Abstract
The linker protein LAT is expressed mainly in T and natural killer (NK) cells. LAT-deficient mice have an arrest of intrathymic T-cell development at the CD4+ CD8+ stage and lack mature T cells in the periphery. However, no gross abnormality in development and function of the B and NK cells has been described. Here we report that LAT is expressed in mouse progenitor B (pro-B) and precursor B (pre-B) cells, but not in immature or mature B cells. LAT in pre-B cells becomes tyrosine phosphorylated upon cross-linking of the pre-B-cell receptor (pre-BCR) by anti-µ antibody. Incubation of 1xN/2b (mouse pre-B-cell line) cells or bone marrow cells from µMT/µMT mice, which lack B cells after the small pre-B-cell stage, with anti-Igβ antibody resulted in the downregulation of LAT expression. Transgenic mice which expressed LAT protein in B-lineage cells showed an increased proportion of pro- and large pre-B cells in the bone marrow and a remarkable reduction in the numbers of mature B cells in peripheral lymphoid tissues. Collectively, the present results indicate that LAT is expressed in the cells at the early stages of B-lineage development, but is absent in immature and mature B cells. LAT may play a crucial role in the negative regulation of B-cell development at the transition from pre-B to mature B-cell stages, and signal(s) via the pre-BCR may extinguish LAT expression, thus allowing pre-B-cell differentiation towards the mature B-cell stage.
Introduction
Bone marrow production begins before birth. B-cell development in mammalian fetal liver and bone marrow is accompanied by a series of ordered gene rearrangements and the differential expression of cell-surface markers. Committed progenitor B (pro-B) cells rearrange V-, D- and J-gene segments in the heavy chain (HC) locus to differentiate into precursor B (pre-B) cells. When a functional VHDHJH rearrangement occurs in a pre-B cell, µHC will be expressed on the cell surface together with the surrogate light chain (sLC), which consists of two polypeptides – VpreB and λ5 – to form the pre-B-cell receptor (pre-BCR). Subsequent rearrangement of a κ or λ LC gene permits pre-B cells to give rise to surface immunoglobulin M (IgM)-positive immature B cells. Following further maturation, B cells leave the bone marrow and migrate to the periphery.1–3
A critical role for the pre-BCR in B-cell development was illuminated by a series of analyses in normal and mutant mice. A defect in the expression of the µHC aborts B-cell development at the transition from the pro-B- to the pre-B-cell stage, resulting in an absence of mature B cells.4 Mutation of the λ5 gene also impairs B-cell development, but does not completely abolish B cells.5 The pre-BCR plays a role in the establishment of allelic exclusion of the HC genes through downregulation of recombination-activating genes (RAG)6,7 and may be involved in the selection of functional µHC with a capacity to assemble with sLC, as pre-B cells expressing certain VH genes are selected for contribution to the B-cell repertoire.8
Despite its crucial role in B-cell development, the biological outcome of pre-BCR-mediated signalling has not yet been defined completely. By utilizing Abelson murine leukaemia virus-transformed mouse pre-B-cell lines and human leukaemia pre-B-cell lines, it has been reported that pre-BCR-mediated signals induce a limited increase of intracellular Ca2+ and tyrosine phosphorylation of the cytoplasmic proteins in a pattern which differs from that induced by cross-linking of the BCR on immature or mature B-cell lines.9–13 Although in vitro Abelson-transformed cell lines have been tremendously valuable for promoting our understanding of immunoglobulin gene rearrangement,14 they are not the best model system for using to analyse the pre-BCR signal because they exhibit a relatively high background of kinase activity, even in the absence of stimulation.15
Recently, the adapter protein, B-cell linker protein (BLNK; also known as SLP-65 or BASH) has been identified as a signalling element of the BCR.16–18 BLNK-deficient mice have shown that this adapter is required for signal transduction in B cells. In the absence of BLNK, B-cell development is severely affected at several stages. Proportions of pre-B cells in the bone marrow and immature B cells in peripheral lymphoid organs are increased in the mutant mice, suggesting that signals from pre-BCR through BLNK may play a crucial role in the differentiation of B cells from the pre-B-cell stage.19,20
In the present study, we analysed an interleukin (IL)-7-dependent early pre-B-cell line, 1xN/2b, which was cloned from mouse bone marrow and expresses the pre-BCR consisting of µHC and Vpre-B/λ5.21 The 1xN/2b cells do not produce any κ or λ LC.22 Cross-linking of the pre-BCR by anti-µ antibody induced tyrosine phosphorylation of an intracellular protein with a molecular weight of 38 000, which we identified as LAT. Thus, LAT was expressed in 1xN/2b cells and tyrosine phosphorylated following pre-BCR engagement. LAT was also detected in other mouse pre-B- and pro-B-cell lines tested, but not in immature or mature B-cell lines. LAT is known to be a substrate of the tyrosine kinase activated by T-cell receptor (TCR) engagement and is essential for T-cell development.23–25 LAT has been reported not to be expressed in B cells. However, in the present study, we show that LAT protein was also present in normal bone marrow pro-B cells and pre-B cells. However, LAT expression was not detected at all in immature or mature B cells. Moreover, LAT expression was downregulated by cross-linking of the pre-BCR. These results suggest that LAT is expressed in mouse B cells at an early developmental stage and its expression may play a role in the negative regulation of B-cell differentiation. In order to test this hypothesis, we produced LAT-transgenic mice in which LAT was expressed in B cells. In the transgenic mice, B-cell development was severely impaired at the stage from pre-B cells to mature B cells. We discuss a possible role for LAT as a negative regulator at early stages of B-cell differentiation.
Materials and methods
Mice
µMT/µMT mice, with an inactivating mutation of the membrane exon of the µ-chain gene,4 were bred and maintained under specific pathogen-free (SPF) conditions in our animal facility and used for analysis at 8–12 weeks of age. C57BL/6 mice were purchased from Clea Japan, Inc. (Tokyo, Japan).
Cell lines and cell culture
The mouse pre-B-cell lines, 1xN/2b and 5.7.10, the mouse pro-B-cell line, DIF9,26 and the mouse B-lymphoma cell line, WEHI231, were cultured in RPMI-1640 (Life Technologies, Rockville, MD) containing 5 × 10−5m 2-mercaptoethanol (2-ME), 100 U/ml penicillin and streptomycin, supplemented with 10% fetal calf serum (FCS). IL-7 was produced, as previously described,26 and used to maintain the IL-7-dependent cell lines. HM79 (a gift from Dr H. Karasuyama) is a hybridoma cell line secreting anti-mouse Igβ monoclonal antibody (mAb) and was cultured in RPMI-1640 supplemented with 20% FCS. mAb against Igβ was purified from culture supernatants using a protein-G affinity column.
Flow cytometric analysis
Single-cell suspensions were prepared from C57BL/6 mouse bone marrow cells. To analyse the intracellular LAT protein in B220-positive cells, 1 × 106 cells were incubated for 30 min on ice with a fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgM antibody (Cappel, Turnhout, Belgium) and a Cy-Chrome-conjugated rat anti-mouse CD45/B220 (PharMingen, San Diego, CA). After washing twice with 2% FCS in phosphate-buffered saline (PBS), cells were fixed with 1% paraformaldehyde overnight at 4°. After fixation, cells were incubated for 30 min on ice with rabbit anti-LAT antibody (Upstate Biotechnology, Lake Placid, NY) or normal rabbit immunoglobulin G (IgG), in PBS containing 0·3% saponin, washed three times with 0·03% saponin/PBS and then incubated with phycoerythrin (PE)-conjugated anti-rabbit IgG antibody (Southern Biotechnology Associates, Birmingham, AL) for 20 min on ice. Stained cells were analysed in a FACScan flow cytometer using CELLQuest software (Becton-Dickinson, Mountain View, CA). Dead cells and debris were gated out based on forward- and side-light scatter characteristics.
Immunoprecipitation and detection of tyrosine-phosphorylation proteins
A total of 1 × 107 cells were resuspended in 100 µl of RPMI-1640. After preincubation at 37° for 5 min, cells were stimulated with a 5-µg/ml affinity-purified goat anti-mouse IgM polyclonal antibody (Jackson ImmunoResearch Laboratories, West Grove, PA). Cells were incubated for 2 min and the reaction was stopped by adding ice-cold PBS containing 2 mm sodium orthvanadate. After washing once with the PBS-orthvanadate, cell pellets were lysed in 500 µl of lysis buffer [10 mm Tris, pH 7·4, 150 mm NaCl, 1 mm NaF, 2 mm EDTA, 1% Nonidet P-40 (NP-40), 1 mm sodium orthvanadate, 0·02 U/ml aprotinin, 1 µg/ml leupeptin, 1 mm phenylmethylsulphonyl fluoride (PMSF)]. The lysates were cleared by centrifugation. Supernatants were then immunoprecipitated using the indicated antibodies. Immunoprecipitates were washed in lysis buffer and lysed in 2× sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) buffer [100 mm Tris–HCl (pH 6·8), 4% SDS, 20% glycerol, 4% 2-ME]. Samples were fractionated by SDS–PAGE (10% gel) and transferred to nitrocellulose filters. After blocking with 3% non-fat milk in 20 mm Tris, 150 mm NaCl, 0·05% Tween-20, pH 8·0 (TBST), containing 2 mm sodium orthvanadate, the filter was incubated with the horseradish peroxidase (HRP)-labelled PY-20 (Transduction Laboratories, Lexington, KY) in the blocking buffer, and developed using the enhanced chemiluminescence (ECL) system (Amersham Corp., Arlington Heights, IL).
Western blot analysis for intracellular LAT protein
To detect the LAT protein, cells were lysed in 2× SDS–PAGE buffer and boiled. The lysates were cleared by centrifugation and 10 µl of each lysate was fractionated by SDS–PAGE (10% gel), then electrotransferred to a nitrocellulose filter, as described above. After blocking with blocking buffer, the filter was incubated with rabbit anti-LAT antibody (Upstate Biotechnology) or normal rabbit IgG in the blocking buffer, followed by incubation with HRP-labelled F(ab′)2 goat anti-rabbit IgG (Zymed Laboratories, Inc., San Francisco, CA), and developed using the ECL system (Amersham Corp.).
LAT gene expression, as determined by reverse transcription–polymerase chain reaction (RT–PCR) analysis
B220+ IgM+ cells and B220+ IgM– cells were separated from mouse bone marrow cells by using Dynabeads (Dynal, Oslo, Norway). RNA was isolated from cells of mouse bone marrow or different cell lines using ISOGEN (Nippon Gene, Chiyoda-ku, Tokyo, Japan) according to the manufacturer's protocol. First-strand cDNA was synthesized using murine Moloney leukaemia virus (MMLV) reverse transcriptase and random hexamer primers. The LAT-encoded cDNA sequences were amplified by PCR using two primers designated as LAT-F (5-AGCCATCCAGCCCCGAAA-3) and LAT-B (5-TCTTAGCGACACCCCAGCAA-3) (Gene Amp PCR system 9700; Applied Biosystems, Foster City, CA).
Transgene construction and generation of transgenic mice
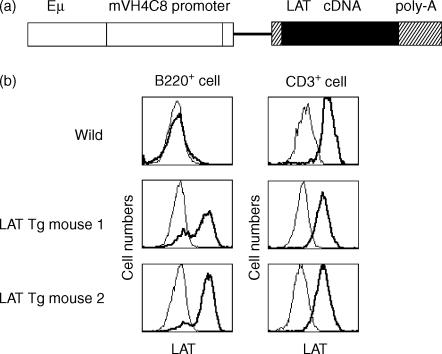
The LAT gene was obtained by PCR from a cDNA library that was generated from 1xN/2b cell lines using a cDNA Synthesis Kit and the ZAP-cDNA Gigapack III Gold Cloning Kit (Stratagene, La Jolla, CA), according to the manufacturer's protocol, with two primers designated as described above (Gene Amp PCR system 9700; Perkin-Elmer Cetus). The resulting LAT fragments (920 bp) were gel-isolated and cloned using the TOPO Cloning Kit (Invitrogen, Carlsbad, CA). Sequence analysis revealed nucleotide differences at positions 699 (G>A) and 714 (A>G) when compared with the published sequence of mouse LAT cDNA. These differences, however, do not alter the amino acid sequence. The BamHI/XhoI LAT cDNA fragment was inserted into the unique XhoI site of the rabbit β-globin gene in the pBluescript II KS(+) cassette, which carries the enhancer Eµ, the promoter of immunoglobulin heavy chain VH4C8, and 3′-untranslated rabbit β-globin gene (Fig. 4a and ref. 27). The resultant vector was transfected to the mouse myeloma cell line, X63Ag8.6.5.3, in order to verify the expression of LAT in B cells. The vector was linearized by using the SacI and KpnI enzymes and microinjected into the pronuclei of fertilized C57BL/6 mouse eggs. Transgenic offspring were identified by using the PCR and Southern blot analysis.
Figure 4.
LAT protein expression on B and T cells of wild-type and transgenic (Tg) mice. (a) Schematic structure of the transgenic construct. mVH4C8, promoter of the mouse immunoglobulin heavy-chain VH4C8 gene; Eµ, the heavy-chain gene intron enhancer. (b) Spleen cells in LAT-transgenic and wild-type mice were stained with the antibodies indicated and analysed by flow cytometry. The experiments were repeated twice and gave similar results on each occasion.
Cell culture with anti-Igβ antibody
1xN/2b cells or µMT/µMT mouse bone marrow cells were cultured with 1 µg/ml or 10 µg/ml anti-Igβ antibody in IL-7-containing medium for 72 hr. Cells were analysed by flow cytometry, as described above. Normal hamster IgG was used as a control.
Proliferation assay
Splenic B cells were treated with anti-mouse Thy-1.2 mAb for 30 min on ice and then with complement for 30 min at 37° for depletion of T cells. To remove adherent cells, cells were incubated at 37° for 1 hr on plastic dishes and non-adherent cells were collected. About 90–95% of the isolated cells were found to express the B-cell marker, B220. Cells were cultured in RPMI-1640 containing 5 × 10−5m 2-ME and 10% heat-inactivated FCS, at 37° in an atmosphere of 5% CO2 in air. Splenic B cells (1 × 106/ml in 100 µl/well) were placed in 96-well flat-bottom plates and cultured for 48 hr with different stimuli. The cultures were pulsed for the final 6 hr with 1 µCi/ml [3H]thymidine.
Results
LAT is expressed in pro- and pre-B cells and is tyrosine phosphorylated upon cross-linking of pre-BCR
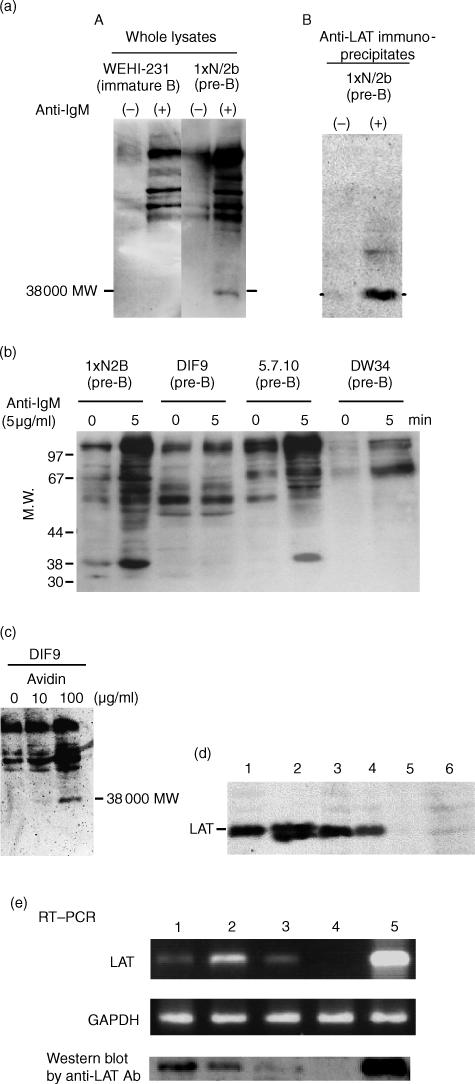
It has been shown that cross-linking of the pre-BCR on pre-B-cell lines induced tyrosine phosphorylation of various cellular proteins.9–13 Signal transduction events mediated via the pre-BCR on large pre-B (1xN/2b) cells and the BCR on immature B (WEHI231) cells were compared upon receptor cross-linking by anti-µ antibody. There was a rapid increase in tyrosine phosphorylation of intracellular proteins in both 1xN/2b and WEHI231 cells. The tyrosine-phosphorylation pattern is illustrated by Western blot analysis in Fig. 1(a)(panel A). The results showed that, similarly to the BCR on WEHI231 cells, the pre-BCR on 1xN/2b cells could transduce signals to activate tyrosine kinases but a distinct spectrum of tyrosine-phosphorylated proteins was observed in 1xN/2b cells. In particular, the species with an apparent molecular weight (MW) of ∼38 000 was prominent in 1xN/2b cells but not in WEHI231 cells. A tyrosine-phosphorylated protein with the same MW was also observed in another pre-B-cell line, 5.7.10, upon stimulation with anti-µ antibody (Fig. 1b).
Figure 1.
Tyrosine phosphorylation of a 38 000-molecular weight (MW) protein in cells of mouse progenitor B (pro-B) and precursor B (pre-B) cell lines. (a) (panel A) 1xN/2b (pre-B) and WEHI231 (immature B) cells were stimulated with 5 µg/ml of goat F(ab′)2 anti-mouse immunoglobulin M (IgM) antibody for 2 min at 37°. Whole-cell lysates were fractionated by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) (10% gel) and transferred to nitrocellulose membrane. Tyrosine-phosphorylated proteins were detected by blotting with the PY20 monoclonal antibody (mAb). (a) (panel B) 1xN/2b cells were stimulated with 5 µg/ml of goat F(ab′)2 anti-mouse IgM antibody for 2 min at 37°. Whole-cell lysates were immunoprecipitated with rabbit anti-mouse LAT antibodies. Immunoprecipitates were fractionated by SDS–PAGE (10% gel), transferred to nitrocellulose membrane and immunoblotted with the PY20 mAb. (b) Cells of 1xN/2b (pre-B), DIF9 (pro-B), 5.7.10 (pre-B) and DW34 (pro-B) were stimulated with 5 µg/ml goat F(ab′)2 anti-mouse IgM antibody for 5 min at 37°. Whole-cell lysates were fractionated by SDS–PAGE (10% gel) and transferred to nitrocellulose membranes. Tyrosine-phosphorylated proteins were detected by blotting with the PY20 mAb. (c) DIF9 cells were preincubated with biotin-conjugated anti-Igβ antibody for 5 min on ice, followed by the addition of avidin (0, 10 or 100 µg/ml) and further incubation at 37° for 2 min. Whole-cell lysates were immunoblotted with the PY20 mAb. (d) LAT protein was detected by Western blot analysis of whole-cell lysates from the indicated cell lines. Lane 1, 1xN/2b (pre-B); lane 2, Jurkat (human T cell); lane 3, DIF9 (pro-B); lane 4, 5.7.10 (pre-B); lane 5, 70z/3 (late pre-B); lane 6, WEHI231 (immature B). (e) Reverse transcription–polymerase chain reaction (RT–PCR) to detect LAT was performed with total RNA prepared from the indicated mouse cell lines. Lane 1, DIF9 (pro-B); lane 2, 1xN/2b (pre-B); lane 3, 70z/3 (late pre-B); lane 4, WEHI231 (immature B); lane 5, EL4 (T cell). The results were confirmed in a separate experiment. GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
The linker protein, LAT, which is essential for TCR-mediated signal transduction and T-cell development, has a MW of 36 000–38 000. It has been reported that LAT is not expressed in mature B cells;23 however, LAT expression had not been examined in mouse pro- or pre-B cells. Whole-cell lysates from anti-µ-stimulated 1xN/2b cells were immunoprecipitated with anti-LAT antibody and blotted with anti-phosphotyrosine antibody (Fig. 1a(panel B). A tyrosine-phosphorylated protein of 38 000 MW was detected. These results suggested that the tyrosine-phosphorylated protein with MW 38 000, found in 1xN/2b or 5.7.10 cells, is LAT. Western blot analysis for cell lysates (Fig. 1d) and RT–PCR analysis for total RNA (Fig. 1e) prepared from mouse B-cell lines, DIF9 (proB-cell line), 1xN/2b (early pre-B-cell line), 5.7.10 (early pre-B-cell line), 70Z/3 (small pre-B-cell line) or WEHI231 (immature B-cell line), clearly showed that LAT protein was detected in pro-B- and pre-B-cell lines, but not in late pre-B- or immature B-cell lines. The products obtained from 1xN/2b were sequenced to confirm the identity of the published sequence of mouse LAT cDNA.
Hyper cross-linking of Igβ on DIF9 cells with biotinylated anti-Igβ antibody and avidin also induced tyrosine phosphorylation of the protein with MW 38 000 (Fig. 1c). These results indicated that LAT protein expression among B-lineage cells appears to be limited to the pro- and early pre-B-cell stages and it appeared to be involved in signalling events from the pre-BCR. In Jurkat cells, LAT was expressed as two forms at apparent MWs of 36 000 and 38 000, as previously described.23 The reason why LAT expression was different in B- and T-cell lines was unknown.
LAT expression in mouse bone marrow cells
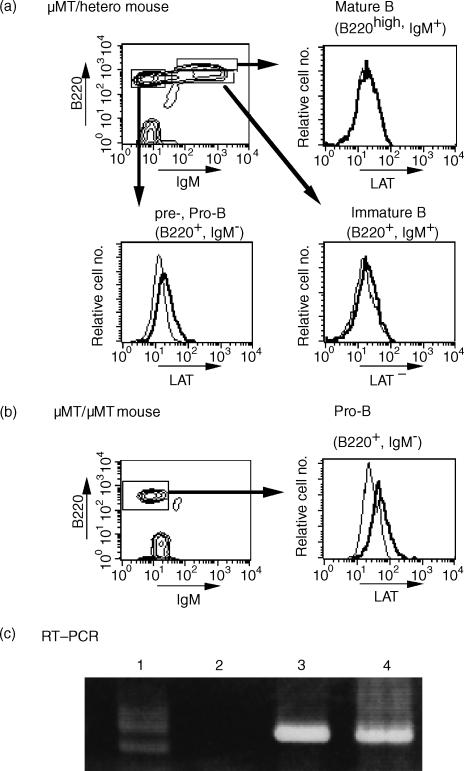
To elucidate stage-specific expression of LAT in B-lineage cells, bone marrow cells of wild-type µMT/+ mice (Fig. 2a) and µMT/µMT mice (Fig. 2b) were analysed. In µMT/+ mice, LAT was expressed by cells in the pro- and pre-B-cell stages, but not by cells in the immature or mature B-cell stages. In µMT/µMT mice, in which the membrane exon of the µ-chain gene has been inactivated and B-cell development is arrested at an early stage of B-cell development before the cells entered the pool of small resting pre-B cells,4 LAT was significantly expressed in B220+ bone marrow cells. The RT–PCR analysis showed that LAT was expressed in IgMlow/– B cells, but not in IgM+ B cells (Fig. 2c). These findings suggested that LAT expression may cease during the transition from an early pre-B-cell stage to a late pre-B- and immature B-cell stage.
Figure 2.
LAT protein expression in bone marrow cells. Bone marrow cells from µMT/+ mouse (a) or µMT/µMT mouse (b) were labelled with a fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse immunoglobulin M (IgM) antibody and a Cy-Chrome-conjugated rat anti-mouse CD45/B220. Cells were fixed with phosphate-buffered saline (PBS) containing 1% paraformaldehyde, permeabilized with saponin, labelled with anti-LAT antibody and analysed by flow cytometry. Background staining with normal rabbit immunoglobulin G (IgG) is shown for comparison (thin lines in the histogram). Results are representative of three different experiments. Reverse transcription–polymerase chain reaction (RT–PCR) was performed on total RNA prepared from the indicated cells (c). Lane 1, whole bone marrow cells; lane 2, B220+ IgM+ cells; lane 3, B220+ IgM– cells; lane 4, EL4 (T cell). The results were confirmed by another, separate, experiment.
Down-regulation of LAT expression following pre-BCR cross-linking
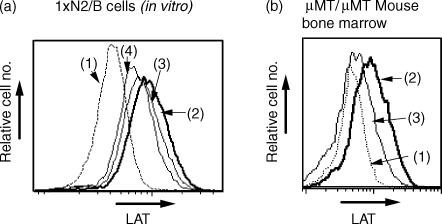
The Igα/Igβ-mediated signal has been reported to elicit the transient and synchronous differentiation of pro-B cells to small pre-B cells in vivo, and a series of intracellular biochemical changes are detectable ex vivo.27 To elucidate the effect of the Igα/Igβ-mediated signals on differentiation of the pre-B-cell line, 1xN/2b, the cells were cultured with 1 or 10 µg/ml anti-Igβ antibody in the presence of IL-7. This treatment did not induce any change of cell-surface markers or cell size (data not shown), but resulted in the downregulation of LAT expression in a dose-dependent manner (Fig. 3a). Similarly, incubation of µMT/µMT mouse bone marrow cells with anti-Igβ antibody resulted in the downregulation of LAT expression (Fig. 3b). These results suggested that signal(s) from the pre-BCR might result in the disappearance of LAT in the cells after the pre-B-cell stage.
Figure 3.
Downregulation of LAT expression after cross-linking of the precursor B-cell receptor (pre-BCR) with Igβ. (a) 1xN/2b cells were incubated in vitro with different concentrations of anti-Igβ antibody for 72 hr: (1) background staining for LAT with normal rabbit serum; (2) with normal hamster immunoglobulin G (IgG) (10 µg/ml); (3) with anti-Igβ antibody (1 µg/ml); or (4) with anti-Igβ antibody (10 µg/ml). Cells were analysed as described in the legend to Fig. 2. Results are representative of three different experiments. (b) Bone marrow cells from µMT/µMT mouse were incubated with normal hamster IgG (2) or with 10 µg/ml of anti-Igβ antibody (3) for 72 hr. (1) background staining for LAT with normal rabbit serum. Cells were analysed as described in the legend to Fig. 2. Results are representative of three different experiments.
Marked reduction of mature B cells in LAT-transgenic mice
We generated several lines of transgenic mice in which the expression of the LAT gene was regulated by the mVH4C8 promoter and the Eµ enhancer (Fig. 4a). Of 13 LAT-gene positive progeny screened by flow cytometry, two lines showed high expression of LAT protein in B cells (Fig. 4b). These two founders were bred with C57BL/6 mice. The other 11 transgenic mice expressed a low level of LAT protein in B cells (data not shown).
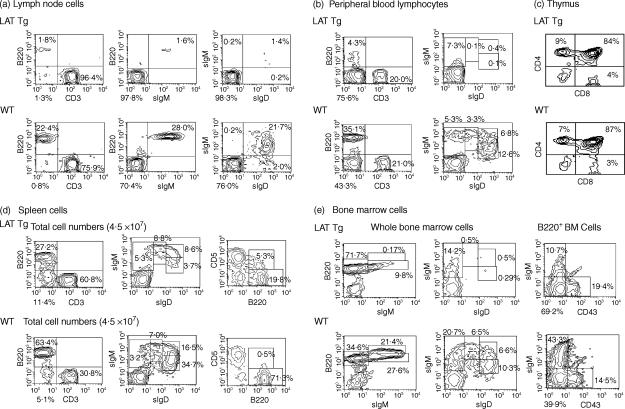
LAT-transgenic mice appeared to be healthy and fertile under SPF conditions. Spleens and lymph nodes from LAT-transgenic mice were rather small in size (data not shown). In 8–12-week-old mice, markedly decreased numbers of IgM+ B cells were observed in lymph nodes (Fig. 5a) and peripheral blood lymphocytes (Fig. 5b). In particular, a proportion of mature (IgD+ IgM+) B cells in LAT-transgenic mice was 6% and 3% of that of wild-type mice in lymph nodes and peripheral blood, respectively. There was a threefold decrease in the numbers of mature (IgD+ IgM+) B cells and a 9–10-fold reduction in the numbers of IgDhi IgMlo B cells in spleen (Fig. 5d). On the other hand, no change in the numbers or ratio of CD4+ and CD8+ T-cell populations were observed in the thymus (Fig. 5c). The reduced numbers of mature B cells in the periphery seemed to result from a defect in maturation at an early stage of B-cell development in bone marrow. As shown in Fig. 5(e), proportions of mature (B220hi IgM+) and immature (B220lo IgM+) B cells in bone marrow cells were markedly reduced in transgenic mice as compared to that of wild-type mice (12·6- and 2·8-fold reduction, respectively). In contrast, the numbers of B220+ IgM– cells increased in the transgenic mouse bone marrow. These data suggested a blockage in development from the pre-B to the mature B-cell stage in bone marrow of LAT-transgenic mice. The proportion and numbers of B1 (B220+ CD5+) cells appeared to be increased in the transgenic mice, suggesting that B1-cell development may not be affected by the appearance of LAT. In the other 11 transgenic mice, a 20–60% decrease was observed in the numbers of IgM+ B cells in peripheral blood lymphocytes. Decreased numbers of B cells corresponded with the level of the expression of LAT protein (data not shown).
Figure 5.
Flow cytometric analysis of B and T cells in primary and secondary lymphoid tissues from LAT transgenic (Tg) and wild-type (WT) mice. Total cells isolated from lymph node (a), peripheral blood (b), thymus (c), spleen (d) and bone marrow (e), from 8–12-week-old WT and LAT Tg mice, were stained simultaneously with the indicated antibodies and analysed by flow cytometry. The number in each quadrant is indicated as a percentage of the total cells plotted. Results are representative of three different experiments. sIgD, surface IgD, sIgM; surface IgM.
In vitro stimulation of splenocytes
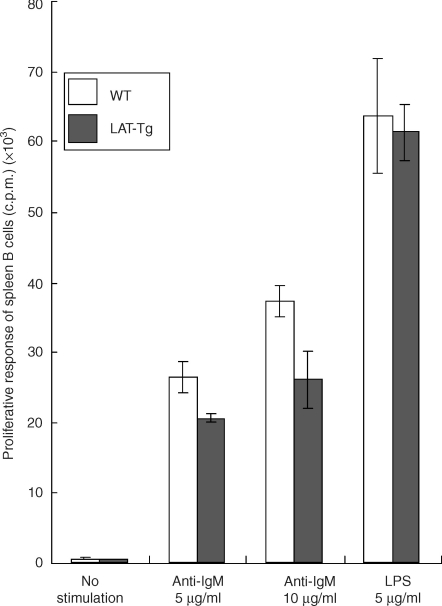
To test the in vitro growth response of LAT-transgenic mouse B cells, splenic B cells derived from transgenic or wild-type mice were cultured in the presence of anti-IgM or lipopolysaccharide (LPS) (Fig. 6). The B cells from LAT-transgenic mice showed a proliferative response against anti-IgM antibody or LPS that was similar to the response of the wild-type mouse B cells. These findings suggested that the presence of LAT protein in B-precursor cells greatly impairs B-cell development, but the presence of LAT protein in mature B cells does not affect their function or activation.
Figure 6.
Proliferative responses [in counts per minute (c.p.m.)] upon stimulation with anti-immunoglobulin M (anti-IgM) antibody or lipopolysaccharide (LPS). Spleen B cells from wild-type (WT) or LAT transgenic (Tg) mice were stimulated with goat F(ab′)2 anti-IgM antibody (5 or 10 µg/ml) or LPS (5 µg/ml). Cells were cultured for 48 hr and pulsed with [3H]thymidine. All assays were performed in triplicate. Bars show the standard deviation (SD). The experiments were repeated twice and gave similar results on each occasion.
Discussion
Initial comparative analysis of biochemical events initiated via the pre-BCR and the BCR indicated significant differences. Tyrosine phosphorylation of a 38 000-MW protein was evident in the pre-B-cell line, 1xN/2b, but not in an immature B-cell line, WEHI231. This indicated that the pre-BCR and the BCR may activate distinct signalling molecules in addition to common ones, even though both receptors utilize the Igα/Igβ heterodimer as a common signal transducer. Analysis by immunoprecipitation with anti-LAT antibody, followed by Western blot with anti-phosphotyrosine antibody, revealed that the rapidly tyrosine phosphorylated, 38 000-MW protein found in the anti-µ-stimulated 1xN/2b cells was LAT. A linker protein, LAT, has been reported to be expressed in T cells, mast cells, natural killer (NK) cells and megakaryocytes,23 and is essential for T-cell development.25 It has been reported that LAT is not expressed in B cells.23 However, expression of LAT in pro- and pre-B cells has not been studied. Furthermore, LAT protein and its message was also detected in other B-precursor cell lines, such as DIF9 (pro-B) cells and 5.7.10 (pre-B) cells, but not in mature B-cell lines. In the case of the 70Z/3 cell line, which is defined as a late pre-B cell, LAT messenger RNA was detected by RT–PCR, but the protein was not evident. Therefore, LAT appeared to be expressed in mouse pro- and pre-B-cell lines, but not in immature or mature B-cell lines. To investigate the stage-specific expression of LAT in mouse B-lineage cells, normal mouse bone marrow cells were analysed. LAT was expressed in the cells of pro-B and pre-B-cell stages, but the expression diminished as the cells developed and was not detected in the immature and mature B-cell stages. It has been shown that the cross-linking of Igα/Igβ heterodimers by anti-Igβ antibody could transduce signals in µ-negative bone marrow pro-B cells and induce early B-cell differentiation.28 Incubation of 1xN/2b cells or bone marrow cells from µMT/µMT mice (in which B-cell development is arrested at the large pre-B stage) with anti-Igβ antibody, induced downregulation of LAT expression (Fig. 3). In contrast, treatment of µMT/µMT mouse bone marrow cells with anti-Igβ antibody has been reported to induce upregulation of the expression of CD25, BP-1 and CD2 on B220+ B-lineage cells and downregulation of λ5 and CD117 (c-kit).29 The same treatment also induced activation of rearrangement at the light-chain gene loci and suppression of the rearrangement at heavy-chain gene loci.29 These findings suggest that the presence of LAT might negatively regulate the differentiation of pre-B cells to immature B cells, and that the disappearance of LAT precedes cell maturation. The signals from the pre-BCR may play a role in shutting off transcription of the LAT gene, thus allowing pre-B cells to differentiate towards immature B cells. However, the exact function of tyrosine-phosphorylated LAT in pre-B cells is not yet clear.
To investigate a role of LAT in B-cell development, LAT-transgenic mice were generated. In the transgenic mice, the LAT gene was mainly expressed in B-lineage cells. Although there was slightly leaky expression of LAT in T-lineage cells, T-cell development seemed to be normal, as shown in Fig. 5(c). High expression of the LAT gene in B-lineage cells resulted in a profound block in B-cell development. B-cell differentiation was mostly arrested at the large pre-B-cell stage in LAT-transgenic mice. Numbers of immature B cells in bone marrow were decreased and a marked reduction of mature B cells was observed in bone marrow, lymph node, peripheral blood and spleen. On the other hand, the proportion of B-precursor cells increased in bone marrow. This result suggested that the downregulation and extinction of LAT expression might be essential for the development of pre-B cells towards immature B cells. However, proliferative responses of residual mature B cells in LAT-transgenic mice against various stimuli were not impaired, suggesting that the presence of LAT in mature B cells does not affect their function. Taken together, the presence of a LAT linker protein in B-lineage cells may specifically intervene in signals from the pre-BCR and block their differentiation from pre-B to immature B cells. The proliferative response of the remaining mature splenic B cells in LAT-transgenic mice was not impaired upon cross-linking of the BCR by anti-IgM antibodies, indicating that the presence of LAT in the membrane lipid raft of mature B cells did not interfere with the signalling pathway from BCR. These data suggest a restricted effect of LAT expression on B cells at early stage of development. One of the molecular mechanisms to explain why B-cell development is arrested in the absence of an intact pre-BCR might be blockade of the differentiation induced by the continuous presence of LAT at the pre-B-cell stage.
B-cell development was reported to be intact in LAT-deficient mice.25 According to the present hypothesis, in the absence of LAT at pro- and pre-B-cell stages, differentiation arrest of pre-B cells towards immature B cells is not induced, but rather B-cell development might be accelerated and the B-cell repertoire might be biased. In LAT-deficient mice, pre-B cells lacking the pre-BCR might also appear, which, however, fail to form intact BCR and will be eliminated at the stage of immature B cells. It has been reported that mice homozygous for a mutation of a single LAT tyrosine residue (LATY136F mice) exhibited an early block in T-cell maturation but later developed a polyclonal proliferation of mature B and plasma cells.30,31 This phenotype, however, appeared to be caused by an indirect effect of exaggerated T helper 2 (Th2) differentiation. Such a B-cell lymphocyte proliferation disappeared in LATY136F × major histocompatibility complex knockout (MHC KO) mice, which lacked CD4 T cells,30,31 indicating that abnormal expansion and activation of mature B cells in LATY136F mice is caused by uncontrolled Th2 differentiation, not by a direct effect of the absence of LAT-mediated signals in pro- or pre-B cells.
The LAT-transgenic mice showed a phenotype similar to that of mice carrying a mutation in BLNK.19,20 Both types of mutant mice show a markedly reduced number of mature B cells in bone marrow and peripheral lymphoid tissues. Whereas the B cells that develop in syk–/– mice express little membrane IgM,32,33 the B cells that accumulate in LAT-transgenic and BLNK–/– mice express normal amounts of membrane IgM. LAT in T cells associates directly with phospholipase C (PLC)-γ1, Grb2 and the p85 subunit of phosphatidylinositol 3 kinase (PI3 kinase), and indirectly with SOS, Vav, Cbl and SLP-76 via Grb2 upon activation.23 BLNK is tyrosine phosphorylated by Syk and associates with numerous signalling elements, including Btk, Grb2, PLCγ2, Vav and Nck, following cross-linking of the BCR.34 Although the phosphorylation pattern and the function of individual LAT tyrosines in B-cell development was not investigated, these data suggest that expression of LAT in early B-lineage cells might block pre-BCR signalling by competing with BLNK, for example, by interfering with the interaction of BLNK with Grb2. However, LAT does not appear to directly interact with BLNK (data not shown). Further investigation is necessary to elucidate a precise mechanism.
BLNK has sequence similarity to the adaptor protein, SLP-76, found in T cells. Thus, BLNK appears to be the B-cell counterpart of SLP-76. Extending this analogy, a counterpart of LAT functioning in T cells should be present in B cells. The tyrosine-phosphorylated 38 000-MW protein found in 1xN/2b cells appeared to be a good candidate for such a counterpart molecule. However, according to our present experiments, the 38 000-MW protein appears to be LAT, although the possibility remains that the anti-LAT antibody we used may cross-react with an unknown protein of 38 000 MW. Recently, NTAL (non-T-cell activation linker) was reported to be a possible homologue of LAT in non-T cells.35 NTAL is expressed in B lymphocytes, NK cells, monocytes and mast cells, but not in resting T lymphocytes. The 38 000-MW protein was detected, by anti-LAT antibody, in pro-B cells, pre-B cells and T cells, but not in immature or mature B cells. Therefore, the anti-LAT antibody appears not to cross-react with NTAL. Whereas BLNK, in B cells, and SLP-76, in T cells, play a similar role in lymphocyte development, LAT might play a different role in each lineage. Expression of LAT in cells at an early stage of T-cell development gives a positive signal for their differentiation, but its expression in early B-cell development might play a crucial role in the negative regulation of B-cell differentiation. These results suggest that LAT in B cells is not a counterpart of LAT in T cells. The reason for the different LAT contribution to early B- or T-lymphocyte development is not known, but a study on the LAT expression in pro-B and pre-B cells may contribute to a better understanding of molecular events triggered by pre-BCR-mediated signals.
Acknowledgments
The authors thank Dr Peter D. Burrows for his critical reading of the manuscript, Dr Hajime Karasuyama for providing us with the hybridoma cell line, HM79, and Mr Toshiro Suzuki of SLC, Hamamatsu, Japan for his help in the generation of LAT transgenic mice.
Abbreviations
- BCR
B-cell receptor
- BLNK
B-cell linker protein
- HC
heavy chain
- IL
interleukin
- LAT
linker for activation of T cells
- PE
phycoerythrin
- PLC
phospholipase C
- pre-BCR
precursor B-cell receptor
- RAG
recombination-activating gene
- sLC
surrogate light chain
References
- 1.Rajewsky K. Clonal selection and learning in the antibody system. Nature. 1996;381:751–8. doi: 10.1038/381751a0. [DOI] [PubMed] [Google Scholar]
- 2.Burrows PD, Cooper MD. B cell development and differentiation. Curr Opin Immunol. 1997;9:239–44. doi: 10.1016/s0952-7915(97)80142-2. [DOI] [PubMed] [Google Scholar]
- 3.Benschop RJ, Cambier JC. B cell development: signal transduction by antigen receptors and their surrogates. Curr Opin Immunol. 1999;11:143–51. doi: 10.1016/s0952-7915(99)80025-9. [DOI] [PubMed] [Google Scholar]
- 4.Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin µ chain gene. Nature. 1991;350:423–6. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 5.Kitamura D, Kudo A, Schaal S, Muller W, Melchers F, Rajewsky K. A critical role of λ5 protein in B cell development. Cell. 1992;69:823–31. doi: 10.1016/0092-8674(92)90293-l. [DOI] [PubMed] [Google Scholar]
- 6.Kitamura D, Rajewsky K. Targeted disruption of µ chain membrane exon causes loss of heavy-chain allelic exclusion. Nature. 1992;356:154–6. doi: 10.1038/356154a0. [DOI] [PubMed] [Google Scholar]
- 7.Grawunder U, Leu TM, Schatz DG, Werner A, Rolink AG, Melchers F, Winkler TH. Down-regulation of RAG1 and RAG2 gene expression in PreB cells after functional immunoglobulin heavy chain rearrangement. Immunity. 1995;3:601–8. doi: 10.1016/1074-7613(95)90131-0. [DOI] [PubMed] [Google Scholar]
- 8.Ye J, McCray SK, Clarke SH. The transition of pre-BI to pre-BII cells is dependent on the VH structure of the µ surrogate L chain. EMBO J. 1996;15:1524–33. [PMC free article] [PubMed] [Google Scholar]
- 9.Takemori T, Mizuguchi J, Miyazoe I, et al. Two types of µ chain complexes are expressed during differentiation from pre-B to mature B cells. EMBO J. 1990;9:2493–500. doi: 10.1002/j.1460-2075.1990.tb07428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Misener V, Downey GP, Jongstra J. The immunoglobulin light chain related protein λ5 is expressed on the surface of mouse pre-B cell lines and can function as a signal transducing molecule. Int Immunol. 1991;3:1129–36. doi: 10.1093/intimm/3.11.1129. [DOI] [PubMed] [Google Scholar]
- 11.Matsuo T, Nomura J, Kuwahara K, Igarashi H, Inui S, Hamaguchi M, Kimoto M, Sakaguchi N. Cross-linking of B cell receptor-related MB-1 molecule induces protein tyrosine phosphorylation in early B lineage cells. J Immunol. 1993;150:3766–75. [PubMed] [Google Scholar]
- 12.Krop I, Shaffer DT, Schlissel MS. The signaling activity of murine CD19 is regulated during B cell development. J Immunol. 1996;157:48–56. [PubMed] [Google Scholar]
- 13.Kuwahara K, Kawai T, Mitsuyoshi S, et al. Cross-linking of B cell antigen receptor-related structure of pre-B cell lines induces tyrosine phosphorylation of p85 and p110 subunits and activation of phosphatidylinositol 3-kinase. Int Immunol. 1996;8:1273–85. doi: 10.1093/intimm/8.8.1273. [DOI] [PubMed] [Google Scholar]
- 14.Rosenberg N. Abl-mediated transformation, immunoglobulin gene rearrangements and arrest of B lymphocyte differentiation. Semin Cancer Biol. 1994;5:95–102. [PubMed] [Google Scholar]
- 15.Baltimore D, Rosenberg N, Witte ON. Transformation of immature lymphoid cells by Abelson murine leukemia virus. Immunol Rev. 1997;48:3–22. doi: 10.1111/j.1600-065x.1979.tb00296.x. [DOI] [PubMed] [Google Scholar]
- 16.Fu C, Turck CW, Kurosaki T, Chan AC. BLNK: a central linker protein in B cell activation. Immunity. 1998;9:93–103. doi: 10.1016/s1074-7613(00)80591-9. [DOI] [PubMed] [Google Scholar]
- 17.Wienands J, Schweikert J, Wollscheid B, Jumaa H, Nielsen PJ, Reth M. SLP-65: a new signaling component in B lymphocytes which requires expression of the antigen receptor for phosphorylation. J Exp Med. 1998;188:791–5. doi: 10.1084/jem.188.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goitsuka R, Fujimura Y, Mamada H, et al. BASH, a novel signaling molecule preferentially expressed in B cells of the bursa of Fabricius. J Immunol. 1998;161:5804–8. [PubMed] [Google Scholar]
- 19.Pappu R, Cheng AM, Li B, et al. Requirement for B cell linker protein (BLNK) in B cell development. Science. 1999;286:1949–54. doi: 10.1126/science.286.5446.1949. [DOI] [PubMed] [Google Scholar]
- 20.Jumaa H, Wollscheid B, Mitterer M, Wienands J, Reth M, Nielsen PJ. Abnormal development and function of B lymphocytes in mice deficient for the signaling adaptor protein SLP-65. Immunity. 1999;11:547–54. doi: 10.1016/s1074-7613(00)80130-2. [DOI] [PubMed] [Google Scholar]
- 21.Namen AE, Schmierer AE, March CJ, Overell RW, Park LS, Urdal DL, Mochizuki DY. B cell precursor growth-promoting activity. Purification and characterization of a growth factor active on lymphocyte precursors. J Exp Med. 1988;167:988–1002. doi: 10.1084/jem.167.3.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park LS, Friend DJ, Schmierer AE, Dower SK, Namen AE. Murine interleukin 7 (IL-7) receptor. Characterization on an IL-7-dependent cell line. J Exp Med. 1990;171:1073–89. doi: 10.1084/jem.171.4.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang W, Sloan-Lancaster J, Kitchen J, Trible RP, Samelson LE. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 1998;92:83–92. doi: 10.1016/s0092-8674(00)80901-0. [DOI] [PubMed] [Google Scholar]
- 24.Finco TS, Kadlecek T, Zhang W, Samelson LE, Weiss A. LAT is required for TCR-mediated activation of PLCγ1 and the Ras pathway. Immunity. 1998;9:617–26. doi: 10.1016/s1074-7613(00)80659-7. [DOI] [PubMed] [Google Scholar]
- 25.Zhang W, Sommers CL, Burshtyn DN, et al. Essential role of LAT in T cell development. Immunity. 1999;10:323–32. doi: 10.1016/s1074-7613(00)80032-1. [DOI] [PubMed] [Google Scholar]
- 26.Wang J, Lin Q, Langston H, Cooper MD. Resident bone marrow macrophages produce type 1 interferons that can selectively inhibit interleukin-7-driven growth of B lineage cells. Immunity. 1995;3:475–84. doi: 10.1016/1074-7613(95)90176-0. [DOI] [PubMed] [Google Scholar]
- 27.Watanabe C, Kumanogoh A, Shi W, Suzuki K, Yamada S, Okabe M, Yoshida K, Kikutani H. Enhanced immune responses in transgenic mice expressing a truncated form of the lymphocyte semaphorin CD100. J Immunol. 2001;167:4321–8. doi: 10.4049/jimmunol.167.8.4321. [DOI] [PubMed] [Google Scholar]
- 28.Nagata K, Nakamura T, Kitamura F, Kuramochi S, Taki S, Campbell KS, Karasuyama H. The Igα/Igβ heterodimer on µ-negative proB cells is competent for transducing signals to induce early B cell differentiation. Immunity. 1997;7:559–70. doi: 10.1016/s1074-7613(00)80377-5. [DOI] [PubMed] [Google Scholar]
- 29.Maki K, Nagata K, Kitamura F, Takemori T, Karasuyama H. Immunoglobulin β signaling regulates locus accessibility for ordered immunoglobulin gene rearrangement. J Exp Med. 2000;191:1333–40. doi: 10.1084/jem.191.8.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aguado E, Richelme S, Nunez-Cruz S, et al. Induction of T helper type 2 immunity by a point mutation in the LAT adaptor. Science. 2002;296:2036–40. doi: 10.1126/science.1069057. [DOI] [PubMed] [Google Scholar]
- 31.Sommers CL, Park CS, Lee J, et al. A LAT mutation that inhibits T cell development yet induces lymphoproliferation. Science. 2002;296:2040–3. doi: 10.1126/science.1069066. [DOI] [PubMed] [Google Scholar]
- 32.Turner M, Mee PJ, Costello PS, et al. Perinatal lethality and blocked B-cell development in mice lacking the tyrosine kinase Syk. Nature. 1995;378:198–302. doi: 10.1038/378298a0. [DOI] [PubMed] [Google Scholar]
- 33.Cheng AM, Rowley B, Pao W, Hayday A, Bolen JB, Pawson T. Syk tyrosine kinase required for mouse viability and B-cell development. Nature. 1995;378:303–6. doi: 10.1038/378303a0. [DOI] [PubMed] [Google Scholar]
- 34.Ishiai M, Kurosaki M, Pappu R, et al. BLNK required for coupling Syk to PLCγ2 and Rac1-JNK in B cells. Immunity. 1999;10:117–25. doi: 10.1016/s1074-7613(00)80012-6. [DOI] [PubMed] [Google Scholar]
- 35.Brdicka T, Imrich M, Angelisova P, et al. Non-T cell activation linker (NTAL): a transmembrane adaptor protein involved in immunoreceptor signaling. J Exp Med. 2002;196:1617–26. doi: 10.1084/jem.20021405. [DOI] [PMC free article] [PubMed] [Google Scholar]