Abstract
B cells and dendritic cells, lacking functional Wiskott–Aldrich syndrome protein (WASP), have aberrant formation of membrane protrusions. We hypothesized that protrusions may play a role in antigen presentation, and consequently, that impaired antigen presentation may be an underlying factor of the immune deficiency in patients with Wiskott–Aldrich syndrome. In this paper, we investigated the antigen presentation capacity of B cells and dendritic cells from WASP knockout mice, using soluble and particulate antigen, to CD4+ T cells from T-cell receptor transgenic DO11.10 mice. As antigen we used soluble ovalbumin (OVA), a peptide thereof (amino acids 323–339) or bacteria expressing OVA. We found that WASP-deficient B cells and dendritic cells efficiently processed and presented soluble OVA protein as well as its peptide in vitro, inducing proliferation and cytokine production from CD4+ T cells. Antigen presentation of soluble protein was efficient also in vivo, because immunization of WASP-deficient mice with OVA elicited proliferation of transferred, fluorescent-labelled, CD4+ T cells. Although we could detect uptake of bacteria in dendritic cells, processing and presentation of bacterial-expressed OVA was impaired in WASP-deficient dendritic cells. In conclusion, our data suggest that WASP is not needed for processing and presentation of soluble antigen, but that efficient presentation of particulate antigen require WASP.
Introduction
The adaptive immune response results from recognition of foreign substances by antigen receptor bearing lymphocytes in the context of host antigen-presenting cells (APCs) that has been triggered by inflammatory signals. Dendritic cells (DCs) circulate and home to tissues where they reside as immature cells with high phagocytic capacity. Upon encounter and recognition of a pathogen, the activated DC migrate to secondary lymphoid organs, where they present peptides on major histocompatibility (MHC) class II molecules to naive CD4+ T cells in the T-cell areas.1,2 Antigen-primed T helper cells move to the edge of the B-cell follicles where they contact antigen-specific B cells.3 T and B cells physically interact and B cells receive help via direct cell–cell contact through CD40 expressed on B cells and CD40 ligand on T cells, and via secretion of cytokines by T cells. One important cytokine for early B-cell activation is interleukin-4 (IL-4), which costimulates B-cell proliferation together with antigen4 and induces increased expression of MHC class II molecules and immunoglobulin class switching. IL-4 also activates cytoskeletal changes such as motility, adhesion and induction of microvilli formation.5–13
Wiskott–Aldrich syndrome (WAS) is a severe immune deficiency disorder characterized by immune dysregulation and thrombocytopenia. WAS is caused by mutations in the gene encoding the WAS protein (WASP), exclusively expressed by haematopoietic cells. Interactions between WASP, the Rho GTPase Cdc42 and the cytoskeletal organizing complex Arp2/3 are important in various cellular functions involving cytoskeletal rearrangements.14–16 Absence of WASP leads to impairment in immune cell migration17–22 a process that requires a dynamic cytoskeleton. Defective signalling in WASP-deficient T and B cells upon surface receptor engagement has also been reported.23–27 Peripheral blood-derived DCs from WAS patients are severely compromised in the formation of dynamic membrane protrusions when coated on fibronectin, whereas healthy DCs express long filopodial protrusions.19 Also, WASP-deficient T and B cells possess distorted microvilli.22,24,25,28 Later complications in WAS include lymphoproliferative disease, especially non-Hodgkin's lymphomas of B-cell origin.29 A significant number of patients also develop autoimmunity.29 This secondary disease and the compromised cytoskeletal regulation in many haematopoietic cells, led us to investigate if an aberrant antigen-presenting capacity may contribute to the development of WAS. We have used two different approaches to explore a potential link between WASP, expression of membrane protrusions and antigen presentation. First, the antigen presenting capacity of WASP-deficient B cells and DCs was compared to that of wildtype APCs. Secondly, we tested differently activated B cells, lacking or possessing long microvilli, in their antigen presenting capacity. Furthermore, we studied presentation of both soluble and particulate antigen. Our results provide evidence that processing and presentation of soluble antigen can occur in the absence of WASP and that expression of membrane protrusions may not be a requirement for efficient antigen presentation. However, WASP is needed for efficient presentation of particulate antigen.
Materials and methods
Mice
A pair of WASP-deficient animals, kindly provided by Dr Frederick Alt and Dr Scott Snapper (Boston, MA26), and DO11.10 mice30 were bred and maintained in the animal facility at the Department of Cell and Molecular Biology (Karolinska Institutet) under conventional conditions. A WASP–/– female (H-2b/b) was mated with a BALB/c male (H-2d/d) (purchased from Charles River, Uppsala, Sweden) to establish breeding in which all F2 animals were homozygous for the MHC haplotype H-2d/d and either WASP+ or WASP−. The MHC haplotype expression was determined by flow cytometry (FACS Calibur, BD Biosciences, Stockholm, Sweden) for H-2d or H-2b on cells from blood samples and the WASP genotype was analysed by polymerase chain reaction (PCR) on DNA prepared from tails. Peripheral blood CD4+ T cells from DO11.10 mice were typed for expression of the transgenic (tg) T-cell receptor (TCR) with flow cytometry. Mice, in which more than 90% of the CD4+ T cells expressed the tg TCR, were used in the study. Females (WASP–/–) and males (WASP–/0 as WASP is expressed on the X chromosome) mice were used at 6–16 weeks of age and denoted as WASP− mice throughout the paper.
Reagents and antibodies
Recombinant murine IL-4 was derived from supernatants of the plasmacytoma X63 Ag8–653 transfected with IL-4 cDNA.31 The amount of IL-4 inducing a half-maximal DNA synthesis response in concanavalin A-activated T cells, was defined as 1 unit. Five units/ml of IL-4 were used in cell cultures. Lipopolysaccharide (LPS) from Escherichia coli O55:B5 and whole chicken ovalbumin (OVA) was purchased from Sigma Aldrich (Stockholm, Sweden). A peptide of OVA corresponding to amino acids 323–339 was purchased from K. J. Ross-Petersen AS (Horsholm, Denmark). Granulocyte–macrophage colony-stimulating factor (GM-CSF) was purchased from Peprotech EC Ltd (London, UK). Batches of fetal calf serum (FCS; Labora, Stockholm, Sweden) were selected for low endotoxin levels (less than 0·05 EU/ml). 5-(and-6)-carboxyfluorescein diacetate, succinimidyl ester (CFSE) was purchased from Molecular Probes (Göteborgs Termometerfabrik, Göteborg, Sweden). Phycoerythrin (PE)-labelled anti-H2Db, anti-H2Dd, anti-IL-2, anti-IL-4, anti-interferon-γ (IFN-γ), rat immunoglobulin G2a (IgG2a), rat IgG2b and cychrome-anti-CD4 antibodies were all purchased from Pharmingen (BD Biosciences).
Cell purification
Small resting B cells were prepared from spleen cell suspensions after removal of T cells and Percoll gradient centrifugation as previously described.11,12 B cells were diluted to 5 × 105 cells/ml and activated with 10 µg/ml LPS or LPS plus 5 units/ml of IL-4 for 48 hr.
Myeloid DCs were differentiated from bone marrow cells obtained from femur and tibia, cultured at 5 × 105 cells/ml, in the presence of 5 ng/ml GM-CSF and 5 units/ml IL-4 (added on day 3). To obtain mature DCs, 5 µg/ml LPS was added on day 6 of culture. On day 7 of culture, non-adherent mature DCs were harvested and used for experiments. CD4+ T cells were isolated from spleens of DO11.10 mice, by selection with anti-CD4 conjugated magnetic beads, following the manufacturer's instructions (Miltenyi Biotec, Göteborgs Termometerfabrik). All cells were cultured in RPMI-1640 supplemented with 2 mm l-glutamine, 1 mm sodium pyruvate, 50 U/ml penicillin, 50 µg/ml streptomycin, 5 µm 2-mercaptoethanol (all from Gibco BRL, Life Technology, Paisley, UK) and 10% of FCS at 37° in a humidified atmosphere containing 5% CO2.
Measurement of T-cell proliferation to soluble antigen
CD4+ T cells from DO11.10 mice express a tg TCR specific for a peptide (amino acids 323–339) of OVA presented on I-Ad MHC class II molecules. APCs were purified from H-2d/d positive mice. The efficiency of antigen presentation was examined as the capacity of irradiated APCs to stimulate CD4+ T cell proliferation, measured by [3H]thymidine incorporation into T cells. Activated B cells and mature DCs (APCs) were pulsed with OVA 323–339 peptides or whole OVA protein for the last 18 hr of culture. APCs were washed twice and irradiated (1000 rad), thereafter washed twice, diluted in fresh medium with FCS and added to 96-well tissue culture plates. CD4+ T cells were isolated, washed, diluted in medium with FCS and added to the APC cultures (2 × 104 T cells per well). T-cell proliferation was measured after 72 hr, by addition of 2 µCi/ml [3H]thymidine for the last 18 hr of culture. Incorporated radioactivity was measured using a Wallac microplate scintillation counter (Wallac Oy, Turku, Finland).
Measurement of T-cell proliferation in vivo
Purified TCR tg CD4+ T cells (107 cells/ml) were labelled with 10 µm CFSE in phosphate-buffered saline (PBS), for 8 min at room temperature. The labelling reaction was stopped by addition of an equal volume of FCS. Cells were centrifuged and washed 3 times before being used. CFSE labelling did not influence cell proliferation as measured by [3H]thymidine incorporation (data not shown and ref. 32). In the in vivo experiments, 12 × 106 CFSE+ CD4+ T cells per mouse were injected intravenously on day 1, followed by 100 µg whole OVA protein in incomplete Freund's adjuvant subcutaneously on the right side of the abdomen on day 2. Mice were killed on day 5 for collection of right and left axillary and inguinal lymph nodes (LNs). LN cells were stained with anti-CD4 antibodies and the extent of injected T-cell proliferation was analysed using flow cytometry.
Cytokine production
Activated B cells and mature DCs were pulsed with OVA peptides (0·5 µg/ml) for the last 18 hr of culture, harvested and washed twice to get rid of excess stimuli. Equal numbers of cells were diluted in fresh medium with FCS and added to 24-well tissue culture plates. CFSE-labelled TCR tg CD4+ T cells were added to the APC cultures and cultured for 120 hr. Phorbol 12-myristate 13-acetate (PMA, 0·5 µg/ml), ionomycin (0·5 µg/ml) and brefeldin A (10 µg/ml) were added for the last 4 hr of culture to enhance cytokine production and detection. Cells were transferred to 96-well tissue cultures, stained with anti-CD4 antibodies for 30 min at 4°, washed and fixed in PBS with 4% formaldehyde, for 20 min at room temperature. Cells were permeabilized in PBS with 0·5% saponin and 0·5% bovine serum albumin for 10 min at room temperature. They were thereafter stained with PE-conjugated anti-IL-2, -IL-4, -IFN-γ or isotype control antibodies in fresh permeabilization buffer, for 30 min at 4°. Finally, cells were washed twice in permeabilization buffer, and once in PBS. Cytokine production was measured using a gate for CFSE+ CD4+ cells. The values for the isotype controls, typically between 1 and 2%, were subtracted from those of the experimental groups.
Measurement of T-cell proliferation to particulate antigen
E. coli harbouring HB101/pOVA (containing full length OVA in a pUC vector) were kindly provided by Dr Mary-Jo Wick (Göteborg, Sweden). Bacteria-processing assays were performed as previously described.33 Briefly, bacterial suspensions were prepared by removing colonies from agar plates into PBS. Suspensions were centrifuged at 1700 r.p.m and quantified spectrophotometrically by determining the OD at 600 nm (assuming that OD600 = 0·4 corresponds to 108 bacteria/ml). Bacteria resuspended in RPMI-1640 with 10% FCS (without antibiotics) were centrifuged onto DCs, precultured for 7 days (1 × 105 per well) and incubated for 3 hr. The cultures were washed to get rid of excess bacteria and fresh medium containing 50 µg/ml gentamicin was added. Twenty-four hr later, TCR tg CD4+ T cells were purified and added to the DC cultures (1 × 105 T cells per well). T-cell proliferation was measured after 144 hr, by addition of 2 µCi/ml [3H]thymidine for the last 18 hr of culture.
Results
WASP-deficient B cells and DCs present OVA 323–339 peptides efficiently
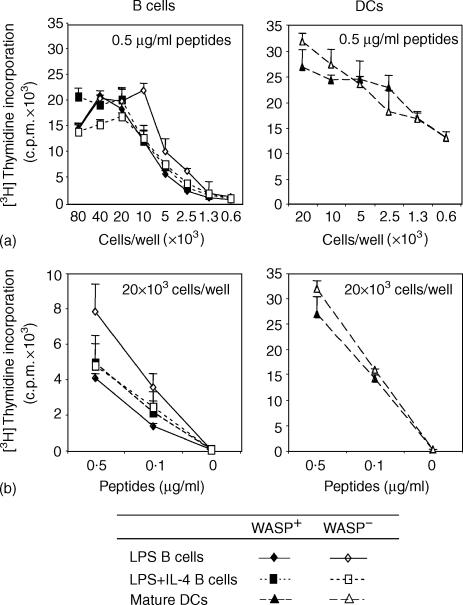
To address if WASP and expression of membrane protrusions play a role for efficient antigen presentation, we used two different approaches. We first tested the ability of B cells and DCs from WASP− mice to present OVA 323–339 peptides to TCR tg CD4+ T cells. WASP− B cells, preactivated with LPS or LPS plus IL-4, were as potent APCs as similarly activated B cells from WASP+ mice, as determined by proliferation of CD4+ T cells (Fig. 1). Similar results were obtained when the number of APCs or the concentration of peptides were reduced (Fig. 1a, b, respectively). Naive B cells from both WASP+ and WASP− mice presented peptides poorly compared to activated B cells (data not shown). Furthermore, DCs differentiated from the bone marrow of WASP− mice were equally potent APCs as DCs of WASP+ mice (Fig. 1a, b). To further support this finding, we also compared the antigen presenting capacity of LPS-activated B cells, lacking expression of microvilli, to that of LPS plus IL-4-activated B cells, possessing many and long microvilli.12,13 LPS- and LPS plus IL-4-activated B cells presented OVA 323–339 peptides equally well to CD4+ T cells (Fig. 1).
Figure 1.
WASP-deficient B cells and DCs efficiently present OVA peptides. B cells or DCs from WASP+ or WASP− mice were used to detect antigen presentation of OVA 323–339 peptides to TCR tg CD4+ T cells. B cells or mature DCs were activated with indicated stimuli and pulsed with the OVA peptides. Excess stimuli and OVA peptides were washed away, cells were irradiated and then cultured with CD4+ T cells. The amount of incorporated [3H]-thymidine into T cells is indicated with cpm-values as a function of APC numbers (a) or titration of OVA peptides (b) Background values (B/DC + whole OVA protein or B/DC/T alone) were less than 500 cpm. Results are presented as mean values of triplicate wells and error bars represent one SD. This experiment is representative of at least three similar experiments.
From the results presented in Fig. 1 we also concluded that DCs were more potent APCs than activated B cells, confirming previously published results.34
WASP-deficient B cells and DCs also process whole OVA protein efficiently
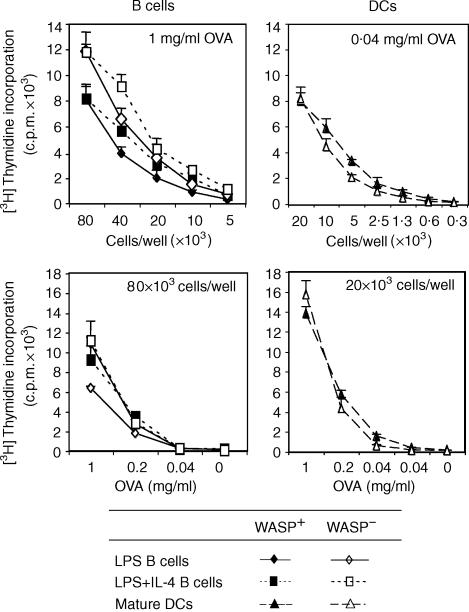
To elucidate the role of WASP-dependent cytoskeletal rearrangements for antigen processing, WASP+ and WASP− B cells and DCs were pulsed with whole OVA protein. LPS and LPS plus IL-4 preactivated B cells from both WASP+ and WASP− mice processed the OVA protein and presented the OVA 323–339 peptide efficiently to TCR tg CD4+ T cells, as determined by proliferation of the CD4+ T cells (Fig. 2). Similar results were obtained when the number of APCs or the concentration of OVA protein were reduced (Fig. 2a, b, respectively). Also, WASP+ and WASP− mature DCs processed the OVA protein and presented the OVA 323–339 peptides efficiently (Fig. 2).
Figure 2.
WASP-deficient B cells and DCs efficiently process whole OVA protein. B cells or DCs from WASP+ and WASP− mice were used to detect processing of whole OVA protein and presentation to TCR tg CD4+ T cells. B cells or mature DCs were activated with indicated stimuli and pulsed with the OVA protein. Excess stimuli and OVA protein were washed away, cells were irradiated and then cultured with CD4+ T cells. The amount of incorporated [3H]thymidine into T cells is indicated with cpm-values as a function of APC numbers (a) or titration of OVA protein (b). Background values (B/DC + whole OVA protein or B/DC/T alone) were less than 500 c.p.m. Results are presented as mean values of triplicate wells and error bars represent one SD. This experiment is representative of three similar experiments.
Together, the results presented in Figs 1 and 2 imply that WASP-deficient B cells and DCs can process soluble antigen and present peptides in vitro.
WASP-deficient mice support an efficient immune response in vivo
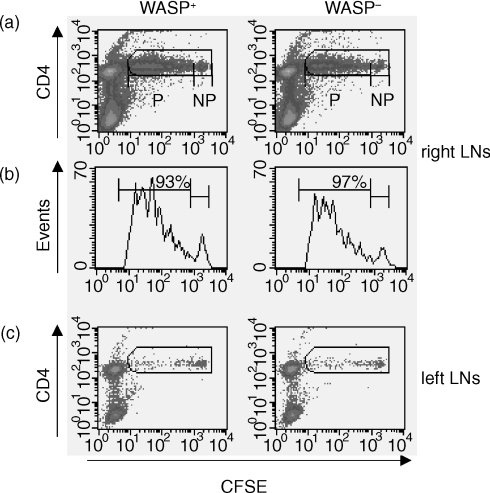
As concluded from the results presented above, WASP-deficient B cells and DCs can present antigen in cell culture systems. To elucidate if the antigen uptake, processing and presentation of peptides was normal also in vivo, we injected TCR tg CD4+ T cells (expressing WASP) into WASP+ and WASP− mice, following injection of whole OVA protein. To be able to track the extent of cell divisions, the TCR tg CD4+ T cells were labelled with the fluorescent dye CFSE, which is equally diluted between the two daughter cells after each subsequent cell division. TCR tg CFSE-labelled CD4+ T cells were injected intravenously on day 1, followed by a subcutanous injection of whole OVA protein (100 µg) at the right abdominal side on day 2. Mice were killed on day 5 for collection of the right and left axillary and inguinal LNs. Identification and proliferation (i.e. dilution of the CFSE staining) of the injected CFSE-labelled CD4+ T cells was assessed with flow cytometry. Both WASP+ and WASP− mice commenced an efficient immune response in vivo, detected as proliferation of CFSE+CD4+ T cells (Fig. 3a, b). The mean percentage values of proliferating cells (P in Fig. 3) from seven WASP+ and seven WASP− mice were similar (92 ± 4% and 95 ± 2%, respectively). When looking at the individual histograms of proliferating CFSE+CD4+ T cells from all WASP+ and WASP− mice (see two representative histograms in Fig. 3b), most cells had gone through at least four cell divisions judging from the intensities of the peaks. As expected, the immune response was site specific, since a large number of the injected CFSE+ CD4+ T cells proliferated in the right LNs, close to the site of antigen injection, but less in the left LNs (compare Fig. 3a and c). Additionally, when testing a lower dose of antigen (20 µg/mouse), we found reduced numbers of CFSE+ CD4+ T cells, but those identified had also gone through at least four cell divisions, both in WASP+ and WASP− mice (data not shown).
Figure 3.
WASP-deficient mice support an efficient immune response of TCR tg CD4+ T cells. WASP+ and WASP− mice were injected with CFSE-labelled TCR tg CD4+ T cells on day 1, followed by injection subcutanously (right abdominal side) of whole OVA protein in incomplete Freund's adjuvant on day 2. The left and right axillary and inguinal lymph nodes (LNs) were taken out on day 5, cells collected and stained with anti-CD4 antibodies. The percentage of proliferating cells in the LNs was assessed using flow cytometry. (a) Representative density plot diagrams of right LN cells from one WASP+ and one WASP− mouse. The gate includes the CFSE+ CD4+ T cells. P indicates proliferating cells and NP nonproliferating cells. (b) A histogram of A, showing only the CFSE+ CD4+ T cells. The percentage of proliferating cells is indicated. (c) Dot plot diagram of cells from the left LNs. Setting of the NP gates was done after injection of cells alone, or cells plus incomplete Freund's adjuvant without antigen. The data from one mouse of each type is shown, but it is representative of data from seven WASP+ and seven WASP− mice.
These results further support the notion that processing of soluble antigen and presentation on MHC class II molecules by APCs can occur efficiently in the absence of WASP.
WASP-deficient B cells and DCs are potent activators of cytokine production by CD4+ T cells
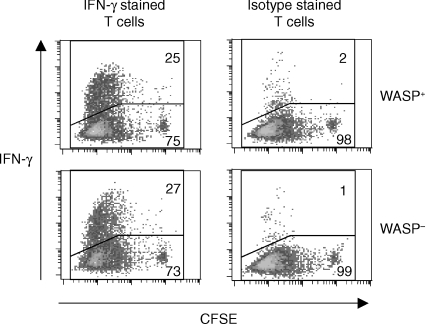
This far, the presented results demonstrated that WASP-deficient APCs are potent activators of CD4+ T cell proliferation both in vitro and in vivo. To elucidate if WASP-deficient APC were also capable of inducing effector functions in T cells, we measured production of cytokines from TCR tg CD4+ T cells activated with WASP-deficient B cells and DCs. Peptide-pulsed APCs were cultured with CD4+ T cells, labelled with CFSE to simultaneously track T-cell proliferation and cytokine production. On day 5, cultures were re-stimulated for 4 hr with PMA and ionomycin followed by intracellular staining of cytokines. LPS and LPS plus IL-4 preactivated B cells from both WASP+ and WASP− mice stimulated high numbers of IL-2-producing CD4+ T cells (Table 1). Only a small percentage of IL-4-producing CD4+ T cells were found, but a high fraction of IFN-γ producers were detected (Fig. 4 and Table 1). WASP+ and WASP− mature DCs also induced a high number of IL-2-producing CD4+ T cells (Table 1). Again, only a small number of IL-4 producers were detected. However, in contrast to B cells, which induced a substantial number of IFN-γ-producing CD4+ T cells, WASP+ and WASP− DCs only induced small numbers of IFN-γ-producing T cells. Thus, although bone marrow derived DCs in this experimental setup were highly efficient in inducing proliferation and IL-2 production from the TCR tg T cells, they were poor in activating the T-cell effector cytokine IFN-γ. Together, these results reveal that WASP-deficient B cells are potent activators of CD4+ effector T cells.
Table 1.
Percentage of cytokine-producing TCR tg CFSE+ CD4+ T cells after stimulation with APCs for 120 hr*
| B cells | DCs | |||
|---|---|---|---|---|
| LPS | LPS + IL-4 | LPS | ||
| WASP+ | IL-2 | 49 | 37 | 67 |
| IL-4 | 3 | 3 | 2 | |
| IFN-γ | 25 | 28 | 4 | |
| WASP− | IL-2 | 49 | 37 | 58 |
| IL-4 | 3 | 3 | 2 | |
| IFN-γ | 27 | 35 | 7 | |
These results are representative of three similar experiments for WASP+ APCs, and two experiments for WASP− APCs. The values from isotype controls are subtracted. They were between 1 and 2%.
Figure 4.
WASP-deficient B cells are potent activators of IFN-γ production by TCR tg CD4+ T cells. IFNγ production of CFSE-labelled TCR tg CD4+ T cells was measured using flow cytometry. LPS activated WASP+ and WASP− B cells were pulsed with OVA 323–339 peptides. Excess stimuli were washed away and cells were then cultured with CFSE-labelled CD4+ T cells for 120 hr. Cells were re-stimulated with PMA and ionomycin for 4 hr, fixed and stained. IFNγ production (upper gate) was measured in a gated population of CFSE+ CD4+ cells. The line that separates IFN-γ-positive and -negative cells was set so that the isotype controls had, at most, 1–2% positive cells in the upper gate.
WASP-deficient DCs are impaired in processing and presentation of bacterial-expressed OVA
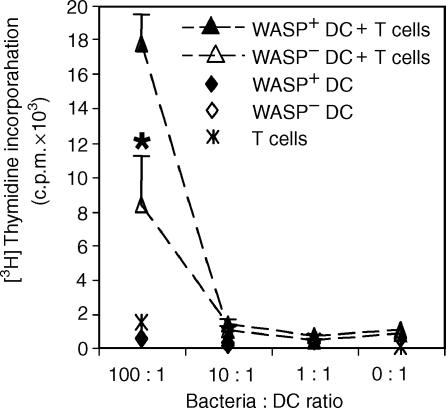
DCs can take up particulate antigen and such uptake is likely to differ from that of soluble antigen. Because it has been shown that WASP-deficient macrophages are impaired in phagocytosis35,36 it was of interest to test if WASP-deficient DCs would be able to process and present a particulate antigen. To this end, we infected DCs from WASP+ and WASP− mice with bacteria expressing the OVA protein. Using fluorescently labelled bacteria and confocal microscopy, we could determine that the majority of bacteria taken up by WASP+ and WASP− DCs were inside the cells (data not shown). We investigated the processing of bacterial-derived OVA protein and presentation of OVA 323–339 peptides to TCR tg CD4+ T cells. As determined by proliferation of the CD4+ T cells, we found that WASP− DCs presented bacterial derived peptides, but the T cell response was reduced to less than half when compared to WASP+ DCs (Fig. 5). These results imply that WASP is needed for efficient processing of particulate antigen in DCs.
Figure 5.
WASP-deficient DCs are impaired in processing and presentation of bacterial-derived OVA. DCs from WASP+ and WASP− mice were cocultured with bacteria, expressing whole OVA protein, for 3 hr. Excess bacteria were washed away and DCs were allowed to process the bacteria overnight and then cultured with TCR tg CD4+ T cells. The amount of incorporated [3H]thymidine into T cells is indicated with c.p.m.-values as a function of bacteria : DC ratio. Results are presented as mean values of triplicate wells and error bars represent 1 SD. Asterisk indicates P < 0·01 using a two-tailed t-test, comparing WASP+ and WASP− DCs. This experiment is representative of three similar experiments.
Discussion
Early studies of WAS leucocytes revealed defective cell surface architecture.28 However, the role of WASP and membrane protrusions, such as microvilli, for processing and presentation of antigen has not been elucidated, neither in cells from healthy donors nor in cells of WAS patients. In the present study we show that WASP was not essential for processing and presentation of soluble antigen. This conclusion is based upon experiments where activated B cells and DCs from WASP-deficient animals process and present OVA, inducing proliferative and cytokine responses in TCR tg CD4+ T cells. This conclusion is further supported by the fact that there is little difference in the antigen processing and presenting capacity between LPS activated B cells, lacking microvilli, and LPS plus IL-4 activated B cells possessing many and long microvilli. In contrast to processing of soluble antigen, WASP-deficient DCs were impaired in presentation of particulate antigen.
During maturation, DCs undergo changes in morphology, including loss of adhesive structures and acquisition of high cellular motility.37 At the same time, they become very potent APCs. When they reach the LN they again change morphology with many dendritic protrusions. This led us to hypothesize that membrane protrusions may help the cluster formation with a large number of T cells observed in the LN, and thus play a role in the antigen-presenting capacity of an APC. We further speculated that impaired antigen presentation can contribute to the WAS phenotype, because WASP-deficient B cells, DCs and T cells are reported to have impaired formation of membrane protrusions.19,22,24,25,28 However, our results did not provide substantial evidence for this hypothesis. Even though results presented here imply that microvilli may not be essential for efficient antigen presentation by B cells, we have indications that certain membrane molecules, among them MHC class II, are preferentially expressed on microvilli of B cells, as detected with immunoelectron microscopy (Greicius et al. submitted for publication). Thus, the function of microvilli and membrane protrusions on APCs for proper activation remains to be elucidated.
Our results on WASP-deficient B cells and DCs being potent APCs when presenting soluble antigen are in line with the finding that DCs and Epstein–Barr virus-transformed B-cell lines, derived from peripheral blood cells of WAS patients, were potent stimulators of allogeneic T cells in a mixed lymphocyte reaction.38 Interaction between APCs and T cells involves intimate contact of cell membranes, including a postulated segregation of proteins into lipid raft-like structures.39 While the present study addressed the function of WASP at the APC side of the immunological synapse (APC : T-cell contact), it does not exclude that WASP may play a more important role on the T-cell side of this interface. Indeed, Cannon et al. found that TCR engagement results in a distinct recruitment of WASP to the T cell side of the B : T-cell contact.40 However, no such recruitment was detected at the B-cell side of the contact site, as WASP was more or less equally distributed in the cytoplasm. In addition, Dupréet al. recently showed that WASP regulates lipid raft dynamics in T cells upon TCR and CD28 activation and furthermore that WAS T cells responded poorly.41 Together, these studies reveal WASP as an important player in T cells for correct formation of the immunological synapse. On the contrary, our results suggest that on the other side of the synapse, APCs can utilize WASP-independent pathways for efficient activation of T cells. However, WASP homologous proteins, such as N-WASP, may substitute for WASP in the WASP-deficient B cells and DCs. Because WASP and N-WASP share 50% homology at the protein level42 it is tempting to speculate that N-WASP can partially substitute for some of the functions of WASP in its absence.
APCs can capture and process antigen through several different pathways.1 In this study, we examined B-cell and DC processing and presentation of soluble and particulate antigen. Although soluble antigen was efficiently processed and presented to T cells, we found a deficiency in the response to particulate antigen. We were able to detect ingested bacteria inside some DCs, but the numbers were too low in both wildtype and WASP-deficient cells to allow quantification. However, earlier studies have shown that WASP has a prominent function for efficient phagocytosis in macrophages. Monocyte-derived macrophages from WAS patients is deficient in Fcγ-receptor mediated phagocytosis.35 Also, murine WASP-deficient macrophages have reduced phagocytosis of apoptotic cells.36 Because most natural antigens are particulate, it is likely that a deficiency in their uptake will contribute to the immunodeficiency in WAS patients. Impaired clearance of apoptotic cells may also lead to the formation of cell debris that could be a major source of auto-antigens. Considerable evidence also suggest that WAS is a disease of immune cell migration, since WASP-deficient macrophages,17,18 dendritic cells,19,20 T cells21 and B cells22 are impaired in directed and, for some cell types, random migration and cell polarization. Our study provides evidence that antigen processing and presentation on MHC class II molecules by B cells and DCs can still occur in the absence of WASP. Development of WAS may be more dependent on spatial and temporal disturbances in the physiological microenvironment, which might lead to aberrant immune cell activation. Further research in the field of immune cell migration and adhesion will tell if impaired cytoskeletal regulation may be a contributing factor also in development of other diseases, such as autoimmunity and lymphoproliferative disorders.
Acknowledgments
We are greatly thankful to Dr Frederick Alt and Dr Scott Snapper for providing the WASP knockout mice. We thank Mary-Jo Wick for kindly giving us OVA-expressing bacteria. We wish to thank Dr Mikael Jondal for helpful discussions and for advice on breeding and usage of the DO11.10 mice. This work was supported by grants from the Swedish Natural Science Research Council, Swedish Cancer Foundation, the ‘Network for Inflammation Research’ funded by the Swedish Foundation for Strategic Research, the Fifth (EC) Framework Program (Contract No. QLRT-1999-01090), the Åke Wiberg Foundation, the Swedish Institute and Karolinska Institutet.
Abbreviations
- APC
antigen-presenting cell
- CFSE
5-(and-6)-carboxyfluorescein diacetate, succinimidyl ester
- DC
dendritic cell
- LN
lymph node
- LPS
lipopolysaccharide
- OVA
ovalbumin
- TCR tg
T cell receptor transgenic
- WAS
Wiskott–Aldrich syndrome
- WASP
Wiskott–Aldrich syndrome protein
References
- 1.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 2.Ingulli E, Mondino A, Khoruts A, Jenkins MK. In vivo detection of dendritic cell antigen presentation to CD4 (+) T cells. J Exp Med. 1997;185:2133–41. doi: 10.1084/jem.185.12.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garside P, Ingulli E, Mercia RR, Johnson JG, Noelle RJ, Jenkins MK. Visualisation of specific B and T lymphocyte interactions in the lymph node. Science. 1998;281:96–9. doi: 10.1126/science.281.5373.96. [DOI] [PubMed] [Google Scholar]
- 4.Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The IL-4 receptor: signaling mechanisms and biologic functions. Annu Rev Immunol. 1999;17:701–38. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- 5.Wilkinson PC, Islam LN. Recombinant IL-4 and IFN-γ activate locomotor capacity in human B lymphocytes. Immunology. 1989;67:237–43. [PMC free article] [PubMed] [Google Scholar]
- 6.Elenström C, Severinson E. Interleukin 4 induces cellular adhesion among B lymphocytes. Growth Factors. 1989;2:73–82. doi: 10.3109/08977198909069083. [DOI] [PubMed] [Google Scholar]
- 7.Clinchy B, Elenström C, Severinson E, Möller G. T and B cells collaboration: induction of motility in small, resting B cells by interleukin 4. Eur J Immunol. 1991;21:1445–51. doi: 10.1002/eji.1830210618. [DOI] [PubMed] [Google Scholar]
- 8.Björck P, Paulie S. Inhibition of LFA-1-dependent human B-cell aggregation induced by CD40 antibodies and interleukin-4 leads to decreased IgE synthesis. Immunology. 1993;78:218–25. [PMC free article] [PubMed] [Google Scholar]
- 9.Komai-Koma M, Liew FY, Wilkinson PC. Interactions between IL-4, anti-CD40, and anti-immunoglobulin as activators of locomotion of human B cells. J Immunol. 1995;155:1110–6. [PubMed] [Google Scholar]
- 10.Santos-Argumedo L, Kincade PW, Partida-Sánchez S, Parkhouse RME. CD44-stimulated dendrite formation (‘spreading’) in activated B cells. Immunology. 1997;90:147–53. doi: 10.1046/j.1365-2567.1997.00126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greicius G, Tamosiunas V, Severinson E. Assessment of the role of leukocyte function-associated antigen-1 in homotypic adhesion of activated B lymphocytes. Scand J Immunol. 1998;48:642–50. doi: 10.1046/j.1365-3083.1998.00442.x. [DOI] [PubMed] [Google Scholar]
- 12.Davey EJ, Thyberg J, Conrad DH, Severinson E. Regulation of cell morphology in B lymphocytes by IL-4: evidence for induced cytoskeletal changes. J Immunol. 1998;160:5366–73. [PubMed] [Google Scholar]
- 13.Davey EJ, Greicius G, Thyberg J, Severinson E. STAT6 is required for the regulation of IL-4-induced cytoskeletal events in B cells. Int Immunol. 2000;12:995–1003. doi: 10.1093/intimm/12.7.995. [DOI] [PubMed] [Google Scholar]
- 14.Symons M, Derry JM, Karlak B, Jiang S, Lemahieu V, Mccormick F, Francke U, Abo A. Wiskott–Aldrich syndrome protein, a novel effector for the GTPase Cdc42Hs, is implicated in actin polymerisation. Cell. 1996;84:723–34. doi: 10.1016/s0092-8674(00)81050-8. [DOI] [PubMed] [Google Scholar]
- 15.Aspenström P, Lindberg U, Hall A. Two GTPases, Cdc42 and rac, binds directly to a protein implicated in the immunodeficiency disorder Wiskott–Aldrich syndrome. Curr Biol. 1996;6:70–5. doi: 10.1016/s0960-9822(02)00423-2. [DOI] [PubMed] [Google Scholar]
- 16.Machesky LM, Insall RH. Scar1 and the related Wiskott–Aldrich syndrome protein, WASP, regulate the actin cytoskeleton through the Arp2/3 complex. Curr Biol. 1998;8:1347–56. doi: 10.1016/s0960-9822(98)00015-3. [DOI] [PubMed] [Google Scholar]
- 17.Badolato R, Sozzani S, Malacarne F, et al. Monocytes from Wiskott–Aldrich patients display reduced chemotaxis and lack of cell polarization in response to monocyte chemoattractant protein-1 and formyl-methionyl-leucyl-phenylalanine. J Immunol. 1998;161:1026–33. [PubMed] [Google Scholar]
- 18.Zicha D, Allen WE, Brickell PM, Kinnon C, Dunn GA, Jones GE, Thrasher AJ. Chemotaxis of macrophages is abolished in the Wiskott–Aldrich syndrome. Br J Haematol. 1998;101:659–65. doi: 10.1046/j.1365-2141.1998.00767.x. [DOI] [PubMed] [Google Scholar]
- 19.Binks M, Jones GE, Brickell PM, Kinnon C, Katz DR, Trasher AJ. Intrinsic dendritic cell abnormalities in Wiskott–Aldrich syndrome. Eur J Immunol. 1998;28:3259–67. doi: 10.1002/(SICI)1521-4141(199810)28:10<3259::AID-IMMU3259>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 20.Burns S, Thrasher AJ, Blundell MP, Machesky L, Jones GE. Configuration of human dendritic cell cytoskeleton by Rho GTPases, the WAS protein, and differentiation. Blood. 2001;98:1142–9. doi: 10.1182/blood.v98.4.1142. [DOI] [PubMed] [Google Scholar]
- 21.Haddad E, Zugaza JL, Louache F, et al. The interaction between Cdc42 and WASP is required for SDF-1-induced T-lymphocyte chemotaxis. Blood. 2001;97:33–8. doi: 10.1182/blood.v97.1.33. [DOI] [PubMed] [Google Scholar]
- 22.Westerberg L, Greicius G, Snapper SB, Aspenström P, Severinson E. Cdc42, Rac1, and the Wiskott–Aldrich syndrome protein are involved in the cytoskeletal regulation of B lymphocytes. Blood. 2001;98:1086–94. doi: 10.1182/blood.v98.4.1086. [DOI] [PubMed] [Google Scholar]
- 23.Simon HU, Mills GB, Hashimoto S, Siminovitch KA. Evidence for defective transmembrane signaling in B cells from patients with Wiskott–Aldrich syndrome. J Clin Invest. 1992;90:1396–405. doi: 10.1172/JCI116006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Molina IJ, Kenney DM, Rosen FS, Remold-O'Donnel E. T cell lines characterize events in the pathogenesis of the Wiskott–Aldrich syndrome. J Exp Med. 1992;176:867–74. doi: 10.1084/jem.176.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gallego MD, Santamaria M, Peña J, Molina IJ. Defective actin reorganization and polymerization of Wiskott-Aldrich T cells in response to CD3-mediated stimulation. Blood. 1997;90:3089–97. [PubMed] [Google Scholar]
- 26.Snapper SB, Rosen FS, Mizoguchi E, et al. Wiskott–Aldrich syndrome protein-deficient mice reveal a role for WASP in T but not B cell activation. Immunity. 1998;9:81–91. doi: 10.1016/s1074-7613(00)80590-7. [DOI] [PubMed] [Google Scholar]
- 27.Zhang BJ, Shehabeldin A, da Cruz LAG, et al. Antigen receptor-induced activation and cytoskeletal rearrangement are impaired in Wiskott–Aldrich syndrome protein-deficient mice. J Exp Med. 1999;190:1329–41. doi: 10.1084/jem.190.9.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kenney D, Cairns L, Remold-O'Donnell E, Peterson J, Rosen FS, Parkman R. Morphological abnormalities in the lymphocytes of patients with the Wiskott–Aldrich syndome. Blood. 1986;68:1329–32. [PubMed] [Google Scholar]
- 29.Sullivan KE, Mullen CA, Blaese RM, Winkelstein JA. A multiinstitutional survey of the Wiskott–Aldrich syndrome. J Pediatr. 1994;125:876–85. doi: 10.1016/s0022-3476(05)82002-5. [DOI] [PubMed] [Google Scholar]
- 30.Murphy KM, Heimberger AB, Loh DY. Induction by antigen of intrathymic apoptosis of CD4+ CD8+ TCRlo thymocytes in vivo. Science. 1990;250:1720–3. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 31.Karasuyama H, Melchers F. Establishment of mouse cell lines which constitutively secrete large quantities of interleukin 2, 3, 4 or 5 using modified cDNA expression vectors. Eur J Immunol. 1988;18:97–104. doi: 10.1002/eji.1830180115. [DOI] [PubMed] [Google Scholar]
- 32.Lyons AB. Analysing cell division in vivo and in vitro using flow cytometric measurement of CFSE dye dilution. J Immunol Methods. 2000;243:147–54. doi: 10.1016/s0022-1759(00)00231-3. [DOI] [PubMed] [Google Scholar]
- 33.Yrlid U, Wick MJ. Antigen presentation capacity and cytokine production by murine splenic dendritic cell subsets upon Salmonella encounter. J Immunol. 2002;169:108–16. doi: 10.4049/jimmunol.169.1.108. [DOI] [PubMed] [Google Scholar]
- 34.Delon J, Bercovici N, Raposo G, Liblau R, Trautmann A. Antigen-dependent and -independent Ca2+ responses triggered in T cells by dendritic cells compared with B cells. J Exp Med. 1998;188:1473–84. doi: 10.1084/jem.188.8.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lorenzi R, Brickell PM, Katz DR, Kinnon C, Thrasher AJ. Wiskott–Aldrich syndrome protein is necessary for efficient IgG-mediated phagocytosis. Blood. 2000;95:2943–6. [PubMed] [Google Scholar]
- 36.Leverrier Y, Lorenzi R, Blundell MP, Brickell P, Kinnon C, Ridley AJ, Thrasher AJ. Cutting edge: the Wiskott–Aldrich syndrome protein is required for efficient phagocytosis of apoptotic cells. J Immunol. 2001;166:4831–4. doi: 10.4049/jimmunol.166.8.4831. [DOI] [PubMed] [Google Scholar]
- 37.Ross R, Ross XL, Schwing J, Langin T, Reske-Kunz AB. The actin-bundling protein fascin is involved in the formation of dendritic processes in maturing epidermal Langerhans cells. J Immunol. 1998;160:3776–82. [PubMed] [Google Scholar]
- 38.Allavena P, Badolato R, Facchetti F, et al. Monocytes from Wiskott–Aldrich patients differentiate in functional mature dendritic cells with a defect in CD83 expression. Eur J Immunol. 2001;31:3413–21. doi: 10.1002/1521-4141(200112)31:12<3413::aid-immu3413>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 39.Dustin ML. Membrane domains and the immunological synapse. keeping T cells resting and ready. J Clin Invest. 2002;109:155–60. doi: 10.1172/JCI14842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cannon JL, Labno CM, Bosco G, Seth A, McGavin MH, Siminovitch KA, Rosen MK, Burkhardt JK. WASP recruitment to the T cell. APC contact site occurs independently of Cdc42 activation. Immunity. 2001;15:249–59. doi: 10.1016/s1074-7613(01)00178-9. [DOI] [PubMed] [Google Scholar]
- 41.Dupre L, Aiuti A, Trifari S, Martino S, Saracco P, Bordignon C, Roncarolo MG. Wiskott–Aldrich syndrome protein regulates lipid raft dynamics during immunological synapse formation. Immunity. 2002;17:157–66. doi: 10.1016/s1074-7613(02)00360-6. [DOI] [PubMed] [Google Scholar]
- 42.Miki H, Miura K, Takenawa T. N-WASP, a novel actin-depolymerizing protein, regulates the cortical cytoskeletal rearrangement in a PIP2-dependent manner downstream of tyrosine kinases. EMBO J. 1996;15:5326–35. [PMC free article] [PubMed] [Google Scholar]