Abstract
ES-62 is a phosphorylcholine (PC)-containing glycoprotein secreted by filarial nematodes, parasites of vertebrates including humans. We have previously demonstrated that pre-exposure to this molecule in vitro interferes with subsequent B-cell receptor (BCR)-dependent activation of murine splenic B lymphocytes. To investigate the significance of this during filarial nematode infection, we now employ mice exposed to ES-62, at concentrations equivalent to those found for PC-containing molecules in the bloodstream of parasitized humans, via release from implanted osmotic pumps. Using this approach, we reveal that splenic and lymph node mononuclear cells, and also purified splenic B cells recovered from these mice have reduced ability ex vivo to proliferate in response to BCR ligation. The effect on BCR-induced proliferation was further investigated with respect to elucidating the mechanism of action of the parasite product and was shown to be associated with impaired signal transduction affecting the ErkMAPkinase pathway. Also, it was found that ES-62 did not act by promoting apoptosis or by priming for apoptosis following subsequent stimulation, but rather, appeared to render cells hyporesponsive to stimulation. ES-62 is thus shown for the first time to be a potent modulator of B lymphocyte function in vivo at a concentration relevant to natural filarial nematode infection. This finding considerably strengthens the idea that ES-62 plays a role in evasion of the immune response during parasitism.
Introduction
Filarial nematodes are arthropod-transmitted parasites of vertebrates including humans. The human parasites infect hundreds of millions of people in the tropics, causing diseases such as elephantiasis and river blindness.1 Although the nematodes are large extracellular parasites that contain multiple immunogens, infection with the worms is commonly lifelong.2 The current consensus of opinion is that the nematodes are directly involved in promoting this situation via molecular secretions that interact with and subvert the host immune system.3,4
An immunomodulatory component of filarial nematode secretions that has been investigated in great detail is ES-62, a phosphorylcholine (PC)-containing glycoprotein.5,6 First discovered in the rodent parasite Acanthocheilonema viteae, this molecule is likely to be secreted by all members of the order Filariata.7 ES-62 has been shown to have a surprisingly wide range of immunomodulatory properties and to target the activities of a number of different cells of the immune system.6,8,9 Particularly well studied have been its effects on B lymphocytes where exposure to ES-62 was shown to drastically inhibit the ability of the majority of these cells to respond to ligation via the B-cell receptor (BCR).8 This effect is a consequence of interaction between ES-62 and certain signal transduction pathways associated with proliferation10–12 and appears to be primarily caused by the parasite product's PC moieties.8,10–12
Although the effects of ES-62 on B lymphocytes would argue for a role in promoting parasite longevity, to date its activity against this particular cell type has been investigated only in vitro.8,10–12 A much stronger case for ES-62's importance could be put forward if the molecule could be shown to be active in vivo. This is the aim of the present investigation and toward this, we have made use of subcutaneously implanted osmotic pumps to release ES-62 at a constant rate in mice. In this way we hope to mirror the release that we assume occurs during natural infection but in the absence of any other worm components. Crucially, information is available on the amount of PC-containing molecules such as ES-62 that are present in the bloodstream during parasitism.13 As the amount of the molecule that can be released from the pump is controllable, it is thus possible to determine the effects of ES-62 in vivo in our model system at a concentration that is consistent with natural infection.
Materials and methods
Animals and parasites
BALB/c mice were bred at the Department of Immunology, University of Strathclyde. All mice used were 6–10-week-old-males and at least 20 g in weight. Animals were used in groups of three to five and serum/cell samples pooled for analyses. The life cycle of the rodent filarial nematode Acanthocheilonema viteae was maintained in the jird, Meriones libycus at the University of Strathclyde as described previously.8 All experiments using animals were undertaken with the permission of the University of Strathclyde Ethical Review Committee.
Preparation of ES-62
ES-62 was prepared from 500 ml spent culture medium (endotoxin-free RPMI-1640, Life Technologies, Paisley, UK with added endotoxin-free glutamine (2 mm), endotoxin-free penicillin (100 U/ml) and endotoxin-free streptomycin (100 mg/ml)) of adult A. viteae. In order to remove larval forms (microfilariae) released by the adult female worms the medium was passed through a 0·22-µm filter (Sigma, Poole, UK). It was then transferred to a stirred cell ultrafiltration unit containing a YM10 membrane (Amicon Ltd, Stonehouse, UK). After reducing the volume of the sample to 5–10 ml and transferring the holding medium to endotoxin-free phosphate-buffered saline (PBS), pH 7·2 (Cambrex Bioscience, Wokingham, UK), it was further concentrated to 200–500 µl using Centricon microconcentrators with a 30 000 MW cut-off membrane (Amicon). The sample was now applied to a 30 × 1 cm Superose 6 column (HR 10/30, Pharmacia, St. Albans, UK), fitted to an isocratic FPLC system (Pharmacia), previously equilibrated with endotoxin-free PBS, pH 7·2 at room temperature. The column was eluted at a flow rate of 0·5 ml/min and monitored for absorbance at 280 nm. More than 95% of the protein elutes as a single peak and this is purified ES-62. Purity and identity of each batch are confirmed by a combination of sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) and Western blotting, the latter employing a rabbit antiserum specific for ES-62. Finally, absence of endotoxin from the ES-62 sample is confirmed using an Endosafe Kit (Charles River Laboratories, Ramsgate, UK).
Priming of osmotic mini-pumps
Alzet Osmotic Mini-Pumps model 2002 (Charles River, Ramsgate, UK) were primed, according to manufacturer's instructions with 200 µl of either 0·1 mg/ml ES-62, 0·4 mg/ml ES-62, 0·4 mg/ml bovine serum albumin (BSA) or PBS, pH 7·4 as a control. The primed pumps were left overnight at room temperature submerged in sterile 0·9% saline solution. Pumps loaded with 0·4 mg/ml of ES-62 release antigen at a rate of ∼0·2 µg/hr. This is the equivalent of that released by two to five mature female worms in vitro.
Surgical implantation of osmotic mini-pumps
Mice were anaesthetized with Halothane-RM (Rhone Merieux Ltd, Dagenham, UK) in an O2/N2O mix. The back of the neck was swabbed with disinfectant (0·1% benzalkonium chloride) and the area shaved. A small incision, approximately 1·5 cm, was made into the skin and connective tissue was severed to create a pocket to insert the mini-pump. The pump was placed, according to manufacturer's instructions with the flow moderator inserted first. The wound was sutured and the animal observed until consciousness was regained. All animals were housed individually, and bled on days 0 and 14 from the superficial tail vein. Animals were killed on day 14 by a rising concentration of CO2.
Disassociation of immune complexes
Serum samples were added to an equal volume of 0·1 m glycine HCl buffer pH 2·4 and left at room temperature for 30 min Samples were then boiled for 5 min and centrifuged at 16 000 g for 15 min, to remove any aggregates formed during treatment. Samples were then returned to neutral pH with an equal volume of 0·5 m sodium phosphate, pH 7·2.
Biotinylation of the mouse myeloma protein TEPC 15
Affinity-purified TEPC 15 (Sigma, Poole, UK) was biotinylated using a BiotinTag™ Micro Biotinylation Kit (Sigma) according to the manufacturer's instructions.
Detection of PC using a one-site sandwich enzyme-linked immunosorbent assay (ELISA)
Flat-bottomed 96 well plates were coated overnight at 4° with PBS pH 9·0 containing 2 µg/ml of purified ascites TEPC 15 antibody (Sigma). Wells were emptied and blocked with 1% haemoglobin in PBS pH 7·4 for 30 min at room temperature. The plate was then washed three times with PBS containing 0·05% Tween-20. Serum samples were added in duplicate at a 1 : 20 dilution in PBS pH 7·4 and a standard curve was obtained using ES-62 starting at 0·5 µg/ml with doubling dilutions prepared in 1% normal mouse serum in PBS pH 7·4. Plates were incubated for 2 h at room temperature. The washing process was repeated and biotin labelled affinity purified TEPC 15 antibody at 2 µg/ml in PBS containing 0·1% haemoglobin was added. Incubation followed for 2 h at room temperature. Following a further wash, extravidin–peroxidase (Sigma) at 1 : 1000 in 0·1% haemoglobin was added and the plate left at room temperature for 1 hr. Washing was repeated and peroxidase substrate (o-phenylenediamine dihydrochloride, Sigma) was added and the plate left in the dark for 15 min after which absorbances were measured on a Titertek Multiskan plate reader at 405 nm.
Cell culture for measurement of DNA synthesis
Murine spleen and nodal mononuclear cells were harvested using routine tissue culture methods. B lymphocytes (>98% surface immunoglobulin positive) were purified from murine BALB/c spleens as described previously12 by negative selection using anti-CD43-coated magnetic beads according to the manufacturer's instructions (Miltenyi Biotec Ltd, Epsom, UK). Briefly, single cell suspensions of splenocytes were centrifuged (400 g) through Ficoll cushions and the enriched lymphocyte fraction harvested from the Ficoll–aqueous interface. The cells were washed and resuspended at 2 × 108/ml in PBS, pH 7·4, containing 5% BSA and 2 mm ethylenediaminetetra-acetic acid (EDTA) and incubated with anti-CD43-coated beads (100 µl/2 × 108 cells) for 20 min at 4°. The cells were then loaded onto a magnetic-activated cell sorter (MACS) negative selection column and the B lymphocytes eluted, washed and resuspended in RPMI-1640 medium containing 5% fetal calf serum (FCS). All cells were cultured in RPMI-1640 Glutamax I (Life Technologies, Paisley, UK) supplemented with 5% heat-inactivated FCS (Life Technologies), 50 µm 2-mercaptoethanol (Sigma), 2 mm glutamine, 1 mm sodium pyruvate, 50 µm non-essential amino acids, 100 U/ml penicillin and 100 µg/ml streptomycin (Life Technologies).
Measurement of DNA synthesis
Cells were seeded into 96-well cell culture plates at 2 × 105 cells/well. Agonists: F(ab′)2 fragments (50 µg/ml) of goat anti-mouse immunoglobulin M (IgM) antibody (Jackson Immunoresearch Laboratories Inc., Stratech Scientific Ltd, Luton, UK), lipopolysaccharide (LPS; 50 µg/ml; Sigma), and zVAD-fmk (N-benzyloxycarbonyl-Val-Ala-Asp(Ome)-fluoromethylketone) (10 µm; Calbiochem, Cambridge, MA)) were added as required to a final volume of 200 µl and the cells incubated at 37° in an atmosphere of 95% air/5% CO2 for 48 hr. Four hr before the end of the stimulation time, 0·0185 MBq [3H]thymidine (specific activity: 185 GBq/mmol; Amersham Pharmacia Biotech, Amersham, UK) was added to each well, and the plate returned to the incubator. On completion of the culture period, the cells were harvested and their DNA bound to filter paper using an automated cell harvester. The filters were dried and incorporated label counted by liquid scintillation using a β-plate reader (Wallac, PE Life Sciences, Salford, UK).
Measurement of ErkMAPkinase activity by flow cytometric analysis of intracellular staining
Splenic mononuclear cells (5 × 105) were incubated with F(ab′)2 fragments of goat anti-mouse IgM (50 µg/ml) or medium alone for 10 min at 37°. The cells were centrifuged at 400 g, 20° for 10 min and then incubated with 4% paraformaldehyde (100 µl) at 20°, for 5 min. They were then washed in 200 µl FACS buffer (PBS/2% FCS/2 mm EDTA), centrifuged at 400 g, 20°, for 10 min before being resuspended in a further 200 µl fluorescence-activated cell sorting (FACS) buffer at 20°, for 10 min. Next, the cells were centrifuged at 400 g, 20° for 10 min and resuspended in 200 µl FACS buffer containing 0·1% saponin, at 4° for 30 min. The cells were then centrifuged at 400 g, 20° for 10 min and then incubated with either rabbit IgG (0·2 µg/ml), rabbit anti-Erk or rabbit anti-dually(thr/tyr)phosphorylated Erk antibodies (1/200 dilution; New England Bio-labs, Hitchin, UK) at 4° for 30 min. The cells were once more washed in FACS buffer and then incubated with anti-rabbit Ig conjugated with fluoroscein isothiocyanate (FITC; 1/80 dilution) at 4° for 30 min. All antibodies were titrated for optimal staining. Samples were finally washed in FACS buffer, resuspended in fresh FACS buffer and analysed using a FACS Calibur System (Becton Dickinson, Oxford, UK).
Flow cytometry analysis of Annexin V binding to phosphatidylserine on the cell surface
Cells to be examined for annexin V expression were washed in PBS and incubated with annexin V–biotin conjugate, in defined calcium and magnesium concentrations, according to manufacturer's instructions (Boehringer Mannheim, Lewis, East Sussex, UK). The cells were washed and then incubated with streptavidin–FITC for 15 min and washed by centrifugation. Cells were immediately analysed using a Becton Dickinson FACScalibur using Lysis II software for analysis.14
Flow cytometry analysis of DNA content and cell cycle analysis
Cells were analysed for propidium iodide (PI) incorporation as described previously.14 At least 104 stained cells were analysed for PI fluorescence on a Becton Dickinson FACScalibur.
Flow cytometry analysis of mitochondrial potential
Incorporation of the cationic lipophilic dye DiOC6 into the mitochondria is proportional to the mitochondrial transmembrane potential, Δψm, with low mitochondrial potential reflecting commitment to apoptosis.14 Briefly, cells were prepared as previously described14 and resuspended at a concentration of 2 × 105 cells/ml. Cells were incubated for 30 min with 50 nm DiOC6 (Molecular Probes, Eugene, OR) and then washed once in PBS, pH 7·2. At least 104 stained cells were analysed using a Becton Dickinson FACScalibur using Lysis II software for analysis.
Flow cytometry of lineage and maturation surface markers
Allophycocyanin (APC)-conjugated rat anti-mouse antibody specific for B220, PE-conjugated rat anti-mouse antibodies specific for CD4 or CD8 and FITC-conjugated rat anti-mouse antibody specific for CD3 were purchased from Pharmingen (Oxford, UK). Germinal centre B cells were detected by peanut agglutinin (PNA) staining using either PNA–FITC or PNA–biotin and streptavidin–APC (Pierce & Warriner, Chester, UK). Flow cytometry was conducted on a Becton Dickinson FACScalibur Immunocytometry System.
Statistics
Statistical analysis was conducted using Student's t-test.
Results
ES-62 release from osmotic mini-pumps can result in serum levels equivalent to those found for PC-containing molecules during natural filarial nematode infection
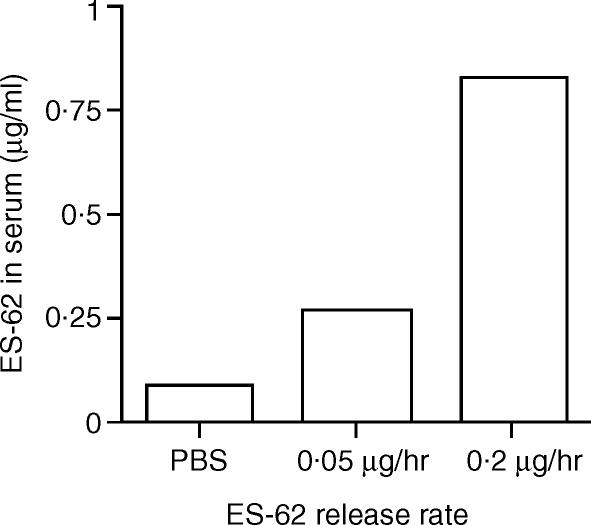
As alluded to earlier, it was important to confirm at the outset that the amount of ES-62 loaded into the pump could produce a concentration in serum that was appropriate to a worm infection. We thus employed a sandwich ELISA based on the PC-specific myeloma protein TEPC 15 to measure ES-62 levels in serum samples. Initially, we were unable to detect the parasite product in the samples. However, once serum was acid-heat treated to disassociate immune complexes, PC on ES-62 was successfully detected by day 14 post-implantation (Fig. 1). Following preliminary testing of a range of loading concentrations, two were employed for the study (loading rates of 0·1 mg/ml and 0·4 mg/ml that result in release rates of 0·05 µg/hr and 0·2 µg/hr, respectively). These routinely resulted in ES-62 serum concentrations within the range for PC-containing molecules in the bloodstream of filariasis patients (range 0·2–2 µg/ml13; Fig. 1).
Figure 1.
Circulating ES-62 levels in serum from mice implanted with osmotic pumps. Serum was taken at day 14 from mice (groups of three to five animals) implanted with osmotic pumps containing ES-62 or PBS as control, pooled and then acid-heat treated. ES-62 concentration was then determined by sandwich ELISA using the myeloma protein TEPC 15 that is reactive for PC. Results are expressed as mean of duplicate determinations and are representative of two other separate experiments. No PC was detected in serum samples taken immediately before implantation of pumps (result not shown).
Exposure to ES-62 in vivo results in defective F(ab′)2 fragments of anti-IgM- and LPS-driven proliferation ex vivo
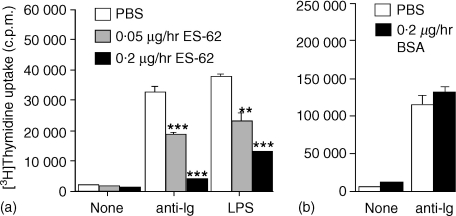
Mice were exposed for 14 days to ES-62 at the two pump concentrations indicated above, spleen cells recovered and the number of B cells determined by staining for B220 using flow cytometry. No significant difference could be determined in B-cell numbers or the proportion of different lymphocytes populations following exposure to ES-62 (Table 1). The spleen cells were stimulated via the BCR using F(ab′)2 fragments of anti-IgM. Analysis of the data clearly indicated that in vivo exposure to ES-62 induced hyporesponsiveness of B lymphocytes to subsequent BCR-driven proliferation in a concentration-dependent manner. A typical experiment is shown in Fig. 2(a). Data pooled from a series of identical but independent experiments showed that F(ab′)2 fragments of anti-IgM-stimulated DNA synthesis was decreased by 40·5 ± 10·3% (n = 6) and 57·1 ± 10·1% (n = 8) following in vivo exposure to ES-62 at release rates of 0·05 and 0·2 µg/hr, respectively (data presented as means ± SEM) relative to the PBS control. A further control in which ES-62 was replaced with BSA at the higher release rate (to allow for non-specific protein effects) failed to mimic the ES-62 effect (Fig. 2b). We also investigated whether continuous in vivo exposure to ES-62 would impact on a subsequent ex vivo response to LPS. Again, this was found to be the case and results of a typical experiment are shown in Fig. 2(a). Data pooled from a series of independent experiments showed that LPS-stimulated DNA synthesis was decreased by 39 ± 10·4% (n = 3) and 54 ± 11·6% (n = 6) following exposure to ES-62 at the two concentrations employed above.
Table 1.
Continuous in vivo exposure to ES-62 does not reduce the recovery of splenic B cells or modulate the relative proportions of lymphocyte populations*
| Cell type | PBS | ES-62 (0·05 µg/hr) | ES-62 (0·2 µg/hr) |
|---|---|---|---|
| B220 | 50·2 ± 7·7 | 42·4 ± 12·0 | 43·9 ± 10·4 |
| CD3 | 29·6 ± 1·8 | 31·5 ± 2·3 | 31·9 ± 11·4 |
| CD4 | 21·9 ± 4·7 | 21·1 ± 8·6 | 22·7 ± 12·0 |
| CD8 | 7·7 ± 0·3 | 8·5 ± 2·0 | 8·1 ± 1·9 |
Cell counts following sacrifice demonstrated no significant differences in the numbers of cells recovered between PBS and ES-62 treatments and indicated that in vivo exposure to ES-62 did not reduce splenic cellularity. In three experiments using five mice per group, post-death spleen cell counts (mean ± SD) were PBS: 142 ± 38 × 106; ES-62 (0·05 µg/hr): 123 ± 27 × 106 and ES-62 (0·2 µg/hr): 143 ± 7 × 106. Determination of the percentage of individual lymphocyte populations by lineage marker analysis demonstrated that relative cell proportions remained unchanged and that B cell numbers recovered were essentially identical in control and ES-62 treated groups. Data are presented as the percentage of cells (mean ± SD, n = 4 independent experiments) and there were no significant differences amongst any of the groups.
Figure 2.
Effect of ES-62 released from osmotic pumps on DNA synthesis of splenic cells stimulated ex vivo. Splenic mononuclear cell cultures from mice implanted with osmotic pumps containing ES-62 (release rates, 0·05 and 0·2 µg/hr) (a) or BSA (release rate 0·2 µg/hr) (b) or PBS (a, b) were stimulated for 48 hr with F(ab′)2 fragments of anti-IgM (50 µg/ml) (a and b) or LPS (50 µg/ml) (a) ex vivo. Culture wells were pulsed with [3H]thymidine 4 hr prior to harvesting and incorporated radioactivity assessed by scintillation counting. Data are expressed as mean ± SD (n = 3) and are representative of a number of other experiments in the case of (a) (see text for details). Significant differences are illustrated as, **P < 0·01; ***P < 0·001 compared with control PBS levels.
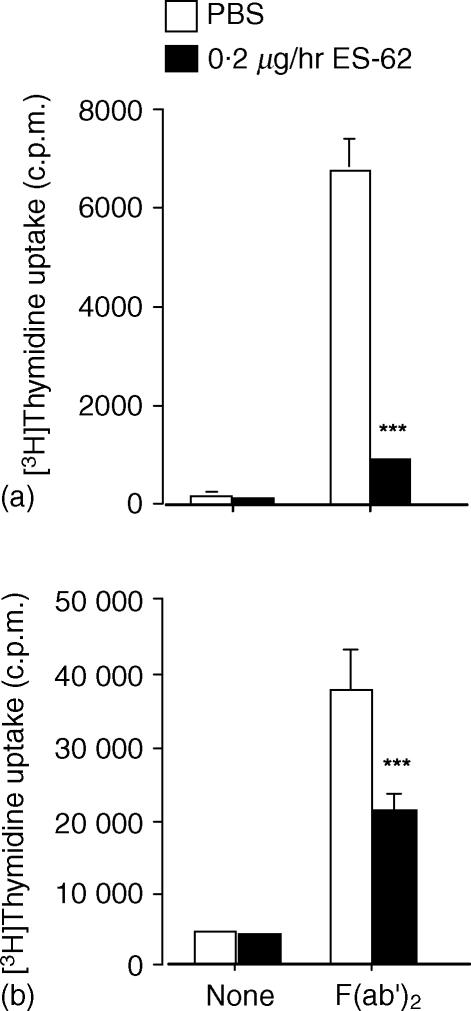
Because of previous indications15,16 that rodent spleen and lymph node cells might vary in their response to filarial nematode antigens we also examined the response to BCR ligation of nodal mononuclear cells ex vivo. However, ES-62 was found to have the same effect on recovered nodal mononuclear cells as on spleen cells (Fig. 3a). We also examined the effect of purifying the B-cell population prior to ex vivo stimulation via the BCR. This population when recovered from mice exposed to ES-62 was likewise found to be unable to respond in a normal manner (Fig. 3b). This latter result indicates that no other cell type is contributing to the effect we are witnessing ex vivo and is consistent with our previous in vitro data.8
Figure 3.
Effect of in vivo exposure to ES-62 on the BCR-induced proliferative responses of lymph node cells and purified splenic B cells, ex vivo. Mice were exposed to PBS or ES-62 in vivo by release from osmotic pumps for two weeks. Mononuclear cells from lymph nodes (a) and purified splenic B cells (b) were stimulated for 48 hr in vitro in the presence of 50 µg/ml F(ab′)2 fragments of anti-mouse IgM as indicated. Cells cultured in the presence of medium alone were included as a control. Culture wells were pulsed with [3H]thymidine 4 hr prior to harvesting and [3H]thymidine-incorporation was assessed by scintillation counting. Data are the mean ± SD of triplicate measurements and are representative of at least two independent experiments. Significant differences are illustrated as ***P < 0·001 compared with control PBS levels.
Exposure to ES-62 in vivo results in defective F(ab′)2 fragments of anti-IgM-driven MAPkinase activation ex vivo
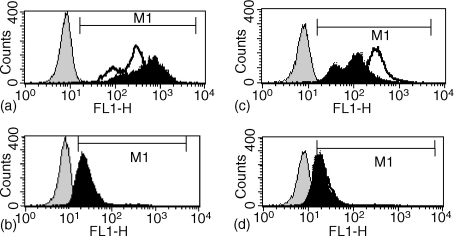
Our previous in vitro investigations revealed that ES-62 pretreatment of B lymphocytes seriously impairs subsequent BCR-dependent activation of signal transduction pathways associated with cell proliferation such as the ErkMAPkinase pathway.11,12 We thus examined whether cells recovered from mice implanted with osmotic pumps containing ES-62 would show a similar defect in Erk activation. As Erk activation correlates with dual phosphorylation of the TEY motif corresponding to T202/Y204 on Erk1, ErkMAPkinase activation was determined by flow cytometric analysis of intracellular staining of active Erk expression in splenic mononuclear cells using anti-dually phosphorylated Erk antibodies. Total Erk (active plus inactive Erk) expression was also measured as an internal control. As shown in Fig. 4(a), in control (PBS) mononuclear cells, F(ab′)2 fragments of anti-IgM stimulated an increase in the mean fluorescence intensity (MFI of 627 versus 257) of phosphoErk expression relative to that observed in unstimulated cells. In contrast, analysis of total Erk expression gave comparable levels of fluorescence (MFI 28 versus 33) in both samples (Fig. 4b), indicating that the increase in phosphoErk staining represented an increased Erk activation. Cells derived from mice pre-exposed to 0·2 µg/hr ES-62 (Fig. 4c, d) exhibited similar levels of phosphoErk (ES-62 MFI: 263; PBS MFI: 257) and Erk (ES-62 MFI: 29; PBS MFI: 33) expression, to those observed in PBS-treated samples. This indicates that long-term in vivo exposure to ES-62 did not substantially modulate basal levels of Erk activation or expression. However, in contrast to that observed with control (PBS) cells (Fig. 4a), ex vivo stimulation of cells pre-exposed to ES-62 with F(ab′)2 fragments of anti-IgM did not induce significant Erk activation but rather led to a decrease in the levels (MFI: 118) of phosphoErk expression (Fig. 4c). Thus these data show that pre-exposure to ES-62 in vivo renders B cells refractory to subsequent coupling of the BCR to ErkMAPkinase activation by inducing Erk dephosphorylation in an analogous manner to that described in our earlier Western blot in vitro studies on purified splenic B cells.11,12
Figure 4.
Effect of ES-62 exposure in vivo on BCR-promoted ErkMAPkinase activation of spleen cells ex vivo. Splenic mononuclear cells derived from mice pre-exposed either to PBS or ES-62 (0·2 µg/hr) were stimulated with medium or F(ab′)2 fragments of anti-IgM (50 µg/ml) for 10 min before processing for flow cytometric analysis of Erk or active, dually phosphorylated Erk expression as described in Materials and Methods. In (a) and (b), PBS-exposed cells were stimulated with medium or F(ab′)2 fragments of anti-IgM and analysed for phosphoErk (a) or Erk (b) expression. In (c) and (d), ES-62-exposed cells were stimulated with F(ab′)2 fragments of anti-IgM and analysed for phosphoErk (c) or Erk expression (d). In all cases, the antibody control plot represented samples stained with rabbit IgG followed by the FITC-conjugated secondary antibody and the M1 gate was set at the 1% cut-off point of the negative cell population. MFI represents mean fluorescence intensity.
ES-62 does not cause or prime for apoptosis
In order to further address how ES-62 was mediating lymphocyte hyporesponsiveness, we investigated whether pre-exposure to the parasite product in vivo induced apoptosis. As a first approach, although we had already established that there was no decrease in spleen cellularity or indeed B-cell recoveries (Table 1), we determined whether there was an increase in the percentage of apoptotic cells observed post sacrifice in mice exposed to ES-62 relative to controls. Apoptosis was assessed both by annexin V binding and propidium iodide staining of subdiploid DNA content of splenic mononuclear cells. The number of apoptotic cells in each group regardless of method of analysis was 1–3% and no significant differences between groups were observed.
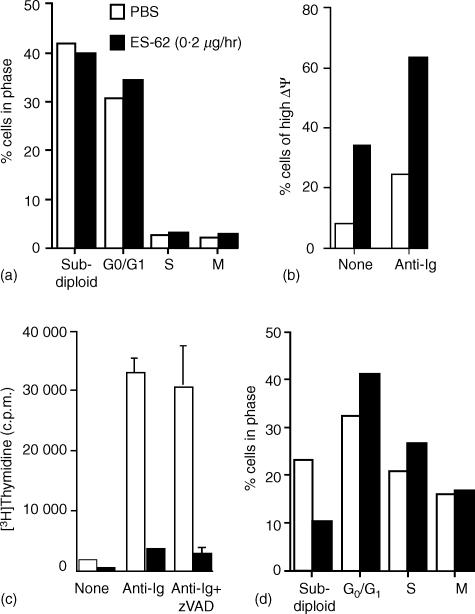
However, since it was possible that exposure to ES-62 could prime lymphocytes for apoptosis following subsequent stimulation via the antigen receptor, we also undertook cell cycle analysis by measuring DNA content following stimulation with F(ab′)2 fragments of anti-IgM for 48 hr ex vivo. This data showed that pre-exposure to ES-62 did not increase the percentage of mononuclear cells exhibiting subdiploid DNA content, and hence undergoing apoptosis, following stimulation (Fig. 5a). Consistent with this, we found that pre-exposure to ES-62 increased the percentage of cells exhibiting high mitochondrial potential (Fig. 5b), a phenotype associated with protection from apoptosis.14 Moreover, culture of cells in the presence of the pan-caspase inhibitor zVAD did not prevent the suppression of BCR-driven DNA synthesis (Fig. 5c), further supporting the proposal that ES-62 does not induce apoptosis. Finally, DNA content analysis of the B220+ population of mononuclear cells confirmed that ES-62 did not mediate selective apoptotic effects, and indeed appeared to confer protective effects, on B cells within the mononuclear cell population (Fig. 5d).
Figure 5.
Pre-exposure to ES-62 does not induce antigen receptor driven apoptosis of B lymphocytes. In (a), splenic mononuclear cells (106) from PBS or ES-62-treated mice were stimulated for 48 hr with F(ab′)2 fragments of anti-IgM before determining DNA content by propidium iodide staining and FACS analysis as described in Materials and Methods. In (b), splenic mononuclear cells (106) from PBS or ES-62-treated mice were stimulated for 24 h in the presence of media or F(ab′)2 fragments of anti-IgM (anti-Ig) before determining mitochondrial potential. Mitochondrial potential was determined by DiOC6(3) staining and FACS analysis as described in Materials and Methods. Data represents DiOC6(3) high (non-apoptotic) cell populations as determined on a logarithmic FL-1 axis and expressed as a percentage of the total number of cells analysed. Proliferation of splenic mononuclear cells from PBS or ES-62-treated mice was assessed by measurement of DNA synthesis (c). Cells (2 × 105 cells/well) were treated for 48 hr with media or F(ab′)2 fragments of anti-IgM (anti-immunoglobulin) in the presence and absence of the pan-caspase inhibitor zVAD-fmk (10 µm). The levels of [3H]thymidine incorporation into DNA were measured and the data expressed as means ± SD (n = 3). In (d), splenic mononuclear cells (106) from PBS or ES-62-treated mice were stimulated for 24 h with F(ab′)2 fragments of anti-IgM before determining DNA content of the B220+ B-cell population by propidium iodide staining and FACS analysis as described in Materials and Methods. All data are representative of at least three independent experiments.
Analysis of the cell recoveries following stimulation with F(ab′)2 fragments of anti-IgM for 48 hr in these ex vivo experiments was undertaken using a combination of cell counts and staining for B-cell lineage and activation markers. Results demonstrated that in vivo exposure to ES-62 for 14 days dramatically reduced the number of splenic mononuclear cells recovered from ex vivo cultures subsequently stimulated for a further 48 hr with F(ab′)2 fragments of anti-IgM (exposure to ES-62 at 0·05 µg/hr and 0·2 µg/hr resulted in recoveries of 73 ± 6% and 63 ± 8%, respectively, relative to PBS-treated animals, n = 3 independent experiments, results expressed as mean ± SEM). Further analysis revealed that the recoveries of B lymphocytes, as indicated by their B220+ expression, were particularly reduced. For example, the relevant cell recoveries of splenic cell populations from the experiment presented in Fig. 5(c) are shown in Table 2. Indeed, the cell recoveries obtained correlate well with the level of DNA synthesis described earlier. Taken together with data that ES-62 did not substantially alter the profiles (G0/G1 and mitotic: S + G2/M phases) of cycling cells in these cultures (Fig. 5a, d), these results suggest that ES-62 does not prime for apoptosis. Rather it interacts with B lymphocytes in such a manner that BCR-mediated clonal expansion is inhibited.
Table 2.
Continuous in vivo exposure to ES-62 reduces the recovery of splenic B cells following culture with (Fab′)2 fragments of anti-IgM ex vivo*
| ES-62 release rate | ||
|---|---|---|
| Cell type | 0·5 µg/hr (%) | 0·2 µg/hr (%) |
| Total splenocyte recovery | 58 | 39 |
| All B220+ B cells | 6·3 | 53 |
| B220+PNA+ GC B cells | 32 | 30 |
| All non-GC B220+ B cells | 4·6 | 54 |
The recovery of cells in control (PBS-exposed mice) cultures were normalized as 100% and the number of B cells recovered in the ES-62-treated cultures were calculated as a percentage of this. These data are the recoveries calculated from the experiment presented in Fig. 5(c) in which the cells were initially seeded at equivalent cell densities prior to ex vivo culture and this profile of reduced splenocyte recoveries correlating with reduced DNA synthesis was found in all experiments.
Discussion
The interaction between the filarial nematode secreted product, ES-62 and murine B lymphocytes has been the subject of a great deal of investigation in our laboratories. We have consistently found that ES-62 is able to interfere with the proliferation of these cells arising from ligation of the antigen receptors. Convincing as this data appears however, the question remains as to whether B lymphocytes are similarly affected by ES-62 during natural infection. This study addressed this question by employing osmotic pumps implanted in mice to release the parasite molecule in isolation, at a constant rate as would be predicted to occur during infection.
It was reasoned that any effects of ES-62 that we were likely to witness in the present study would almost certainly be caused by its PC moieties. This claim is based not just on our previous in vitro work.6,8,11 It is also dependent on experiments in which we showed that administering mice weekly subcutaneous injections of PC conjugated to BSA but not BSA alone resulted in hyporesponsiveness to BCR ligation of spleen cells when tested ex vivo.6 It was therefore important to establish at the outset that the amount of PC to which the murine immune system was being exposed in our study was comparable to that confronted during a natural infection. Initially when we tried to do this we ran into problems as we could not in fact detect any PC. However, treatment of the serum samples with acid to dissociate immune complexes resulted in its detection: PC-containing ES-62 was indeed present and in concentrations related to pump-loading concentrations. A number of previous studies have had to resort to dissociation of immune complexes to detect PC-containing filarial nematode molecules in serum (reviewed in 17). Importantly, even when undertaking dissociation, levels of PC detected in the present study are at the lower range for PC-containing molecules noted in the serum of filariasis patients in a previous study.13 We believe therefore that the rates of release we are producing can be used to obtain data that can be compared with a natural infection.
Ex vivo analysis of splenic (and nodal) B cells from mice implanted with pumps releasing ES-62 did indeed reveal impaired ability to proliferate in response to ligation of the antigen receptor. The effect we have repeatedly observed in vitro18 therefore is confirmed as occurring in vivo. Likewise, the previously noted concentration-dependence of the effect is also now observed. Interestingly, we also found that pre-exposure to ES-62 released from osmotic pumps is also able to inhibit proliferation induced by LPS. Our previous attempt to do this in vitro was unsuccessful.8 The success in the present study may reflect prolonged pre-exposure to the parasite molecule (the earlier in vitro study simply involved costimulation for 48–72 hr).
The impairment of B-cell proliferation ex vivo following exposure to ES-62 in vivo does not appear to be a result of apoptosis or induction of commitment to apoptosis following further stimulation. Rather, all of our data – cell counts, lineage-marker measurement, apoptosis and cell-cycle determination – points to the parasite molecule inducing hyporesponsiveness in B cells such that clonal expansion is blocked. Our previous in vitro investigations revealed that ES-62 pretreatment of B lymphocytes seriously impaired subsequent BCR-induced activation of signal transduction pathways associated with cell proliferation such as the ErkMAPkinase pathway.10–12 Our present investigations indicate similar impairment of BCR-coupling to ErkMAPkinase in cells recovered from ES-62-containing pump-implanted mice. Thus, not only for the first time have we shown that a single filarial nematode product at concentrations found during parasitism can interact with lymphocytes in vivo to subvert their subsequent ability to be activated. We have also provided information relating to the nature of the parasite product-induced defect at the molecular level.
The results we have obtained in this study would argue strongly for a role for ES-62 in modulation of B-cell responses during filarial nematode infection. If this is the case, however, one would perhaps expect to witness poor antibody responses to parasite molecules in infected individuals. Examination of the literature provides evidence for this in the sense that many human studies reveal an indirect association between the presence of circulating filarial nematode products and levels of the IgG1, IgG2 and IgG3 antibody subclasses against parasite antigens (see for example 19,20). However this is not the whole picture as the IgG4 subclass is usually elevated and often to a considerable degree (see for example 19,20). How can we explain this latter finding if PC-containing molecules such as ES-62 are interfering with B lymphocyte function? One possible answer relates to the generation of interleukin-4 (IL-4), a cytokine that would promote IgG4 production21 and which is a frequently recorded feature of active filariasis.20 We have previously shown during in vitro studies that IL-4 actually synergizes with ES-62 to cause B lymphocyte proliferation rather than hyporesponsiveness.6 This appears to arise as a consequence of exposure to IL-4 preventing the degradation of protein kinase Cα (an important enzyme in activation of B lymphocytes) that is normally induced by ES-62·10 Thus, it is possible that although ES-62 renders B lymphocytes hyporesponsive in vivo such that their ability to produce antibody is impaired, in an environment that contains IL-4, they may in fact be induced to produce antibodies of the IgG4 subclasses. Such a scenario could also help explain why total, in addition to specific, levels of this subclass are greatly increased in filariasis patients.22
The main effect of PC-containing molecules such as ES-62 on antibody responses during filarial nematode infection may thus not be so much to inhibit them as to polarize them. Consistent with this, we have recently shown that the murine antibody response to ES-62 is converted from solely IgG1 to mixed IgG1/IgG2a when the PC moiety is removed.23 The availability of the osmotic pump system now gives us an opportunity to test this hypothesis by measuring antibody responses to heterologous antigens in the presence/absence of ES-62. Furthermore there is much current interest in the idea that filarial nematode infection impacts on the nature of immune responses to vaccines. The osmotic pump system will also enable us to establish any role for PC-containing nematode products such as ES-62, in relation to this intriguing possibility.
Acknowledgments
This work was supported by joint awards to W.H and M.M.H. from the Leverhulme Trust (award number F/179/AT) and the Wellcome Trust (award number 060440).
References
- 1.WHO. Filariasis. Geneva: World Health Organization; 2000. [Google Scholar]
- 2.Vanamail P, Ramaiah KD, Pani SP, Das PK, Grenfell BT, Bundy DA. Estimation of the fecund life span of Wuchereria bancrofti in an endemic area. Trans R Soc Trop Med Hyg. 1996;90:119–21. doi: 10.1016/s0035-9203(96)90106-6. [DOI] [PubMed] [Google Scholar]
- 3.Allen JE, MacDonald AS. Profound suppression of cellular proliferation mediated by the secretions of nematodes. Parasite Immunol. 1998;20:241–7. doi: 10.1046/j.1365-3024.1998.00151.x. [DOI] [PubMed] [Google Scholar]
- 4.Harnett W, Parkhouse RME. Structure and function of nematode surface and excretory-secretory products. In: Sood ML, editor. Perspectives in Nematode Physiology and Biochemistry. New Delhi: M/S Narendra Publication House; 1995. pp. 207–42. [Google Scholar]
- 5.Harnett W, Grainger M, Kapil A, Worms MJ, Parkhouse RME. Origin, kinetics of circulation and fate in vivo of the major excretory-secretory product of Acanthocheilonema viteae. Parasitology. 1989;99:229–39. doi: 10.1017/s0031182000058686. [DOI] [PubMed] [Google Scholar]
- 6.Harnett W, Deehan MD, Houston KM, Harnett MM. Immunomodulatory properties of a phosphorylcholine-containing secreted filarial glycoprotein. Parasite Immunol. 1999;21:601–8. doi: 10.1046/j.1365-3024.1999.00267.x. [DOI] [PubMed] [Google Scholar]
- 7.Stepek G, Auchie M, Tate R, Watson K, Russell DG, Devaney E, Harnett W. Expression of the filarial nematode phosphorylcholine-containing glycoprotein, ES-62, is stage-specific. Parasitology. 2002;125:155–64. doi: 10.1017/s0031182002001920. [DOI] [PubMed] [Google Scholar]
- 8.Harnett W, Harnett MM. Inhibition of murine B cell proliferation and down-regulation of protein kinase C levels by a phosphorylcholine-containing filarial excretory-secretory product. J Immunol. 1993;151:4829–37. [PubMed] [Google Scholar]
- 9.Harnett MM, Deehan MR, Williams DM, Harnett W. Induction of signalling anergy via the T-cell receptor in cultured Jurkat T cells by pre-exposure to a filarial nematode secreted product. Parasite Immunol. 1998;20:551–63. doi: 10.1046/j.1365-3024.1998.00181.x. [DOI] [PubMed] [Google Scholar]
- 10.Deehan M, Harnett M, Harnett W. A filarial nematode secreted product differentially modulates expression and activation of protein kinase C isoforms in B lymphocytes. J Immunol. 1997;159:6105–11. [PubMed] [Google Scholar]
- 11.Deehan MR, Frame MJ, Parkhouse RM, Seatter SD, Reid SD, Harnett MM, Harnett W. A phosphorylcholine-containing filarial nematode-secreted product disrupts B lymphocyte activation by targeting key proliferative signaling pathways. J Immunol. 1998;160:2692–9. [PubMed] [Google Scholar]
- 12.Deehan MR, Harnett W, Harnett MM. A filarial nematode-secreted phosphorylcholine-containing glycoprotein uncouples the B cell antigen receptor from extracellular signal-regulated kinase-mitogen-activated protein kinase by promoting the surface Ig-mediated recruitment of Src homology 2 domain-containing tyrosine phosphatase-1 and Pac-1 mitogen-activated kinase-phosphatase. J Immunol. 2001;166:7462–8. doi: 10.4049/jimmunol.166.12.7462. [DOI] [PubMed] [Google Scholar]
- 13.Lal RB, Paranjape RS, Briles DE, Nutman TB, Ottesen EA. Circulating parasite antigen(s) in lymphatic filariasis – use of monoclonal-antibodies to phosphocholine for immunodiagnosis. J Immunol. 1987;138:3454–60. [PubMed] [Google Scholar]
- 14.Katz E, Deehan MR, Seatter S, Lord C, Sturrock RD, Harnett MM. B cell receptor-stimulated mitochondrial phospholipase A2 activation and resultant disruption of mitochondrial membrane potential correlate with the induction of apoptosis in WEHI-231 B cells. J Immunol. 2001;166:137–47. doi: 10.4049/jimmunol.166.1.137. [DOI] [PubMed] [Google Scholar]
- 15.Weiss N. Dipetalonema viteae: in vitro blastogenesis of hamster spleen and lymph node cells to phytohemagglutinin and filarial antigens. Exp Parasitol. 1978;46(2):283–99. doi: 10.1016/0014-4894(78)90142-x. [DOI] [PubMed] [Google Scholar]
- 16.Lammie PJ, Katz SP, Anderson WH. Serosuppression in experimental filariasis. Clin Exp Immunol. 1984;55:602–10. [PMC free article] [PubMed] [Google Scholar]
- 17.Harnett W. Molecular approaches to the diagnosis of Onchocerca volvulus in man and the insect vector. In: Kennedy MW, editor. Parasitic Nematodes – Antigens, Membranes and Genes. London: Taylor & Francis; 1991. p. 195. [Google Scholar]
- 18.Harnett W, Harnett MM. Modulation of the host immune system by phosphorylcholine-containing glycoproteins secreted by parasitic filarial nematodes. Biochimica Biophysica Acta. 2001;1539:7–15. doi: 10.1016/s0167-4889(01)00101-x. [DOI] [PubMed] [Google Scholar]
- 19.Mohanty MC, Satapathy AK, Sahoo PK, Ravindran B. Human bancroftian filariasis – a role for antibodies to parasite carbohydrates. Clin Exp Immunol. 2001;124:54–61. doi: 10.1046/j.1365-2249.2001.01484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maizels RM, Sartono E, Kurniawan A, Partono F, Selkirk ME, Yazdanbakhsh M. T-cell activation and the balance of antibody isotypes in human lymphatic filariasis. Parasitol Today. 1995;11:50–6. doi: 10.1016/0169-4758(95)80116-2. [DOI] [PubMed] [Google Scholar]
- 21.Wierenga EA, Snoek M, de Groot C, Chretien I, Bos JD, Jansen HM, Kapsenberg ML. Evidence for compartmentalization of functional subsets of CD2+ T lymphocytes in atopic patients. J Immunol. 1990;144:4651–6. [PubMed] [Google Scholar]
- 22.Ottesen EA, Skvaril F, Tripathy SP, Poindexter RW, Hussain R. Prominence of IgG4 in the IgG antibody response to human filariasis. J Immunol. 1985;134:2707–12. [PubMed] [Google Scholar]
- 23.Houston K, Wilson EH, Eyres L, Brombacher F, Harnett MM, Alexander J, Harnett W. The presence of phosphorylcholine on a filarial nematode protein influences the IgG subclass response to a molecule and by a mechanism dependent on IL-10. Infection Immunity. 2000;68:5466–8. doi: 10.1128/iai.68.9.5466-5468.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]