Abstract
2B4 (CD244) is a member of the CD2 subset of the immunoglobulin superfamily and functions as a triggering molecule on natural killer (NK) cells. Previously, we have found that 2B4-mediated activation of NK cells involves complex interactions involving LAT, Ras, Raf, ERK and p38 and that cytolytic function and cytokine production may be regulated by distinct pathways. Here we assessed the role of protein kinase C (PKC) in 2B4-mediated cytotoxicity of YT cells, a human NK cell line. Our data indicate that PKC-δ is activated upon stimulation with monoclonal antibody against 2B4. Treatment with the PKC inhibitor, bisindolylmaleimide I (Gö6850), of YT cells or YT cells depleted of Ca2+-dependent isoforms of PKC prior to 2B4 stimulation, resulted in inhibition of natural cytotoxicity and redirected antibody-dependent cellular cytotoxicity. However, inhibition of PKC failed to block 2B4 stimulation of interferon-γ secretion as opposed to pretreatment with LY294002, a phosphoinositide 3-kinase inhibitor. We also examined the effect of phorbol 12-myristate 13-acetate (PMA) induction on 2B4 gene transcription. PMA induction resulted in a more than two-fold increase of 2B4 transcription. However, when we introduced a three-base substitution mutation to disrupt the activator protein-1 binding site at (−106 to −100) in the 2B4 promoter, we found complete loss of transcriptional activity, including the two-fold increase due to PMA induction of PKC. The present study indicated that PKC may play an important role in 2B4 signalling and activator protein-1 activation.
Introduction
Natural killer (NK) cells are bone-marrow-derived lymphocytes that function as key players in innate immunity by recognizing viral, bacterial and parasitic infections and neoplastic target cells.1,2 The major effector functions of NK cells are cytotoxicity and cytokine release, including interferon-γ (IFN-γ), tumour necrosis factor-α, granulocyte–macrophage colony-stimulating factor as well as matrix metalloproteinases.3–6 NK cell recognition is regulated by specific receptors that, upon interaction with their respective ligands, may send stimulating or inhibitory signals.7–9 An important activating receptor expressed on NK cells is 2B4 (CD244).10 2B4 is a member of the CD2 subset of the immunoglobulin superfamily.11,12 2B4 is expressed on NK cells, monocytes, basophils and on subsets of T-cell receptor (TCR) γδ+ T cells and CD8+ T cells.13 Ligation of 2B4 either by a monoclonal antibody (mAb) or by its natural ligand, CD48, on NK cells results in increased cytotoxicity and secretion of IFN-γ.6,13–15 Recent findings indicate that 2B4 may function as an inhibitory molecule at early stages of NK cell differentiation.16
Previously, we investigated the possible role of various signalling molecules that may be involved in the activation of NK cells via 2B4. We found through the treatment of YT cells with various specific inhibitors that 2B4-stimulation of YT cells in spontaneous and antibody-dependent cytotoxicity is Ras/Raf dependent and involves multiple mitogen-activated protein kinase (MAPK) signalling pathways [extracellular regulated kinase1/2 (ERK1/2) and p38].17 Inhibition of transcription also inhibited 2B4-mediated cytotoxicity, implying that there are transcriptional events critical in regulating NK cell function. When we examined the effect of these inhibitors on 2B4-mediated secretion of IFN-γ, only inhibitors of transcription and p38 inhibited 2B4-mediated IFN-γ release. These results indicate that 2B4-mediated activation of NK cell cytolytic function and cytokine production may be regulated by several distinct pathways. Thus, our studies on the signalling of 2B4 revealed that NK cell cytolytic function and cytokine production may be regulated by distinct pathways in activated NK cells.17 Another recent study also indicates that receptor signalling in NK cells can be functionally complex depending on the state of NK cells.18 Rajagopalan et al. observed that the killer cell immunoglobulin-like receptor KIR2DL4 induced IFN-γ production, but not cytotoxicity by resting NK cells, whereas in activated NK cells both cytokine production and cytotoxicity were induced by KIR2DL4 stimulation. This indicates that resting NK cells may behave differently to activated NK cells, upon stimulation through specific receptors.
In the current study, we examined the possible role of protein kinase C (PKC) in the 2B4 signalling pathway in YT cells, a human leukaemia cell line. Pretreatment using a general PKC inhibitor, bisindolylmaleimide I (Gö6850), on YT cells or YT cells depleted of Ca2+ -dependent isoforms of PKC prior to 2B4 stimulation resulted in varying levels of inhibition of cytotoxicity and redirected antibody-dependent cellular cytotoxicity (rADCC). However, when we examined the effect of PKC inhibition on 2B4-mediated secretion of IFN-γ, there was no decrease as opposed to pretreatment with LY294002, a phosphoinositide 3-kinase (PI3K) inhibitor. We also examined the effect of PKC activation through phorbol ester induction on 2B4 gene transcription. Phorbol 12-myristate 13-acetate (PMA) induction resulted in a more than two-fold general increase of 2B4 transcription. However, base substitution mutations of the activator protein-1 (AP-1) binding site at (−106 to −100) in the 2B4 promoter resulted in the complete loss of transcriptional activity, including the two-fold increase due to PMA induction of PKC.
Materials and methods
Cell lines, antibodies and chemicals
YT (human NK cell line), K562 (human erythroleukaemia cell line) and P815 (mouse lymphoma cell line) cells were maintained in complete medium [RPMI-1640 supplemented with 10% fetal bovine serum (Hyclone, Logan, UT), 2 mm glutamine, 100 U/ml penicillin, 100 U/ml streptomycin, 10 mm HEPES and 10 mm non-essential amino acids]. Cells were maintained at 37° in a humidified 5% CO2/95% air incubator. Cell culture reagents were obtained from Life Technologies (Gaithersburg, MD) unless otherwise noted. The mAb that specifically recognizes human 2B4 (C1.7),19 was purchased from Coulter (Orlando, FL). All enzymes were purchased from New England Biolabs (Beverly, MA) unless otherwise stated. Poly(dI-dC) was purchased from Amersham Pharmacia Biotech (Piscataway, NJ). All custom synthesized oligonucleotides used in this study were supplied by Integrated DNA Technologies (Coralville, IA). All inhibitors used in this study were purchased from Calbiochem (San Diego, CA).
51Cr release cytotoxicity assay
Target cells were labelled with (NEN Research Products, Boston, MA) for 90 min at 37° under 5% CO2 in air. The target cells were then washed three times in culture media. Ten thousand labelled target cells (in 100 μl) were incubated with varying amounts of effector YT cell suspension (100 μl), with and without anti-2B4 mAb (200 ng/ml). After incubation for 4 hr at 37° under 5% CO2 in air, the cells were pelleted at 250 g for 5 min. One hundred microlitres of the supernatant was removed and the radioactivity was measured. The percentage of specific lysis was calculated by the following equation: (a − b/c − b) × 100, where a is the radioactivity of the supernatant of target cells mixed with effector cells, b is that in the supernatant of target cells incubated alone, and c is that in the supernatant after lysis of target cells with 1% Nonidet P-40. All data points in each graph represent the average of at least four independent trials with similar results. Determination of statistical significance was determined on each data point representing 2B4-mediated cytotoxicity assays performed with inhibitor-treated effector cells or target cells compared to assays conducted with non-treated effector and target cells with the Student's t-test. Data groups were considered significantly different when P < 0·05.
Treatment of cells
In assays using inhibitors, both effector YT cells and target K562 and P815 cells were subjected to treatment in culture media as indicated. Cells were incubated in PKC inhibitor, bisindolylmaleimide I (Gö6850) in varying concentrations or PI3K inhibitor, LY249002 (1 μm), for 1 hr at 37° under 5% CO2 in air. To deplete PMA-sensitive isoforms of PKC, YT cells were incubated in complete media with PMA (100 ng/ml) for 20 hr prior to use and designated PMA-treated YT (P-YT).
Immunoblot analysis
YT cells were incubated (1 × 107/100 μl; 37°) for the indicated times with the C1.7 mAb (10 μg/ml). After stimulation, the cells were lysed with 900 μl of lysis buffer (1% Nonidet P-40, 0·5% deoxycholate, 0·1% sodium dodecyl sulphate, 10 mm HEPES (pH 7·5), 0·15 m NaCl, 10% glycerol, 1 mm phenylmethylsulphonyl fluoride, 1 mm Na3VO4, 50 mm NaF, 1 mm ethylenediaminetetraacetic acid and 10 μg/ml each of aprotinin and leupeptin). Forty micrograms of protein lysate was analysed in 8% sodium dodecyl sulphate–polyacrylamide gel electrophoresis (reducing conditions). Western blots were performed according to manufacturer's chemiluminescence detection system instructions (Kirkegaard & Perry Laboratories, Gaithersburg, MD). Western blots were hybridized with anti-phospho-PKC-δ, anti-phospho-PKC-ζ (Cell Signaling Tech. Inc., Beverly, MA) and anti-phospho-PKC-ε mAbs (Upstate, Lake Placid, NY) to detect phosphorylated forms of PKC-δ, PKC-ζ, and PKC-ε, respectively. The Western blots were then stripped and re-probed with anti-PKC-δ, anti-PKC-ζ and anti-PKC-ε mAbs (Santa Cruz Biotechnology Inc., Santa Cruz, CA) to detect total amounts of PKC-δ, PKC-ζ and PKC-ε, respectively.
IFN-γ release assay
Inhibitor-treated or untreated YT cells (500 000) were stimulated or unstimulated with C1.7 mAb (200 ng/ml) in flat-bottomed 96-well plates for 1 hr at 37° under 5% CO2 in air. Target K562 cells (50 000) were then added. After incubation for 16 hr at 37° under 5% CO2 in air, 100 μl of cell-free supernatant was collected. IFN-γ concentration was then quantified with an enzyme-linked immunosorbent assay kit according to manufacturer's instructions (CLB, Amsterdam, the Netherlands). Each condition was tested in at least four independent trials.
Transfection and luciferase assays
Nested promoter fragments were derived by polymerase chain reaction using Taq polymerase (Promega, Madison, WI) and using a genomic DNA clone (F5A1) containing the 5′ flanking sequence of the h2B4 gene as template.20 The nested promoter fragments were then cloned upstream of a promoterless and enhancerless firefly luciferase gene in the pGL2-B vector (Promega). The numbering of the constructs refers to the first nucleotide of each promoter construct relative to the start of transcription. All constructs were verified by nucleotide sequencing. Substitution mutant promoter constructs were generated by a two-step polymerase chain reaction procedure using overlapping internal primers that contain a mutant sequence, as described previously.20 A Renilla luciferase reporter plasmid, pRL-CMV driven by an upstream cytomegalovirus immediate-early enhancer/promoter region (Promega) was co-transfected with the pGL2 promoter constructs to adjust firefly luciferase activity for transfection efficiency. All plasmid DNA used in transient transfection assays was purified by two rounds of caesium chloride centrifugation.
YT cells were transfected with each of the 4 μg firefly luciferase reporter constructs, and 400 ng pRL-CMV for internal normalization of transfection efficiency. The cells were transfected using the DMRIE-C reagent at a 2·2 : 3 ratio of μg DNA : μl DMRIE-C reagent following the manufacturer's instructions (Gibco Life Technologies, Gaithersburg, MD). Cells were stimulated with 50 ng/ml PMA 5 hr post-transfection. Cell lysates were then harvested 40 hr post-stimulation. Firefly and Renilla luciferase assays were performed using the Dual-Luciferase reporter assay system following the manufacturer's instructions (Promega). Each test promoter construct was co-transfected with pRL-CMV into YT cells in at least four independent trials.
Results
PKC is involved in 2B4-mediated NK cell cytotoxicity and rADCC
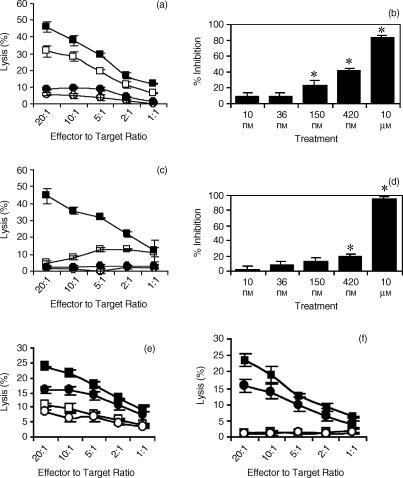
2B4 stimulation of NK cell lines increases spontaneous cytolytic and rADCC activity.6,13,21,22 Recently, immunoprecipitation studies identified linker for activation of T cells (LAT) to be constitutively associated with the 2B4.23,24 Ligation of 2B4 not only results in phosphorylation of 2B4 and LAT but also LAT-associated proteins including phospholipase Cγ (PLCγ) and Grb2.23 PLCγ activity can lead to the activation of PKC through diacylglycerol and calcium flux. Thus, we examined whether PKC modulated 2B4-mediated cytotoxicity. The inhibitor bisindolylmaleimide I (Gö6850), specifically inhibits many isoforms of PKC, possibly by competing with ATP binding.25 Pre-treatment of YT cells with Gö6850 (10 μm) prior to 2B4 stimulation and subsequent use in 51Cr release cytotoxicity assays examining both spontaneous and rADCC lysis resulted in diminished activities in both scenarios (Fig. 1a,c). Varying amounts of Gö6850 can inhibit specific isoforms of PKC.25 We examined the effect of incubating YT cells in varying concentrations of Gö6850 and found that only higher concentrations of Gö6850 resulted in significant inhibition (P < 0·05) of YT cytolytic activity (Fig. 1b,d). This suggests that the Gö6850-sensitive PKC isoforms PKCα and PKCβI may not be part of the signal transduction pathway used by 2B4 in activating cytolytic function because of their sensitivity to Gö6850 at 8·4 nm and 18 nm concentrations, respectively. To determine whether inhibition by Gö6850 is specific for 2B4 signalling or is general for inhibition of NK cell cytotoxicity, we determined the lysis of target cells at low concentrations of Gö6850. It is evident from Fig. 1(e,f), that 2B4-mediated signalling is significantly reduced at lower concentrations of Gö6850 compared with the effect on natural cytotoxicity. However, even at low concentrations, Gö6850 shows some inhibition of NK cell cytotoxicity. Therefore, at high concentrations, in addition to inhibiting 2B4 signalling, Gö6850 may have a general effect on NK cell cytolytic function.
Figure 1.
Effect of PKC inhibition on 2B4-mediated cytotoxicity. YT cells were incubated (1 hr, 37°) with anti-2B4 mAb, C1.7 (200 ng/ml) (▪) or without (□). YT cells were preincubated (1 hr, 37°) with the indicated concentrations of Gö6850 prior to incubation with C1.7 (•) or without (○). The YT cells were incubated with each target cell for 4 hr. (a) Cytotoxicity against K562 target cells. YT cells were incubated with Gö6850 (10 μm) followed by C1.7 stimulation prior to introduction of the K562 target cells. (b) Percent inhibition of the cytotoxicity against K562 target cells. (c) rADCC against P815 target cells. YT cells were incubated with Gö6850 (10 μm) followed by C1.7 stimulation prior to introduction of P815 target cells. (d) Percent inhibition of the cytotoxicity against P815 target cells. All data points in each graph represent the average of four independent trials with similar results. In (e) and (f) specific inhibition of signalling via 2B4 is demonstrated at lower concentrations of Gö6850 (1 μm). (e) YT cells incubated with Gö6850 (1 μm) followed by C1.7 stimulation prior to introduction of K562 target cells. (f) rADCC against P815 target cells. YT cells incubated with Gö6850 (1 μm) followed by C1.7 stimulation prior to introduction of P815 target cells. All data points in each graph represent the average of four independent trials with similar results. * indicates significance of P < 0·001.
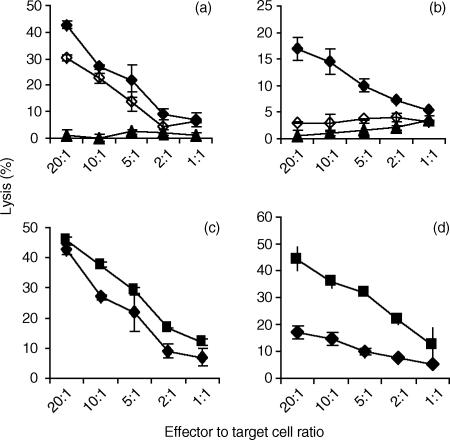
Several isoforms of PKC are responsive to PMA.25 However, prolonged exposure of YT cells to PMA (20 hr) results in the depletion of PMA-responsive PKC isoenzymes.26 Using YT cells whose PMA-responsive PKC isoenzymes were depleted (P-YT) cells, Gö6850 (10 μm) pretreatment prior to 2B4 stimulation resulted in abrogation in cytolytic activity against both target K562 cells and in rADCC against target P815 cells (Fig. 2). 2B4-stimulation of both YT cells and P-YT cells resulted in increased cytolytic activity against both K562 cells and P815 cells (Figs 1 and 2). However, when we compared the levels of cytolytic activity between YT cells and P-YT cells, we observed a decrease in cytolytic activity in P-YT cells against target K562 cells (Fig. 2c). In rADCC, the effect was even more evident (Fig. 2d). Taken together, these data suggest that there are multiple isoforms of PKC, both PMA-sensitive and insensitive, that participate in 2B4 stimulation of NK cell cytolytic activity. In contrast, it has been reported that the PKC inhibitor, GF109293X (bisindolylmaleimide I or Gö6850) did not affect anti-CD16-induced cytolysis.27
Figure 2.
2B4-mediated cytotoxicity of YT cells depleted of PMA-sensitive PKC isoforms can still be inhibited by Gö6850. To deplete PMA-sensitive isoforms of PKC, YT cells were incubated in complete media with PMA (100 ng/ml) for 20 hr prior to use. PMA-treated YT (P-YT) cells were incubated (1 hr, 37°) with (♦) or without (⋄) C1.7 mAb (200 ng/ml), prior to incubation with target cells. P-YT cells incubated with 10 μm Gö6850 were subsequently incubated with C1.7 mAb (200 ng/ml) (▴). (a) K562 cells were used as target cells. (b) P815 cells were used as target cells. Effect of PMA depletion of PMA responsive forms of PKC on 2B4-mediated cytolytic activity is shown in (c) and (d). YT (□) or P-YT (♦) cells were incubated with anti-2B4 mAb, C1.7, for 45 min prior to 4 hr incubation with target cells. (c) K562 cells were used as target cells. (d) P815 cells were used as target cells. All data points in each graph represent the average of four independent trials with similar results.
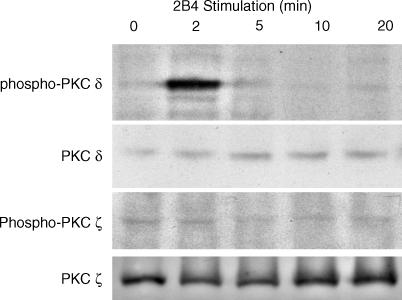
To identify specific isoforms of PKC that are involved in 2B4 signalling, we observed changes in phosphorylation states. YT cells were stimulated with anti-2B4 mAb (200 ng/ml) and then lysed at varying time points and analysed by Western blot. 2B4 engagement by anti-2B4 mAb failed to change the phosphorylation states of PKC-ζ (Fig. 3). However, 2B4 stimulation did result in the tyrosine phosphorylation of PKC-δ, 2 min after 2B4 ligation (Fig. 3). Experiments performed examining the phosphorylation state of PKC-ε in response to 2B4 stimulation showed no change in phosphorylation of PKC-ɛ (data not shown).
Figure 3.
2B4 stimulation results in phosphorylation of PKC-δ. YT cells were stimulated with anti-2B4 mAb for the indicated times at 37°. Cell lysates were then prepared and analysed by Western blotting with anti-PKC-δ or anti-PKC-ζ as indicated. The membrane was stripped and reprobed with anti-PKC-δ and PKC-ζ to check for total protein amounts.
2B4-mediated secretion of IFN-γ involves PI3K, but not PKC
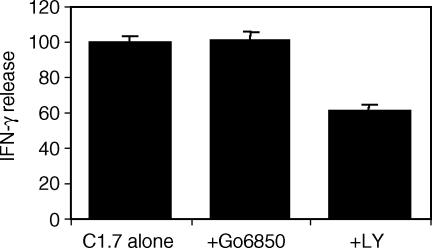
Production and secretion of IFN-γ is a major function of NK cells.1 We have shown that 2B4 stimulation of NK cells results in the secretion of IFN-γ.6,28 Using similar inhibitor treatment protocols in the 51Cr release cytotoxicity assays, we investigated whether PKC was involved in 2B4-induced IFN-γ secretion. Pretreatment of YT cells with Gö6850 (10 μm) failed to inhibit IFN-γ production (Fig. 4). However, pretreatment of YT cells with LY249002 (1 μm), a phosphoinositide 3-kinase (PI3K) inhibitor, for 1 hr at 37°, prior to 2B4 stimulation resulted in a significant (P < 0·001) decrease in IFN-γ secretion. This indicates that PI3K, not PKC is required for 2B4 induction of IFN-γ production.
Figure 4.
PKC inhibition fails to inhibit 2B4-mediated secretion of IFN-γ. YT cells (1 × 106) were preincubated in 1 ml of culture media containing PKC inhibitor, Gö6850 (10 μm), PI3K inhibitor LY294002 (1 μm) or alone, followed by the addition of anti-2B4 mAb (200 ng/ml). After 20 hr, the cells were then spun down and 100 μl of supernatant was assayed for the presence of IFN-γ by enzyme-linked immunosorbent assay. Values are relative activity of IFN-γ secreted from 2B4-stimulated cells in the absence of any inhibitors.
Up-regulation of transcription of 2B4 by PMA is through AP-1
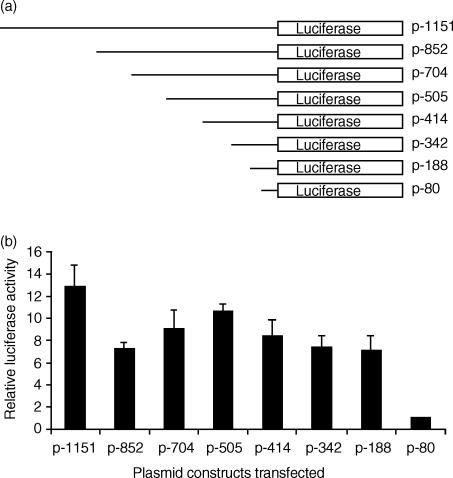
Previously, our studies on the transcriptional regulation of the 2B4 gene revealed that promoter regions (−188 to −80) and (−1151 to −704) had a positive effect on transcription whereas the promoter regions (−414 to −342) and (−704 to −505) had a negative effect.20 Transfection of YT cells revealed that maximal luciferase activity was achieved with the promoter fragment (p-342), which produced luciferase activity that was almost four-fold higher than that produced by the smallest promoter fragment (p-80).20 Here, we performed similar transfection experiments with the same 2B4 promoter constructs and added PMA (50 ng/ml) 5 hr post-transfection and incubated the transiently transfected cells for an additional 40 hr before determining the luciferase activity. We observed a general increase in luciferase activity from all promoter constructs of at least seven-fold over the luciferase activity recorded for the p-80 construct (Fig. 5). The p-342 construct produced a two-fold increase in luciferase activity under PMA stimulation as compared to transfection experiments conducted without PMA.
Figure 5.
PMA stimulation of YT cells during transient transfection assays results in elevated transcriptional activity from the 2B4 promoter. (a) YT cells were transfected with a series of 5′ promoter deletion mutants in the pGL2B reporter vector, along with a pRL3-CMV control plasmid. The 5′ 2B4 promoter base position relative to +1 is denoted on the right. Each construct ends at the +126 nucleotide position. Transfected cells were cultured in complete media with PMA (50 ng/ml) for 40 hr. (b) Firefly luciferase activity, following normalization to Renilla luciferase activity, are expressed as the mean relative luciferase activity and SE (from a minimum of four independent experiments) to when the p-80 construct (relative activity = 1) was transfected into YT cells.
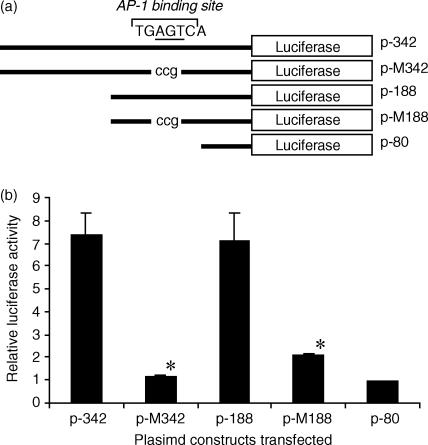
Previously, we have identified a functional AP-1 site that lies between −106 and −100 of the h2B4 promoter that strongly activates transcription. Many studies in different systems have shown that activation of transcription can involve signalling pathways that include PKC.29–31 To examine whether PKC influenced 2B4 gene transcription through AP-1, we mutated the (−111 to −89) sequence of the h2B4 promoter with the AP-1 binding site from TGAGTCA to TGccgCA and synthesized mutant promoter constructs p-M188 and p-M342 (Fig. 6a). Transient transfections with the mutant promoter constructs revealed that the mutation of the AP-1 site significantly reduced the luciferase activity (P < 0·001) of both constructs to levels similar to p-80 despite PMA stimulation of the transfected cells (Fig. 6b). The data suggest that PMA activation of 2B4 gene transcription involves AP-1.
Figure 6.
The mutation in the AP-1 binding site inhibits h2B4 promoter function despite PMA stimulation. (a) AP-1 binding site mutant constructs (TGccgCA) were created and used in the transient promoter reporter assay in the context of the p-342 and p-188 promoter constructs. Lower case letters are nucleotides used to mutate the underlined letters within the AP-1 binding site. (b) Each promoter fragment was inserted in front of the firefly luciferase gene. Transfected cells were cultured in complete media with PMA (50 ng/ml) for 40 hr before assaying for luciferase activity. Firefly luciferase activities, following normalization to Renilla luciferase activity, are expressed as the mean relative luciferase activity and SE (from four to six independent experiments) when the p-80 construct (relative activity = 1) was transfected into YT cells. * indicates significance of P < 0·001 from the respective wild-type counterpart.
Discussion
2B4 on NK cells is an activating receptor that increases cytotoxicity and IFN-γ release, as well as perforin degranulation and increased matrix metalloproteinase expression.6,13 2B4 stimulation has also been shown to induce calcium flux and phosphorylation of PLCγ.14,23 It has been well established that members of the PKC family can be activated by an increase in intracellular levels of calcium, diacylglycerol, or phosphatidylserine.25 Activation of PLCγ results in PLCγ hydrolysing phosphatidylinositol 4,5-bisphosphate to generate the second messengers, inositol 1,4,5-trisphosphate and diacylglycerol. Thus, several members of the PKC family may be involved in 2B4 signalling in NK cells. Since NK cell-mediated cytotoxicity has been found to involve PKC,32,33 we examined whether 2B4-mediated cytolytic activation of NK cells was also dependent on PKC. In the present study, we have shown that ligation of 2B4 receptors on YT cells induces cytotoxicity following the activation of PKC. Inhibition of PKC in YT cells and P-YT cells, in which the phorbol ester-sensitive PKC isoforms were depleted, resulted in a significant decrease in 2B4-induced cytolytic activity. When we examined 2B4-mediated cytolytic activity in rADCC, PKC inhibitor pretreatment resulted in even more pronounced decrease using both YT and P-YT cells. These results suggest that there are multiple isoforms of PKC that are involved in the signal transduction of 2B4. Western blot analysis revealed PKC-δ become activated in response to 2B4 stimulation of YT cells but PKC-ζ and PKC-ɛ do not. There may be other PKC isoforms that become activated by 2B4. PKC-δ belongs to the novel PKC group and possesses regulatory domains that interact with diacylglycerol and phorbol esters. Our cytotoxicity studies have found that 2B4 could still increase cytolytic activity in YT cells depleted of PMA-responsive PKC isoenzymes and still be inhibited by Gö6850. Thus other isoforms of PKC may be involved in 2B4 signalling.
We also examined whether PKC played a role in regulating IFN-γ production in response to 2B4 stimulation. With preincubation of effector cells with PKC inhibitors, we did observe a decrease in IFN-γ secretion when the effector cells were pretreated with PI3K inhibitors (Fig. 4). Activation of 2B4 induces phosphorylation of the 2B4 cytoplasmic domain.19,23,34,35 This results in the recruitment of SLAM-associated protein/SH2D1A (SAP/SH2D1A) to the phosphorylated tyrosine residues on the 2B4 cytoplasmic tail. Recently, it has been found that PI3K plays an important role in the recruitment of SAP to the 2B4 cytoplasmic tail.36 The inhibition of PI3K not only prevents the recruitment of SAP but also subsequently results in the inhibition of cytotoxicity. SAP is expressed in both NK cells and T lymphocytes. Mutations in the SH2 domain of SAP/SH2D1A have been identified as the genetic defect in X-linked lymphoproliferative disease and are known to cause 2B4 defective signalling in this disease.37–43 Thus, our data showing that inhibition of 2B4-mediated IFN-γ production by PI3K inhibitors correlates well with these findings, revealing the importance of PI3K in 2B4 signalling.
Although a number of molecules have been shown to play a role in 2B4-mediated activation of NK cell cytolytic function, the signal transduction pathway used by 2B4 is not fully understood. Our earlier studies indicated that 2B4-mediated activation of NK cells involved complex interactions with LAT, Ras, Raf, ERK and p38.17 Recent findings show that LAT may not be required for natural cytotxicity but is essential for redirected lysis triggered by 2B4 cross-linking.24 Association of SAP with the cytoplasmic tail of 2B4 is essential for the activating role of 2B4, as evident from studies using NK cells from patients with X-linked lymphoproliferative disease.40 Activation of NK cells via 2B4 requires the phosphorylation of tyrosine residues present in the cytoplasmic domain. Engagement of NK cell inhibitory receptors prevents the phosphorylation of 2B4 and thus blocks activation.34 It has been reported that in the absence of SAP, 2B4 functions as an inhibitory receptor.43 This inhibitory role of 2B4 is implicated to prevent the killing of autologous cells by differentiating NK cells which do not express inhibitory receptors for major histocompatibility complex class I molecules.16 All these data indicate that 2B4 signalling in NK cells is complex and is mediated by several signalling molecules.
Previously, we have identified a functional AP-1 site that lies between −106 and −100 of the h2B4 promoter that strongly activates transcription. AP-1 can be activated by the active c-Jun NH2-terminal kinase (JNK) pathway, which can be activated by the PKC pathways. In fact, PKC-θ is selectively expressed in T lymphocytes and leads to activation of JNK, NFAT and interleukin-2 genes.44 It has been also reported that PKC-δ can activate the JNK pathway in human thyroid cells.45 In our study, PMA stimulation resulted in an increase of promoter activity and these activities were abolished by mutation of the AP-1 binding site. We could conclude that PKC signalling is also involved in the regulation of 2B4 transcription.
The results presented in this study reveal the functional role of PKCs in the signal transduction of 2B4-mediated NK cell cytotoxicity. While previous studies have implicated PKCs in other receptor-triggered cytotoxicity such as CD28 and NC1.1,46–48 we have found that PKC-δ becomes activated in response to 2B4 stimulation of YT cells. While 2B4-mediated cytotoxicity was dependent on PKC, IFN-γ production was dependent on PI3K. In addition, this study demonstrates that PMA stimulation of 2B4 gene transcription is mainly mediated by the AP-1 signal transduction pathway.
Acknowledgments
We thank Dr P. R. Kumaresan and Swapnil Vaidya for their comments and for review of the manuscript and Dr Richard Kitson for help with the figures. This work was supported in part by the National Institutes of Health grant CA 85753.
Abbreviations
- AP-1
activator protein-1
- LAT
linker for activation of T cells
- PI3K
phosphoinositide 3-kinase
- PKC
protein kinase C
- PLCγ
phospholipase Cγ
- PMA
phorbol 12-myristate 13-acetate
- P-YT
PMA-treated YT [cells]
- rADCC
redirected antibody-dependent cellular cytotoxicity
- SAP
SLAM-associated protein
References
- 1.Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cerwenka A, Lanier LL. Natural killer cells, viruses and cancer. Nat Rev Immunol. 2001;1:41–9. doi: 10.1038/35095564. [DOI] [PubMed] [Google Scholar]
- 3.Biron CA. Activation and function of natural killer cell responses during viral infections. Curr Opin Immunol. 1997;9:24–34. doi: 10.1016/s0952-7915(97)80155-0. [DOI] [PubMed] [Google Scholar]
- 4.Naume B, Espevik T. Immunoregulatory effects of cytokines on natural killer cells. Scand J Immunol. 1994;40:128–34. doi: 10.1111/j.1365-3083.1994.tb03441.x. [DOI] [PubMed] [Google Scholar]
- 5.Kitson RP, Appasamy PM, Nannmark U, Albertsson P, Gabauer MK, Goldfarb RH. Matrix metalloproteinases produced by rat IL-2-activated NK cells. J Immunol. 1998;160:4248–53. [PubMed] [Google Scholar]
- 6.Chuang SS, Kim MH, Johnson LA, Albertsson P, Kitson RP, Nannmark U, Goldfarb RH, Mathew PA. 2B4 stimulation of YT cells induces natural killer cell cytolytic function and invasiveness. Immunology. 2000;100:378–83. doi: 10.1046/j.1365-2567.2000.00031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Long EO. Regulation of immune responses through inhibitory receptors. Annu Rev Immunol. 1999;17:875–904. doi: 10.1146/annurev.immunol.17.1.875. [DOI] [PubMed] [Google Scholar]
- 8.Lanier LL. Turning on natural killer cells. J Exp Med. 2000;191:1259–62. doi: 10.1084/jem.191.8.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moretta A, Biassoni R, Bottino C, Mingari MC, Moretta L. Natural cytotoxicity receptors that trigger human NK-cell-mediated cytolysis. Immunol Today. 2000;21:228–34. doi: 10.1016/s0167-5699(00)01596-6. [DOI] [PubMed] [Google Scholar]
- 10.Boles KS, Stepp SE, Bennett M, Kumar V, Mathew PA. 2B4 (CD244) and CS1: novel members of the CD2 subset of the immunoglobulin superfamily molecules expressed on natural killer cells and other leukocytes. Immunol Rev. 2001;181:234–49. doi: 10.1034/j.1600-065x.2001.1810120.x. [DOI] [PubMed] [Google Scholar]
- 11.Garni-Wagner BA, Purohit A, Mathew PA, Bennett M, Kumar V. A novel function-associated molecule related to non-MHC-restricted cytotoxicity mediated by activated natural killer cells and T cells. J Immunol. 1993;151:60–70. [PubMed] [Google Scholar]
- 12.Mathew PA, Garni-Wagner BA, Land K, Takashima A, Stoneman E, Bennett M, Kumar V. Cloning and characterization of the 2B4 gene encoding a molecule associated with non-MHC-restricted killing mediated by activated natural killer cells and T cells. J Immunol. 1993;151:5328–37. [PubMed] [Google Scholar]
- 13.Nakajima H, Cella M, Langen H, Friedlein A, Colonna M. Activating interactions in human NK cell recognition: the role of 2B4-CD48. Eur J Immunol. 1999;29:1676–83. doi: 10.1002/(SICI)1521-4141(199905)29:05<1676::AID-IMMU1676>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 14.Valiante NM, Trinchieri G. Identification of a novel signal transduction surface molecule on human cytotoxic lymphocytes. J Exp Med. 1993;178:1397–406. doi: 10.1084/jem.178.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kubin MZ, Parshley DL, Din W, et al. Molecular cloning and biological characterization of NK cell activation-inducing ligand, a counterstructure for CD48. Eur J Immunol. 1999;29:3466–77. doi: 10.1002/(SICI)1521-4141(199911)29:11<3466::AID-IMMU3466>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 16.Sivori S, Falco M, Marcenaro E, Parolini S, Biassoni R, Bottino C, Moretta L, Moretta A. Early expression of triggering receptors and regulatory role of 2B4 in human natural killer cell precursors undergoing in vitro differentiation. Proc Natl Acad Sci USA. 2002;99:4526–31. doi: 10.1073/pnas.072065999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chuang SS, Kumaresan PR, Mathew PA. 2B4 (CD244) -mediated activation of cytotoxicity and IFN-gamma release in human NK cells involves distinct pathways. J Immunol. 2001;167:6210–16. doi: 10.4049/jimmunol.167.11.6210. [DOI] [PubMed] [Google Scholar]
- 18.Rajagopalan S, Fu J, Long EO. Cutting edge: induction of IFN-gamma production but not cytotoxicity by the killer cell Ig-like receptor KIR2DL4 (CD158d) in resting NK cells. J Immunol. 2001;167:1877–81. doi: 10.4049/jimmunol.167.4.1877. [DOI] [PubMed] [Google Scholar]
- 19.Tangye SG, Lazetic S, Woollatt E, Sutherland GR, Lanier LL, Phillips JH. Cutting edge: human 2B4, an activating NK cell receptor, recruits the protein tyrosine phosphatase SHP-2 and the adaptor signaling protein SAP. J Immunol. 1999;162:6981–85. [PubMed] [Google Scholar]
- 20.Chuang SS, Pham H-T, Kumaresan PR, Mathew PA. A prominent role for activator protein-1 in the transcription of the human 2B4 (CD244) gene in natural killer cells. J Immunol. 2001;166:6188–95. doi: 10.4049/jimmunol.166.10.6188. [DOI] [PubMed] [Google Scholar]
- 21.Sivori S, Parolini S, Falco M, Marcenaro E, Biassoni R, Bottino C, Moretta L, Moretta A. 2B4 functions as a co-receptor in human NK cell activation. Eur J Immunol. 2000;30:787–93. doi: 10.1002/1521-4141(200003)30:3<787::AID-IMMU787>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 22.Boles KS, Nakajima H, Colonna M, Chuang SS, Stepp SE, Bennett M, Kumar V, Mathew PA. Molecular characterization of a novel human natural killer cell receptor homologous to mouse 2B4. Tissue Antigens. 1999;54:27–34. doi: 10.1034/j.1399-0039.1999.540103.x. [DOI] [PubMed] [Google Scholar]
- 23.Bottino C, Augugliaro R, Castriconi R, Nanni M, Biassoni R, Moretta L, Moretta A. Analysis of the molecular mechanism involved in 2B4-mediated NK cell activation: evidence that human 2B4 is physically and functionally associated with the linker for activation of T cells. Eur J Immunol. 2000;30:3718–22. doi: 10.1002/1521-4141(200012)30:12<3718::AID-IMMU3718>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 24.Klem J, Verrett PC, Kumar V, Schatzle JD. 2B4 is constitutively associated with linker for the activation of T cells in glycolipid-enriched microdomains: properties required for 2B4 lytic function. J Immunol. 2002;169:55–62. doi: 10.4049/jimmunol.169.1.55. [DOI] [PubMed] [Google Scholar]
- 25.Way KJ, Chou E, King GL. Identification of PKC-isoform-specific biological actions using pharmacological approaches. Trends Pharmacol Sci. 2000;21:181–87. doi: 10.1016/s0165-6147(00)01468-1. [DOI] [PubMed] [Google Scholar]
- 26.Leibson PJ, Midthun DE, Windebank KP, Abraham RT. Transmembrane signaling during natural killer cell-mediated cytotoxicity. Regulation by protein kinase C activation. J Immunol. 1990;145:1498–504. [PubMed] [Google Scholar]
- 27.Shibuya A, Lanier LL, Phillips JH. Protein kinase C is involved in the regulation of both signaling and adhesion mediated by DNAX accessory molecule-1 receptor. J Immunol. 1998;161:1671–6. [PubMed] [Google Scholar]
- 28.Johnson LA, Goldfarb RH, Mathew PA. Regulation of IFN-γ production following 2B4 activation in human NK cells. In Vivo. 2000;14:625–30. [PubMed] [Google Scholar]
- 29.Guo TL, White KL, Jr, Brown RD, Delclos KB, Newbold RR, Weis C, Germolec DR, McCay JA. Genistein modulates splenic natural killer cell activity, antibody-forming cell response, and phenotypic marker expression in F(0) and F(1) generations of Sprague-Dawley rats. Toxicol Appl Pharmacol. 2002;181:219–27. doi: 10.1006/taap.2002.9418. [DOI] [PubMed] [Google Scholar]
- 30.Ladekjaer-Mikkelsen AS, Nielsen J. A longitudinal study of cell-mediated immunity in pigs infected with porcine parvovirus. Viral Immunol. 2002;15:373–84. doi: 10.1089/08828240260066297. [DOI] [PubMed] [Google Scholar]
- 31.Tarazona R, Casado JG, Delarosa O, et al. Selective depletion of CD56 (dim) NK cell subsets and maintenance of CD56 (bright) NK cells in treatment-naive HIV-1-seropositive individuals. J Clin Immunol. 2002;22:176–83. doi: 10.1023/a:1015476114409. [DOI] [PubMed] [Google Scholar]
- 32.Peter I, Nawrath M, Kamarashev J, Odermatt B, Mezzacasa A, Hemmi S. Immunotherapy for murine K1735 melanoma: combinatorial use of recombinant adenovirus expressing CD40L and other immunomodulators. Cancer Gene Ther. 2002;9:597–605. doi: 10.1038/sj.cgt.7700475. [DOI] [PubMed] [Google Scholar]
- 33.Bonnema JD, Karnitz LM, Schoon RA, Abraham RT, Leibson PJ. Fc receptor stimulation of phosphatidylinositol 3-kinase in natural killer cells is associated with protein kinase C-independent granule release and cell-mediated cytotoxicity. J Exp Med. 1994;180:1427–35. doi: 10.1084/jem.180.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watzl C, Stebbins CC, Long EO. NK cell inhibitory receptors prevent tyrosine phosphorylation of the activation receptor 2B4 (CD244) J Immunol. 2000;165:3545–58. doi: 10.4049/jimmunol.165.7.3545. [DOI] [PubMed] [Google Scholar]
- 35.Nakajima H, Colonna M. 2B4: an NK cell activating receptor with unique specificity and signal transduction mechanism. Hum Immunol. 2000;61:39–43. doi: 10.1016/s0198-8859(99)00170-6. [DOI] [PubMed] [Google Scholar]
- 36.Aoukaty A, Tan R. Association of the X-linked lymphoproliferative disease gene product SAP/SH2D1A with 2B4, a natural killer cell-activating molecule, is dependent on phosphoinositide 3-kinase. J Biol Chem. 2002;277:13331–7. doi: 10.1074/jbc.M112029200. [DOI] [PubMed] [Google Scholar]
- 37.Nichols KE, Harkin DP, Levitz S, et al. Inactivating mutations in an SH2 domain-encoding gene in X-linked lymphoproliferative syndrome. Proc Natl Acad Sci USA. 1998;95:13765–70. doi: 10.1073/pnas.95.23.13765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coffey AJ, Brooksbank RA, Brandau O, et al. Host response to EBV infection in X-linked lymphoproliferative disease results from mutations in an SH2-domain encoding gene [see comments] Nat Genet. 1998;20:129–35. doi: 10.1038/2424. [DOI] [PubMed] [Google Scholar]
- 39.Sayos J, Wu C, Morra M, et al. The X-linked lymphoproliferative-disease gene product SAP regulates signals induced through the co-receptor SLAM. Nature. 1998;395:462–9. doi: 10.1038/26683. [DOI] [PubMed] [Google Scholar]
- 40.Tangye SG, Phillips JH, Lanier LL, Nichols KE. Functional requirement for SAP in 2B4-mediated activation of human natural killer cells as revealed by the X-linked lymphoproliferative syndrome. J Immunol. 2000;165:2932–6. doi: 10.4049/jimmunol.165.6.2932. [DOI] [PubMed] [Google Scholar]
- 41.Benoit L, Wang XP, Abstract HF, Dutz J, Tan R. Defective NK cell activation in X-linked lymphoproliferative disease. J Immunol. 2000;165:3549–53. doi: 10.4049/jimmunol.165.7.3549. [DOI] [PubMed] [Google Scholar]
- 42.Nakajima H, Cella M, Bouchon A, Grierson HL, Lewis J, Duckett CS, Cohen JI, Colonna M. Patients with X-linked lymphoproliferative disease have a defect in 2B4 receptor-mediated NK cell cytotoxicity. Eur J Immunol. 2000;30:3309–18. doi: 10.1002/1521-4141(200011)30:11<3309::AID-IMMU3309>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 43.Parolini S, Bottino C, Falco M, et al. X-linked lymphoproliferative disease. 2B4 molecules displaying inhibitory rather than activating function are responsible for the inability of natural killer cells to kill Epstein–Barr virus-infected cells. J Exp Med. 2000;192:337–46. doi: 10.1084/jem.192.3.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Isakov N, Altman A. Protein kinase C (theta) in T cell activation. Annu Rev Immunol. 2002;20:761–94. doi: 10.1146/annurev.immunol.20.100301.064807. [DOI] [PubMed] [Google Scholar]
- 45.Mitsutake N, Namba H, Shklyaev SS, et al. PKC delta mediates ionizing radiation-induced activation of c-Jun NH (2) -terminal kinase through MKK7 in human thyroid cells. Oncogene. 2001;20:989–96. doi: 10.1038/sj.onc.1204179. [DOI] [PubMed] [Google Scholar]
- 46.Holmgreen SP, Wang X, Clarke GR, Noltorp RS, Roberts TK, Burton RC, Robinson PJ, Smart YC. Phosphorylation of the NC-1.1 receptor and regulation of natural cytotoxicity by protein kinase C and cyclic GMP-dependent protein kinase. J Immunol. 1997;158:2035–41. [PubMed] [Google Scholar]
- 47.Masuda M, Takahashi H. [Increase of soluble Fc gamma RIIIa derived from macrophages in plasma from patients with atherosclerosis] Rinsho Byori. 2002;50:502–5. [PubMed] [Google Scholar]
- 48.Verneris MR, Baker J, Edinger M, Negrin RS. Studies of ex vivo activated and expanded CD8+ NK-T cells in humans and mice. J Clin Immunol. 2002;22:131–6. doi: 10.1023/a:1015415928521. [DOI] [PubMed] [Google Scholar]