Abstract
(C57BL/6 × DBA/2) F1-hybrid mice injected with lymphoid cells from wild-type, C57BL/6 donors develop acute, lethal graft-versus-host disease (GVHD) in which the intestine is a major target. In its destructive phase intestinal GVHD is characterized by apoptosis of intestinal crypt epithelial cells and the development of endotoxaemia. Injection of as little as 10 μg endotoxin is lethal in mice with acute GVHD, and associated with the release of large amounts of tumour necrosis factor-α (TNF-α) into the serum. To explore the role of interferon-γ (IFN-γ) in the pathogenesis of intestinal GVHD we used IFN-γ gene knockout (gko) mice as donors. Recipients of grafts from these donors did not develop intestinal GVHD and, unlike recipients of wild-type grafts, did not die when injected with lipopolysaccharide (LPS). We also found that injection 10 μg LPS into recipients of wild-type grafts induced apoptosis of intestinal epithelial crypt cells and was associated with a burst of nitric oxide production in the intestine. Administration of Nωnitro l-arginine methyl ester blocked this response. In contrast, LPS did not induce either intestinal epithelial cell apoptosis or increased nitric oxide production in recipients of IFN-γ gko grafts. These findings indicate that donor-derived IFN-γ is instrumental for the development of intestinal GVHD. In a previous study we showed that recipients of IFN-γ gko grafts develop high levels of LPS-induced TNF-α release. When our current data are viewed in the context of this observation, they suggest that intestinal epithelial cell apoptosis in the parent→F1-hybrid model of acute GVHD is mediated primarily by nitric oxide rather than TNF-α, and that this depends on donor-derived IFN-γ.
Introduction
Graft-versus-host disease (GVHD) is a serious and potentially fatal complication of allogeneic bone marrow transplantation. Acute GVHD is a rapidly progressive syndrome characterized by profound wasting,1,2 immunosuppression3–5 and tissue injury in a number of organs including the skin, liver, intestinal mucosa6 and lung.7 Without major immunosuppressive therapy, GVHD is almost invariably fatal.
Endotoxin is purported to play a major role in the pathogenesis of acute GVHD. This was first recognized in a study showing that gnotobiotic mice housed in pathogen-free conditions do not develop acute GVHD when infused with allogeneic lymphoid cell grafts.8 It has since been shown that antimicrobial chemotherapy targeted to intestinal, anaerobic, Gram-negative bacteria significantly reduces the severity of acute GVHD in human bone marrow transplant recipients.9,10 Further evidence of endotoxin's importance in acute GVHD comes from the observation that GVH mice die when injected with a dose of endotoxin too low to have any discernible effect in control mice.11 This phenomenon is associated with the appearance of high levels of tumour necrosis factor-α (TNF-α) in the circulation and the development of endotoxaemic shock. It has been suggested that the heightened sensitivity to lipopolysaccharide (LPS) in GVHD is the result of interferon-γ (IFN-γ) -induced priming of macrophages for TNF-α release. This has been further supported by the observation that GVH mice do not develop this sensitivity if IFN-γ production is inhibited by the use of polarized T helper type 2 (Th2) cells.12
Bacterial LPS can be detected within the liver of GVH mice as early as day 2 post-induction13 and somewhat later in the serum, at a time when mice begin to succumb to the disease.14,15 The translocation of enteric endotoxin into the systemic circulation is caused by lesions that compromise the barrier function of the intestinal epithelium to endotoxin.14,16 Both TNF-α1,17–19 and nitric oxide (NO)20 have been implicated as mediators of intestinal epithelial cell injury in acute GVHD. There is evidence suggesting that IFN-γ may also be involved in the development of intestinal GVHD.21 Our previous work has shown that recipients of IFN-γ gene knockout (gko) grafts develop chronic, rather than acute GVHD and survive for 45 to >100 days post-induction.22 They also develop lesions in a number of target organs, including the salivary gland, lung, liver and skin. These infiltrates are much larger than those seen in recipients of wild-type grafts and are rich in eosinophils. GVHD developing in IFN-γ gko recipients is associated with high levels of the Th2 cytokines interleukin-4 (IL-4), IL-5 and IL-13. Despite the absence of significant IFN-γ levels, recipients of IFN-γ gko grafts still demonstrate macrophage priming for LPS-induced TNF-α release at a level comparable to that seen in recipients of wild-type grafts, suggesting that in GVH mice, cytokines other than IFN-γ have the capacity to prime macrophages for LPS-induced TNF-α release and that TNF-α may not be the only mediator of septic shock in GVH mice.
Several studies have demonstrated that levels of both NO and its metabolites increase in mice with acute GVHD. One study showed that treatment with aminoguanidine (AG), an NO synthesis inhibitor, significantly reduces mortality in the C57BL/6→(C57BL/6 × DBA/2) F1-hybrid model of acute, lethal GVHD, the model we also employ.23 This finding firmly supports a role for NO in the pathogenetic mechanism of acute GVHD. A more specific role for NO in the development of GVHD-associated enteropathy was identified in experiments demonstrating that AG treatment prolongs survival and reduces the levels of both NO production and bacterial translocation across the intestine in rats with GVHD produced as a result of small bowel transplantation.24 This finding is corroborated by results from other experiments showing that treatment of GVH mice with l-NG-monomethyl arginine (l-NMMA), another inhibitor of NO synthesis, abrogates GVHD-associated enteropathy and reduces lymphocytic infiltration in the intestinal epithelium.20
In a pilot study, we found that we can consistently induce intestinal epithelial cell apoptosis in GVH mice as early as day 8 post-induction by injecting them with as little as 10 μg LPS intravenously. This lesion develops approximately 90 min after LPS injection and is never seen in controls. We do not entirely understand how LPS induces this lesion, but we believe that it short circuits the spontaneous mechanism by triggering mucosal macrophages to release NO. The purpose of our current study was to use this experimental approach to further explore the roles of NO and IFN-γ in the development of intestinal epithelial cell death in acute GVHD.
Materials and methods
Mice
Female B6·129S7-Ifngtm1Ts (IFN-γ gko) mice were obtained from The Jackson Laboratory (Bar Harbor, ME), housed in filter-capped cages and used at 13–16 weeks of age. Female C57BL/6J (H-2b, hereafter referred to as wild-type) donors and (C57BL/6J × DBA/2J) F1-hybrid recipients (H-2b/d, hereafter referred to as B6D2F1) were also obtained from The Jackson Laboratory and used at 13–16 weeks of age. All experimentation was performed according to the guidelines outlined by the Canadian Council on Animal Care.
Induction of GVHD
Graft-versus-host reactions were induced in 13–16-week-old B6D2F1-hybrid recipients using either wild-type or IFN-γ gko donors. Donors were age- and gender-matched to the recipients. The method used to induce GVHD has been described in detail previously.25 Briefly, lymph nodes and spleens were harvested from donors, pooled and dissociated into a cell suspension using a stainless steel wire mesh. The suspension was then washed and filtered through gauze. Recipients were injected via the tail vein with 100 × 106 donor cells suspended in 300 μl Hanks' balanced salt solution (HBSS).
Identification of apoptotic crypt cells in intestinal tissue from GVH mice
Recipient mice were monitored daily for weight loss and signs of morbidity, including ruffled fur, a hunched posture and a marked reduction in activity level. Recipients of wild-type grafts were killed on day 15 post-induction or later, when they appeared moribund. Intestinal sections were then collected for histopathological analysis. Intestinal sections were also collected from recipients of IFN-γ gko grafts on days 1, 4, 8, 17, 35, 70 and 100 post-induction for histopathological analysis. On days 8 and 15 post-induction, recipients of wild-type grafts and recipients of IFN-γ gko grafts and B6D2F1-hybrid control mice were given a single intravenous (i.v.) injection of 10 μg LPS in phosphate-buffered saline (PBS). Ninety minutes later, mice were killed by CO2 asphyxiation. Sections of ileum, jejunum and colon were collected, cut longitudinally, pinned open on Styrofoam blocks, fixed in 10% neutral-buffered formalin for 24 hr, machine-processed through graded alcohol, and embedded in paraffin. Four-micrometre sections were cut, stained with haematoxylin & eosin (H & E) and examined by light microscopy. Apoptotic intestinal epithelial crypt cells were identified by the presence of the following features: nuclear condensation, nuclear fragmentation, vacuolation and loss of cytoplasmic integrity. We counted the number of apoptotic cells per 100 intestinal epithelial crypt cells in sections of jejunum collected from recipients of wild-type grafts, recipients of IFN-γ gko grafts and B6D2F1-hybrid controls that either had, or had not been injected with LPS 90 min before being killed. We also performed this analysis using sections of jejunum collected from recipients of wild-type grafts that had been injected with l-NAME and then with LPS, 1 hr later.
To confirm our morphological findings, we employed the TUNEL technique using the Apop Tag Kit (Oncor, Gaithersburg, MD). A summary of the procedure, performed according to the manufacturer's instructions, follows. Unstained intestinal sections were prepared as described for histopathological analyses. Paraffin was removed by washing the sections, sequentially, in xylene, graded alcohol and PBS. A 15-min incubation, at 25°, in Proteinase K (Mallinkrodt, Phillipsburg, NJ) was performed and sections were washed in double-distilled water (ddH2O). Endogenous peroxidase was quenched by incubating sections with 2% hydrogen peroxide for 5 min at 25°. Sections were washed in PBS, incubated in Equilibration Buffer for 10–15 seconds at 25°, and incubated at 37° for 1 hr in terminal deoxynucleotide transferase. Sections were washed in Stop/Wash Buffer for 30 min at 37° and then in PBS for 5 min at 25°. A 30-min incubation, at 25°, in anti-digoxigenin-conjugated–peroxidase antibody followed. Sections were washed in PBS at 25° and incubated for 4 min at 25° in substrate containing 0·05% wt/v diaminobenzidine (Sigma, St Louis, MO) in PBS and 0·02% v/v hydrogen peroxide. Three washes in ddH2O followed. Sections were incubated, for 10 min at 25°, with 0·5% w/v methylene green (Sigma) in 0·1 m sodium acetate (pH 4·0), washed in ddH2O and then in 100% butanol, dehydrated in xylene, and mounted in Permount (Fischer Scientific, Ottawa, ON, Canada).
Measurement of NO in intestinal tissue
In a separate experiment, performed on days 4, 8 and 13 post-induction, recipients of wild-type grafts and recipients of IFN-γ gko grafts were injected with NO spin-trapping agents 30 min before being killed. The level of NO was measured in intestinal tissue using electron paramagnetic resonance (EPR) spectroscopy. This method has been used previously to measure NO levels in hepatic tissue26 but, to our knowledge, has never been used to measure NO in intestinal tissue. To determine whether LPS would increase NO levels within the intestines of GVH mice, another series of experiments was performed in which recipients of wild-type grafts and recipients of IFN-γ gko grafts were injected with 10 μg LPS, i.v., 60 min before the injection of NO spin-trapping agents. They were killed 30 min later and intestinal tissue was collected for the measurement of trapped NO. Levels of NO were also determined, using the same method, in intestinal tissue from B6D2F1 control mice.
NO spin-trapping agents consisted of diethyldithiocarbamate (DETC; Sigma) and FeSO4 (Sigma)/citrate. DETC was administered in saline, intraperitoneally (i.p.), at a dose of 400 mg/kg. FeSO4/sodium citrate (trisodium salt; formula weight 294·1) was mixed in water and administered subcutaneously, at a dose of 40 mg/kg FeSO4 and 200 mg/kg sodium citrate. A section of intestine extending from the distal end of the duodenum to an area approximately 2 cm proximal to the ileocaecal valve was removed immediately post-mortem, cut longitudinally and rinsed in cold HBSS. The tissue was placed in a pre-cooled Petri dish, minced with scissors, packed into a pre-cooled 5-cm3 syringe, and injected into a pre-cooled, Suprasil, synthetic quartz tube (2·4 mm, inner diameter; Heraeus Amersil, Atlanta, GA). Each tube containing intestinal tissue was immediately frozen in liquid nitrogen. Samples were stored in liquid nitrogen until EPR spectroscopy was performed.
EPR spectroscopy, used for the measurement of trapped ON-Fe2+–(DETC)2 complex, was carried out at 125 K (− 158°; temperature controller Model BVT-3000; Bruker Spectrospin Ltd, Karlsruhe, Germany). The spectra were measured with a Bruker Model EMX EPR X-band spectrometer system operating at 9·25 GHz with 100 KHz modulation. The instrument settings were as follows: microwave power, 5 mW; modulation amplitude, 5 Gauss (G) and signal level, 1 × 103; and scan range, 500 G. The amount of the ON-Fe2+–(DETC)2 complex in each sample was assumed to be proportional to the signal amplitude (peak-to-trough) of the triplet-hyperfine structure (hyperfine splitting of 13 G) observed at g = 2·04 (where g is the absolute magnetic field position of the line of the EPR spectrum). Data were expressed as relative EPR signal intensities (arbitrary units) after subtracting the Cu2+–(DETC)2 complex signal observed in all samples. win-epr software (Bruker Spectrospin, Ltd) was employed for nearly all EPR data manipulation on a Compaq Deskpro P500 computer. We were able to convert the measured EPR peak-to-trough signal amplitudes into nanomoles of trapped ON-Fe2+–(DETC)2 per gram of wet tissue before freezing. This calculation involved the double integration of the EPR spectrum from a sample in which the ON-Fe2+–(DETC)2 produced a strong signal relative to that produced by a standard sample containing a known amount of NO that had been quantified under similar conditions. The method used for this calibration, which is associated with a 20% error, has been used previously.26 Our results indicate that a value of 495 relative EPR units is equal to 100 nmol ON-Fe2+–(DETC)2 per gram of wet tissue.
Inhibition of NO synthesis by l-NAME and l-NIL
On day 8 post-induction, recipients of wild-type grafts were injected with NωNitro l-arginine methyl ester (l-NAME; Sigma), in PBS, i.p., at a dose of 5 mg/kg. Some recipients of wild-type grafts were injected with N6-(1-Iminoethyl)-l-lysine (l-NIL; Tocris Cookson Limited, Bristol, UK), instead of l-NAME, i.p., at a dose of 5 mg/kg. l-NIL is 30-fold more selective for the inducible form of NO synthase (iNOS) than for the constitutive forms.27,28 This was followed, 60 min later, by an injection of 10 μg LPS, i.v. Sections of ileum, jejunum and colon were collected and examined, histopathologically, 90 min later, for the presence of apoptotic crypt cells within the intestine.
In a separate experiment, recipients of wild-type grafts were injected first with l-NAME, second with LPS (60 min later), and third with NO spin-trapping agents (60 min later). Recipients were killed 30 min after injection of the NO trapping agents, and intestinal tissue was collected for the measurement of NO using EPR spectroscopy.
Quantification of intestinal IFN-γ mRNA levels by RNAse protection assay (RPA)
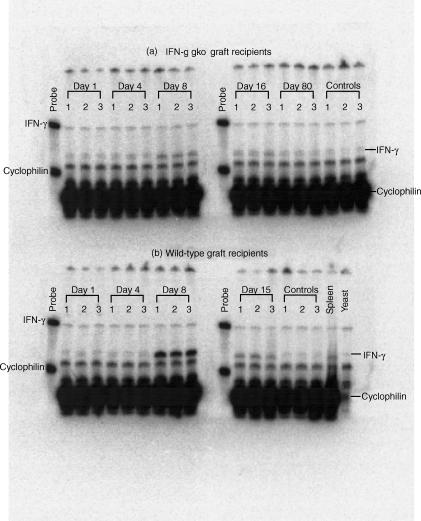
Intestinal tissues from recipients of wild-type grafts and recipients of IFN-γ gko grafts were collected immediately post-mortem. A section of intestine extending from the distal end of the duodenum to an area approximately 2 cm proximal to the ileocaecal valve was removed and snap-frozen in liquid nitrogen. Total RNA was extracted with STAT 60 (Tel-Test ‘B’, Friendswood, TX) and quantified using OD260/280. An aliquot was run on denaturing agarose gel to assess RNA integrity. 32P-labelled murine probes were synthesized from linearized plasmid templates using α32P-UTP and the appropriate RNA polymerase, and gel purified. The IFN-γ probe corresponded to nucleotides 379–557 from Gb: M28621. The cyclophilin probe was obtained from Ambion (Austin, TX). A standard RPA protocol (RPAII kit, Ambion) was used. Fifty micrograms RNA and 3·3 × 105 counts per min of each probe were hybridized at 55° overnight followed by RNase digestion and precipitation. Samples were run on a 6% TBE urea gel, which was then dried at 80°, exposed on a phosphorscreen overnight and scanned in a phosphorimager (Molecular Dynamics, Sunnyvale, CA). Bands produced by IFN-γ mRNA were normalized to cyclophilin bands and quantified with image quant software using local average background correction.
Results
Apoptotic lesions are absent from the intestines of recipients of IFN-γ gko grafts
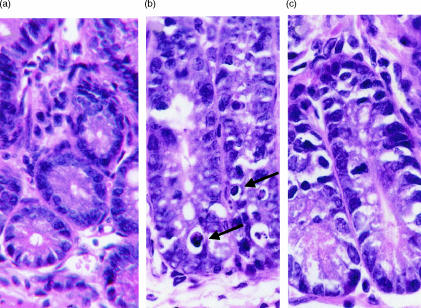
Intestinal lesions typically seen in the later stages of acute GVHD consist of apoptotic intestinal crypt cells.15 We were unable to identify the lesions in intestinal sections from B6D2F1 control mice that did not receive grafts. A representative photomicrograph of a section of tissue from a control mouse is shown in Fig. 1(a). Figure 1(b) shows a lesion of this type in a section of jejunum collected from a wild-type graft recipient on day 17 post-induction. The lesions were also observed in sections of ileum and colon collected from moribund, recipients of wild-type grafts (data not shown). Figure 1(c) shows a representative photomicrograph of a section of jejunum collected from an IFN-γ gko graft recipient on day 17 post-induction. The lesions seen in recipients of wild-type grafts were consistently absent from sections of jejunum, ileum and colon that had been collected from recipients of IFN-γ gko grafts on days 1, 4, 8, 17, 35, 70 and 100 post-induction (data not shown).
Figure 1.
Photomicrographs of H & E-stained sections of jejunum from B6D2F1 control mice (a), recipients of wild-type grafts (b), and recipients of IFN-γ gko grafts (c). Both (b) and (c) are from sections that were harvested from recipient mice on day 17 post-induction. Magnifications were ×400. Arrows in (b) indicate examples of apoptotic epithelial crypt cells.
Recipients of IFN-γ gko grafts do not succumb to a sublethal dose of LPS
Table 1 shows that 100% of the recipients of wild-type grafts died following the injection of 10 μg LPS i.v., a finding which is consistent with that of Nestel and colleagues who first performed this experiment.11 Death occurred within 24 hr of the injection in these recipients, whereas none of the recipients of IFN-γ gko grafts died in response to the LPS injection. Our observation that none of the B6D2F1-hybrid mice died following the injection of 10 μg LPS demonstrates that this dose is sublethal in healthy mice that do not have GVHD.
Table 1.
Number of mice surviving 36 hr after the injection of LPS
| Experimental group | No. of survivors |
|---|---|
| Control B6D2F1-hybrids* | 6 |
| Recipients of wild-type grafts† | 0 |
| Recipients of IFN-γ gko grafts† | 6 |
B6D2F1-hybrid control mice were age- and sex-matched to recipient mice but did not receive grafts.
Recipient mice were injected with 10 μg LPS i.v. on day 15 post-induction.
LPS induces apoptosis in the intestines of recipients of wild-type grafts, but not in the intestines of recipients of IFN-γ gko grafts
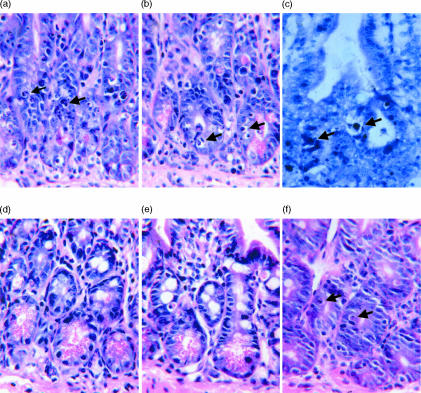
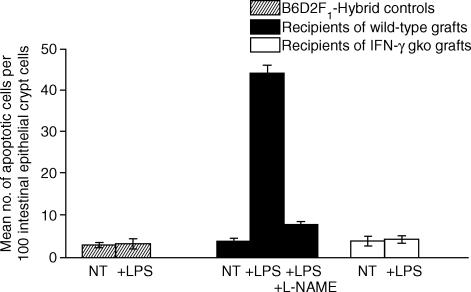
We found that we could induce the development of apoptotic intestinal lesions in GVH mice on either day 8 post-induction, or day 13 post-induction (data not shown) by injecting them with a small amount of LPS (10 μg i.v.). The lesions, as seen in the section stained with H & E (Fig. 2a,b), and in a section stained with the Apop Tag Kit (Fig. 2c), were present 90 min after the injection of LPS. Apoptotic lesions of this type and of the magnitude observed in recipients of wild-type grafts were not seen in intestinal sections from LPS-injected recipients of IFN-γ gko grafts (Fig. 2d), in sections from LPS-injected B6D2F1-hybrid control mice (Fig. 2e), or in sections from LPS-injected recipients of wild-type grafts that received l-NAME prior to the LPS injection (Fig. 2f). A small number of apoptotic intestinal epithelial crypt cells were, however, present in sections from the latter three treatment groups. When we counted the number of apoptotic intestinal epithelial crypts cells present within sections collected on day 8 post-induction, we found that the number present in sections from LPS-injected recipients of wild-type grafts was significantly higher than that for all of the other treatment groups (P < 0·001). These results are shown in Fig. 3.
Figure 2.
Photomicrographs of sections of jejunum-collected recipients of wild-type grafts (a–c), recipients of IFN-γ gko grafts (d), B6D2F1-hybrid control mice (e) and recipients of wild-type grafts that were injected with l-NAME (f). All mice received an injection of LPS 90 min before they were killed. In (b) apoptotic cells were stained using the Apop Tag peroxidase kit (Oncor). Arrows indicate examples of apoptotic cells. Sections were collected from recipient mice on day 8 post-induction. Magnification ×400.
Figure 3.
Comparison of the mean number of apoptotic crypt epithelial cells in sections of jejunum collected from groups of B6D2F1-hybrid control mice, recipients of wild-type grafts and recipients of IFN-γ gko grafts on day 8 post-induction. The mean number of apoptotic cells counted per 100 crypt epithelial cells are shown for groups of mice receiving either no treatment (NT) or an injection of 10 μg LPS i.v. (+ LPS). Also shown is the mean number counted for recipients of wild-type grafts that were injected with l-NAME before LPS (+ LPS + l-NAME). Error bars represent the mean SEM determined for three individual mice in each group. The number of apoptotic cells counted in the LPS-injected recipients of wild-type grafts was significantly greater than those counted in all of the other treatment groups, either with or without LPS injection (P < 0·001). It was also significantly higher than the number counted in recipients of wild-type grafts that were injected with l-NAME before the LPS injection (P < 0·001). Statistical analysis were performed using anova followed by a Tukey–Kramer multiple comparison test.
l-NAME blocks LPS-induced apoptosis in the intestines of recipients of wild-type grafts
We wished to determine whether NO is involved in the development of LPS-induced apoptosis by treating recipients of wild-type grafts with l-NAME (5 mg/kg i.p.), 60 min before LPS (10 μg, i.v.) was injected. l-NAME is an l-arginine analogue that inhibits the production of NO by both constitutive and inducible NOS enzymes. Our findings indicate that recipients of wild-type grafts treated with l-NAME do not develop the typical apoptotic response in the intestine following the injection of LPS (Fig. 2f).
LPS induces NO release in the intestines of recipients of wild-type grafts, but not in the intestines of recipients of IFN-γ gko grafts
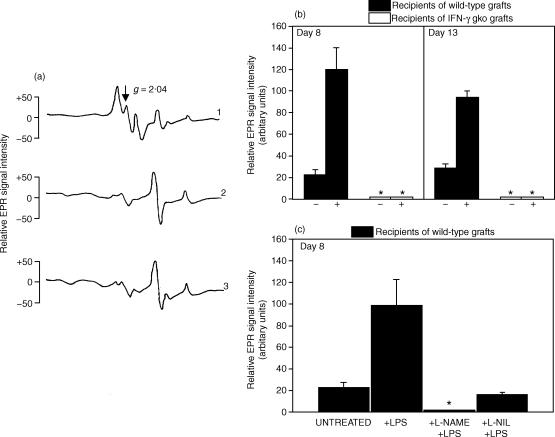
Figure 4(a; trace 1) is a representative EPR spectroscopic plot showing the triplet-hyperfine structure, which indicates the presence of ON-Fe2+–(DETC)2 complex within the intestinal tissue sample. This plot was produced by the analysis of intestinal tissue from recipients of wild-type grafts that had been injected with LPS (10 μg, i.v.), followed by NO-trapping agents (subcutaneous and i.p., 60 min later), on day 8 post-induction. The triplet-hyperfine structure is not seen in Fig. 4(a; trace 2), a representative plot produced by the analysis of intestinal tissue from an untreated B6D2F1 mouse that did not receive a graft. It is also absent in Fig. 4(A; trace 3), a representative plot produced by the analysis of intestinal tissue from recipients of IFN-γ gko grafts that were killed on day 8 post-induction.
Figure 4.
Representative plots of the EPR spectroscopy signals observed in intestinal tissue (a). Mice were injected with the NO spin trapping agents DETC and FeSO4. The relative EPR spectroscopy signal intensities of ON-Fe2+–(DETC)2 complexes were expressed in arbitrary units, which were calculated by measuring the distance from the peak to the trough of the triplet hyperfine structure at the g = 2·04 location. Plot 1 shows the signal from an LPS-injected wild-type graft recipient on day 8. The background signal observed in intestinal tissue from a B6D2F1 control mouse is shown in plot 2. Plot 3 shows the signal from an IFN-γ gko graft recipient on day 8. The upper bar graph (b) shows the NO levels in intestinal tissue from recipients of wild-type grafts and recipients of IFN-γ gko grafts on days 8 and 13 post-induction. The levels for mice that either did (+), or did not (–) receive LPS are shown. The lower bar graph (c) presents results from another experiment in which NO levels were measured in intestinal tissue from recipients of wild-type grafts that were either untreated or that had been injected with either LPS only, l-NAME followed by LPS, or l-NIL followed by LPS. The experiment in (c) was performed on day 8 and all recipients were injected with NO spin-trapping agents 30 min before they were killed. In (b) and (c), an asterisk indicates that a triplet-hyperfine structure produced by the NO-Fe2+–(DETC)2 complex could not be detected by EPR spectroscopy. Error bars in (b) and (c) represent the standard error of the mean NO level determined for three individual mice and the data are expressed as the mean, relative EPR spectroscopy signal intensities of each ON-Fe2+–(DETC)2 complex after subtraction of the Cu2+–(DETC)2 signal. In (b), the LPS-induced NO level was significantly higher than the constitutive level on days 8 (P < 0·001) and 15 (P < 0·01). Similarly, the LPS-induced NO level was significantly higher than the constitutive level in (c) (P < 0·01). It was also significantly higher than the levels observed when l-NAME (P < 0·01) and l-NIL (P < 0·01) were used. Statistical analyses were performed using anova followed by a Tukey–Kramer multiple comparison test.
The ability of LPS to induce NO release in recipients of wild-type grafts is demonstrated in the bar graph shown in Fig. 4(b). On day 8, the increase in the level of NO was approximately six-fold following LPS injection when compared to that seen in uninjected, recipients of wild-type grafts. On day 13 this increase was approximately five-fold following LPS stimulation. The difference between the constitutive and LPS-induced NO levels was statistically significant on both of these days (P < 0·001 and P < 0·01, respectively; anova followed by a Tukey–Kramer multiple comparison test). We did not observe the triplet-hyperfine structure in EPR spectroscopic plots from either untreated or LPS-injected recipients of wild-type grafts on day 4 post-induction (data not shown). Nitric oxide was undetectable in intestinal tissue from recipients of IFN-γ gko grafts on either days 8 or 13 post-induction and the injection of LPS did not alter this result (Fig. 4b). It was also absent from the intestines of recipients of IFN-γ gko grafts on later days in the GVH reaction (day 70 post-induction; data not shown).
NO synthase inhibitors block NO release in intestinal tissue from GVH mice
Our finding that l-NAME blocks LPS-induced apoptosis in intestinal tissue from recipients of wild-type grafts was consistent with results from our EPR spectroscopic analysis showing that intestinal NO was undetectable in recipients of wild-type grafts that had been injected with l-NAME before the LPS injection (Fig. 4c). When l-NIL, a NO synthase inhibitor with a high specificity for the iNOS, was used instead of l-NAME, a small amount of NO was measured in the tissue. This level was similar to that seen in untreated GVH mice, suggesting that iNOS is responsible for the augmentation of NO production that occurs in response to LPS.
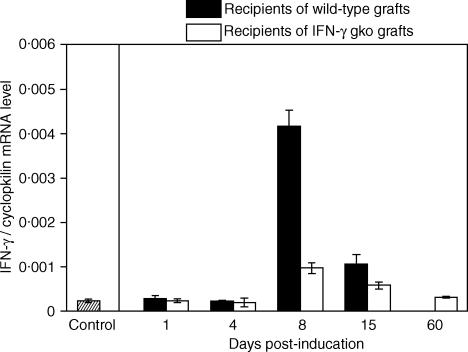
Intestinal IFN-γ mRNA levels increase significantly in recipients of wild-type grafts, but not in recipients of IFN-γ gko grafts
The gel from a representative RPA analysis is shown in Fig. 5. Figure 6 summarizes this data graphically, and shows a marked increase in the mean level of IFN-γ mRNA detected in intestinal tissue collected from recipients of wild-type grafts on day 8 post-induction. This level also increased, to a much lesser extent, in tissue collected from recipients of IFN-γ gko grafts on day 8, and was found to be significantly higher than that seen in control tissue collected from B6D2F1 mice (Student's t-test, P < 0·001). On day 15 post-induction, the level of IFN-γ mRNA observed in tissue from recipients of wild-type grafts decreased by approximately 75%, and was not significantly different from the level seen in recipients of IFN-γ gko grafts on day 15 (Student's t-test, P > 0·05). The mean level observed on day 15 in recipients of IFN-γ gko grafts was lower than that seen on day 8 and was very similar to control values by day 60 post-induction.
Figure 5.
Phosphorimage showing results from an RNase protection assay used to determine the level of IFN-γ mRNA in intestinal tissue from recipients IFN-γ gko grafts (a) and recipients of wild-type grafts (b). Tissue was collected from three mice at each time-point. Mice were killed on days 1, 4, 8 and 15 post-induction in both recipient groups, and on day 80 post-induction in recipients of IFN-γ gko grafts. Control intestinal tissue was harvested from six B6D2F1-hybrid mice that did not receive grafts. Spleen tissue and yeast were run as positive controls. Bands were quantified with image quant software using the local average background correction.
Figure 6.
Kinetics of IFN-γ mRNA production in intestinal tissue from recipients of wild-type grafts and recipients of IFN-γ gko grafts. IFN-γ levels were measured using an RNase protection assay. Controls consisted of intestinal tissue from B6D2F1 control mice that did not receive grafts. Error bars represent the standard error of the mean IFN-γ levels determined for three individual mice. On day 8 post-induction, the mean level of IFN-γ mRNA level seen in IFN-γ gko graft recipients was significantly higher than that seen in the control (Student's t-test, P < 0·001). On day 15 post-induction, the levels seen in recipients of wild-type grafts and recipients of IFN-γ gko grafts were not significantly different (Student's t-test P > 0·05).
Discussion
In this study we found that recipients of allogeneic lymphoid cell grafts from IFN-γ gko donors do not develop lesions characteristic of intestinal GVHD and that, unlike recipients of wild-type grafts, they do not die when injected with a sublethal dose of LPS. We also observed that the administration of exogenous LPS induces apoptosis of intestinal epithelial crypt cells in recipients of wild-type grafts and is associated with a burst of NO production in the intestine. Administering l-NAME before the LPS is injected can block both of these responses. On the other hand, if LPS is given to recipients of IFN-γ gko grafts, it does not induce either intestinal epithelial cell apoptosis or increased NO production. Furthermore, recipients of IFN-γ gko grafts do not demonstrate an increase in the number of proliferating intestinal basal crypt cells (Ellison and Gartner, unpublished observations), a characteristic feature of intestinal GVHD.29,30 These findings indicate that donor-derived IFN-γ is intimately involved in the mechanism of intestinal GVHD.
The heightened sensitivity of GVH mice to small amounts of endotoxin is an important component in the pathogenesis of acute GVHD.11 When the phenomenon was first observed, it was attributed to the release of large amounts of TNF-α into the circulation and the subsequent development of endotoxaemic shock. It was hypothesized that IFN-γ-primed macrophages were the source of TNF-α in GVH mice. One of the aims of our investigation was to study the relationship between LPS, IFN-γ and mediators such as TNF-α and NO. Both of these mediators have been implicated in the pathogenesis of intestinal GVHD.1,17–20 In a previous study, we showed that recipients of IFN-γ gko grafts develop a form of chronic GVHD associated with the production of Th2 cytokines (IL-4, IL-5 and IL-13) and that, despite the absence of donor-derived IFN-γ, large amounts of TNF-α are still released following the injection of a sublethal dose of LPS. This suggests that some factor other than IFN-γ is responsible for macrophage priming and that priming for LPS-induced TNF-α release can occur in chronic GVHD as well. In our present study, we found that, even with this LPS-induced TNF-α burst, recipients of IFN-γ gko grafts do not die in response to LPS given in a dose that is invariably lethal in recipients of wild-type grafts. These findings provide strong evidence that TNF-α, in and of itself, is not responsible for the lethal effects of LPS in GVH mice.
Intestinal lesions play a pivotal role in the development of endotoxaemic shock in acute GVHD by providing a portal of entry for Gram-negative bacteria and LPS to enter the host. Mowat has described three phases of intestinal GVHD.30 The first, known as the ‘proliferative’ phase, is characterized by increased crypt cell mitotic activity, crypt lengthening and infiltration of the epithelium by lymphocytes. In the second, known as the ‘destructive’ phase, crypt cell hyperplasia, crypt stem cell apoptosis and necrosis, villus atrophy, and the loss of mucosal lymphoid cells are seen. Mucosal thinning, crypt hypoplasia and mucosal lymphopenia characterize the third, or ‘atrophic’ phase of the disease. Depending on the severity of GVHD, the enteropathy may not progress beyond the first or second phases. The factors responsible for the initial proliferative response in crypts cells are not known, but transforming growth factor-β has been suggested as a possible mediator.30 Results from other experiments aimed at identifying the factors that mediate intestinal epithelial cell destruction have been equivocal. Some studies have suggested that TNF-α may be involved,1,17–19 but all of these have employed an experimental model of GVHD in which irradiation was used as a preconditioning regimen. Using a murine model, Hill and colleagues showed that increasing the dose of total body irradiation from 900 cGy to 1300 cGy increased the severity of GVHD, and was associated with more severe, early intestinal GVHD.31 Both mortality and morbidity decreased in recipients given the higher dose of total body irradiation when they were treated with recombinant human TNF receptor:Fc to block the effects of TNF-α. This study suggests that higher doses of irradiation might cause more severe intestinal injury by augmenting TNF-α release. Whether the lower doses of total body irradiation that are sometimes used in murine models could also contribute to the development of intestinal injury through a mechanism involving TNF-α is not known. Although our findings indicate that NO is the principal mediator of intestinal epithelial cell apoptosis, we cannot exclude the possibility that TNF-α may act synergistically with IFN-γ, NO and LPS to induce this response.
We did not observe evidence of either the proliferative or the destructive phases of intestinal GVHD in recipients of IFN-γ gko grafts. This finding is consistent with that of Mowat, who showed that antibody neutralization of IFN-γ prevents all aspects of enteropathy in GVH mice.21 These observations suggest that IFN-γ plays a pivotal role in the pathogenesis of intestinal GVHD, but how it is specifically involved is not known. Our observation that exogenous LPS triggers intestinal epithelial crypt cell apoptosis in recipients of wild-type grafts, but not in recipients of IFN-γ gko grafts, may help to elucidate the role of IFN-γ in this process. We found that injection of LPS is associated not only with the development of apoptotic lesions in the intestine, but also with a burst of NO production. We were able to block both of these responses by administering l-NAME before LPS was injected. Garside and colleagues previously showed that the administration of l-NMMA, another specific inhibitor of NO synthesis, abolishes the development of intestinal pathology.20 This work was also performed using a parent→F1-hybrid model of acute GVHD in which no irradiation was used to condition the recipient. Results from both this study and our own firmly support a role for NO in the development of intestinal injury. Our finding that LPS could not induce either intestinal epithelial cell apoptosis or an NO burst in recipients of IFN-γ gko grafts, indicate that both of these responses depend on donor-derived IFN-γ. We hypothesize that IFN-γ primes macrophages to release NO in response to LPS and that macrophage-derived NO is the principal mediator of intestinal injury. Our findings also suggest that NO may have a more important role in the development of intestinal epithelial cell injury than TNF-α does in a model in which irradiation is not employed.
In our study, we have chosen to focus on the mechanism underlying intestinal epithelial cell apoptosis in GVH mice, rather than on other parameters of intestinal pathology, such as villus damage or the infiltration of mononuclear cells. Intestinal crypt cell apoptosis is seen only rarely in GVH mice, and, since the point at which individual GVH mice succumb is highly variable, finding this lesion during the course of the disease is fortuitous. Because the intestine undergoes post-mortem autolysis very quickly and because sequential intestinal mucosal biopsies are not feasible in mice, it is impossible to study the lesion either morphologically or functionally in mice that die spontaneously. We have shown that we can consistently induce apoptosis of intestinal epithelial cells in GVH mice as early as day 8 post-induction, by injecting them with 10 μg LPS i.v. The resulting lesions are indistinguishable from those occurring spontaneously later in the reaction. We propose that exogenous LPS short circuits the development of the lesion that would occur spontaneously by triggering the release of NO from IFN-γ-primed macrophages within the lamina propria. The released NO then induces the apoptosis of intestinal crypt cells, compromising the integrity of the intestinal epithelium and making it more permeable to endotoxin. As this occurs, endotoxin accumulates within the lamina propria and a vicious cycle of intestinal epithelial injury is established in which more endotoxin triggers the release of more NO, and so on. Endotoxin travelling to the liver via the portal circulation eventually reaches the systemic circulation and fatal endotoxaemic shock ensues. While it is possible that this may not be a true representation of the events that trigger intestinal epithelial cell death in GVH mice naturally, our ability to consistently reproduce the pathognomonic lesion of intestinal GVHD using this method has provided us with a reliable ‘experimental window’ through which to study this process.
To summarize, other investigators have shown that intestinal injury associated with acute GVHD can be abrogated by inhibiting either IFN-γ or NO production.20,21 Results from our experiments provide the first evidence that systemically administered LPS induces the release of large amounts of NO in the small intestine, and that this is associated with the apoptosis of intestinal epithelial crypt cells and can be blocked by l-NAME, a NO synthase inhibitor. This technical approach to inducing this cardinal feature of intestinal GVHD is novel and provides a valuable tool for elucidating the precise sequence of events that occurs during the development of this apoptotic response. This is particularly important because it is very difficult to study the spontaneous lesion experimentally. While the participation of LPS in this process may not be unexpected, our data provide the first evidence, albeit indirect, that LPS can trigger the apoptosis of intestinal epithelial cells through a mechanism involving NO. The actual source of NO remains unknown. We have hypothesized that it comes from IFN-γ-primed macrophages, but it is also possible that it may be derived from the epithelial cells themselves, which have also been shown to release NO production in response to LPS.32,33 We have also demonstrated that IFN-γ plays a central role in the pathogenesis of intestinal GVHD, affecting both the proliferative and destructive phases of the disease. Our data suggest that IFN-γ works to potentiate the release of NO, which from our results appears to be the primary mediator of intestinal cell injury in a model in which no irradiation was used.
Acknowledgments
We acknowledge the excellent technical assistance provided by Jacqie Fischer.
Abbreviations
- AG
aminoguanidine
- B6D2F1-hybrid
(C57BL/6 × DBA/2) F1-hybrid
- DETC
diethyldithiocarbamate
- eNOS
endothelial nitric oxide synthase
- EPR
electron paramagnetic resonance
- gko
gene knockout
- GVHD
graft-versus-host disease
- GVH
graft-versus-host
- iNOS
inducible nitric oxide synthase
- l-NAME
Nωnitro l-arginine methyl ester
- l-NMMA
l-NG-monomethyl arginine
- nNOS
neuronal nitric oxide synthase
- NOS
nitric oxide synthase
- TBI
total body irradiation
References
- 1.Piguet PF, Grau GE, Allet B, Vassalli P. Tumor necrosis factor/cachectin is an effector of skin and gut lesions of the acute phase of graft-vs.-host disease. J Exp Med. 1987;166:1280–9. doi: 10.1084/jem.166.5.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holler E, Kolb HJ, Hintermeier-Knabe R, Mittermuller J, Thierfelder S, Kaul M, Wilmanns W. Role of tumor necrosis factor alpha in acute graft-versus-host disease and complications following allogeneic bone marrow transplantation. Transplant Proc. 1993;25:1234–6. [PubMed] [Google Scholar]
- 3.Howard JG, Woodruff MFA. Effect of the graft-versus-host reaction on the immunological responsiveness of the mouse. Proc R Soc Lond. 1961;154:532–9. [Google Scholar]
- 4.Blaese M, Martinez C, Good RA. Immunological incompetence of immunologically runted animals. J Exp Med. 1964;119:211–24. doi: 10.1084/jem.119.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lapp WS, Ghayur T, Mendes M, Seddik M, Seemayer TA. The functional and histological basis for graft-versus-host-induced immunosuppression. Immunol Rev. 1985;88:107–33. doi: 10.1111/j.1600-065x.1985.tb01155.x. [DOI] [PubMed] [Google Scholar]
- 6.Ferrara JL, Deeg HJ. Graft-versus-host disease. N Engl J Med. 1991;324:667–74. doi: 10.1056/NEJM199103073241005. [DOI] [PubMed] [Google Scholar]
- 7.Piguet PF, Grau GE, Collart MA, Vassalli P, Kapanci Y. Pneumopathies of the graft-versus-host reaction. Alveolitis associated with an increased level of tumor necrosis factor mRNA and chronic interstitial pneumonitis. Lab Invest. 1989;61:37–45. [PubMed] [Google Scholar]
- 8.Jones JM, Wilson R, Bealmear PM. Mortality and gross pathology of secondary disease in germfree mouse radiation chimeras. Radiat Res. 1971;45:577–88. [PubMed] [Google Scholar]
- 9.Beelen DW, Elmaagacli A, Muller KD, Hirche H, Schaefer UW. Influence of intestinal bacterial decontamination using metronidazole and ciprofloxacin or ciprofloxacin alone on the development of acute graft-versus-host disease after marrow transplantation in patients with hematologic malignancies: final results and long-term follow-up of an open-label prospective randomized trial. Blood. 1999;93:3267–75. [PubMed] [Google Scholar]
- 10.Beelen DW, Haralambie E, Brandt H, et al. Evidence that sustained growth suppression of intestinal anaerobic bacteria reduces the risk of acute graft-versus-host disease after sibling marrow transplantation. Blood. 1992;80:2668–76. [PubMed] [Google Scholar]
- 11.Nestel FP, Price KS, Seemayer TA, Lapp WS. Macrophage priming and lipopolysaccharide-triggered release of tumor necrosis factor alpha during graft-versus-host disease. J Exp Med. 1992;175:405–13. doi: 10.1084/jem.175.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fowler DH, Kurasawa K, Husebekk A, Cohen PA, Gress RE. Cells of Th2 cytokine phenotype prevent LPS-induced lethality during murine graft-versus-host reaction. Regulation of cytokines and CD8+ lymphoid engraftment. J Immunol. 1994;152:1004–13. [PubMed] [Google Scholar]
- 13.Price KS, Nestel FP, Lapp WS. Progressive accumulation of bacterial lipopolysaccharide in vivo during murine acute graft-versus-host disease. Scand J Immunol. 1997;45:294–300. doi: 10.1046/j.1365-3083.1997.d01-404.x. [DOI] [PubMed] [Google Scholar]
- 14.Nestel F, Kichian KY-TK, Desbarats J, Price K, Ponka P, Lapp W, Seemayer T. The role of endotoxin in the pathogenesis of acute graft-versus-host disease. In: Ferrara JLM, Deeg HJ, Burakoff SJ, editors. Graft-Vs-Host Disease. New York: Marcel Dekker; 1997. pp. 501–23. [Google Scholar]
- 15.Ellison CA, Amadeo RJ, Gartner JG. GVHD-associated enteropathy and endotoxemia in F1-hybrid recipients of NK1.1-depleted grafts. Scand J Immunol. 2001;54:375–82. doi: 10.1046/j.1365-3083.2001.00963.x. [DOI] [PubMed] [Google Scholar]
- 16.Koltun WA, Bloomer MM, Colony P, Kauffman GL. Increased intestinal permeability in rats with graft versus host disease. Gut. 1996;39:291–8. doi: 10.1136/gut.39.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stuber E, Buschenfeld A, von Freier A, Arendt T, Folsch UR. Intestinal crypt cell apoptosis in murine acute graft versus host disease is mediated by tumour necrosis factor alpha and not by the FasL–Fas interaction: effect of pentoxifylline on the development of mucosal atrophy. Gut. 1999;45:229–35. doi: 10.1136/gut.45.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown GR, Lee E, Thiele DL. TNF–TNFR2 interactions are critical for the development of intestinal graft-versus-host disease in MHC class II-disparate (C57BL/6J → C57BL/6J × bm12), F1 mice. J Immunol. 2002;168:3065–71. doi: 10.4049/jimmunol.168.6.3065. [DOI] [PubMed] [Google Scholar]
- 19.Brown GR, Lindberg G, Meddings J, Silva M, Beutler B, Thiele D. Tumor necrosis factor inhibitor ameliorates murine intestinal graft-versus-host disease. Gastroenterology. 1999;116:593–601. doi: 10.1016/s0016-5085(99)70181-2. [DOI] [PubMed] [Google Scholar]
- 20.Garside P, Hutton AK, Severn A, Liew FY, Mowat AM. Nitric oxide mediates intestinal pathology in graft-vs.-host disease. Eur J Immunol. 1992;22:2141–5. doi: 10.1002/eji.1830220827. [DOI] [PubMed] [Google Scholar]
- 21.Mowat AM. Antibodies to IFN-gamma prevent immunologically mediated intestinal damage in murine graft-versus-host reaction. Immunology. 1989;68:18–23. [PMC free article] [PubMed] [Google Scholar]
- 22.Ellison CA, Fischer JM, HayGlass KT, Gartner JG. Murine graft-versus-host disease in an F1-hybrid model using IFN-gamma gene knockout donors. J Immunol. 1998;161:631–40. [PubMed] [Google Scholar]
- 23.Hoffman RA, Nussler NC, Gleixner SL, Zhang G, Ford HR, Langrehr JM, Demetris AJ, Simmons RL. Attenuation of lethal graft-versus-host disease by inhibition of nitric oxide synthase. Transplantation. 1997;63:94–100. doi: 10.1097/00007890-199701150-00018. [DOI] [PubMed] [Google Scholar]
- 24.Langrehr JM, Machens C, Zill E, Leder K, Nussler A, Hoffman R, Neuhaus P. Bacterial translocation during graft-versus-host disease after small bowel transplantation is reduced following inhibition of inducible nitric oxide synthesis. Transplantation. 2000;69:2415–21. doi: 10.1097/00007890-200006150-00035. [DOI] [PubMed] [Google Scholar]
- 25.Gartner JG, Merry AC, Smith CI. An analysis of pulmonary natural killer cell activity in F1-hybrid mice with acute graft-versus-host reactions. Transplantation. 1988;46:879–86. doi: 10.1097/00007890-198812000-00018. [DOI] [PubMed] [Google Scholar]
- 26.Wang HH, McIntosh AR, Hasinoff BB, Rector ES, Ahmed N, Nance DM, Orr FW. B16 melanoma cell arrest in the mouse liver induces nitric oxide release and sinusoidal cytotoxicity: a natural hepatic defense against metastasis. Cancer Res. 2000;60:5862–9. [PubMed] [Google Scholar]
- 27.Moore WM, Webber RK, Jerome GM, Tjoeng FS, Misko TP, Currie MG. L-N6-(1-iminoethyl) lysine: a selective inhibitor of inducible nitric oxide synthase. J Med Chem. 1994;37:3886–8. doi: 10.1021/jm00049a007. [DOI] [PubMed] [Google Scholar]
- 28.Stenger S, Thuring H, Rollinghoff M, Manning P, Bogdan C. L-N6-(1-iminoethyl)-lysine potently inhibits inducible nitric oxide synthase and is superior to NG-monomethyl-arginine in vitro and in vivo. Eur J Pharmacol. 1995;294:703–12. doi: 10.1016/0014-2999(95)00618-4. [DOI] [PubMed] [Google Scholar]
- 29.Wall AJ, Rosenberg JL, Reilly RW. Small intestinal injury in the immunologically runted mouse: morphologic and autoradiographic studies. J Lab Clin Med. 1971;78:833–4. [PubMed] [Google Scholar]
- 30.Mowat AM. Intestinal graft-versus-host disease. In: Ferrara JLM, Deeg HJ, Burakoff SJ, editors. Graft-Vs-Host Disease. New York: Marcel Dekker; 1997. pp. 337–84. [Google Scholar]
- 31.Hill GR, Crawford JM, Cooke KR, Brinson YS, Pan L, Ferrara JL. Total body irradiation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood. 1997;90:3204–13. [PubMed] [Google Scholar]
- 32.Crouser ED, Julian MW, Weinstein DM, Fahy RJ, Bauer JA. Endotoxin-induced ileal mucosal injury and nitric oxide dysregulation are temporally dissociated. Am J Respir Crit Care Med. 2000;161:1705–12. doi: 10.1164/ajrccm.161.5.9907043. [DOI] [PubMed] [Google Scholar]
- 33.Lamarque D, Moran AP, Szepes Z, Delchier JC, Whittle BJ. Cytotoxicity associated with induction of nitric oxide synthase in rat duodenal epithelial cells in vivo by lipopolysaccharide of Helicobacter pylori: inhibition by superoxide dismutase. Br J Pharmacol. 2000;130:1531–8. doi: 10.1038/sj.bjp.0703468. [DOI] [PMC free article] [PubMed] [Google Scholar]