Abstract
We have previously shown that oestradiol treatment of ovariectomized rats for 3 days inhibits antigen presentation by uterine stromal cells at a time when oestradiol increases the numbers of antigen-presenting cells (APC) in the uterine stroma. In the present study, we found that oestradiol treatment for 1 day is sufficient to inhibit antigen presentation by stromal cells. To define the mechanism(s) of this inhibition, we examined the effect of cytokines and found that exogenous transforming growth factor-β (TGF-β) inhibits antigen presentation when stromal cells from saline- but not oestradiol-treated animals are incubated with ovalbumin (OVA)-specific T cells and OVA. In contrast, antigen presentation by uterine epithelial cells was not affected by TGF-β. In other studies, the acute inhibitory effect of oestradiol (1 day) on stromal antigen presentation is fully reversed when anti-TGF-β antibody is added to the culture media. When given for 3 days, oestradiol inhibition of antigen presentation is partially reversed by anti-TGF-β antibody at a time when antibodies to tumour necrosis factor-α and interleukin-10 have no effect. To determine whether uterine epithelial cells produce TGF-β, epithelial cells were grown to confluence on transwell inserts. Our findings indicate that uterine epithelial cells produce biologically active TGF-β which is preferentially released basolaterally in the direction of underlying stromal cells. When oestradiol is given to ovariectomized rats 1 day before sacrifice, TGF-β production by epithelial cells increases within 24 hr in culture, relative to saline controls. Taken together, these results suggest that oestradiol inhibition of stromal cell antigen presentation is mediated through the stimulatory effect of oestradiol on TGF-β production by epithelial cells.
Introduction
The mucosal immune system in the female reproductive tract consists of immune cells which migrate into the uterus, cervix and vagina as well as resident epithelial cells and supportive stromal cells.1,2 Sex hormones have been shown to influence the migration of antigen-presenting cells (APC) such as macrophages and dendritic cells as well as T and B cells by affecting the expression of adhesion molecules and chemotactic factors.3–7 Epithelial cells, in addition to expressing polymeric immunoglobulin receptor (pIgR), which moves polymeric IgA from tissue to lumen,8–10 also produce defensins which are both bactericidal and virucidal.11–14 Through the production of cytokines and chemokines, epithelial cells can enhance or suppress immune protection, depending on the pathogen involved and the endocrine balance.5
Previous studies from our laboratory have reported that oestradiol regulates antigen presentation in the uterus and vagina.15,16,18 In these studies we found that APC in the uterine stroma and vagina are under hormonal control.18,19 Elevated levels of oestradiol both during the reproductive cycle and in response to exogenous hormone treatment of ovariectomized animals inhibited antigen presentation by APC (macrophages, dendritic cells and B cells) in the uterus and vagina. In contrast, under the same endocrine conditions, antigen presentation by uterine epithelial cells as well as APC in the spleen and thymus is enhanced.15–17 These findings suggest that oestradiol acts locally in the uterine and vaginal stroma to decrease antigen presentation. This hypothesis is further supported by our findings that, in response to oestradiol, APC numbers either remained unchanged or increased in the uterus and vagina at a time when antigen presentation was inhibited.5,19,20
Cytokines expressed in the reproductive tract during the reproductive cycle and following exogenous hormone treatment include interleukin (IL)-1α, IL-1β, tumour necrosis factor-α (TNF-α), IL-6, transforming growth factor-β (TGF-β) and granulocyte–macrophage colony-stimulating factor as well as IL-4, IL-5 and IL-10.21–26 Considering the multitude of cytokines produced and the recognition that many can influence immune function, our laboratory examined the effect of oestradiol on vaginal APC and found that TGF-β mediates the inhibitory effect of oestradiol on vaginal antigen presentation.27 When vaginal cells were incubated with TGF-β or anti-TGF-β antibody, antigen presentation was affected; this response was specific in that IL-6, IL-10 and TNF-α had no effect. In the uterus, TGF-β expression increases in response to hormone treatment and during the peri-implantation period of pregnancy.25,28 In response to diethylstilbesterol, a synthetic oestrogen, epithelial TGF-β1, -2, -3 mRNAs increase transiently (0.5–3 hr) with TGF-β1, -2, -3 proteins elevated for longer times in the uterine tissues of immature mice.29 Similarly, TNF-α and IL-10 are produced in the murine and human uterus.23,30–32 Taken together, these studies suggest that TGF-β might be playing a central role in regulating antigen presentation in the uterus of the adult rat.
The overall goal of the present study was to examine the mechanism(s) whereby oestradiol regulates antigen presentation in the uterine stroma of the rat. Our objectives were to: (1) establish whether TGF-β, TNF-α and IL-10 inhibit uterine antigen presentation; (2) measure the effects of antibody neutralization of cytokines (TGF-β, TNF-α and IL-10) on oestradiol inhibition of antigen presentation by uterine cells and (3) determine if uterine epithelial cells produce TGF-β in culture and whether production of TGF-β is under hormonal control.
Materials and methods
General procedures
Adult female Lewis rats (150–200 g) were purchased from Charles River Breeding Laboratories, Kingston, NY. Animals were housed in a constant-temperature room with fixed light/dark intervals (12 hr each) and provided food and water ad libitum. Rats were ovariectomized 7–10 days before each experiment. Animals were killed by decapitation and uterine tissues were recovered to prepare epithelial and stromal cells. All procedures involving animals were conducted after approval of the Dartmouth College Institutional Animal Care and Use Committee.
Preparation of uterine epithelial and stromal cells
Uterine cells from ovariectomized rats were prepared by slitting uteri lengthwise to expose the luminal surface, cutting tissues into four sections or mincing to 2–5 mm pieces, and incubating with bovine trypsin (46 500 Units/ml; Sigma Chemical Co., St. Louis, MO), porcine pancreatin (25 mg/ml; Invitrogen/Gibco, Grand Island, NY) and bovine pancreas DNase (400 Units/ml; Worthington Biochemical Co., Freehold, NJ) at a volume of 19·5 ml/g tissue for 60 min at 4°, with rotation (120 r.p.m.) for the first 10 min, and for an additional 60 min at 22° as previously described.18 Epithelial sheets were released by vortexing (one to two times at high speed for 5 s) in Hank's balance salt solution (HBSS) and grinding on a 250-µm mesh nylon screen. Epithelial cells were purified from this suspension by filtering through a 20-µm screen and collecting cell sheets retained by the filter. To isolate stromal cells, uteri were incubated a second time, in 0·05% trypsin and 0·02% ethylenediamine tetra-acetic acid (Sigma) with 400 Units DNase/ml at 2 ml per tissue, for 30 min at 37° then ground on a 40-µm mesh screen. Cells passing through the screen and recovered were the stromal population. All cells were resuspended in complete RPMI-1640 medium (Invitrogen/Gibco) containing 25 mm HEPES, supplemented with 10% fetal bovine serum (FBS; Hyclone, Logan, UT), 5% NCTC-109 (BioWhittaker, Walkersville, MD), 50 µm 2-mercaptoethanol (Sigma), 2 mml-glutamine (Mediatech, Inc., Herndon, VA), 100 µg/ml streptomycin and 100 Units/ml penicillin (Mediatech). For antigen presentation experiments, cells were aspirated through 18 and 20 g needles to prepare single cell suspensions. When analysed by immunohistochemistry, very few class II positive and immune cells were present in the uteri of saline-treated rats. In contrast, when animals were treated with oestradiol, macrophages, dendritic cells, granulocytes and T cells increased throughout the uterine endometrium.5
Epithelial cell sheets were either grown directly in wells of 24-well plates (3·5–5·0 × 105 cells/900 µl per well) or on insert culture chambers placed in 24-well companion plates (BD Biosciences/Falcon, Franklin Lakes, NJ). Cell culture inserts (0·4-µm pore, 6 mm diameter, transparent membrane; BD Biosciences/Falcon) were coated with Matrigel (growth factor reduced, without phenol red; BD Biosciences) diluted 1 : 3 with serum-free RPMI-1640. Epithelial sheets were plated at a density of two to three wells/uterus in 300 µl medium, into the top (apical) compartment of the culture chamber; 850 µl medium was added to the bottom (basolateral) compartment. Supernatants were collected from apical and basolateral chambers and replaced with fresh medium at 24 or 48 hr intervals. Following collection, supernatants were centrifuged at 10 000 g for 5 min, and stored at −20° until assayed.
Transepithelial resistance (TER), which is used as an indication of tight junction formation in the epithelial cell monolayer10 was monitored daily using an EVOM electrode and Voltohmmeter (World Precision Instruments, Inc, Sarasota, FL).
Antigen presentation assays
For antigen presentation studies, ovalbumin (OVA)-specific T cells (1 × 105 cells/100 µl) in complete RPMI-1640 medium were cultured in triplicate wells in 96-well flat-bottom microtitre plates with irradiated epithelial or stromal APC (1 × 105 cells/100 µl) in the presence of OVA (50 µl, 1500 µg/ml) (APC + T + OVA).18 Briefly, ovalbumin-specific T-cell lines for in vitro studies were prepared from the lymph nodes of Lewis rats injected with OVA–complete Freund's adjuvant (CFA) emulsion into the footpads (100 µg OVA in 100 µl phosphate-buffered saline and 100 µl CFA per rat) as previously described.18 Epithelial and stromal cells were irradiated prior to the start of antigen presentation with 4000 rad to prevent their proliferation. Controls included in all experiments were APC incubated with T cells in the absence of ovalbumin (APC + T), APC incubated with ovalbumin (APC + OVA) and T cells incubated with ovalbumin (T + OVA). Following 48 hr of incubation at 37°, T-lymphocyte proliferation was measured by [3H]thymidine uptake. Individual wells received 1 µCi of [3H]thymidine (50 µl medium) 20–24 hr before the termination of each experiment. Cells from each well were transferred onto glass fibre filtermats with a cell harvester (Skatron, Sterling, VA). Radioactivity incorporated into cells was measured in a liquid scintillation counter (Packard, Meriden, CT).
Hormone treatment, antibodies and cytokines
Oestradiol-17β (Calbiochem, La Jolla, CA) was dissolved in 100% ethanol, evaporated to dryness, and resuspended in 0·9% saline. Daily injections of 2 µg/rat were given in a 100-µl volume, control animals received only saline. To correct for the alcohol present in the oestradiol preparation, an equivalent amount of ethanol was evaporated in flasks used to prepare saline. Antibodies were purchased from R & D Systems (Minneapolis, MN). Antibodies (mouse anti-human TGF-β1, -2, -3, mouse anti-rat TNF-α, and goat anti-rat IL-10) were used at the concentrations indicated in the figure legends. P3 myeloma supernatant (mouse IgG1; a gift from Dr M. Fanger, Department of Microbiology) and goat IgG (R & D Systems) were used as isotype controls at the appropriate concentrations. Recombinant human TGF-β1 and TGF-β2 as well as IL-10 and TNF-α were purchased from R & D Systems and used at the concentrations indicated in the figure legends.
Measurement of TGF-β
Biologically active (mature) TGF-β was measured in a bioassay which utilizes mink lung epithelial (MLE) cells transfected with a plasminogen activator inhibitor-1 (PAI-1) promoter fused to the luciferase (L) reporter gene.33 This PAI/L cell line measures TGF-β in picogram quantities and is based on the ability of TGF-β to induce PAI-1 expression, resulting in a dose-dependent increase in luciferase activity.33 Frozen transfected MLE cells were thawed and washed in cold complete RPMI-1640 medium. Cells were seeded at 1 × 105/100 µl per well in a 96-well flat-bottom white opaque plate (USA Scientific, Inc., Ocala, FL), centrifuged at 800 g (Beckman GC-6R centrifuge with a swinging bucket rotor) for 15 s and incubated for 3 hr at 37° in 5% CO2 to allow cells to adhere. Following incubation, cells were centrifuged and medium was replaced with 50 µl fresh medium plus 50 µl of cell culture supernatant or serially diluted TFG-β1 for a standard curve. Following an additional 17–20 hr incubation, cells were washed twice in 100 µl HBSS (Invitrogen/Gibco). Cell lysates were prepared by treating cells with cell culture Lysis Reagent (50 µl; Promega, Madison, WI) for 15 min at room temperature. Luciferase activity of the lysates was measured using luciferase assay reagent (100 µl; Promega) added to each well. Illumination was recorded for 10 s following a 2-s delay in a Microplate Luminometer model LB96V (EG & G Berthold, Gaithersburg, MD).
Statistics
Data were compared by one-way anova, followed by a Tukey multiple comparison post test. Differences of P = 0·05 were considered significant. All values are expressed as mean ± SEM. Numbers in parenthesis in the legends indicate the number of times each experiment was carried out.
Results
Effect of oestradiol on antigen presentation by rat uterine stromal cells
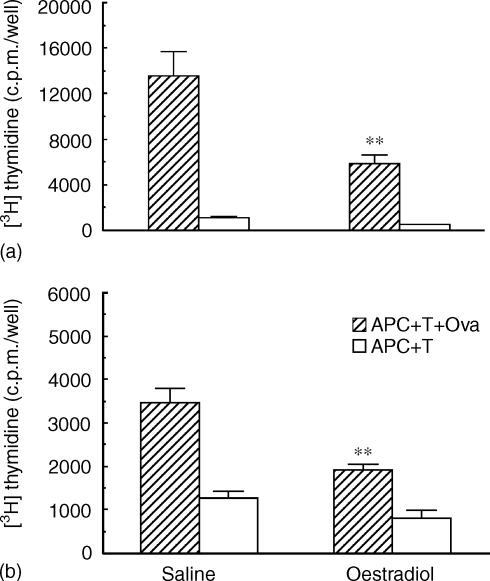
Previous studies from our laboratory have shown that oestradiol inhibits stromal cell antigen presentation when animals are treated with oestradiol for 3 days prior to death 24 hr after the last daily injection.16 As seen in Fig. 1(a), when ovariectomized rats are treated with daily injections of oestradiol for 3 days (2 µg/rat), antigen presentation by uterine stromal APC to OVA-specific T cells is inhibited by approximately 60% of that seen with APC from saline controls. Antigen presentation is measured as the difference between 3H-thymidine incorporation when stromal APC are incubated with T cells in the presence versus the absence of OVA. To more fully define the influence of oestradiol, ovariectomized animals were treated with a single injection of oestradiol and killed 24 hr later. Figure 1(b) demonstrates that a single exposure to oestradiol also inhibits stromal cell antigen presentation in that antigen presentation of saline controls is reduced by oestradiol from approximately 2200 c.p.m./well to 1120 c.p.m./well (APC + T + OVA) − (APC + T).
Figure 1.
Influence of oestradiol on antigen presentation by uterine stromal cells from ovariectomized rats treated with saline (100 µl) or oestradiol (2 µg) for 3 days (a) or 1 day (b) prior to death. Uteri (seven to eight animals/group) were pooled and stromal APC were prepared as described in Materials and Methods. Stromal APC (1 × 105 cells/100 µl) were incubated with OVA-sensitized T cells (1 × 105 cells/100 µl) and OVA (300 µg/ml) for 3 days. [3H]Thymidine was added for the last 24 h of incubation. Values shown are mean ± SE of three wells per group. (Number of experiments: n = 3–5). **Significantly different from saline control (P < 0.01).
As seen in Table 1, treatment of rats with a single injection of oestradiol has no effect on antigen presentation by uterine epithelial cells. In previous studies, we found that 3 days of oestradiol treatment stimulated uterine epithelial cell antigen presentation.15,16 Our finding that stromal cell antigen presentation is inhibited with a single injection of oestradiol (Fig. 1b), at a time when epithelial cells are non-responsive, indicates a difference between uterine epithelial cells and stromal cells in their ability to respond to oestradiol.
Table 1.
Lack of effect of oestradiol on antigen presentation by uterine epithelial cells following a single injection of oestradiol
| Treatment | APC + T + OVA | APC + T | (APC + T + OVA) − (APC + T) |
|---|---|---|---|
| Saline | 21 832 ± 232 | 5540 ± 113 | 16 292 |
| Oestradiol | 19 292 ± 1496 | 6572 ± 354 | 12 720 |
Seven to eight animals/group. Oestradiol: 2 µg/0·1 ml/rat, animals were killed 24 hr after injection. Saline: 0·1 ml/rat.
Effect of TGF-β on antigen presentation by stromal cells
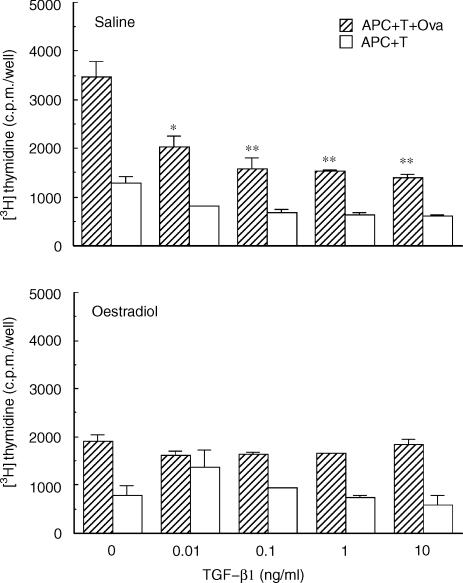
To explore the possibility that TGF-β affects antigen presentation by APC in the uterine stroma, animals were ovariectomized and treated with either saline or oestradiol (2 µg) for 1 day prior to death. As seen in Fig. 2, when TGF-β1 is added to the incubation media, antigen presentation by stromal cells from saline-treated rats (Saline) is inhibited. This inhibitory effect was observed over a dose range from 0·01 to 10 ng/ml of TGF-β1. The effects of TGF-β on 3H-thymidine incorporation in the absence of OVA (APC + T) as well as in the absence of APC (T + OVA; data not shown) were minimal compared to that seen when antigen was present (APC + T + OVA). In contrast, when TGF-β1 was added to the culture media of stromal cells from oestradiol-treated rats (Oestradiol), no inhibitory effect was observed. To examine the effects of different isoforms of TGF-β on antigen presentation, isolated stromal cells were incubated with TGF-β2 (data not shown). These results were identical to that seen with TGF-β1 in that antigen presentation by stromal cells from saline- but not oestradiol-treated rats was inhibited by TGF-β2 over the same dose range (0·01–10 ng/ml).
Figure 2.
Effect of TGF-β on antigen presentation by uterine stromal cells from saline- and oestradiol-treated rats. Isolated stromal cells (APC) from ovariectomized rats (seven to eight animals/group) treated with saline (100 µl) or oestradiol (2 µg) for 1 day were prepared and incubated with OVA-sensitized T cells and OVA for 3 days. TGF-β (0·01, 0·1, 1·0 and 10 ng/ml, final concentration) was added to each well at the start of culture. [3H]Thymidine was added for last 24 hr of incubation. Values shown are mean ± SE of three wells per group. (n = 3) *Significantly different from control (P < 0·05). **Significantly different from control (P < 0·01).
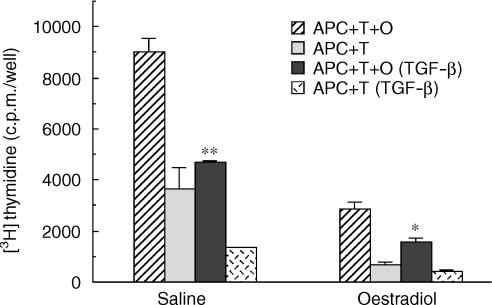
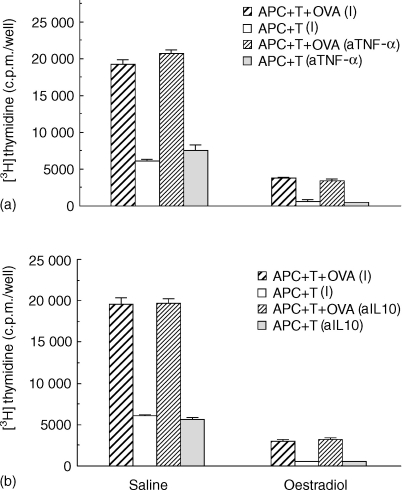
To examine the effect of TGF-β on antigen presentation by uterine stromal cells from rats treated with oestradiol for 3 days prior to death, ovariectomized animals received either saline or oestradiol (2 µg/day for 3 days) prior to stromal cell preparation and incubation with T cells and OVA. As seen in Fig. 3, addition of TGF-β1 (10 ng/ml) significantly inhibited antigen presentation by stromal APC from both saline- and oestradiol-treated rats. These findings indicate that antigen presentation by stromal cells from rats treated with oestradiol for 3 days, but not 1 day (Fig. 2), are further inhibited with TGF-β, beyond that seen with oestradiol alone.
Figure 3.
Effect of TGF-β on antigen presentation by stromal cells from rats treated with oestradiol for 3 days. Ovariectomized rats (seven to nine animals/group) received three injections daily (100 µl) of oestradiol (2 µg/rat) or saline prior to death 24 hr after the third injection. Uterine stromal cells from saline- and oestradiol-treated rats were incubated with OVA-specific T cells and OVA, along with TGF-β (10 ng/ml) for 3 days with the addition of [3H]thymidine for the last 24 hr. Values are mean ± SE of three wells per group. (n = 2) *Significantly lower than control group (P < 0·05). **Significantly lower than control (P < 0·01).
Effect of anti-TGF-β antibody on antigen presentation by uterine stromal cells
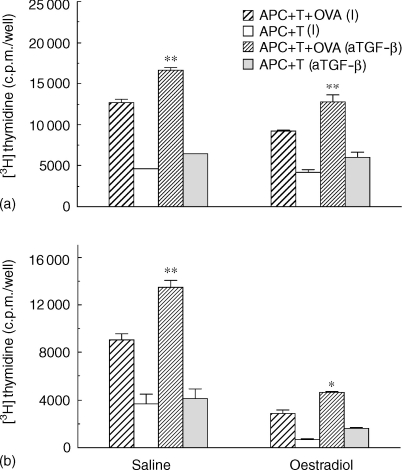
The inhibitory effect of TGF-β on antigen presentation by uterine stromal APC prompted us to examine the possibility that endogenous TGF-β production by epithelial and/or stromal cells might be influencing uterine stromal cell antigen presentation. To examine this possibility, stromal cells from ovariectomized rats treated with either saline (0·1 ml) or oestradiol 2 µg/day for 1 day (a) or 3 days (b) were prepared and incubated with T cells and OVA in the presence or absence of anti-TGFβ antibody. Anti-TGF-β1, -2, -3 antibody (1 µg/ml) was added at a concentration recommended by the manufacturer to be effective at neutralizing TGF-β. Controls used in these studies were matched in terms of isotype (I) and amount of antibody added to the incubation media. In preliminary studies, we found that the presence of an isotype control had no effect on antigen presentation when compared to incubations in the absence of immunoglobulin. As shown in Fig. 4(a), the addition of neutralizing antibody to TGF-β increased antigen presentation by stromal cells from saline-treated rats and fully reversed the inhibitory effect of oestradiol on stromal cell antigen presentation at 1 day post-hormone treatment. In contrast, when animals were treated with oestradiol for 3 days (Fig. 4b), whereas antibody to TGF-β increased antigen presentation by stromal cells from saline-treated animals, anti-TGF-β antibody only partially reversed the inhibitory effect seen with stromal cells from rats treated with oestradiol. Our finding that stromal cell antigen presentation is enhanced with anti-TGF-β antibody suggests either that epithelial cells present in our stromal cell preparation are producing TGF-β or that other cells in the stroma are a source of TGF-β.
Figure 4.
Effect of anti-TGF-β1, -2, -3 antibody on antigen presentation by uterine stromal cells from rats treated with oestradiol for either 1 day (a) or 3 days (b) prior to death. Ovariectomized rats received one to three injections daily (100 µl) of oestradiol (2 µg/rat) or saline prior to death 24 hr after the last injection. Stromal cells from saline- and oestradiol-treated rats were incubated with OVA-specific T cells and OVA, along with anti-TGF-β antibody (1 µg/ml) or an isotype (I) control (IgG1; 1 µg/ml), for 3 days with the addition of [3H]thymidine for the last 24 hr. Values are mean ± SE of three wells per group. (n = 2). *Significantly higher than isotype-control group (P < 0·05). **Significantly higher than isotype control (P < 0·01).
To determine whether other cytokines are involved in the regulation of antigen presentation, stromal cells from ovariectomized rats treated with saline or oestradiol for 3 days were incubated with T cells and OVA in the presence of (a) anti-TNFα antibody (80 Units/ml); or (b) anti-IL-10 antibody (2·5 µg/ml). As seen in Fig. 5, antibodies against TNF-α and IL-10 had no effect on antigen presentation by stromal cells irrespective of prior hormone treatment. These experiments, which are representative of two studies in which a range of antibody concentrations was used (not shown), demonstrate the specificity of the anti-TGF-β neutralizing response seen in these studies. In other experiments, TNF-α and IL-10, when added to the culture media of stromal, had little or no effect on antigen presentation (data not shown).
Figure 5.
Influence of anti-TNF-α antibody and anti-IL-10 antibody on antigen presentation by uterine stromal cells from saline- and oestradiol-treated rats. Ovariectomized rats received three injections at 24-hr intervals of oestradiol (2 µg/rat) or saline (100 µl) prior to death 24 hr after the last injection. APC and T cells were incubated with OVA for 3 days in the presence of (a) anti-TNF-α antibody (80 Units/ml); or (b) anti-IL-10 antibody (2·5 µg/ml). Anti-TNF-α antibody purchased from Genzyme was neat hyperimmune rabbit antiserum which was diluted 1 : 1250 to correspond to an antibody binding capacity of 80 Units/ml. Control normal rabbit serum was similarly diluted. Anti-IL-10 control wells received matched isotype at the same concentration. 3H-thymidine was added for last 24 hr of incubation. Values shown are mean ± SE of three wells per group. (n = 2).
Effect of oestradiol on TGF-β production by uterine epithelial cells
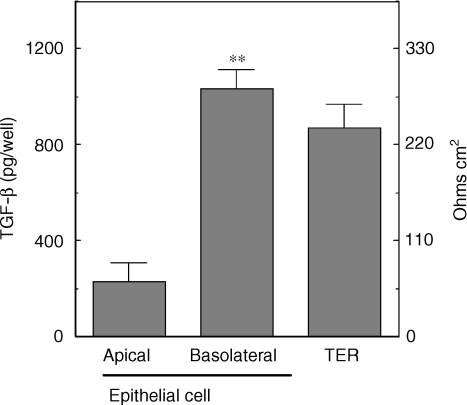
To determine whether uterine epithelial cells produce TGF-β that is biologically active, purified cells were grown to confluence on cell inserts prior to collection of apical and basolateral media. Previously, we reported the purity of uterine epithelial cells prepared by enzymatic digestion prior to filter capture of epithelial sheets.10,18 Homogeneity of epithelial cells was determined by immunofluorescent localization of pIgR. As seen in Fig. 6, by the fourth day in culture, epithelial cells on inserts grew to confluence and formed tight junctions as indicated by high transepithelial resistances (TER) in excess of 110 ohms cm2. In the absence of epithelial cells, baseline resistance ranged between 55 and 60 ohms cm2. Analysis of TGF-β in the apical and basolateral chambers following 48 hr of incubation indicates that TGF-β is preferentially secreted into the basolateral chamber, in a direction which in vivo would bathe stromal cells. Preferential release to the basolateral compartment was apparent irrespective of whether results were expressed as TGF-β per well (Fig. 6) or as TGF-β per ml (apical versus basolateral: 754 ± 58 pg/ml versus 1214 ± 93 pg/ml). As determined by our assay32 we conclude that the TGF-β produced by epithelial cells is biologically active.
Figure 6.
Secretion of TGF-β by polarized uterine epithelial cells in culture. Epithelial cells from intact adult rats were isolated and cultured for 4 days in cell inserts (eight inserts) as described in Materials and Methods. Culture media in apical and basolateral compartments was replaced at 48 h intervals prior to collection on day 4. Following centrifugation at 10 000 g for 5 min and storage at −20°, samples were assayed for TGF-β by bioassay as described in Materials and Methods. Uterine epithelial cells formation of tight junctions was determined by TER measurement in which baseline resistance was 55–60 ohms cm2. (n = 3) **Significantly higher than TGF-β present in apical media (P < 0·001).
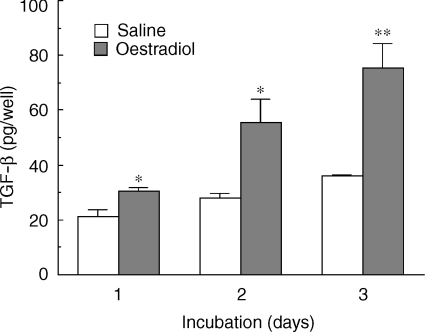
Previous reports have indicated that in response to diethylstilbestrol, a known potent synthetic oestrogen, epithelial cells in the uteri of immature mice produce increasing amounts of TGF-β.29 To determine whether adult rat uterine epithelial cells are hormonally responsive, ovariectomized rats were treated with one injection of saline or oestradiol (2 µg/day) prior to death 24 hr after the last injection. As seen in Fig. 7, oestradiol treatment in vivo significantly increased the production of TGF-β by isolated epithelial cells in culture media beyond that seen with epithelial cells from saline controls measured on days 1, 2 and 3 of culture. In other experiments (not shown), we have found that stimulation of TGF-β production by uterine epithelial cells occurs with as little as 12 hr of in vivo exposure to oestradiol.
Figure 7.
Effect of oestradiol on TGF-β production by uterine epithelial cells in culture. Ovariectomized rats received one injection (100 µl) of oestradiol (2 µg/rat) or saline prior to death 24 hr later. Isolated epithelial cells (3·5 × 105 cells/900 µl) from saline- and oestradiol-treated rats were cultured for 1, 2 and 3 days in RPMI-1640 media with 10% FBS. Media was collected and replaced from four wells per group at 24 hr intervals, centrifuged at 10 000 g for 5 min and stored at −20° until assayed for TGF-β. (n = 2) *Significantly greater than saline controls (P < 0·05). **Significantly greater than control wells (P < 0·005).
Discussion
The studies presented demonstrate that oestradiol regulation of antigen presentation by uterine stromal cells is mediated through the actions of TGF-β. We show that uterine epithelial cells are a primary source of TGF-β which is preferentially secreted at the basolateral surface by cells grown on transwells. In culture, uterine epithelial cells from oestradiol-treated rats produce significantly more biologically active TGF-β than do cells from control animals. These studies demonstrate that the addition of TGF-β to uterine stromal APC inhibits antigen presentation and that antibody neutralization of TGF-βin vitro partially reverses the inhibitory effects of oestradiol on antigen presentation. Overall, these findings suggest a key role for TGF-β produced by uterine epithelial cells in mediating the inhibitory effects of oestradiol on antigen presentation by APC in the stroma of the rat uterus.
An effective immune response in the female reproductive tract is dependent upon antigen presenting cells (APC) processing antigen and presenting it to T cells to induce T-cell activation.34,35 For an immune protection to be initiated, antigen is initially internalized, processed and returned to the cell surface in association with major histocompatibility complex class II for recognition by CD4+ T cells.36 In response to antigen presentation, lymphocytes are activated in a way to affect cytokine production, cytotoxicity, and antibody synthesis. Within the female reproductive tract, both the recognition and response arms of the immune system are present. Central to immune protection and reproductive function is the recognition that female sex hormones act directly and indirectly through a number of immunologically important cytokines to affect most aspects of immune function. Because of the close proximity of the epithelium to the underlying stroma, it is not surprising that both interact to maintain a uterine environment that is both protective against potential pathogens as well as supportive of reproductive function. Previous studies from our laboratory have shown that oestradiol, when given to ovariectomized rats, inhibits antigen presentation by APC in the uterine stroma and stimulates antigen presentation by the epithelial cells that line the uterine lumen.15,18 This paradoxical observation led us to examine the underlying mechanism(s) involved in the regulation by oestradiol of stromal antigen presentation. Previously, we looked at the infiltration of APC into the uterus in response to oestradiol treatment and found that the number of class II positive cells is not affected by a single injection of oestradiol but increases with 2 and 3 days of hormone treatment.5 The recognition that oestradiol stimulates antigen presentation by uterine epithelial cells as well as APC in the spleen15,16 and thymus (Wira, unpublished observation) led us to focus on effects occurring locally in the uterus for an explanation as to the mechanism involved in inhibiting stromal cell antigen presentation.
The inhibition by oestradiol of stromal cell antigen presentation in the uterus parallels that seen in the vagina. Previously, we reported that inhibition of uterine stromal and vaginal antigen presentation occurred with repeated (three daily injections) oestradiol treatment.16,19 The present study extends these findings by demonstrating that uterine stromal inhibition occurs within 24 hr following a single injection of oestradiol (Fig. 3). Moreover, we found that inhibition of antigen presentation in the uterine stroma at 24 hr coincides exactly with that seen in the vagina in that a single treatment with oestradiol leads to inhibition of antigen presentation in the vaginal APC.19 As previously reported27 oestradiol stimulates the production of TGF-β by vaginal cells which, in turn, down regulates vaginal antigen presentation. The findings of Takahashi et al. as determined by in situ hybridization, demonstrated in mice, that epithelial cells lining both the uterus and vagina are the primary sources of TGF-β in the female reproductive tract.28 Our findings in the present study suggest that oestradiol regulation of uterine stromal antigen presentation is also mediated through biologically active TGF-β, which is produced by uterine epithelial cells. To the best of our knowledge, our studies are the first demonstration that oestradiol acts through TGF-β as a common denominator, most likely produced by uterine columnar- and vaginal squamous-epithelial cells, to regulate the afferent arm of the immune system in the uterus and vagina. Taken together, these findings suggest that oestradiol acts through TGF-β for regulating the recognition arm of the immune system throughout the female reproductive tract.
Our data demonstrate that uterine epithelial cells grown to confluence on inserts, polarize, form tight junctions and preferentially release TGF-β into the basolateral chamber. It is likely therefore, that these cells in situ produce TGF-β, which is released into the stroma to regulate uterine APC (macrophages, dendritic cells, B cells).5,19,20 Others have shown that uterine epithelial cells from immature mice treated with a synthetic oestrogen, diethylstilboestrol, respond by increasing both TGF-β mRNA uterine epithelial cell and protein expression.29 Our findings extend these studies by showing that, in response to a single in vivo injection of oestradiol, isolated uterine epithelial cells in culture produce significantly more biologically active TGF-β than do epithelial cells from saline controls.
Stromal cells in the uterus play a central role in regulating the effects of oestradiol on epithelial cell function.37,38 In seminal studies, Cooke et al. used the oestradiol receptor knockout mouse in tissue recombination experiments to demonstrate that the stimulatory effects of oestradiol on epithelial cell proliferation are mediated through stromal cell oestradiol receptor α.37 Studies in our laboratory have shown that stromal cells produce a soluble mediator that inhibits polymeric IgA receptor (pIgR) production, stimulates epithelial cell transepithelial resistance and inhibits TNF-α without affecting TGF-β production (39 and Grant and Wira, unpublished observations). Less recognized is the role of epithelial cells in regulating stromal cell function. For example, uterine epithelial cells produce soluble factors which stimulate stromal cell protein synthesis as well as prostaglandin (PGE2 and PGF2α) secretion by stromal cells.40 Our findings extend these observations by demonstrating that epithelial cells, through the production of TGF-β, modulate uterine stromal cell immune function. Taken together, these findings support the hypothesis that stroma and epithelium work as an integrated unit, each producing factors which regulate the growth, differentiation, and immune function of each other.
Unexpectedly, we found that whereas antibody to TGF-β completely reverses the inhibitory effect of oestradiol (1 day) on stromal cell antigen presentation, it only partially reverses the inhibitory effect of oestradiol given to ovariectomized rats for 3 days (Fig. 1). One explanation for these differences is that prolonged exposure to oestradiol (1 versus 3 days) irreversibly alters stromal APC in their ability to present antigens. Alternatively, an additional contributing factor might be the amount of TGF-β APC are exposed to in situ. As shown in Fig. 6, significantly more TGFβ is produced by epithelial cells following 3 days of hormone relative to that seen with a single dose of oestradiol. Further studies are needed to account for these differences.
Communication between epithelial cells and stromal cells during the reproductive cycle is essential for both successful reproduction and immune protection. Antigen presentation by uterine stromal APC is downregulated in response to oestradiol as is antigen presentation by vaginal cells.19 This inhibition parallels our in vivo studies showing that at proestrus, when ovulation is imminent and serum oestradiol levels are known to be elevated, uterine and vaginal antigen presentation is transiently suppressed, only to rise again at diestrous, when serum progesterone levels are predominant.16 While the physiological significance of these findings remains to be established, our vaginal antigen presentation results, as discussed elsewhere27 parallel the time (pro-oestrus/oestrus) when sperm would be deposited in the vagina. This correlation suggests that local inhibition of antigen presentation might reduce the possibility of generating an immune response against sperm antigens with infertility the end result. Less clear is our understanding of why uterine stromal cell antigen presentation is inhibited by oestradiol. Work by others has shown that epithelial cells lining the uterine lumen are critical for the induction of the uterine decidualization reaction (DCR).41,42 Irrespective of optimal hormone priming and mechanical stimulation, in the absence of epithelial cells, stromal cells will not respond to a decidual stimulus. These results indicate that the luminal epithelium is an obligatory transmitter of the stimulus to DCR that cannot be by-passed by trauma. Our findings indicate that signals such as TGF-β are sent from epithelia to stroma possibly to immunologically prepare the stroma for implantation and the attachment/invasion of a conceptus that is allogeneic to the female. While significant evidence has accumulated to demonstrate that local immunosuppression occurs at the implantation site, our findings suggest that the afferent arm of the immune system, which initiates an adaptive immune response, is transiently suppressed in anticipation of fertilization and implantation. Further studies are needed to more fully define the physiological significance of these findings.
In conclusion, our findings indicate that the afferent arm of the mucosal immune system in the rat uterus is under hormonal control. These studies suggest that control of antigen presentation by oestradiol in the uterus is most likely mediated through local cytokine production rather than through direct hormone action on APC. Further, these findings suggest that endocrine balance is an important determinant in the recognition and response of the mucosal immune system to potential pathogens.
Acknowledgments
The authors gratefully thank Dr James Gorham, Department of Pathology, Dartmouth Hitchcock Medical Center for his assistance in setting up the TGF-β assay used in this study. This work was supported by research grants AI-13541 and AI-34478 from NIH and in part by the Norris Cotton Cancer Center Support Grant CA-23108.
Abbreviations
- APC
antigen-presenting cells
- OVA
ovalbumin
References
- 1.Parr MB, Parr EL. Langerhans cells and T lymphocyte subsets in the murine vagina and cervix. Biol Reprod. 1991;44:491. doi: 10.1095/biolreprod44.3.491. [DOI] [PubMed] [Google Scholar]
- 2.Givan AL, White HD, Stern JE, Colby E, Gosselin EJ, Guyre PM, Wira CR. Flow cytometric analysis of leukocytes in the human female reproductive tract. Comparison of Fallopian tube, uterus, cervix, and vagina. Am J Reprod Immunol. 1997;38:350. doi: 10.1111/j.1600-0897.1997.tb00311.x. [DOI] [PubMed] [Google Scholar]
- 3.Bonatz G, Hansmann ML, Buchholz F, Mettler L, Radzun HJ, Semm K. Macrophage- and lymphocyte-subtypes in the endometrium during different phases of the ovarian cycle. Int J Gynecol Obstet. 1992;37:29. doi: 10.1016/0020-7292(92)90974-n. [DOI] [PubMed] [Google Scholar]
- 4.Yeaman GR, Guyre PM, Fanger MW, et al. Unique CD8+ T cell-rich lymphoid aggregates in human uterine endometrium. J Leukocyte Biol. 1997;61:427. [PubMed] [Google Scholar]
- 5.Wira CR, Kaushic C, Richardson JM. Role of sex hormones and cytokines in regulating the mucosal immune system in the female reproductive tract. In: Ogra PL, Mestecky J, Lamm ME, Strober W, McGhee JR, Bienenstock J, editors. Mucosal Immunology. New York: Academic Press; 1999. p. 1449. [Google Scholar]
- 6.Jones RL, Kelly RW, Critchley HO. Chemokine and cyclooxygenase-2 expression in human endometrium coincides with leukocyte accumulation. Hum Reprod. 1997;12:1300. doi: 10.1093/humrep/12.6.1300. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Velasco JA, Arici A. Chemokines and human reproduction. Fertil Steril. 1999;71:983. doi: 10.1016/s0015-0282(99)00120-x. [DOI] [PubMed] [Google Scholar]
- 8.Mestecky J, McGhee JR. Immunoglobulin A (IgA). Molecular and cellular interactions involved in IgA biosynthesis and immune response. Adv Immunol. 1987;40:153. doi: 10.1016/s0065-2776(08)60240-0. [DOI] [PubMed] [Google Scholar]
- 9.Underdown BJ, Schiff JM. Immunoglobulin A. Strategic defense initiative at the mucosal surface. Annu Rev Immunol. 1986;4:389. doi: 10.1146/annurev.iy.04.040186.002133. [DOI] [PubMed] [Google Scholar]
- 10.Richardson JM, Kaushic C, Wira CR. Polymeric immunoglobulin (Ig) receptor production and IgA transcytosis in polarized primary cultures of mature rat uterine epithelial cells. Biol Reprod. 1995;53:488. doi: 10.1095/biolreprod53.3.488. [DOI] [PubMed] [Google Scholar]
- 11.Wahl S, McNeely T, Janoff E, Shugars DC, Worley P, Tucker C, Orenstein JM. Secretory leukocyte protease inhibitor (SLPI) in mucosal fluids inhibits HIV-1. Oral Dis. 1997;3(Suppl. 1):S64. doi: 10.1111/j.1601-0825.1997.tb00377.x. [DOI] [PubMed] [Google Scholar]
- 12.Moriyama A, Shimoya K, Ogata I, et al. Secretory leukocyte protease inhibitor (SLPI) concentrations in cervical mucus of women with normal menstrual cycle. Mol Human Reprod. 1999;5:656. doi: 10.1093/molehr/5.7.656. [DOI] [PubMed] [Google Scholar]
- 13.King AE, Critchley HOD, Kelly RW. Presence of secretory leukocyte protease inhibitor in human endometrium and first trimester decidua suggests an antibacterial protective role. Mol Hum Reprod. 2000;6:191. doi: 10.1093/molehr/6.2.191. [DOI] [PubMed] [Google Scholar]
- 14.Fahey JV, Wira CR. Effect of menstrual status on anti-bacterial activity and secretory leukocyte protease inhibitor production by human uterine epithelial cells in culture. J Infect Dis. 2002;185:1606. doi: 10.1086/340512. [DOI] [PubMed] [Google Scholar]
- 15.Prabhala RH, Wira CR. Sex hormone and IL-6 regulation of antigen presentation by epithelial and stromal cells in the uterus of the rat. J Immunol. 1995;155:5566. [PubMed] [Google Scholar]
- 16.Wira CR, Rossoll RM. Antigen presenting cells in the female reproductive tract: Influence of sex hormones on antigen presentation in the vagina. Immunology. 1995;84:505. [PMC free article] [PubMed] [Google Scholar]
- 17.Wira CR, Fahey JV, Abrahams VM, Rossoll RM. Influence of stage of the reproductive cycle and oestradial on thymus cell antigen presentation. J Steroid Biochem Mol Biol. 2003;84:79. doi: 10.1016/s0960-0760(03)00002-5. [DOI] [PubMed] [Google Scholar]
- 18.Wira CR, Rossoll RM. Antigen presenting cells in the female reproductive tract: Influence of the estrous cycle on antigen presentation by uterine epithelial and stromal cells. Endocrinology. 1995;136:4526. doi: 10.1210/endo.136.10.7664673. [DOI] [PubMed] [Google Scholar]
- 19.Wira CR, Rossoll RM, Kaushic C. Antigen presenting cells in the female reproductive tract: Influence of estradiol on antigen presentation by vaginal cells. Endocrinology. 2000;141:2877. doi: 10.1210/endo.141.8.7594. [DOI] [PubMed] [Google Scholar]
- 20.Kaushic C, Frauendorf E, Rossoll RM, Richardson JM, Wira CR. Influence of estrous cycle on the presence and distribution of immune cells in the rat reproductive tract. Am J Reprod Immunol. 1998;39:209. doi: 10.1111/j.1600-0897.1998.tb00355.x. [DOI] [PubMed] [Google Scholar]
- 21.Tabibzadeh S, Sun XZ. Cytokine expression in human endometrium throughout the menstrual cycle. Hum Reprod. 1992;7:1214. doi: 10.1093/oxfordjournals.humrep.a137829. [DOI] [PubMed] [Google Scholar]
- 22.Simon C, Piquette GN, Frances A, Polan ML. Localization of interleukin-1 type I receptor and interleukin-1 beta in human endometrium throughout the menstrual cycle. J Clin Endocrinol Metab. 1993;77:549. doi: 10.1210/jcem.77.2.8345061. [DOI] [PubMed] [Google Scholar]
- 23.Hunt JS. Expression and regulation of the tumour necrosis factor-alpha gene in the female reproductive tract. Reprod Fertil Dev. 1993;5:141. doi: 10.1071/rd9930141. [DOI] [PubMed] [Google Scholar]
- 24.Chegini N, Zhao Y, Williams RS, Flanders KC. Human uterine tissue throughout the menstrual cycle expresses transforming growth factor-beta 1 (TGF beta 1), TGF beta 2, TGF beta 3, and TGF beta type II receptor messenger ribonucleic acid and protein and contains [125I]TGF beta 1-binding sites. Endocrinology. 1994;135:439. doi: 10.1210/endo.135.1.8013382. [DOI] [PubMed] [Google Scholar]
- 25.Marascalco BA, Flanders KC, Simon JA, Roberts AB, Sporn MB. Symposium on Growth Factors in Reproduction, Serono Symposia. New York: Springer Verlag Inc.; 1990. Immunodetection of 3 isoforms of transforming growth factor Β in the rat uterus in response to steroids; p. 34. [Google Scholar]
- 26.Hua-Lin Chen K, Yelavarthi KK, Hunt JS. 1993 Identification of transforming growth factor Β1 mRNA in virgin and pregnant rat uteri by in situ hybridization. J Reprod Immunol. 1990;25:221. doi: 10.1016/0165-0378(93)90065-p. [DOI] [PubMed] [Google Scholar]
- 27.Wira CR, Roche MA, Rossoll RM. Antigen presentation by vaginal cells. Role of TGFβ as a mediator of estradiol inhibition of antigen presentation. Endocrinology. 2002;143:2872. doi: 10.1210/endo.143.8.8938. [DOI] [PubMed] [Google Scholar]
- 28.Tamada H, McMaster MT, Flanders KC, Andrews GK, Dey SK. Cell type-specific expression of transforming growth factor-beta 1 in the mouse uterus during the periimplantation period. Mol Endocrinol. 1990;4:965. doi: 10.1210/mend-4-7-965. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi T, Eitzman B, Bossert NL, Walmer D, Sparrow K, Flanders KC, McLachlan J, Nelson KG. Transforming growth factors beta 1, beta 2, and beta 3 messenger RNA and protein expression in mouse uterus and vagina during estrogen-induced growth: a comparison to other estrogen-regulated genes. Cell Growth Differ. 1994;5:919. [PubMed] [Google Scholar]
- 30.Tabibzadeh S. Human endometrium. An active site of cytokine production and action. Endocr Rev. 1991;12:272. doi: 10.1210/edrv-12-3-272. [DOI] [PubMed] [Google Scholar]
- 31.Hunt JS, Miller L, Roby KF, Huang J, Platt JS, DeBrot BL. Female steroid hormones regulate production of pro-inflammatory molecules in uterine leukocytes. J Reprod Immunol. 1997;35:87. doi: 10.1016/s0165-0378(97)00060-0. [DOI] [PubMed] [Google Scholar]
- 32.Chen HL, Yang YP, Hu XL, Yelavarthi KK, Fishback JL, Hunt JS. Tumor necrosis factor alpha mRNA and protein are present in human placental and uterine cells at early and late stages of gestation. Am J Pathol. 1991;139:327. [PMC free article] [PubMed] [Google Scholar]
- 33.Abe M, Harpel JG, Metz CN, Nunes I, Loskutoff DJ, Rifkin DB. An assay for transforming growth factor-Β using cells transfected with a plasminogen activator inhibitor-1 promoter–luciferase construct. Anal Biochem. 1994;216:276. doi: 10.1006/abio.1994.1042. [DOI] [PubMed] [Google Scholar]
- 34.Ziegler K, Unanue ER. Identification of a macrophage antigen-processing event required for Ia region-restricted antigen presentation to T-lymphocytes. J Immunol. 1981;127::1869. [PubMed] [Google Scholar]
- 35.Ashwell JD, DeFranco AL, Paul WE, Schwartz RH. Antigen presentation by resting B cells. Radiosensitivity of the antigen-presentation function and two distinct pathways of T cell activation. J Exp Med. 1984;159:881. doi: 10.1084/jem.159.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weinberger O, Herrmann S, Mescher MF, Benacerraf B, Burakaff SJ. Antigen presenting cell function in induction of helper T cells for cytotoxic T-lymphocyte responses: Evidence for antigen processing. Proc Natl Acad Sci USA. 1981;78::1796. doi: 10.1073/pnas.78.3.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cooke PS, Buchanan DL, Young P, et al. Stromal estrogen receptors mediate mitogenic effects of estradiol on uterine epithelium. Proc Natl Acad Sci USA. 1997;94:6535. doi: 10.1073/pnas.94.12.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iruela-Arispe ML, Rodriguez-Manzaneque JC, Abu-Jawdeh G. Endometrial endothelial cells express estrogen and progesterone receptors and exhibit a tissue specific response to angiogenic growth factors. Microcirculation. 1999;6:127. [PubMed] [Google Scholar]
- 39.Richardson JM, Wira CR. Uterine stromal cell suppression of pIgR production by uterine epithelial cells in vitro: a mechanism for regulation of pIgR production. J Reprod Immunol. 1997;33:95. doi: 10.1016/s0165-0378(97)00015-6. [DOI] [PubMed] [Google Scholar]
- 40.Jacobs AL, Carson DD. Uterine epithelial cell secretion in interleukin-1α induces prostaglandin E2 (PGE2) and PGF2α secretion by uterine stromal cells in vitro. Endocrinology. 1993;132:300. doi: 10.1210/endo.132.1.8419129. [DOI] [PubMed] [Google Scholar]
- 41.Lejeune B, Leroy F. Role of uterine epithelium in inducing the decidual cell reaction. Prog Reprod Biol. 1980;7:92. [Google Scholar]
- 42.Lejeune B, van Hoek J, Leroy F. Transmitter role of the luminal uterine epithelium in the induction of decidualization in the rat. J Reprod Fertil. 1981;61:235. doi: 10.1530/jrf.0.0610235. [DOI] [PubMed] [Google Scholar]