Abstract
Apolipoprotein E (ApoE) is synthesized by a variety of cells including macrophages. These cells activate T lymphocytes by antigen presentation, while the T-cell cytokine, interferon-γ, inhibits macrophage ApoE expression. ApoE inhibits T-cell proliferation in culture but its role in immune responses has been unclear. The ApoE-deficient (E0) mouse permits an evaluation of the immunological role of ApoE. We have analysed T-cell responses to an exogenous antigen (ovalbumin) and polyclonal mitogen (concanavalin A) in E0 and ApoE+/+ mice. Macrophages of E0 mice stimulated T-cell activation more effectively as antigen-presenting cells than macrophages from ApoE+/+ mice. Both proliferation and interferon-γ secretion were enhanced in T cells activated in the context of antigen-presenting cells from E0 mice. Since the macrophage–T-cell interaction depends on interactions between cell surface molecules, we assessed the expression of such molecules after in vivo stimulation with interferon-γ. This treatment caused an increased expression of the co-stimulatory surface proteins CD40 and CD80, and also of the major histocompatibility complex class II molecules I-Ab on macrophages of E0 mice compared with ApoE+/+. Our data suggest that ApoE inhibits T-cell activation by reducing the density of immune stimulatory proteins on antigen-presenting cells.
Introduction
Apolipoprotein E (ApoE) is a multifunctional component of plasma lipoproteins. It is found on very-low-density (VLDL), low-density (LDL) and high-density (HDL) lipoprotein particles and mediates their cellular uptake via the B- and E-receptor as well as the LDL receptor-related protein (LRP) receptor. ApoE is synthesized not only by liver cells but also by a variety of other cells, including those in the intestine, adrenal gland, kidney, lung, spleen, testes, ovary and brain. This broad pattern of expression suggests that ApoE may exert other functions in addition to those relating to lipoprotein metabolism. Indeed, Curtiss and colleagues have shown that ApoE inhibits both luteinizing hormone-stimulated androgen production by ovarian theca cells and the proliferation of activated lymphocytes.1–4 The latter activity may be pathophysiologically important because activated lymphocytes are abundant in atherosclerotic plaques,5–9 that also contain ApoE-secreting macrophages.10–15
The lymphocyte modulatory action of ApoE was originally discovered as an immune-inhibitory activity of LDL in vitro.16–22 This activity could be ascribed to ApoE, and both ApoE-containing lipoproteins and multimers of synthetic ApoE peptides inhibit mitogen- and antigen-induced proliferation of cultured lymphocytes.23–26 ApoE is produced by macrophages,10–15 which activate T lymphocytes by antigen presentation, while activated T cells secrete interferon-γ (IFN-γ), which in turn inhibits macrophage ApoE expression.25 This implies an intricate, ApoE-mediated feedback regulation of immune activation. However, our understanding of ApoE as an immune modulator is based on cell culture experiments and its role in integrative immune regulation has been unclear.
The generation of ApoE-deficient (E0) mice by targeted gene disruption27,28 permits an evaluation of the immunological role of ApoE. We have analysed T-cell responses to antigen and polyclonal mitogen in E0 mice. The lack of ApoE was associated with augmented cellular immune responses as a result of enhanced antigen-presenting activity of macrophages.
Materials and methods
Mice and immunization protocol
Female E0 mice, backcrossed for 10 generations to a C57BL/6 (B6) with H-2b background and female wild-type C57BL/6 (H-2b) mice were obtained from Bomholtgaard Breeding and Research Center, Denmark. E0 mice were derived from the construct generated by Piedrahita et al.28 The mice were fed with standard mouse chow and used for experiments at 8–10 weeks of age. For experiments, five mice were included in each group. Both ovalbumin (OVA, analytic grade) and Concanavalin A (Con A) were purchased from Sigma, St Louis, MO. Recombinant murine IFN-γ was bought from R&D, Abingdon, UK. The E0 and B6 mice were immunized with 100 μg OVA in complete Freund's adjuvant by subcutaneous injections in the heel footpads. The animals were killed 1 week after immunization using an overdose of carbon dioxide. The draining lymph nodes were collected for assay.
Lymphocyte and macrophage preparation
Monocellular suspensions were obtained from inguinal lymph nodes. T cells were enriched on nylon wool columns. The preparations contained around 90% T cells as determined by fluorescence-activated cell sorter using fluorescein isothiocyanate (FITC) conjugated anti-mouse CD3 monoclonal antibody (PharMingen, San Diego, CA). Monocytes/macrophages were harvested from the peritoneal cavity using a standard lavage technique. The eluted cells were adhered to Petri dishes for 120 min in a 37° incubator, detached with a rubber policeman and suspended in cell culture medium. The cells were then irradiated with 50 Gy γ-irradiation at 4° and used as antigen-presenting cells (APC) in T-cell proliferation analysis.
T-cell proliferation assay
Enriched T cells (1·8 × 105 cells/well) were suspended in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal calf serum (FCS) and added in duplicate to round-bottom microtitre wells together with irradiated APC (3 × 104 cells/well). Alternatively, mixed cells were prepared from draining lymph nodes (3 × 105 cells/well). Both preparations were incubated with OVA (100 μg/ml or 1 mg/ml) or Con A (2 μg/ml) for 2–3 days and after addition of 1 μCi/well [3H]thymidine (Amersham, Buckinghamshire, UK) overnight. The cultures were then harvested using an Inotech cell harvester. Radioactivity was determined in a Wallac microtitre beta counter.
T-cell cytokine determination
T cells were enriched and co-cultured with irradiated APC in the presence of OVA (100 μg/ml) for 3 days as described above. Both IFN-γ and interleukin-4 (IL-4) levels in the supernatant were analysed by using sandwich enzyme-linked immunosorbent assay with OptEIA™ antibody sets (PharMingen) and avidin–horseradish peroxidase detection (Vector, Burhingame, CA).
Macrophage activation in vivo
Either sex- and age-matched E0, B6, or B6 background based LDL receptor knockout mice (LDLR0, Bomholtgaard Breeding and Research Center, Denmark) were injected peritoneally with recombinant murine IFN-γ (100 unit/mouse) or phosphate-buffered saline. The peritoneal cells were collected 18 hr after injection using a standard lavage technique, and were directly incubated with biotin-conjugated anti-I-Ab, CD80 (B7-1) or CD40 (PharMingen), followed by binding with avidin-FITC (Dako, Glostrup, Denmark) at 4°. Molecular expression was measured in a FACS Calibur flow cytometer using a monocyte/macrophage gate.
Statistical analysis
Since the distribution of the data was unknown, skewed variables were analysed by Kruskal–Wallis nonparametric anova and the nonparametric Mann–Whitney test. The significance level was set at P = 0.05.
Results
We immunized both ApoE knockout (E0) and wild-type C57BL/6 (B6) mice with a conventional antigen, OVA, and evaluated the proliferative T-cell response by challenging the cells in vitro with this antigen, as well as with the polyclonal mitogen, Con A. Since E0 mice on the B6 background were used, we could compare the responses with those obtained in major histocompatibility complex (MHC) haplotype identical wild-type mice.
Enhanced primary immune response in E0 mice
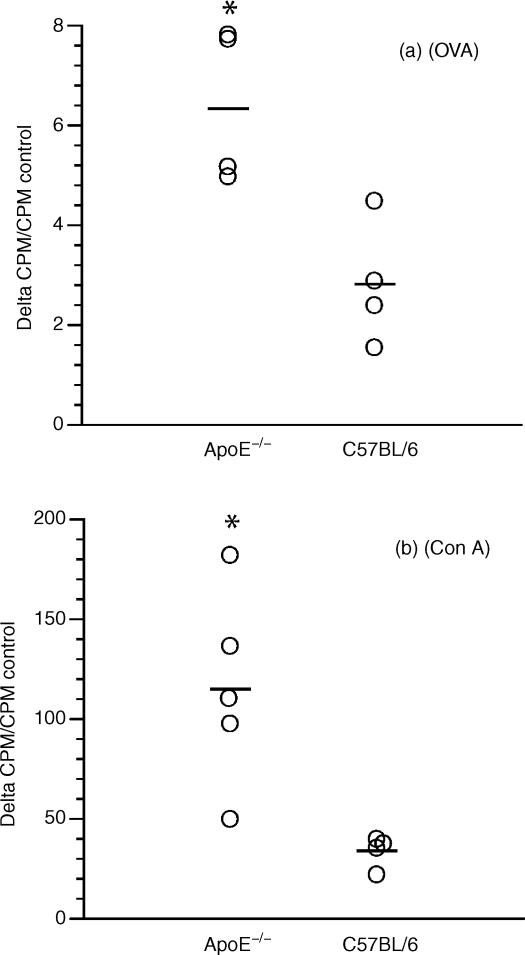
E0 and wild-type B6 mice were immunized once with OVA in complete Freund's adjuvant. When cells from draining lymph nodes were exposed to the antigen, there was an increased proliferation in cultures derived from E0 mice compared to B6 (Fig. 1a). Similarly, proliferation in response to the T-cell mitogen, Con A, was significantly increased in E0 mice. This was not the result of a general growth-stimulatory activity by adding (ApoE-containing) FCS to the cells because fibroblast proliferation was not different from that of wild-type cells (data not shown). This experiment therefore suggested that T cells of E0 mice proliferate more vividly upon activation than those of wild-type mice.
Figure 1.
Dot scatter plot showing the proliferation of cells from E0 (ApoE–/– mice) and ApoE+/+ C57BL/6 mice 1 week after primary immunization with OVA in complete Freund's adjuvant. The cells were isolated from draining inguinal lymph nodes and challenged in vitro with OVA (a) or Con A (b). Since the cells were isolated from different strains with different basal (control) values, delta counts per min (c.p.m.)/c.p.m. control was used instead of delta c.p.m. to truly reflect the response in the different strains after stimulation. *P < 0·05.
APC function is enhanced in E0 mice
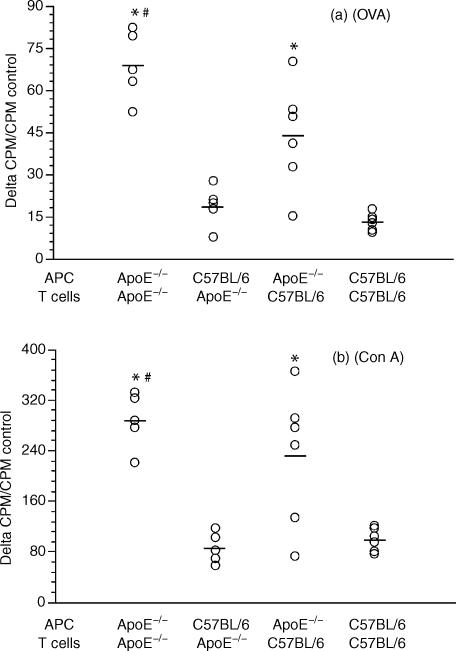
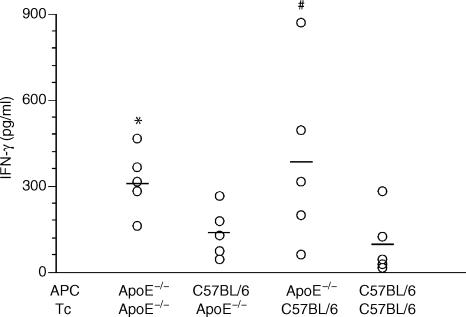
Since ApoE is produced by macrophages and reported to inhibit T-cell proliferation,1,4 we separated these two cell types and evaluated the role of the E0 phenotype by combining APC and T cells from OVA-primed E0 and wild-type mice. T-cell proliferation in response to OVA was enhanced in E0 mice (Fig. 2a). Interestingly, proliferation was lower when E0 T cells were activated in the presence of B6 APC. In other words, E0 APC supported the proliferation of the antigen-specific T cells more effectively than B6 APC did. This suggests that the macrophage is important for ApoE-dependent immune regulation. Con A was also tested on E0 and B6 cells. Again, E0 APC supported not only E0 but also B6 T-cell proliferation better than B6 APC (Fig. 2b). The cytokines IFN-γ and IL-4, which are secreted from activated T cells in response to OVA, were measured after co-incubation of irradiated APCs and T cells. Again, E0 APC supported an enhanced response as reflected in a significantly increased IFN-γ release (Fig. 3). The level of IL-4 was undetectable (data not shown).
Figure 2.
Dot scatter plot showing the proliferation of enriched T cells from draining inguinal lymph nodes of primed E0 (ApoE–/–) and ApoE+/+ C57BL/6 mice to antigens presented by peritoneal macrophages from E0 or E+ mice. The cells were incubated with OVA (a) or Con A (b). * indicates significant difference between the proliferation of E0/E0 (APC/T) to C57BL/6/E0 or E0/C57BL/6 to C57BL/6/C57BL/6, P < 0·05. # indicates significant difference between the proliferation of E0/E0 (APC/T) to C57BL/6/C57BL/6, P < 0·05.
Figure 3.
Dot scatter plot showing the IFN-γ level in the supernatant of enriched T cells from draining inguinal lymph nodes of primed E0 (ApoE–/–) and ApoE+/+ C57BL/6 mice to antigens presented by peritoneal macrophages from E0 or C57BL/6 mice. The cells were incubated with OVA. * indicates significant difference between the proliferation of E0/E0(APC/T) to C57BL/6/E0, P < 0·05. # indicates E0/C57BL/6 (APC/T) to C57BL/6/C57BL/6, P = 0·0556.
It has been shown that exogenous human ApoE inhibits proliferation of activated human peripheral lymphocytes.3,4 We therefore performed an in vitro test to observe if adding ApoE can restore the defect. However, mouse ApoE was unfortunately not available. When human ApoE was added in the mouse spleen cell culture, the defect could be reduced marginally (data not shown). We believe that this is the result of species differences or of autocrine/paracrine effects that cannot be mimicked by the addition of ApoE to the culture.
Enhanced antigen presentation and co-stimulation after in vivo activation of E0 APC
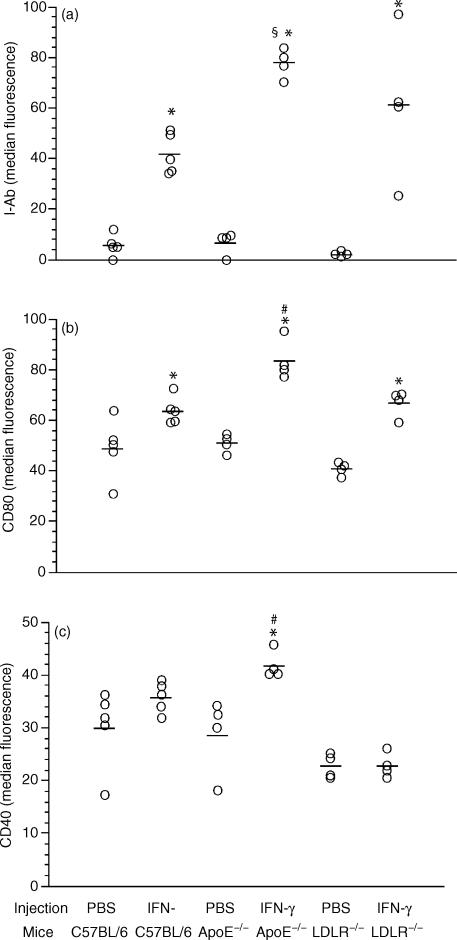
To explore the molecular mechanism underlying the enhanced E0 APC function, we analysed and compared the expression of co-stimulators (CD80 and CD40) and MHC class II (I-Ab) on the surface of monocytes/macrophages from E0 and B6 mice after IFN-γ stimulation in vivo. This is based on the fact that IFN-γ up-regulates the expression of MHC class II,29 CD4030 and CD80 on the surface of the APC.31 Our data showed that E0 APC were more sensitive to IFN-γ stimulation than B6 APC, with a higher expression of MHC class II molecules and co-stimulators (Fig. 4). The test was repeated in LDL receptor knockout mice (LDLR0), another hypercholesterolemic murine model, to explore if the increased response to IFN-γ in E0 APC was a result of the ApoE deficiency or of the inflammation associated with hypercholesterolemia in E0 mice. APC of IFN-γ treated E0 mice displayed increased expression of MHC class II molecules compared to the APC from identically treated B6 and LDLR0 mice (Fig. 4a). As shown in Fig. 4(b,c), E0 APC exhibited significantly higher expression of co-stimulatory molecules in comparison with the LDLR0 APC. The difference in APC response between LDLR0 and B6 mice was not statistically significant (Fig. 4). Taken together, ApoE directly affects the immune function of APC by down-regulating their expression of MHC class II and co-stimulatory molecules after IFN-γ treatment
Figure 4.
Dot scatter plot showing the expression of MHC Class II (a) and co-stimulators (b,c) on gated peritoneal monocytes/macrophages from the mice 18 hr after peritoneal injection of recombinant murine IFN-γ. * indicates significant difference in comparison with PBS control within the same strain, P < 0·05. # indicates significant difference compared with all other groups, P < 0·05. § indicates significant difference in comparison with corresponding C57BL/6 group, P < 0·05.
Discussion
Although it has been known for many years that ApoE can modulate immune activation, its immunological mechanism of action has remained unclear. We now show that ApoE deficiency results in enhanced expression of immunostimulatory cell surface molecules by the activated APC. The expression of MHC molecules that present T-cell antigens, together with the co-stimulatory surface proteins CD40 and CD80 are crucial for activation of the T cells. By modulating the level of these molecules, ApoE may time-tune the T-cell response to antigens.
ApoE is produced by macrophages.10–15 Previous studies of ApoE as an immune modulator have been performed in cell culture systems and show that ApoE attenuates T-cell proliferation.1 However, the role of this apolipoprotein in integrative immunology has been unclear. The APC provides a complex of surface molecules that are needed for antigen-dependent T-cell activation. It includes MHC molecules with associated antigen-derived peptides and co-stimulatory surface proteins. The most important of the latter are CD80 (B7-1), which ligates CD28 on the T-cell surface, and CD40, which binds to CD40 ligand (CD154). The conventional antigen OVA is taken up and processed by APC and finally presented as a complex of OVA-derived peptide and MHC class II molecules on the surface of the APC. The complex is recognized by the OVA-specific T cells, resulting in T-cell proliferation and IFN-γ secretion. This cytokine further activates APC to express more MHC class II and co-stimulatory molecules and therefore enhanced APC–T-cell interaction. The lack of ApoE caused stronger proliferation of the T cells and higher secretion of IFN-γin vitro and increased expression of MHC class II and co-stimulatory molecules after IFN-γ stimulation in vivo. It is known that macrophages can produce ApoE and also have receptors that can recognize ApoE protein.32 The ApoE secreted by macrophages may therefore dampen immune activation by inhibiting the expression of MHC and co-stimulatory molecules via an autocrine loop.
In addition to its effects on T-cell activation, ApoE modulates the innate immune response in mice. As shown by Roselaar33 and De Bont34 and their colleagues, E0 mice are more sensitive to infection with the intracellular bacterium, Listeria monocytogenes and Klebsiella pneumoniae, suggesting that ApoE promotes the macrophage-dependent defence against micro-organisms. ApoE may therefore exert the paradoxical effects of enhancing innate and dampening adaptive immune responses. Such effects could perhaps be explained by ApoE-dependent control of the differentiation and/or activation of macrophages. These immunomodulatory effects of ApoE could be important in atherosclerosis, a vascular disease that is characterized by lipoprotein accumulation and immune/inflammatory activation. Indeed, reconstruction of macrophage-specific expression of ApoE reduces atherosclerosis in E0 mice,35 while reconstitution of C57BL/6 mice with macrophages from E0 mice increases atherosclerosis.36 In conclusion, our results imply that ApoE controls T-cell activation by down-regulating the expression of MHC class II and co-stimulating molecules on the antigen-presenting cell.
Acknowledgments
We thank Dr Göran Hansson for stimulating discussions and Ingrid Törnberg for excellent technical assistance. This work was supported by the Swedish Medical Research Council (project 6816 and 14053), Heart-Lung Foundation, Gun/Bertil Stohnes Foundation, Gamla Tjänarinnor Foundation, Loo/Hans Foundation and Lars Hiertas Minne Foundations.
References
- 1.Curtiss LK, Forte TM, Davis PA. Cord blood plasma lipoproteins inhibit mitogen-stimulated lymphocyte proliferation. J Immunol. 1984;133:1379–84. [PubMed] [Google Scholar]
- 2.Dyer CA, Curtiss LK. Apoprotein E-rich high density lipoproteins inhibit ovarian androgen synthesis. J Biol Chem. 1988;263:10965–73. [PubMed] [Google Scholar]
- 3.Avila EM, Holdsworth G, Sasaki N, Jackson RL, Harmony JA. Apoprotein E suppresses phytohemagglutinin-activated phospholipid turnover in peripheral blood mononuclear cells. J Biol Chem. 1982;257:5900–9. [PubMed] [Google Scholar]
- 4.Pepe MG, Curtiss LK. Apolipoprotein E is a biologically active constituent of the normal immunoregulatory lipoprotein, LDL-In. J Immunol. 1986;136:3716–23. [PubMed] [Google Scholar]
- 5.Hansson GK, Holm J, Jonasson L. Detection of activated T lymphocytes in the human atherosclerotic plaque. Am J Pathol. 1989;135:169–75. [PMC free article] [PubMed] [Google Scholar]
- 6.Jonasson L, et al. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis. 1986;6:131–8. doi: 10.1161/01.atv.6.2.131. [DOI] [PubMed] [Google Scholar]
- 7.Stemme S, Holm J, Hansson GK. T lymphocytes in human atherosclerotic plaques are memory cells expressing CD45RO and the integrin VLA-1. Arterioscler Thromb. 1992;12:206–11. doi: 10.1161/01.atv.12.2.206. [DOI] [PubMed] [Google Scholar]
- 8.Zhou X, Stemme S, Hansson GK. Evidence for a local immune response in atherosclerosis. CD4+ T cells infiltrate lesions of apolipoprotein-E-deficient mice [see comments] Am J Pathol. 1996;149:359–66. [PMC free article] [PubMed] [Google Scholar]
- 9.Roselaar SE, Kakkanathu PX, Daugherty A. Lymphocyte populations in atherosclerotic lesions of apoE-/- and LDL receptor-/- mice. Decreasing density with disease progression. Arterioscler Thromb Vasc Biol. 1996;16:1013–18. doi: 10.1161/01.atv.16.8.1013. [DOI] [PubMed] [Google Scholar]
- 10.Basu SK, Brown MS, Ho YK, Havel RJ, Goldstein JL. Mouse macrophages synthesize and secrete a protein resembling apolipoprotein E. Proc Natl Acad Sci USA. 1981;78:7545–9. doi: 10.1073/pnas.78.12.7545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basu SK, Ho YK, Brown MS, Bilheimer DW, Anderson RG, Goldstein JL. Biochemical and genetic studies of the apoprotein E secreted by mouse macrophages and human monocytes. J Biol Chem. 1982;257:9788–95. [PubMed] [Google Scholar]
- 12.Basu SK, Goldstein JL, Brown MS. Independent pathways for secretion of cholesterol and apolipoprotein E by macrophages. Science. 1983;219:871–3. doi: 10.1126/science.6823554. [DOI] [PubMed] [Google Scholar]
- 13.Werb Z, Chin JR. Endotoxin suppresses expression of apoprotein E by mouse macrophages in vivo and in culture. A biochemical and genetic study. J Biol Chem. 1983;258:10642–8. [PubMed] [Google Scholar]
- 14.Werb Z, Chin JR. Onset of apoprotein E secretion during differentiation of mouse bone marrow-derived mononuclear phagocytes. J Cell Biol. 1983;97:1113–18. doi: 10.1083/jcb.97.4.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takemura R, Werb Z. Modulation of apoprotein E secretion in response to receptor-mediated endocytosis in resident and inflammatory macrophages. J Exp Med. 1984;159:167–78. doi: 10.1084/jem.159.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hui DY, Harmony JA. Phosphatidylinositol turnover in mitogen-activated lymphocytes. Suppression by low-density lipoproteins. Biochem J. 1980;192:91–8. doi: 10.1042/bj1920091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hui DY, Harmony JA. Inhibition by low density lipoproteins of mitogen-stimulated cyclic nucleotide production by lymphocytes. J Biol Chem. 1980;255:1413–19. [PubMed] [Google Scholar]
- 18.Akeson AL, Scupham DW, Harmony JA. The phosphatidylinositol response and proliferation of oxidative enzyme-activated human T lymphocytes: suppression by plasma lipoproteins. J Lipid Res. 1984;25:1195–205. [PubMed] [Google Scholar]
- 19.Cuthbert JA, Lipsky PE. Immunoregulation by low density lipoproteins in man: low density lipoprotein inhibits mitogen-stimulated human lymphocyte proliferation after initial activation. J Lipid Res. 1983;24:1512–24. [PubMed] [Google Scholar]
- 20.Cuthbert JA, Lipsky PE. Immunoregulation by low density lipoproteins in man. Inhibition of mitogen-induced T lymphocyte proliferation by interference with transferrin metabolism. J Clin Invest. 1984;73:992–1003. doi: 10.1172/JCI111325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Macy M, Okano Y, Cardin AD, Avila EM, Harmony JA. Suppression of lymphocyte activation by plasma lipoproteins. Cancer Res. 1983;43(5 Suppl):2496s–502s. [PubMed] [Google Scholar]
- 22.Harmony JAK, Akeson AL, McCarthy BM, Morris RE, Scumpham DW, Gruup SA. Immunoregulation by plasma lipoproteins. In: Scanu AM, Spector AA, editors. Biochemistry and Biology of Plasma Lipoprotein. New York: Dekker; 1986. pp. 403–52. [Google Scholar]
- 23.Dyer CA, Smith RS, Curtiss LK. Only multimers of a synthetic peptide of human apolipoprotein E are biologically active. J Biol Chem. 1991;266:15009–15. [PubMed] [Google Scholar]
- 24.Kelly ME, Clay MA, Mistry MJ, Hsieh LH, Harmony JA. Apolipoprotein E inhibition of proliferation of mitogen-activated T lymphocytes: production of interleukin 2 with reduced biological activity. Cell Immunol. 1994;159:124–39. doi: 10.1006/cimm.1994.1302. [DOI] [PubMed] [Google Scholar]
- 25.Mistry MJ, Clay MA, Kelly ME, Steiner MA, Harmony JA. Apolipoprotein E restricts interleukin-dependent T lymphocyte proliferation at the G1A/G1B boundary. Cell Immunol. 1995;160:14–23. doi: 10.1016/0008-8749(95)80004-3. [DOI] [PubMed] [Google Scholar]
- 26.Laskowitz DT, Lee DM, Schmechel D, Staats HF. Altered immune responses in apolipoprotein E-deficient mice. J Lipid Res. 2000;41:613–20. [PubMed] [Google Scholar]
- 27.Plump AS, Smith JD, Hayek T, Aalto SK, Walsh A, Verstuyft JG, Rubin EM, Breslow JL. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E- deficient mice created by homologous recombination in ES cells. Cell. 1992;71:343–53. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- 28.Piedrahita JA, Zhang SH, Hagaman JR, Oliver PM, Maeda N. Generation of mice carrying a mutant apolipoprotein E gene inactivated by gene targeting in embryonic stem cells. Proc Natl Acad Sci USA. 1992;89:4471–5. doi: 10.1073/pnas.89.10.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glimcher LH, Kara CJ. Sequences and factors: a guide to MHC class-II transcription. Annu Rev Immunol. 1992;10:13–49. doi: 10.1146/annurev.iy.10.040192.000305. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen VT, Benveniste EN. Involvement of STAT-1 and ets family members in interferon-gamma induction of CD40 transcription in microglia/macrophages. J Biol Chem. 2000;275:23674–84. doi: 10.1074/jbc.M002482200. [DOI] [PubMed] [Google Scholar]
- 31.Ding L, Linsley PS, Huang LY, Germain RN, Shevach EM. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. J Immunol. 1993;151:1224–34. [PubMed] [Google Scholar]
- 32.Brown MS, Goldstein JL. Lipoprotein metabolism in the macrophage: implications for cholesterol deposition in atherosclerosis. Annu Rev Biochem. 1983;52:223–61. doi: 10.1146/annurev.bi.52.070183.001255. [DOI] [PubMed] [Google Scholar]
- 33.Roselaar SE, Daugherty A. Apolipoprotein E-deficient mice have impaired innate immune responses to Listeria monocytogenes in vivo. J Lipid Res. 1998;39:1740–3. [PubMed] [Google Scholar]
- 34.De Bont N, Netea MG, Demacker PN, Kullberg BJ, Van Der Meer JW, Stalenhoef AF. Apolipoprotein E-deficient mice have an impaired immune response to Klebsiella pneumoniae[In Process Citation] Eur J Clin Invest. 2000;30:818–22. doi: 10.1046/j.1365-2362.2000.00715.x. [DOI] [PubMed] [Google Scholar]
- 35.Bellosta S, Mahley RW, Sanan DA, Murata J, Newland DL, Taylor JM, Pitas RE. Macrophage-specific expression of human apolipoprotein E reduces atherosclerosis in hypercholesterolemic apolipoprotein E-null mice. J Clin Invest. 1995;96:2170–9. doi: 10.1172/JCI118271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fazio S, Babaev VR, Murray AB, Hasty AH, Carter KJ, Gleaves LA, Atkinson JB, Linton MF. Increased atherosclerosis in mice reconstituted with apolipoprotein E null macrophages. Proc Natl Acad Sci USA. 1997;94:4647–52. doi: 10.1073/pnas.94.9.4647. [DOI] [PMC free article] [PubMed] [Google Scholar]