK). In some assays, final concentrations of 0·2 µg/ml indomethacin or 1 mm NMMA were added to wells.
Antigen presentation in vitro and in vivo
Various DC populations were isolated, pulsed with 100 µg/ml keyhole limpet haemocyanin (KLH) (Sigma), washed extensively with PBS containing 50 mm EDTA and then injected into the footpads of syngeneic rats. After 10 days, popliteal lymph node cells were removed from primed rats, made into single-cell suspensions and incubated in 96-well plates with soluble antigen (100 µg/ml KLH in R5 media with 5% normal rat serum replacing FCS). Four days later, proliferation was measured, as described above, for MLR.
Various populations of DC were incubated with 100 µg/ml KLH for 3 hr at 37°, washed extensively and then graded numbers were cultured with 2 × 105/well nylon wool-enriched splenic T cells from rats primed to KLH 2 weeks previously. After 5 days, proliferation was measured as described for the MLR experiments.
Generation of cytotoxic T cells in vivo
Cytotoxic T-lymphocyte (CTL) responses were primed to a maternally transmitted mitochondrial-associated minor histocompatibility antigen (MTA; ref. 31). In short, MTA− rats were injected i.p. with lymphocytes from MTA+ rats. After at least 3 weeks, rats were killed, spleen and lymph nodes were removed and single-cell suspensions were prepared, Primed MTA− cells were cultured at 2 × 105 cells per well in 96-well plates with 2 × 105 MTA+ irradiated (20 Gy) spleen or lymph node cells. Five to 6 days later, cells were harvested and tested for CTL activity against MTA− and MTA+ concanavalin A (Con A)-stimulated blasts in a 3-hr JAM cytotoxicity assay.32 Con A blasts were produced by culturing spleen or lymph node cells with Con A (2·5–10 µg/ml) for 24 hr and then pulsing overnight with [3H]TdR. Cells were washed, centrifuged over Histopaque to remove dead cells and plated at 104 cells/well in 96-well plates. Effector cells were added in graded doses and, after 3 hr, cells were harvested and residual radioactivity measured as described above. The percentage specific cytotoxicity was calculated as follows:
where E = counts per minute (c.p.m.) retained in the presence of effector cells and S = c.p.m. retained in the absence of effector cells.
Results
BMDC comprise FcR+ and FcR− subpopulations and are not Mφs
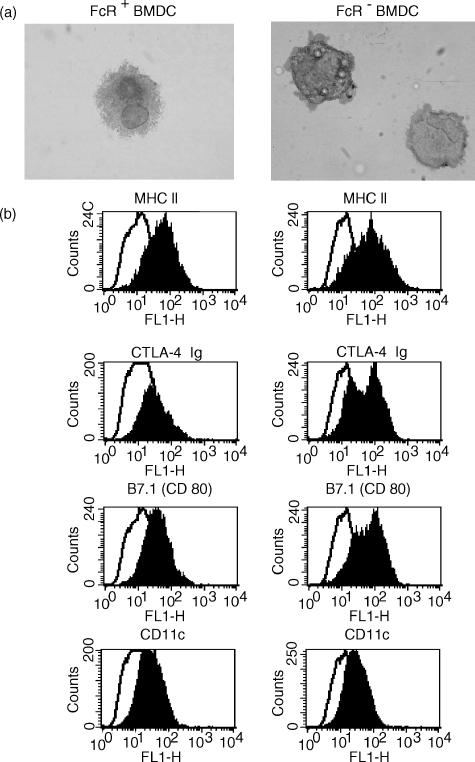
Bone marrow cells were cultured for 7 days in the presence of GM–CSF and IL-4, and the resulting cells (BMDC) were examined by immunoperoxidase labelling on cytospins or by flow cytometry. Most BMDC displayed typical DC morphology and expressed MHC class II, intracellular adhesion molecule-1 (ICAM-1), B7 and CD80 molecules (Fig. 1). BMDC did not express the rat lymph DC marker OX62 (data not shown, also reported in ref. 12). In contrast to lymph DC, most BMDC expressed OX41 but not CD4 (data not shown). A subpopulation of LDC have been shown to express both of these markers.17 Flow cytometry showed that the BMDC expressed heterogeneous levels of MHC class II molecules, suggesting different maturation stages.
Figure 1.
Phenotype of bone marrow-derived dendritic cells (BMDC) separated into FcR+ and FcR− populations and labelled by (a) immunocytochemistry [labelling of anti-major histocompatibility complex (MHC) class II; magnification × 25] and (b) flow cytometry. BMDC were cultured from lymphocyte/MHC class II-depleted bone marrow cells. After 7 days, cells were harvested and separated into FcR+ and FcR− subpopulations by rosetting. Cells were labelled for MHC class II, B7 (CTLA-4 Ig), B7.1 (CD80) or CD11c molecules (filled histograms) or irrelevant antibody (open histograms).
As immature DC may express Fc receptors,33 BMDC were separated by Fc rosetting using opsonized SRBC (no mAb to rat FcR is available). Two populations were recovered: after 7 days of culture about 75% of BMDC were FcR+, but by 14 days, this proportion had dropped to 35–40%. Flow cytometry showed that FcR− BMDC and FcR+ BMDC expressed similar levels of MHC class II and CD11c. FcR+ BMDC expressed low levels of B7 (visualized by CTLA-4 fusion protein and anti-CD80),26 but expression of B7 by FcR− BMDC was biphasic, with a proportion expressing relatively high levels (Fig. 1b). Although expression of MHC class II was heterogeneous, on cytospins it appeared that in FcR+ BMDC, a larger proportion of MHC class II was cytoplasmic (Fig. 1a, and data not shown).
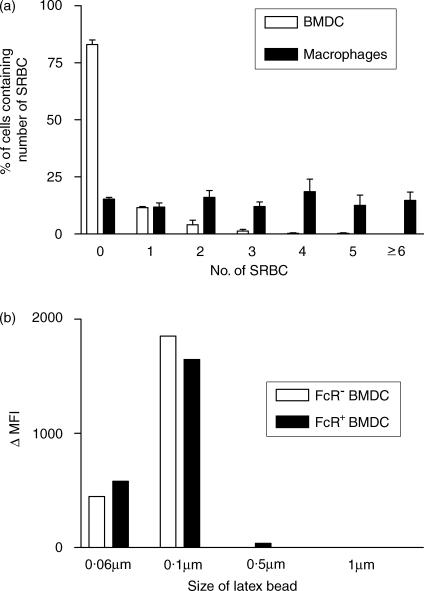
To show that the FcR+ cells were not Mφs, phagocytosis of opsonized SRBC and endocytosis of latex beads were assessed (Fig. 2a). Although 20–30% of FcR+ BMDC phagocytosed SRBC, positive cells contained only one or two SRBC (Fig. 2a). In contrast, thioglycollate-elicited peritoneal Mφs (Thio-Mφs) phagocytosed much larger numbers of SRBC. Both FcR+ and FcR− BMDC were only able to endocytose small beads of ≤ 0·1 µm in diameter (Fig. 2b). We have previously found that peritoneal Mφs endocytose large numbers of particles of a range of sizes tested. In addition, FcR+ BMDC did not adhere to plastic.
Figure 2.
Phagocytosis and endocytosis by bone marrow-derived dendritic cells (BMDC) and macrophages. BMDC were harvested after 7 days of culture and used either as whole cells or as subpopulations separated by FcR rosetting. (a) BMDC or thioglycollate-elicited peritoneal macrophages (Mφs) were cultured with opsonized sheep red blood cells (SRBC), non-phagocytosed SRBC were lysed and cytospins were prepared and labelled with OX6 [anti-major histocompatibility complex (MHC) class II] or OX42 (anti-CD11b/c). The numbers of SRBC within 200 cells were counted. (b) FcR− and FcR+ BMDC were cultured with 0·06–1 µm fluorescein isothiocyanate (FITC)-labelled latex beads for 30 min at 37° or 4° and, after washing, the mean fluorescence intensity (MFI) was measured by flow cytometry. Numbers represent the difference between the MFI at 4° and 37°. The data shown represent the results from a representative experiment repeated four times.
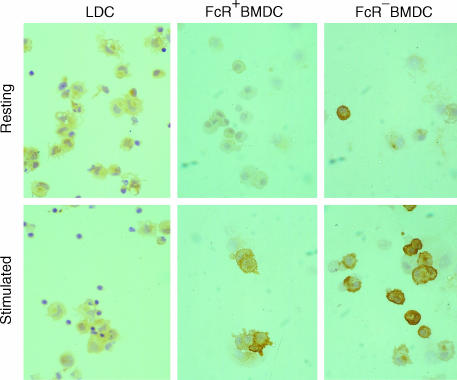
BMDC, but not LDC, express iNOS
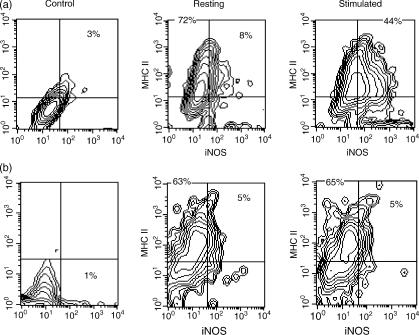
BMDC harvested after 6 days were separated into FcR+ and FcR− populations and cultured overnight with or without IFN-γ and LPS. Expression of iNOS by BMDC was examined by immunocytochemistry, and by flow cytometry following permeabilization. Both FcR− and FcR+ BMDC expressed iNOS when cultured overnight with IFN-γ and LPS (Fig. 3, stimulated), but not when cultured with media alone (Fig. 3, resting). In contrast, LDC cultured in an identical manner, with or without IFN-γ and LPS, did not express detectable iNOS (Fig. 3, resting and stimulated LDC). The expression of iNOS by BMDC was found to vary, with 30–60% of cells being positive in different experiments. iNOS-positive cells displayed typical DC morphology. Flow cytometry of BMDC cultured with IFN-γ and LPS showed that 60% of BMDC co-expressed iNOS and MHC class II molecules (Fig. 4a) and that iNOS-positive BMDC also expressed the typical DC marker, CD11c (data not shown). LDC cultured under the same conditions did not express detectable iNOS (Fig. 4b). To confirm the specificity of the anti-iNOS mAbs, BMDC were cultured with or without IFN-γ and LPS, and cell lysates were immunoblotted using ANOS5 and ANOS10 mAbs. Both mAbs recognized a band migrating at 150 kDa under reducing conditions (the expected molecular mass of iNOS23) in lysates from stimulated, but not resting, BMDC (data not shown).
Figure 3.
Immunocytochemical detection of inducible nitric oxide synthase (iNOS) expression by dendritic cells. FcR+ and FcR− bone marrow-derived dendritic cells (BMDC) (harvested on day 7), and fresh pseudo-afferent lymph DC (LDC), were cultured overnight with interferon-γ (IFN-γ) and lipopolysaccharide (LPS) (stimulated), or medium alone (resting), and cytospins were labelled with anti-iNOS mAbs (ANOS 5 and 11). Fresh DC show little, if any, reactivity. Cultured BMDC, but not LDC, show conspicuous up-regulation of expression. The data shown represent the results from a representative experiment repeated at least four times. Magnification: × 25.
Figure 4.
Flow cytometric analysis of inducible nitric oxide synthase (iNOS) expression by DC. Bone marrow-derived dendritic cells (BMDC) (a) and pseudo-afferent lymph DC (LDC) (b), prepared as described in the Materials and methods, were cultured overnight in the presence (stimulated) or absence (resting) of interferon-γ (IFN-γ) and lipopolysaccharide (LPS). Cells were permeabilized and then labelled with anti-iNOS monoclonal antibodies (mAbs) and rabbit anti-mouse-conjugated fluorescein isothiocyanate (FITC), followed by biotin-conjugated OX6 [major histocompatibility complex (MHC) class II] and streptavidin-Quantum Red. Resting cells show little, if any, iNOS reactivity. After culture with IFN-γ and LPS, MHC class II+ BMDC, but not LDC show marked up-regulation of expression.
BMDC, but not LDC or SDC, secrete NO in a regulated manner
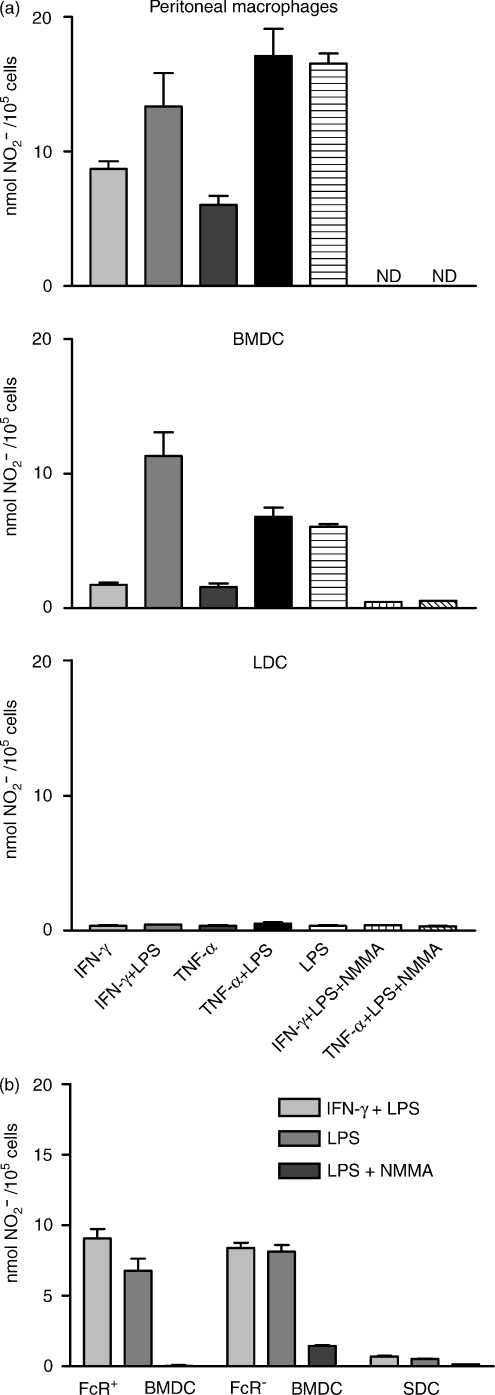
To show that iNOS expression correlated with NO secretion, BMDC were cultured for 48 hr with different stimuli, in parallel with peritoneal Mφs, LDC and SDC. NO secretion was assessed by nitrite accumulation in the supernatant (Fig. 5). Mφs and BMDC secreted similar amounts of NO, whereas LDC and SDC did not secrete detectable NO. NO secretion was completely inhibited by NMMA (Mφ data not shown). Separated FcR− and FcR+ BMDC were cultured for 48 hr with IFN-γ and LPS, and nitrite production was measured. SDC were cultured in an identical manner. Both FcR− and FcR+ BMDC secreted similar amounts of NO, whereas SDC did not secrete detectable NO (Fig. 5b).
Figure 5.
Release of nitric oxide (NO) by DC and macrophages (Mφs). Bone marrow-derived dendritic cells (BMDC) (harvested on day 7), pseudo-afferent lymph DC (LDC), spleen DC (SDC) and peritoneal Mφs were cultured for 48 hr in the presence of interferon-γ (IFN-γ) and lipopolysaccharide (LPS), or with medium alone. NO release was assessed (using the Greiss reagent) as nitrite accumulation in the supernatants. BMDC and Mφs, but not LDC or SDC, show considerable NG-monomethyl l-Arginine (NMMA)-inhibitable nitrite accumulation. (a) Peritoneal Mφs, BMDC, and LDC. (b) FcR+ BMDC, FcR− BMDC, and SDC. The data shown represent the results from a representative experiment repeated at least three times. ND, not done in a particular experiment. NMMA reproducibly inhibited NO release by Mφs in other experiments.
Therefore, both FcR+ and FcR− BMDC, as well as peritoneal Mφs, can be stimulated to secrete similar amounts of NO, but LDC and SDC are unable to secrete detectable NO.
BMDC are weak stimulators of an allogeneic MLR
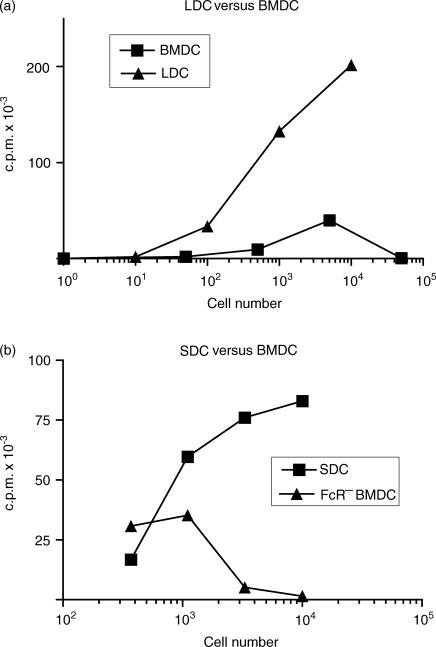
BMDC harvested at 7 days were compared with SDC and LDC as stimulators in an allogeneic MLR (Fig. 6). Consistently, LDC and SDC were almost always considerably more potent than BMDC. However, small numbers of BMDC show relatively efficient stimulation, whereas when larger numbers are used (>5 × 103), thymidine incorporation was reduced. Direct cell counting showed that this reduction reflects decreased cell proliferation (data not shown). In contrast, SDC and LDC do not show such inhibition, even when 2 × 105 LDC are used as stimulators. These differences are not explained by kinetics of the MLR, as proliferation stimulated by all DC peaked at 3 days (data not shown).
Figure 6.
Allogeneic mixed lymphocyte reaction (MLR) stimulation by ex vivo DC and bone marrow-derived dendritic cells (BMDC). Graded numbers of irradiated BMDC (harvested on day 7), pseudo-afferent lymph DC (LDC) and spleen DC (SDC) were added to 2 × 105 allogeneic CLN cells. Three days later, [3H]thymidine ([3H]TdR) was added for 16 hr, following which the cells were harvested and [3H]TdR measured by scintillation counting. (a) Comparison of LDC and BMDC. (b) SDC and FcR− BMDC. BMDC are weaker stimulators of an MLR than LDC and SDC, and when larger numbers of BMDC are used, [3H]TdR incorporation is progressively reduced. c.p.m., counts per minute.
As the weak stimulatory ability of BMDC might reflect immaturity, we attempted to mature the DC by replating and culturing them overnight,12 with or without LPS and/or IFN-γ. BMDC treated as such remained weak stimulators at low numbers in comparison with SDC and still inhibited proliferation at high numbers (data not shown).
FcR+ BMDC do not stimulate a significant MLR and inhibit stimulation by other DC
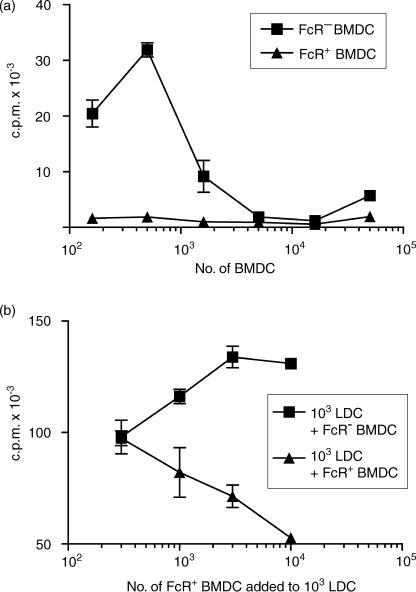
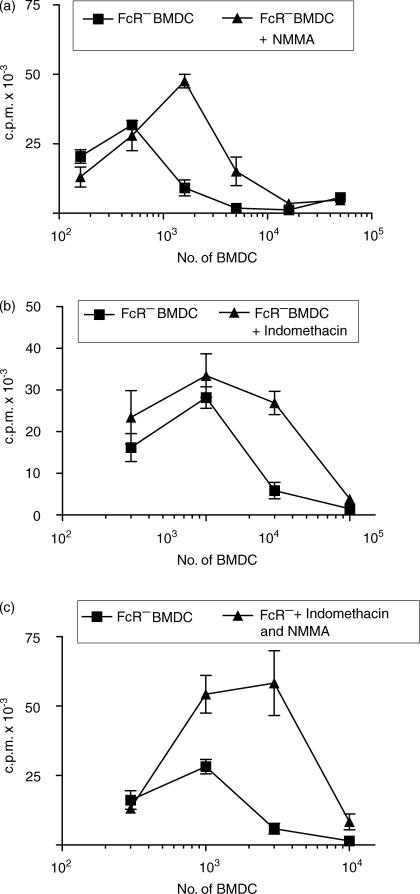
BMDC harvested at 7 days were separated by Fc rosetting and used to stimulate an allogeneic MLR with either CLN cells or purified CD4+ T cells as responders. In all assays the FcR− BMDC were far more potent than FcR+ BMDC (Fig. 7a), but purified FcR− BMDC still inhibited T-cell proliferation at high DC : T-cell ratios (Fig. 7a). Direct counting of cells showed that the reduced thymidine incorporation reflects decreased proliferation and the culture media does not become exhausted (data not shown). Examination of cultures showed that there was little or no clustering of FcR+ BMDC and lymphocytes in comparison with FcR− BMDC, LDC or SDC (not shown). When graded numbers of FcR+ BMDC were added to a constant number of LDC in the MLR, dose-dependent inhibition of proliferation was seen (Fig. 7b). A similar experiment, using a fixed number of LDC and adding graded numbers of FcR− BMDC, resulted in enhanced proliferation (Fig. 7b). Inhibition of proliferation at high numbers might be the result of NO or prostaglandin secretion.34,35 Both NMMA, an inhibitor of iNOS, and indomethacin, when added to a BMDC-stimulated MLR, partially reduced the dose-dependent inhibition (Fig. 8a,8b). A combination of the NMMA and prostaglandin did not remove inhibition (Fig. 8c). Addition of NMMA and/or indomethacin did not result in FcR+ BMDC stimulating significant proliferation (data not shown). We conclude that the inhibition of T-cell proliferation by high numbers of FcR− BMDC is partly caused by NO and prostaglandin secretion, but that other, as-yet-unidentified, factors are also involved.
Figure 7.
Allogeneic mixed lymphocyte reaction (MLR) stimulation by FcR+ and FcR− bone marrow-derived dendritic cells (BMDC). (a) Graded doses of irradiated FcR− and FcR+ BMDC were added to 2 × 105 allogeneic CLN cells and cultured for 96 hr. [3H]Thymidine ([3H]TdR) was added for the final 16 hr. (b) Graded doses of FcR− BMDC and FcR+ BMDC were added to a fixed number (103) of pseudo-afferent lymph DC (LDC) and cultured with allogeneic CLN cells, as described in the Materials and methods. c.p.m., counts per minute.
Figure 8.
Partial abrogation of mixed lymphocyte reaction (MLR) inhibitory effects of bone marrow-derived dendritic cells (BMDC) by NG-monomethyl l-Arginine (NMMA) and indomethacin. Graded doses of irradiated FcR− BMDC were added to 2 × 105 allogeneic CLN cells and cultured for 96 hr, with or without the addition of (a) NMMA, (b) indomethacin or (c) NMMA+indomethacin. Proliferation was measured as described in the Materials and methods. The data shown represent the results from representative experiments repeated at least four times. c.p.m., counts per minute.
BMDC can present Ag to T cells in vitro and in vivo
BMDC and SDC were compared as APC for naive T cells in vivo. BMDC were harvested at 7 days, separated into FcR+ and FcR− populations, pulsed with KLH and injected into the footpads of syngeneic naïve rats. Freshly isolated SDC were treated similarly as a positive control. Ten days later, rats were killed and popliteal lymph node cells incubated with KLH, HSA or no Ag, and proliferation measured. SDC, FcR+ and FcR− BMDC are all able to prime T cells to KLH (Fig. 9a). SDC and both subpopulations of BMDC are able to prime naïve T cells to comparable levels. To show that the effects of BMDC were not the result of cross-priming (host processing), parental strain (DA) DC were used to prime (LEW × DA) F1 recipients. Purified popliteal node T cells were then restimulated with KLH-pulsed SDC from LEW or DA donors. Only DC from DA donors stimulated significant proliferation, showing that priming was restricted to DA MHC and must have occurred by direct presentation of Ag by DC (data not shown). The high background for FcR− BMDC in Fig. 9(a), was not seen in other experiments (data not shown).
Ag presentation by DC in vitro to primed CD4+ T cells was assessed by restimulation of T cells primed to KLH in vivo 2 weeks previously. Both FcR− and FcR+ BMDC were able to stimulate primed T cells, but FcR− BMDC were again much more potent than FcR+ BMDC (Fig. 9b). Interestingly, in this assay SDC were less potent than FcR− BMDC when small numbers were used, but were more potent when larger numbers were used. FcR− BMDC inhibited proliferation when large numbers were used. FcR+ BMDC were less potent than FcR− BMDC, but similar to SDC when small numbers were used.
SDC, but not BMDC, can prime CTL to minor histocompatibility Ags
In view of the discrepancy between the ability of BMDC to stimulate an MLR and to activate T cells to KLH, we examined the ability of BMDC to prime CTL to a mitochondria-associated minor histocompatibility antigen (miHA) MTA.31 Injection of MTA− rats with MTA+ SDC stimulates CTL which are able to kill MTA+ Con A blast targets, but not MTA− targets. When 2 × 106 SDC or 4 × 107 spleen cells were used to prime this CTL response, significant killing was observed, yet when BMDC were used under identical conditions, no priming of CTL was seen (Fig. 9c).
Discussion
DC have central roles in the induction and regulation (both positive and negative) of specific immune responses.1,36 In keeping with the complexity of their roles, it is apparent that at least two distinct lineages of DC exist in vivo, and that within these lineages DC may exist in different stages of maturation or differentiation, expressing distinct functional and phenotypic characteristics. To help understand DC properties and life histories, systems have been developed for generating DC in vitro, including growth from BM precursors.8,12 While BMDC show many functional and phenotypic similarities to DC extracted from animals,8 our preliminary studies in the rat suggested that BMDC showed significant differences from ex vivo DC. Because we felt that it was not clear how BMDC relate to any population of DC identified in vivo, we performed a detailed comparison of BMDC with DC extracted from spleen or intestinal pseudo-afferent lymph.5,30
BMDC express some, but not all, markers expressed by ex vivo DC (e.g. BMDC express OX41, but not OX62). BMDC, identified by morphology and MHC class II expression, are themselves functionally heterogeneous. A proportion of BMDC bound, but did not internalize, IgG-coated SRBC and we were thus able to separate BMDC using FcR expression. Surprisingly, there were few differences in surface marker expression between the two populations; in particular, levels of surface MHC class II were similar. FcR+ cells did, however, express low levels of B7 whereas some FcR− BMDC expressed high levels. LDC, in contrast to BMDC, express higher levels of B7.17 In contrast to Mφs, neither BMDC population was able to phagocytose significant numbers of opsonized SRBC. Both populations were only able to endocytose small (0·1 µm) latex beads. Although it has not been formally proven that FcR+ BMDC are precursors of FcR− BMDC, this is probably the case because when cultured, FcR+ BMDC give rise to populations containing both FcR+ and FcR− DC (T. J. Powell & G. G. MacPherson, unpublished). Two subpopulations of lung DC have been identified that differ in Fc receptor expression and function with respect to stimulation of naïve and memory T cells.37
DC can exist at different stages of maturation and, as well as expression of FcR (characteristic of some immature DC),33 expression of MHC class II and B7 suggests that most FcR+ BMDC, and some FcR− cells, are immature. In contrast to another report,12 a variety of potential maturation signals (replating, TNF-α, LPS) did not lead to up-regulation of surface MHC class II expression or B7, neither did they increase the ability of BMDC to stimulate an allogeneic MLR in comparison with SDC or LDC.
BMDC also differ markedly from ex vivo DC in that BMDC, but not ex vivo DC, can be stimulated to express iNOS and secrete NO. Secretion of NO by DC is controversial. Murine BMDC have been shown to express iNOS and secrete NO when stimulated with IFN-γ and LPS.34,35 We found that rat BMDC behaved similarly, and that the amounts of NO they secreted were comparable to those secreted by peritoneal Mφs. Interestingly, both FcR+ and FcR− BMDC expressed similar levels of iNOS and secreted similar amounts of NO. BMDC that expressed iNOS also expressed the characteristic DC marker, CD11c. A recent report has found that FLT3 ligand increases yields of rat BMDC, but comparisons with SDC were not attempted38 and there is no evidence that FLT3 ligand affects NO secretion by BMDC. Rat GM–CSF was not available to us when we carried out these studies.
The secretion of NO by ex vivo DC appears to be very variable. Langerhans' cells have been stimulated to express39 or not express40 iNOS/NO, and thymic DC appear to express iNOS constitutively.41 DC obtained from rats recovering from experimental allergic encephalomyelitis can secrete NO,42 while freshly isolated human blood DC express iNOS and can secrete NO.43 In contrast, we could not detect significant iNOS expression or NO secretion by freshly isolated SDC or LDC, or after their culture with LPS and IFN-γ. These in vivo and ex vivo differences may reflect different lineages or activation states of DC. Apart from the special case of the thymus, those DC in or isolated from solid tissues, which can secrete NO, are associated with inflammation, whereas DC isolated in the absence of inflammation cannot secrete NO. There is a rapid influx of DC into inflamed tissues in the rat21,44 and these ‘inflammatory’ DC may be able to secrete NO. It may also be relevant that it has been shown recently that CD2+ monocytes are DC precursors;45 these may represent the source of ‘inflammatory’ DC.
BMDC have always been considered to be a paradigm for ‘in vivo’ DC and direct comparison has indicated that MLR stimulation by BMDC and spleen DC are similar.8,12 However, we found that rat BMDC differ markedly from SDC and LDC as APC. BMDC are almost always relatively weak stimulators of an allogeneic MLR compared with SDC or LDC. In addition, the two subpopulations of BMDC differed markedly as stimulators. Whole BMDC inhibited T-cell proliferation when large numbers were used, and separation of FcR+ and FcR− cells showed that despite expressing MHC class II and low levels of B7, the FcR+ DC could barely stimulate an MLR at all, and inhibited stimulation by FcR− BMDC and SDC or LDC. This was unlikely to be caused by the presence of Mφs in the FcR+ population as it did not contain cells capable of significant FcR-mediated phagocytosis or of adhesion to plastic. In contrast, FcR− BMDC stimulated a moderate MLR and did not inhibit stimulation by other DC populations. The molecular basis of inhibition is partially explained by NO and prostaglandin secretion by BMDC. Murine BMDC can be stimulated to secrete NO, which inhibits an allogeneic MLR,34,35 but blocking NO secretion by NMMA only partially restored MLR stimulation by rat BMDC. Similarly indomethacin, a prostaglandin inhibitor, had only a minimal effect and the two agents together did not remove inhibition. When BMDC were tested as APC in vitro to primed T cells, FcR− BMDC performed as well as or better than SDC in their ability to stimulate the proliferation of T cells. FcR+ BMDC were weaker stimulators of primed T cells compared with FcR− BMDC, but showed similar amounts of proliferation to SDC at lower cell numbers. However, there was still inhibition at high numbers of BMDC that was not present with SDC. It is generally accepted that only DC can activate naïve T cells, whereas activated T cells can be stimulated by many MHC class II+ cells. FcR+ DC cannot activate naïve T cells in vitro although they do indeed stimulate naïve T cells in vivo. Overall, we show that FcR+ BMDC are generally worse than other DC at stimulating T cells.
In contrast to these in vitro results, when BMDC were used to prime naïve T cells in vivo, both subpopulations were able to generate T cells that proliferated strongly in a recall response. This could have been a result of the phenomenon of cross-priming.46 When parental strain A BMDCs were used to prime (A × B) F1 recipients, the sensitized T cells only responded significantly to Ag presented by parental strain A DC, showing that sensitization was by direct presentation by the injected DC and not a result of cross-priming. The most probable interpretation of these data is that FcR+ DC received maturation signals in vivo that we have not been able to identify or reproduce in vitro. However, recent reports that MHC class II molecules may be transferred intact between APC47–49 bring into question the validity of this approach to identifying cross-priming. In addition, rat monocyte-derived DC have been shown to home to peripheral lymph nodes.50
In contrast to the priming of CD4+ T cells described above, neither population of BMDC was able to stimulate CTL to a minor histocompatibility antigen. The reasons for this are unclear but may reflect low levels of the mitochondrial peptide expressed by the BMDC.
In conclusion, rat BMDC, grown in IL-4 and GM–CSF, express some (but not all) surface markers, similar to those of ex vivo SDC and LDC, but differ markedly in that BMDC are weak APC and can secrete NO. At present it is not possible to determine how BMDC relate to any DC populations identified in vivo, but we suggest that they may be equivalent to monocyte-derived DC and/or the DC that accumulate at inflammatory sites. These results illustrate the complexity of DC subpopulations and the need for caution when extrapolating results generated in vitro with BMDC to concepts in vivo.
Acknowledgments
We thank Don Mason and other members of the MRC Cellular Immunology Unit for kind gifts of reagents. We also wish to thank Michelle Wykes, Fang-Ping Huang, James Major and Jim Kaufman for many discussions and Launce Tomlinson for photography. This work was supported by the Medical Research Council, UK, and the Edward Abraham Research Trust.
Abbreviations
- BMDC
bone marrow-derived DC
- CLN
cervical lymph node
- DC
dendritic cell
- LDC
pseudo-afferent lymph DC
- iNOS
inducible nitric oxide synthase
- SDC
spleen DC
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Steinman RM, Witmer MD. Lymphoid dendritic cells are potent stimulators of the primary mixed leukocyte reaction in mice. Proc Natl Acad Sci USA. 1978;75:5132–6. doi: 10.1073/pnas.75.10.5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reis-e-Sousa C, Stahl PD, Austyn JM. Phagocytosis of antigens by Langerhans' cells in vitro. J Exp Med. 1993;178:509–19. doi: 10.1084/jem.178.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu LM, MacPherson GG. Rat intestinal dendritic cells: immunostimulatory potency and phenotypic characterization. Immunology. 1995;85:88–93. [PMC free article] [PubMed] [Google Scholar]
- 5.Pugh CW, MacPherson GG, Steer HW. Characterization of nonlymphoid cells derived from rat peripheral lymph. J Exp Med. 1983;157:1758–79. doi: 10.1084/jem.157.6.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howard CJ, Sopp P, Brownlie J, Kwong LS, Parsons KR, Taylor G. Identification of two distinct populations of dendritic cells in afferent lymph that vary in their ability to stimulate T cells. J Immunol. 1997;159:5372–82. [PubMed] [Google Scholar]
- 7.Bujdoso R, Hopkins J, Dutia BM, Young P, McConnell I. Characterization of sheep afferent lymph dendritic cells and their role in antigen carriage. J Exp Med. 1989;170:1285–301. doi: 10.1084/jem.170.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santiago Schwarz F, Belilos E, Diamond B, Carsons SE. TNF in combination with GM–CSF enhances the differentiation of neonatal cord blood stem cells into dendritic cells and macrophages. J Leukoc Biol. 1992;52:274–81. [PubMed] [Google Scholar]
- 10.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–18. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Young JW, Szabolcs P, Moore MA. Identification of dendritic cell colony-forming units among normal human CD34+ bone marrow progenitors that are expanded by c-kit-ligand and yield pure dendritic cell colonies in the presence of granulocyte/macrophage colony-stimulating factor and tumor necrosis factor alpha. J Exp Med. 1995;182:1111–9. doi: 10.1084/jem.182.4.1111. [published erratum appears in J Exp Med 1996 March 1; 183 (3): 1283]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Talmor M, Mirza A, Turley S, Mellman I, Hoffman LA, Steinman RM. Generation of large numbers of immature and mature dendritic cells from rat bone marrow cultures. Eur J Immunol. 1998;28:811–7. doi: 10.1002/(SICI)1521-4141(199803)28:03<811::AID-IMMU811>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 13.Ardavin C, Wu L, Li CL, Shortman K. Thymic dendritic cells and T cells develop simultaneously in the thymus from a common precursor population. Nature. 1993;362:761–3. doi: 10.1038/362761a0. [DOI] [PubMed] [Google Scholar]
- 14.Traver D, Akashi K, Manz M, Merad M, Miyamoto T, Engleman EG, Weissman IL. Development of CD8alpha-positive dendritic cells from a common myeloid progenitor. Science. 2000;290:2152–4. doi: 10.1126/science.290.5499.2152. [DOI] [PubMed] [Google Scholar]
- 15.Caux C, Vanbervliet B, Massacrier C, et al. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to GM–CSF+TNF alpha. J Exp Med. 1996;184:695–706. doi: 10.1084/jem.184.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cella M, Sallusto F, Lanzavecchia A. Origin, maturation and antigen presenting function of dendritic cells. Curr Opin Immunol. 1997;9:10–6. doi: 10.1016/s0952-7915(97)80153-7. [DOI] [PubMed] [Google Scholar]
- 17.Liu LM, Zhang M, Jenkins C, MacPherson GG. Dendritic cell heterogeneity in vivo. Two functionally different dendritic cell populations in rat intestinal lymph can be distinguished by CD4 expression. J Immunol. 1998;161:1146–55. [PubMed] [Google Scholar]
- 18.Huang FP, Platt N, Wykes M, Major JR, Powell TJ, Jenkins CD, MacPherson GG. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J Exp Med. 2000;191:435–44. doi: 10.1084/jem.191.3.435. [see comments]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cumberbatch M, Kimber I. Dermal tumour necrosis factor-alpha induces dendritic cell migration to draining lymph nodes, and possibly provides one stimulus for Langerhans' cell migration. Immunology. 1992;75:257–63. [PMC free article] [PubMed] [Google Scholar]
- 20.Roake JA, Rao AS, Morris PJ, Larsen CP, Hankins DF, Austyn JM. Dendritic cell loss from nonlymphoid tissues after systemic administration of lipopolysaccharide, tumor necrosis factor, and interleukin 1. J Exp Med. 1995;181:2237–47. doi: 10.1084/jem.181.6.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McWilliam AS, Napoli S, Marsh AM, et al. Dendritic cells are recruited into the airway epithelium during the inflammatory response to a broad spectrum of stimuli. J Exp Med. 1996;184:2429–32. doi: 10.1084/jem.184.6.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garrigan K, Moroni Rawson P, McMurray C, Hermans I, Abernethy N, Watson J, Ronchese F. Functional comparison of spleen dendritic cells and dendritic cells cultured in vitro from bone marrow precursors. Blood. 1996;88:3508–12. [PubMed] [Google Scholar]
- 23.Sato K, Miyakawa K, Takeya M, et al. Immunohistochemical expression of inducible nitric oxide synthase (iNOS) in reversible endotoxic shock studied by a novel monoclonal antibody against rat iNOS. J Leukoc Biol. 1995;57:36–44. [PubMed] [Google Scholar]
- 24.Brenan M, Puklavec M. The MRC OX-62 antigen: a useful marker in the purification of rat veiled cells with the biochemical properties of an integrin. J Exp Med. 1992;175:1457–65. doi: 10.1084/jem.175.6.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brenan M, Rees DJ. Sequence analysis of rat integrin alpha E1 and alpha E2 subunits: tissue expression reveals phenotypic similarities between intraepithelial lymphocytes and dendritic cells in lymph. Eur J Immunol. 1997;27:3070–9. doi: 10.1002/eji.1830271145. [DOI] [PubMed] [Google Scholar]
- 26.Maeda K, Sato T, Azuma M, Yagita H, Okumura K. Characterization of rat CD80 and CD86 by molecular cloning and mAb. Int Immunol. 1997;9:993–1000. doi: 10.1093/intimm/9.7.993. [DOI] [PubMed] [Google Scholar]
- 27.Powell TJ, Major JR, MacPherson GG. Generation of dendritic cells from rat bone marrow. In: Robinson SP, Stagg AJ, editors. Methods in Molecular Medicine. Vol. 64. Totowa: Humana Press; 2001. pp. 199–205. Dendritic Cell Protocols. [DOI] [PubMed] [Google Scholar]
- 28.Mason DW, Penhale WJ, Sedgwick JD. Preparation of lymphocyte subpopulations. In: Klaus GGB, editor. LymphocytesA Practical Approach. Oxford: IRL Press; 1987. pp. 35–54. [Google Scholar]
- 29.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973;137:1142–62. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wykes M, Pombo A, Jenkins C, MacPherson GG. Dendritic cells interact directly with naive B-lymphocytes to transfer antigen and intiate class-switching in a primary T-dependent response. J Immunol. 1998;161:1313–9. [PubMed] [Google Scholar]
- 31.Bhuyan PK, Young LL, Lindahl KF, Butcher GW. Identification of the rat maternally transmitted minor histocompatibility antigen. J Immunol. 1997;158:3753–60. [PubMed] [Google Scholar]
- 32.Matzinger P. The JAM test. A simple assay for DNA fragmentation and cell death. J Immunol Methods. 1991;145:185–92. doi: 10.1016/0022-1759(91)90325-a. [DOI] [PubMed] [Google Scholar]
- 33.Schuler G, Steinman RM. Murine epidermal Langerhans' cells mature into potent immunostimulatory dendritic cells in vitro. J Exp Med. 1985;161:526–46. doi: 10.1084/jem.161.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonham CA, Lu L, Li Y, Hoffman RA, Simmons RL, Thomson AW. Nitric oxide production by mouse bone marrow-derived dendritic cells: implications for the regulation of allogeneic T cell responses. Transplantation. 1996;62:1871–7. doi: 10.1097/00007890-199612270-00033. [DOI] [PubMed] [Google Scholar]
- 35.Lu L, Bonham CA, Chambers FG, Watkins SC, Hoffman RA, Simmons RL, Thomson AW. Induction of nitric oxide synthase in mouse dendritic cells by IFN-gamma, endotoxin, and interaction with allogeneic T cells: nitric oxide production is associated with dendritic cell apoptosis. J Immunol. 1996;157:3577–86. [PubMed] [Google Scholar]
- 36.Hart DN. Dendritic cells: unique leukocyte populations which control the primary immune response. Blood. 1997;90:3245–87. [PubMed] [Google Scholar]
- 37.Kradin RL, Xia W, McCarthy K, Schneeberger EE. FcR+/– subsets of Ia+ pulmonary dendritic cells in the rat display differences in their abilities to provide accessory co-stimulation for naive (OX-22+) and sensitized (OX-22–) T cells. Am J Pathol. 1993;142:811–9. [PMC free article] [PubMed] [Google Scholar]
- 38.Brissette-Storkus CS, Kettel JC, Whitham TF, Giezeman-Smits KM, Villa LA, Potter DM, Chambers WH. Flt-3 ligand (FL) drives differentiation of rat bone marrow-derived dendritic cells expressing OX62 and/or CD161 (NKR-P1) J Leukoc Biol. 2002;71:941–9. [PubMed] [Google Scholar]
- 39.Qureshi AA, Hosoi J, Xu S, Takashima A, Granstein RD, Lerner EA. Langerhans' cells express inducible nitric oxide synthase and produce nitric oxide. J Invest Dermatol. 1996;107:815–21. doi: 10.1111/1523-1747.ep12330572. [DOI] [PubMed] [Google Scholar]
- 40.Blank C, Bogdan C, Bauer C, Erb K, Moll H. Murine epidermal Langerhans' cells do not express inducible nitric oxide synthase. Eur J Immunol. 1996;26:792–6. doi: 10.1002/eji.1830260410. [DOI] [PubMed] [Google Scholar]
- 41.Aiello S, Noris M, Piccinini G, et al. Thymic dendritic cells express inducible nitric oxide synthase and generate nitric oxide in response to self- and alloantigens. J Immunol. 2000;164:4649–58. doi: 10.4049/jimmunol.164.9.4649. [DOI] [PubMed] [Google Scholar]
- 42.Xiao BG, Hy M, Xl Y, Ishikawa M, Link H. Mechanisms of recovery from experimental allergic encephalomyelitis induced with myelin basic protein peptide 68–86 in Lewis rats: a role for dendritic cells in inducing apoptosis in CD4+ T cells. J Neuroimmunol. 1999;97:25–36. doi: 10.1016/s0165-5728(99)00041-7. [DOI] [PubMed] [Google Scholar]
- 43.Huang YM, Xb G, Westerlund I, Link H. Phenotypic and functional properties of dendritic cells isolated from human peripheral blood in comparison with mononuclear cells and T cells. Scand J Immunol. 1999;49:177–83. doi: 10.1046/j.1365-3083.1999.00491.x. [DOI] [PubMed] [Google Scholar]
- 44.McWilliam AS, Nelson D, Thomas JA, Holt PG. Rapid dendritic cell recruitment is a hallmark of the acute inflammatory response at mucosal surfaces. J Exp Med. 1994;179:1331–6. doi: 10.1084/jem.179.4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crawford K, Gabuzda D, Pantazopoulos V, Xu J, Clement C, Reinherz E, Alper CA. Circulating CD2+ monocytes are dendritic cells. J Immunol. 1999;163:5920–8. [PubMed] [Google Scholar]
- 46.Liu LM, MacPherson GG. Antigen acquisition by dendritic cells: intestinal dendritic cells acquire antigen administered orally and can prime naive T cells in vivo. J Exp Med. 1993;177:1299–307. doi: 10.1084/jem.177.5.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161–72. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arnold PY, Mannie MD. Vesicles bearing MHC class II molecules mediate transfer of antigen from antigen-presenting cells to CD4+ T cells. Eur J Immunol. 1999;29:1363–73. doi: 10.1002/(SICI)1521-4141(199904)29:04<1363::AID-IMMU1363>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 49.Bedford P, Garner K, Knight SC. MHC class II molecules transferred between allogeneic dendritic cells stimulate primary mixed leukocyte reactions. Int Immunol. 1999;11:1739–44. doi: 10.1093/intimm/11.11.1739. [DOI] [PubMed] [Google Scholar]
- 50.Richters CD, Mayen I, Havenith CE, Beelen RH, Kamperdijk EW. Rat monocyte-derived dendritic cells function and migrate in the same way as isolated tissue dendritic cells. J Leukoc Biol. 2002;71:582–7. [PubMed] [Google Scholar]