Abstract
In this study we investigated whether berberine-mediated induction of interleukin-12 (IL-12) production in antigen-presenting cells could regulate a cytokine profile of antigen-primed CD4+ T helper (Th) cells. Pretreatment with berberine induced IL-12 production in both macrophages and dendritic cells, and significantly increased the levels of IL-12 production in lipopolysaccharide-stimulated macrophages and in CD40 ligand-stimulated dendritic cells. Importantly, berberine pretreatment of macrophages increased their ability to induce interferon-γ (IFN-γ) and reduced their ability to induce IL-4 in antigen-primed CD4+ T cells. Berberine did not influence the macrophage cell surface expression of the class II major histocompatibility complex molecule, the co-stimulatory molecules CD80 and CD86, and intracellular adhesion molecule-1. Addition of neutralizing anti-IL-12p40 monoclonal antibody to cultures of berberine-pretreated macrophages and CD4+ T cells restored IL-4 production in antigen-primed CD4+ T cells. The in vivo administration of berberine resulted in the enhanced induction of IL-12 production by macrophages when stimulated in vitro with lipopolysaccharide or heat-killed Listeria monocytogenes, leading to the inhibition of the Th type 2 cytokine profile (decreased IL-4 and increased IFN-γ production) in antigen-primed CD4+ T cells. These findings may point to a possible therapeutic use of berberine or medicinal plants containing berberine in the Th type 2 cell-mediated immune diseases such as allergic diseases.
Introduction
The existence of T helper type 1 (Th1)/Th2 subsets in Th lymphocytes that differ in their cytokine secretion patterns and effector functions provides a framework for understanding normal and pathological immune responses.1,2 Recent studies indicate that the ratio of these two Th cell types, Th1 and Th2, is closely correlated with the outcome of many diseases3,4 and controlling this Th1 : Th2 ratio has been demonstrated as a therapeutic strategy of these diseases.5 Induction of Th1 immune responses plays a critical role in protecting against various intracellular micro-organisms and tumours,6,7 and also in reversing Th2 cell-facilitating diseases such as allergic inflammation.8 However, the nature of Th1 polarizing signals is not yet fully understood. The cytokines that are present in the environment of the CD4+ T cell at the time it encounters the antigen significantly regulate the differentiation of Th cells into Th1 cells.9
Interleukin-12 (IL-12), a heterodimeric cytokine secreted by macrophages and other antigen-presenting cells (APC), is critical for the development of Th1 cells and the initiation of the cell-mediated immune response.10 Recent evidence showed that the administration of exogenous recombinant IL-12 may be a key strategy in the treatment of Th2-dominated diseases such as infectious diseases and allergic diseases.11,12 Rempel and colleagues reported that administration in vivo of recombinant IL-12 induced profound but transient commitment to Th1-associated patterns of cytokine and antibody production.13 Repeated injections of recombinant IL-12 at relatively high doses showed severe toxicities, including increase in transaminase concentration, pulmonary toxicity, and leucopenia.14 As an alternative method of IL-12 administration, a number of microbial products, including endotoxin, superantigen exotoxins, and microbial DNA with CpG oligonucleotide motifs (CpG ODN), have been reported to induce the endogenous production of Th1 cytokines such as IL-12 and IL-18 by macrophages, resulting in down-regulation of Th2 responses in various allergic mouse models.15 Recently, the imidazoquinolines also augmented Th1 cytokine production and inhibited Th2 cytokine production, and they have been considered as attractive candidates of Th1-inducing adjuvants because they are chemically defined, low-molecular weight compounds.16 Therefore, pharmacological modulation of IL-12 may be a therapeutic target in immune-mediated and inflammatory diseases.17
The mechanism by which these immunomodulators act as adjuvants for inducing Th1 immune responses and suppressing Th2 responses is in part mediated by cytokines produced following stimulation of APC. For example, the ability of polyinosinic acid : polycytidylic acid to skew the response toward a Th1 response seems to be mediated through the production of the cytokines interferon-γ (IFN-γ) and IL-12.18 A similar mechanism is probably involved in the ability of CpG ODN to promote a Th1 response.19
Berberine is a benzodioxoloquinolizine alkaloid that has been isolated from Hydrastis canadensis (goldenseal), Coptis chinensis (Coptis or goldenthread), Berberis aquifolium (Oregon grape), Berberis vulgaris (barberry), and Berberis aristata (tree turmeric).20 Berberine has shown a number of beneficial effects including immunostimulation and macrophage activation. Recently, we reported that berberine induced the production of IL-12p40, a larger subunit of IL-12, in mouse macrophages via activation of p38 mitogen-activated protein kinase.21
In this study we have demonstrated that pretreatment with berberine induced IL-12 production in both macrophages and dendritic cells (DCs), and strongly enhanced IL-12 production when subsequently stimulated with either lipopolysaccharide (LPS) or heat-killed Listeria monocytogenes (HKL), two well-known inducers of IL-12 production. Importantly, the increased levels of IL-12 production in berberine-treated macrophages deviated CD4+ T cells from the Th2 to the Th1 pathway.
Materials and methods
Materials, cell culture and mice
Berberine and LPS (from Escherichia coli 0111:B4) were purchased from Sigma (Sigma Chemical Co., St. Louis, MO), and keyhole limpet haemocyanin (KLH) was purchased from Calbiochem (San Diego, CA). Anti-murine IL-4 (BVD4 and BVD6) and anti-murine IFN-γ monoclonal antibodies (mAbs) (R46A2 and XMG1.2) were purified from ascitic fluids by ammonium sulphate precipitation followed by diethylaminoethyl–Sephacel chromatography (Sigma). Anti-mouse IL-12 (p35/p70) (Red-T/G297-289), anti-CD80, anti-CD86, anti-intracellular adhesion moelcule-1 (ICAM-1) and anti-H-2I-Ad mAbs were obtained from PharMingen (San Diego, CA), and rat anti-mouse IL-12p40 mAbs C17.8 and 15.6 were kindly donated by Dr G. Trinchieri (Wistar Institute, Philadelphia, PA). Chinese hamster ovary cells transfected with murine CD40 ligand (CD40L–CHO)22 were kindly provided by Dr H. Yagita (Tokyo, Japan), and used after fixation with 1% paraformaldehyde in phosphate-buffered saline (PBS). The cells were maintained at 37° in humidified 5% CO2 in RPMI-1640 or Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum and antibiotics (Gibco BRL, Grand Island, NY). Six- to eight-week-old female DBA/2 mice were obtained from SLC Japan (Tokyo, Japan), and maintained in pathogen-limited conditions. The mice were maintained and treated according to National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Preparation of splenic macrophages stimulated with either LPS or HKL
Splenic macrophages were isolated from DBA/2 mice and stimulated, as previously described.23 In brief, spleen cells were cultured at 106 cells/ml for approximately 3 hr in DMEM containing 10% fetal bovine serum at 37° in a 5% CO2 humidified air atmosphere. The non-adherent cells were removed by washing with warm DMEM until visual inspection revealed a lack of lymphocytes (>98% of the cell population). The adherent cells were removed from plates by incubating for 15 min with ice-cold PBS and rinsing repeatedly. The isolated adherent cell population was treated with varying concentrations of berberine at 1 × 105 cells per well in 96-well culture plates for 6–48 hr. For some experiments, the cells were further stimulated in vitro with either 5 μg/ml LPS or HKL at 2 × 106 bacteria per well.
Preparation of splenic DCs stimulated with CD40L–CHO
Mouse DCs were prepared from spleens, as previously described.24 In brief, the isolated adherent cells as mentioned above were incubated for an additional 18 hr to allow the DCs to detach. After this incubation, floating cells were collected, and DCs were positively purified using anti-CD11c MicroBeads and a MACS column (Miltenyi Biotech, Bergisch Gladbach, Germany). Purified cells were routinely >95% CD11c+. Freshly isolated DCs were induced to mature by cultivation in the culture medium overnight. The isolated DCs (1 × 105 cells) were stimulated with 3 × 104 fixed CD40L–CHO cells per well in the absence or presence of varying concentrations of berberine at 1 × 105 cells per well in 96-well culture plates for 24 hr.
Purification and induction of cytokine synthesis in antigen-primed CD4+ T cells
Draining axillary, popliteal, and inguinal lymph nodes were removed from mice 9 days after priming with 100 μg KLH in complete Freund's adjuvant in the footpads, as previously described.25 Lymph node cells were depleted of B cells by adherence to goat anti-mouse immunoglobulin-coated dishes for 1 hr at 4°. Non-adherent cells were depleted of CD8+ T cells and other APCs by treating the cells with a mixture of anti-CD8 and anti-class II mAbs on ice for 30 min, followed by addition of low-toxicity rabbit complement (Pel Freeze, Rogers, AR) and incubation at 37° for 45 min. More than 95% of the cells were CD4+ T cells, as demonstrated by cytofluorometric analysis using anti-CD4 mAb (PharMingen). Purified CD4+ T cells were incubated in 96-well plates at 4 × 105 cells per well with macrophages (1 × 105 cells per well) and KLH (10 μg/ml). Culture supernatants were harvested after 2 days (for IL-12 p70) or after 4 days (for IFN-γ and IL-4), and assayed by an enzyme-linked immunosorbent assay (ELISA).
Cytokine assays
The quantities of IFN-γ, IL-4, IL-12p40 and IL-12 p70 in culture supernatants were determined by a sandwich ELISA using mAbs specific for each cytokine as previously described.26 The mAbs for coating the plates and the biotinylated second mAbs were as follows: for IFN-γ, HB170 and XMG1.2; for IL-4, BVD4-1D11 and BVD6; for IL-12p40, C17.8 and C15.6; for IL-12 p70, anti-mouse IL-12 (p35/p70) (Red-T/G297-289) and rat anti-mouse IL-12p40 (C17.8). Standard curves were generated using recombinant cytokines (purchased from PharMingen). The lower limits of detection were 125 pg/ml for IFN-γ, 3 pg/ml for IL-4, 30 pg/ml for IL-12p40 and 25 pg/ml for IL-12 p70, respectively.
Reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was prepared from the cells and reverse-transcribed into cDNA, and then PCR amplification of the cDNA was performed. The sequences of PCR primers are as follows: mouse IL-12p40 (sense, 5′-CAGAAGCTAACCATCTCCTGGTTTG-3′; anti-sense, 5′-TCCGGAGTAATTTGGTG CTTCACAC-3′), IL-10 (sense, 5′-ATGCAGGACTTTAAGGGTTACTTGGGT-3′; anti-sense, 5′-ATTTCGGAGAGAGGTACAAACGAGGTTT-3′), tumour necrosis factor-α (sense, 5′-GGCAGGTCTACTTTGGAGTCATTG-3′; anti-sense, 5′-ACATTCGAGGCTCCAGTGAATTCGG-3′) and β-actin (sense, 5′-TGGAATCCTGTGGCATCCATGAAAC-3′; anti-sense, 5′-TAAAACGCAGCTCAGTAACAGTCCG-3′). The PCR reactions were run for 35 cycles of 94° (30 seconds), 58° (45 seconds), and 72° (30 seconds) using an MJ Thermal Cycler (Watertown, MA). After the amplification, 6 μl of the RT-PCR products was separated in 1·5% (w/v) agarose gels and stained with ethidium bromide.
Immunofluorescent staining and cytofluorometric measurements
Quantitative immunofluorescence measurements were performed in an Epic V flow cytofluorograph equipped with a multiparameter data acquisition and display system as previously described.27 Briefly, single-cell suspensions were collected from the various cultures and washed twice with ice-cold PBS (pH 7·4). Afterwards, fluorescein isothiocyanate-conjugated anti-H-2I-Ad, anti-CD80, anti-CD86, or anti-ICAM-1 mAbs were added and incubated at 4° for 1 hr. After incubation, the cells were washed with PBS and were fixed in PBS containing 1% paraformaldehyde, and cytofluorometric analysis was performed. Background staining was determined by staining cells with fluorescein isothiocyanate-conjugated isotype control mAbs. One parameter fluorescence histograms were generated by analysing at least 1 × 104 cells.
Statistical analysis
Student's t-test and one-way analysis of variance (anova) were used to determine the statistical differences between values for various experimental and control groups. P values <0·05 were considered statistically significant.
Results
Pretreatment with berberine induced IL-12 production from mouse macrophages and CD40L-stimulated DCs
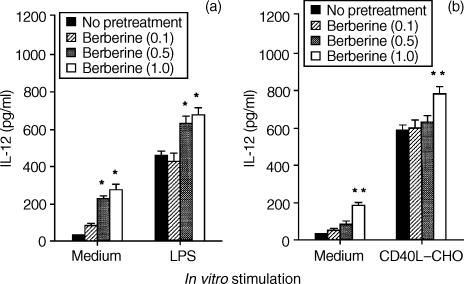
To determine whether berberine could affect the production of T-cell cytokine indirectly via an effect on IL-12 production by APCs such as macrophages and DCs, we first investigated the effect of berberine on IL-12 production in mouse macrophages and DCs. As shown in Fig. 1(a), pretreatment of macrophages with berberine induced IL-12 production in a dose-dependent manner and, furthermore, significantly enhanced IL-12 production when subsequently stimulated in vitro with LPS, a well-known inducer of IL-12 production.
Figure 1.
Pretreatment with berberine stimulates IL-12 production in mouse macrophages and dendritic cells. Mouse macrophages (a) or dendritic cells (b) were pretreated with berberine (0·1, 0·5, and 1·0 μg/ml) or untreated. After 6 hr, the cells were washed and stimulated in vitro with medium alone, or with either LPS (5 μg/ml) or CD40L–CHO cells for 48 hr. The culture supernatants were harvested, and the levels of IL-12 were evaluated by ELISA. The results are presented as the means ± SEM (n = 3). *P < 0·01, relative to groups which were not pretreated with berberine. **P < 0·05, relative to groups which were not pretreated with berberine.
We also investigated whether IL-12 production by in vitro-generated DCs is affected by berberine. DCs are the professional effective APCs and their secretion of immunoregulatory and pro-inflammatory cytokines plays a crucial role during T-cell priming.28 As shown in Fig. 1(b), pretreatment of DCs with berberine resulted in the induction of IL-12 production, which was significantly enhanced by the stimulation with CD40L–CHO cells. The stimulation with untransfected CHO as a control cell-line did not induce detectable amounts of IL-12 production. Furthermore, berberine significantly increased mRNA levels of the IL-12 gene in a dose-dependent manner, indicating that the induction of IL-12 production by berberine occurred at the transcriptional level. In contrast, pretreatment with berberine did not influence mRNA levels of IL-10 and tumour necrosis factor-α in macrophages, suggesting that the induction of IL-12 production by berberine was not the result of a general activation of cells (data not shown).
Pretreatment of macrophages with berberine enhanced IFN-γ production and inhibited IL-4 production by antigen-primed CD4+ T cells
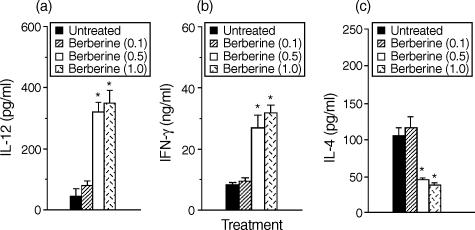
Since IL-12 has been known to enhance IFN-γ strongly and to inhibit IL-4 in CD4+ T cells, we asked if the cytokine profiles of CD4+ T cells responding to antigen presented by berberine-treated macrophages would be altered. Direct effects of berberine on CD4+ T cells were eliminated by pretreatment of macrophages in vitro with berberine for 6 hr and washing them to remove the berberine, before culture with syngeneic CD4+ T cells purified from lymph nodes of KLH-primed mice and the antigen KLH. Figure 2(a) shows that IL-12 production in cultures of berberine-treated macrophages was significantly increased in comparison with that in untreated macrophages. In the absence of berberine treatment, stimulation with KLH resulted in the development of T cells producing relatively low levels of IFN-γ. However, pretreatment of macrophages with berberine for 6 hr greatly increased their capacity to induce IFN-γ production by KLH-primed CD4+ T cells (Fig. 2b) and significantly decreased IL-4 production (Fig. 2c). No cytokine production by CD4+ T cells was detected in the absence of macrophages, demonstrating that the berberine-treated macrophages regulated the cytokine production by KLH-primed CD4+ T cells. Thus, pretreatment of macrophages with berberine enhances their capacity to inhibit Th2 and enhance Th1 cytokine synthesis.
Figure 2.
Macrophages pretreated with berberine enhance IFN-γ and inhibit IL-4 production by antigen-primed CD4+ T cells. Macrophages (1 × 105 cells/well) were pretreated with medium alone or 1·0 μg/ml berberine. After 6 hr, the cells were washed and incubated with KLH-primed CD4+ T cells (5 × 105 cells/well) and KLH (10 μg/ml). Supernatants were harvested after 2 days for IL-12 (a) or after 4 days for IFN-γ (b) and IL-4 (c), and assayed by cytokine-specific ELISA. The results are presented as the means ± SEM (n = 3). *P < 0·01, relative to an untreated group.
Pretreatment with berberine does not affect the expression of surface molecules on macrophages
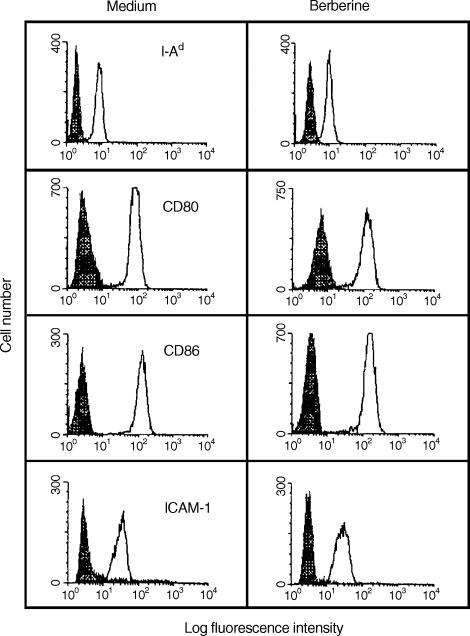
To determine whether pretreatment of macrophages with berberine affects their cell surface expression of the class II major histocompatibility complex (MHC) molecule H-2I-Ad, the co-stimulatory molecules CD80 and CD86, and the adhesion molecule ICAM-1, mouse macrophages were treated with medium alone (control) or 1·0 μg/ml berberine for 24 hr and the expression of the cell surface molecules was determined by cytofluorometric analysis. As shown in Fig. 3, treatment with berberine did not affect the expression of surface molecules on mouse macrophages, compared with that of the control cells.
Figure 3.
Cytofluorometric analysis of cell surface molecules on berberine-treated macrophages. Mouse macrophages were treated for 24 hr with medium alone or 1·0 μg/ml berberine and analysed for the expression of cell surface molecules by flow cytometry. Data are representative of three independent experiments.
Addition of neutralizing IL-12 mAb to cultures of berberine-pretreated macrophages and CD4+ T cells restored the levels of IL-4 production in CD4+ T cells
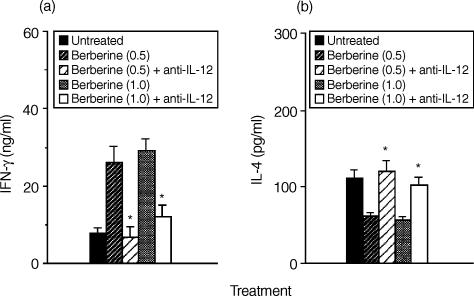
To determine whether the reduced ability of berberine-pretreated macrophages to induce IL-4 synthesis in CD4+ T cells was a result of their increased production of IL-12, we added neutralizing anti-IL-12p40 mAb (10 μg/ml) into the cultures of berberine-pretreated macrophages and CD4+ T cells. As shown in Fig. 4, addition of anti-IL-12p40 mAb to the cultures of berberine-pretreated macrophage and CD4+ T cells significantly increased IL-4 production and reduced that of IFN-γ. These results suggest that induction of IL-12 synthesis by berberine-treated macrophages was a major effect that affected the ability of macrophages to regulate cytokine synthesis in CD4+ T cells.
Figure 4.
Addition of neutralizing IL-12p40 mAb restores the decreased IL-4 production of T cells in cultures of berberine-pretreated macrophages and antigen-primed CD4+ T cells. KLH-primed CD4+ T cells were cultured with berberine (1·0 μg/ml)-pretreated macrophages in the presence of anti-IL-12p40 (10 μg/ml) and KLH (10 μg/ml). Culture supernatants were harvested 4 days later and assayed for IFN-γ (a) and IL-4 (b) by ELISA. The results are presented as the means ± SEM (n = 3). *P < 0·01, relative to each berberine-treated group in the absence of anti-IL-12p40.
Macrophages from mice treated in vivo with berberine inhibited the Th2 cytokine profile of antigen-primed CD4+ T cells
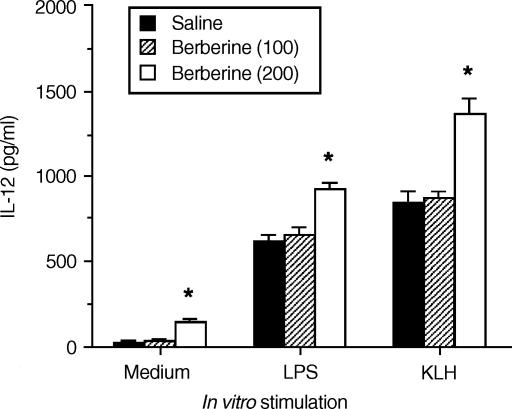
To demonstrate that berberine had a consequential effect on macrophages in an in vivo system, mice were injected intraperitoneally (i.p.) with 200 μg berberine per mouse. After 24 hr, splenic macrophages were purified from the berberine-treated mice, or from saline-injected control mice. As shown in Fig. 5, macrophages from berberine-treated mice significantly enhanced the levels of IL-12 production in response to either LPS or HKL compared with macrophages from the control mice.
Figure 5.
Macrophages exposed to berberine in vivo increase levels of IL-12 production. Mice were injected in vivo with berberine (200 μg per mouse, i.p.). After 24 hr, macrophages were purified and stimulated with medium only, or with either LPS (5 μg/ml) or HKL (2 × 106 bacteria per well). Culture supernatants were harvested 48 hr later and IL-12 levels were determined by ELISA. The results are presented as the means ± SEM (n = 3). *P < 0·05, relative to a saline-injected group.
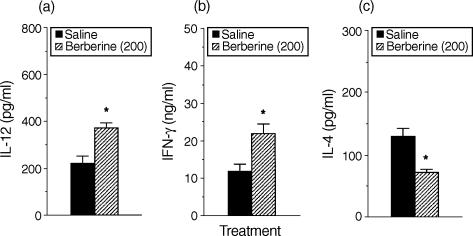
To determine whether cytokine production by antigen-primed CD4+ T cells would differ in the presence of antigen presented by macrophages from mice treated in vivo with berberine, splenic macrophages from DBA/2 mice were purified 24 hr following i.p. injection with berberine (200 μg per mouse), and cultured with CD4+ T cells from KLH-primed mice, and KLH. As shown in Fig. 6, production of IL-12 in cultures containing KLH-primed CD4+ T cells and macrophages from berberine-treated mice was greatly increased compared with those from saline-injected control mice. Moreover, macrophages from mice injected with berberine significantly induced lower amounts of IL-4 and higher amounts of IFN-γ than macrophages from the control mice. These results show that in vivo treatment with berberine regulates the ability of macrophages to control IFN-γ and IL-4 production in CD4+ T cells.
Figure 6.
Macrophages purified from mice treated in vivo with berberine regulate cytokine production in antigen-primed CD4+ T cells. DBA/2 mice were injected in vivo with either berberine (200 μg per mouse, i.p.) or saline. After 24 hr, macrophages were purified and incubated with KLH-primed CD4+ T cells and KLH (10 μg/ml). After 4 days, culture supernatants were harvested and assayed for IL-12 (a), IFN-γ (b) and IL-4 (c) levels by ELISA. The results are presented as the means ± SEM (n = 3). *P < 0·05, relative to a saline-injected group.
Discussion
In this study we have demonstrated that treatment of macrophages and DCs with berberine, a benzodioxoloquinolizine alkaloid present in medicinal plants, significantly induced IL-12 production and, furthermore, strongly enhanced the levels of IL-12 production when subsequently treated with either LPS or KLH. Importantly, enhanced IL-12 production in berberine-treated macrophages resulted in an increased ability to induce IFN-γ and a decreased ability to induce IL-4 in antigen-primed CD4+ T cells. These results suggest that berberine-mediated induction of IL-12 production led to the inhibition of Th2 and enhancement of Th1 cytokine synthesis in CD4+ T cells. Since the cytokine profile of Th cells plays an important role in determining the outcome of many diseases, berberine may have therapeutic potential to treat Th2-mediated diseases including allergic diseases.
In allergic diseases, there is a polarization of T-lymphocyte responses into Th2 predominance and enhanced secretion of Th2 cytokines, ultimately leading to inflammation and disease.29 Th1 responses tend to antagonize the allergic responses. The microenvironment in which the DCs and macrophages prevail is an important determinant of the balance between IFN-γ-secreting Th1 cells and pro-allergic Th2 cells.30 IL-12 is a cytokine which induces preferential induction of Th1 cells. IL-12 may play an important role in inhibiting inappropriate immunoglobulin E (IgE) synthesis and allergic inflammation as a result of allergen exposure.31 Indeed, treatment of mice with IL-12 during the active sensitization period reduced the antigen-induced influx of eosinophils in bronchoalveolar lavage fluid, inhibited IgE synthesis, and abolished antigen-induced bronchial hyperresponsiveness.32 Furthermore, the production of IL-12 and IL-12-induced IFN-γ release is reduced in whole blood cultures from patients with atopic asthma compared with normal subjects.33 Allergen immunotherapy results in an increase in IL-12 expression.34 These studies strongly suggest that IL-12 and the resultant Th1 cell response are importantly involved in the immunotherapy of atopic asthma.
The use of systemically administered IL-12 in patients with asthma has been limited because of cytokine toxicity.35 Another treatment option that has the potential to induce a Th1 cytokine response is the use of endogenous inducers of IL-12 with encouraging results in both mice and humans. Chitin (polymers of N-acetyl-d-glucosamine) particles, which are recognized and ingested by macrophages through the mannose receptor, strongly down-regulated Th2-facilitated IgE production and lung eosinophilia in the allergic mouse by inducing the production of Th1 cytokines.36 Similar studies showed that the imidazoquinoline family such as imiquimod and resiquimod inhibited IgE production and Th2 responses by inducing Th1 cytokines in mouse spleen cells or human peripheral mononuclear cells.16,37,38 CpG ODN inhibited the development of Th2-mediated allergic asthma in mice by stimulation of IL-12 production through a p38 MAPK-dependent process.39 In this report, we added berberine to the lists of compounds that induce IL-12 production in macrophages and DCs.
Although berberine may affect cytokine production in CD4+ T cells in several ways, we believe that induction of IL-12 production in macrophages and DCs is a major mechanism by which berberine affects cytokine production in CD4+ T cells, particularly since IL-12 is extremely potent in enhancing IFN-γ and inhibiting IL-4 in CD4+ T cells.40,41 In our cultures, the effect of berberine on cytokine production in CD4+ T cells was indirect since the CD4+ T cells in these cultures were never directly exposed to the berberine.
Induction of IL-12 production in berberine-treated macrophages may be accompanied by an enhancement of their maturation and antigen-presenting capacity. Resiquimod, an inducer of IL-12 production, is known to increase the cell surface expression of molecules involved in co-stimulation and antigen presentation.42 However, our data show that the expression of the class II MHC molecule H-2I-Ad and the co-stimulatory molecules CD80 and CD86 remained unaffected after the preincubation of macrophages with berberine. In alveolar macrophages, LPS induced IL-12 but did not increase the cell surface expression of MHC class II, CD80 and CD86 molecules. In the presence of IFN-γ, LPS strongly enhanced IL-12 production and also increased the expression of the cell surface molecules, suggesting that IFN-γ secretion from natural killer cells and T cells might be important in enhancing the cell maturation and antigen-presenting capacity.43
In addition, berberine may indirectly induce IL-12 production via the down-regulation of IL-10, which is known to reduce IL-12 synthesis.44 Although this inductive pathway may be relevant, the levels of IL-10 expression in both berberine-treated and untreated macrophages were very low (data not shown), suggesting that the inductive effect of berberine on IL-12 production by mouse macrophages cannot be explained by the indirect effect of down-regulation of IL-10.
In the inhibition experiment of berberine-treated macrophages with anti-IL-12 mAb, neutralizing anti-IL-12 mAb could effectively reverse cytokine production by the activated CD4+ T cells if present at the initiation of cultures. Addition of anti-IL-12 mAb to cultures of mannose-binding lectin-treated macrophages restored the induction of IL-4 and inhibited the induction of IFN-γ synthesis by T cells.45 Delayed addition of anti-IL-12 mAb to the cultures or animal might be significantly less effective because the corresponding T cells became more activated and the capacity to produce IL-4 or IFN-γ became more established in vitro. Early Th2 cells maintain the ability to signal for some time after they have acquired high IL-4 synthesis, and addition or induction of IL-12 can reverse the development of the Th2 phenotype. Fully mature Th2 effectors appear to be irreversibly committed and cannot be switched away from the Th2 phenotype.46 The apparent irreversibility of the Th phenotype at the clonal level does not necessarily, however, spell this down for immunotherapy approaches aimed at reversing the phenotype of the response at the population level. Numerous studies have established that the cytokine milieu can direct the development of newly recruited autoimmune cells to a nonpathogenic phenotype. In chronic autoimmune disease new clones continue to be primed and recruited into the antigen-specific effector pool, and those newly emerging effectors could conceivably be shifted away from the pathogenic phenotype by immunotherapy designed to provide an appropriate cytokine milieu.
Addition of anti-IL-12 mAb might inhibit IL-4 production in CD4+ T cells by decreasing IL-10 production in the cultures of the berberine-treated macrophages and T cells. IL-12 has been reported to increase IL-10 production by T cells in systems using established T-cell lines.47 However, IL-10 production increased by IL-12 appears to occur mainly when very high concentrations of IL-12 (10 ng/ml, significantly higher than the amounts produced by berberine-treated macrophages, 200–300 pg/ml) are used.48 Therefore, induction of IL-12 production by berberine was a major immunoregulatory mechanism that led to the inhibition of the Th2 cytokine profile in CD4+ T cells.
In conclusion, we have shown that treatment of macrophages with berberine induced IL-12 production in a dose-dependent manner, leading to the inhibition of the Th2 cytokine profile in CD4+ T cells. These results suggest that berberine-mediated induction of IL-12 production in APC may explain some known biological effects of berberine, including its anti-tumour effect and berberine may also be useful in the treatment of Th2-mediated immunological disorders.
Acknowledgments
We would like to thank Drs Y. K. Choe, I. Choi, S. Wolf and G. Trinchieri for providing valuable reagents and helpful discussion. This work was supported by a grant from the HMP (HMP-01-D-0021).
Abbreviations
- Ag
antigen
- APC
antigen-presenting cell
- CHO
Chinese hamster ovary cell
- DC
dendritic cell
- ELISA
enzyme-linked immunosorbent assay
- HKL
heat-killed Listeria monocytogenes
- IFN-γ
interferon-γ
- IL
interleukin
- KLH
keyhole limpet haemocyanin
- LPS
lipopolysaccharide
- mAb
monoclonal antibody
- RT-PCR
reverse transcription-polymerase chain reaction
- Th
T helper
References
- 1.Mosmann TR, Coffman RL. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;9:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 2.Chtanova T, Mackay CR. T cell effector subsets: extending the Th1/Th2 paradigm. Adv Immunol. 2001;78:233–66. doi: 10.1016/s0065-2776(01)78005-4. [DOI] [PubMed] [Google Scholar]
- 3.Singh VK, Mehrotra S, Agarwal SS. The paradigm of Th1 and Th2 cytokines: its relevance to autoimmunity and allergy. Immunol Res. 1999;20:147–61. doi: 10.1007/BF02786470. [DOI] [PubMed] [Google Scholar]
- 4.Spellberg B, Edwards JE., Jr Type 1/type 2 immunity in infectious diseases. Clin Infect Dis. 2001;32:76–102. doi: 10.1086/317537. [DOI] [PubMed] [Google Scholar]
- 5.Boothby M, Mora AL, Aronica MA, Youn J, Sheller JR, Goenka S, Stephenson L. IL-4 signaling, gene transcription regulation, and the control of effector T cells. Immunol Res. 2001;23:179–91. doi: 10.1385/IR:23:2-3:179. [DOI] [PubMed] [Google Scholar]
- 6.Kang BY, Chung SW, Lim YS, Kim EJ, Kim SH, Hwang SY, Kim TS. Interleukin-12-secreting fibroblasts are more efficient than free recombinant interleukin-12 in inducing the persistent resistance to Mycobacterium avium complex infection. Immunology. 1999;97:474–80. doi: 10.1046/j.1365-2567.1999.00796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dredge K, Marriott JB, Todryk SM, Dalgleish AG. Adjuvants and the promotion of Th1-type cytokines in tumor immunotherapy. Cancer Immunol Immunother. 2002;51:521–31. doi: 10.1007/s00262-002-0309-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohn L, Ray A. T-helper type 2 cell-directed therapy for asthma. Pharmacol Ther. 2000;88:187–96. doi: 10.1016/s0163-7258(00)00091-7. [DOI] [PubMed] [Google Scholar]
- 9.Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2:933–44. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 10.Trinchieri G. Proinflammatory and immunoregulatory functions of interleukin-12. Int Rev Immunol. 1998;16:365–96. doi: 10.3109/08830189809043002. [DOI] [PubMed] [Google Scholar]
- 11.Gavett SH, O'Hearn DJ, Li X, Huang SK, Finkelman FD, Wills-Karp M. Interleukin 12 inhibits antigen-induced airway hyperresponsiveness, inflammation, and Th2 cytokine expression in mice. J Exp Med. 1995;182:1527–36. doi: 10.1084/jem.182.5.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yap G, Pesin M, Sher A. IL-12 is required for the maintenance of IFN-γ production in T cells mediating chronic resistance to the intracellular pathogen, Toxoplasma gondii. J Immunol. 2000;165:628–31. doi: 10.4049/jimmunol.165.2.628. [DOI] [PubMed] [Google Scholar]
- 13.Rempel JD, Wang M, Hayglass KT. In vivo IL-12 administration induces profound but transient commitment to T helper cell type 1-associated patterns of cytokine and antibody production. J Immunol. 1997;159:1490–6. [PubMed] [Google Scholar]
- 14.Colombo MP, Trinchieri G. Interleukin-12 in anti-tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002;13:155–68. doi: 10.1016/s1359-6101(01)00032-6. [DOI] [PubMed] [Google Scholar]
- 15.Ma X, Trinchieri G. Regulation of interleukin-12 production in antigen-presenting cells. Adv Immunol. 2001;79:55–92. doi: 10.1016/s0065-2776(01)79002-5. [DOI] [PubMed] [Google Scholar]
- 16.Vasilakos JP, Smith RMA, Gibson SJ, Lindh JM, Pederson LK, Reiter MJ, Smith MH, Tomai MA. Adjuvant activities of immune response modifier R-848: comparison with CpG ODN. Cell Immunol. 2000;204:64–74. doi: 10.1006/cimm.2000.1689. [DOI] [PubMed] [Google Scholar]
- 17.Hasko G, Szabo C. IL-12 as a therapeutic target for pharmacological modulation in immune-mediated and inflammatory diseases: regulation of T helper 1/T helper 2 responses. Br J Pharmacol. 1999;127:1295–304. doi: 10.1038/sj.bjp.0702689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manetti R, Annunziato F, Tomasevic I, Gianno V, Parronchi P, Romagnani S, Maggi E. Polyinosinic acid: polycytidylic acid promotes T helper type 1-specific immune responses by stimulating macrophage production of interferon-γ and interleukin-12. Eur J Immunol. 1995;25:2656–60. doi: 10.1002/eji.1830250938. [DOI] [PubMed] [Google Scholar]
- 19.Jakob T, Walker PS, Krieg AM, von Stebut E, Udey MC, Vogel JC. Bacterial DNA and CpG-containing oligodeoxynucleotides activate cutaneous dendritic cells and induce IL-12 production: implications for the augmentation of Th1 responses. Int Arch Allergy Immunol. 1999;118:457–61. doi: 10.1159/000024163. [DOI] [PubMed] [Google Scholar]
- 20.Fukuda K, Hibiya Y, Mutoh M, Koshiji M, Akao S, Fujiwara H. Inhibition by berberine of cyclooxygenase-2 transcriptional activity in human colon cancer cells. J Ethnopharmacol. 1999;66:227–33. doi: 10.1016/s0378-8741(98)00162-7. [DOI] [PubMed] [Google Scholar]
- 21.Kang BY, Chung SW, Cho D, Kim TS. Involvement of p38 mitogen-activated protein kinase in the induction of interleukin-12 p40 production in mouse macrophages by berberine, a benzodioxoloquinolizine alkaloid. Biochem Pharmacol. 2002;63:1901–10. doi: 10.1016/s0006-2952(02)00982-6. [DOI] [PubMed] [Google Scholar]
- 22.Inaba M, Inaba K, Fukuba Y, et al. Activation of thymic B cells by Signals of CD40 molecules plus interleukin-10. Eur J Immunol. 1995;25:1244–8. doi: 10.1002/eji.1830250517. [DOI] [PubMed] [Google Scholar]
- 23.Chung SW, Kang BY, Kim SH, Pak YK, Cho D, Trinchieri G, Kim TS. Oxidized low density lipoprotein inhibits interleukin-12 production in lipopolysaccharide-activated macrophages via direct interactions between peroxisome proliferator-activated receptor-γ and nuclear factor-κB. J Biol Chem. 2000;275:32681–7. doi: 10.1074/jbc.M002577200. [DOI] [PubMed] [Google Scholar]
- 24.Fukao T, Koyasu S. Expression of functional IL-2 receptors on mature splenic dendritic cells. Eur J Immunol. 2000;30:1453–7. doi: 10.1002/(SICI)1521-4141(200005)30:5<1453::AID-IMMU1453>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 25.Kim TS, DeKruyff RH, Rupper R, Maecker HT, Levy S, Umetsu DT. An ovalbumin–IL-12 fusion protein is more effective than ovalbumin plus free recombinant IL-12 in inducing a T helper cell type 1-dominated immune response and inhibiting antigen-specific IgE production. J Immunol. 1997;158:4137–44. [PubMed] [Google Scholar]
- 26.Na S-Y, Kang BY, Chung SW, et al. Retinoids inhibit interleukin-12 production in macrophages through physical associations of retinoid X receptor and ΝF-κβ. J Biol Chem. 1999;274:7674–80. doi: 10.1074/jbc.274.12.7674. [DOI] [PubMed] [Google Scholar]
- 27.Kang SN, Lee MH, Kim K-M, Cho D, Kim TS. Induction of human promyelocytic leukemia HL-60 cell differentiation into monocytes by silibinin: involvement of protein kinase C. Biochem Pharmacol. 2001;61:1487–95. doi: 10.1016/s0006-2952(01)00626-8. [DOI] [PubMed] [Google Scholar]
- 28.Peters JH, Gieseler N, Thiele B, Steinbach F. Dendritic cells: from ontogenetic orphans to myelomonocytic descendants. Immunol Today. 1996;17:273–8. doi: 10.1016/0167-5699(96)80544-5. [DOI] [PubMed] [Google Scholar]
- 29.Romagnani S. The role of lymphocytes in allergic disease. J Allergy Clin Immunol. 2000;105:399–408. doi: 10.1067/mai.2000.104575. [DOI] [PubMed] [Google Scholar]
- 30.Kalinski P, Hilkens CM, Wierenga EA, Kapsenberg ML. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol Today. 1999;20:561–7. doi: 10.1016/s0167-5699(99)01547-9. [DOI] [PubMed] [Google Scholar]
- 31.Chung KF, Barnes PJ. Cytokines in asthma. Thorax. 1999;54:825–57. doi: 10.1136/thx.54.9.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kips JC, Brusselle GJ, Joos GF, Peleman RA, Tavernier JH, Devos RR, Pauwels RA. Interleukin-12 inhibits antigen-induced airway hyperresponsiveness in mice. Am J Respir Crit Care Med. 1996;153:535–9. doi: 10.1164/ajrccm.153.2.8564093. [DOI] [PubMed] [Google Scholar]
- 33.van der Pouw Kraan TC, Boeije LC, de Groot ER, Stapel SO, Snijders A, Kaspenberg ML, van der Zee JS, Aarden LA. Reduced production of IL-12, IL-12-dependent IFN-γ release in patients with allergic asthma. J Immunol. 1997;158:5560–5. [PubMed] [Google Scholar]
- 34.Hamid QA, Schotman E, Jacobson MR, Walker SM, Durham SR. Increases in IL-12 messenger RNA+ cells accompany inhibition of allergen-induced late skin responses after successful grass pollen immunotherapy. J Allergy Clin Immunol. 1997;99:254–60. doi: 10.1016/s0091-6749(97)70106-4. [DOI] [PubMed] [Google Scholar]
- 35.Leonard P, Sur S. Interleukin-12: potential role in asthma therapy. Biodrugs. 2003;17:1–7. doi: 10.2165/00063030-200317010-00001. [DOI] [PubMed] [Google Scholar]
- 36.Shibata Y, Foster LA, Bradfield JF, Myrvik QN. Oral administration of chitin down-regulates serum IgE levels and lung eosinophilia in the allergic mouse. J Immunol. 2000;164:1314–21. doi: 10.4049/jimmunol.164.3.1314. [DOI] [PubMed] [Google Scholar]
- 37.Frotscher B, Anton K, Worm M. Inhibition of IgE production by the imidazoquinoline resiquimod in nonallergic and allergic donors. J Invest Dermatol. 2002;119:1059–64. doi: 10.1046/j.1523-1747.2002.19531.x. [DOI] [PubMed] [Google Scholar]
- 38.Brugnolo F, Sampognaro S, Liotta F, et al. The novel synthetic immune response modifier R-848 (resiquimod) shifts human allergen-specific CD4 Th2 lymphocytes into IFN-γ-producing cells. J Allergy Clin Immunol. 2003;111:380–8. doi: 10.1067/mai.2003.102. [DOI] [PubMed] [Google Scholar]
- 39.Choudhury BK, Wild JS, Alam R, Klinman DM, Boldogh I, Oharajiya N, Mileski WJ, Sur S. In vivo role of p38 mitogen-activated protein kinase in mediating the anti-inflammatory effects of CpG oligodeoxynucleotide in murine asthma. J Immunol. 2002;169:5955–61. doi: 10.4049/jimmunol.169.10.5955. [DOI] [PubMed] [Google Scholar]
- 40.Marshall JD, Secrist H, DeKruyff RH, Wolf SF, Umetsu DT. IL-12 inhibits the production of IL-4 and IL-10 in allergen-specific human CD4+ T lymphocytes. J Immunol. 1995;155:111–17. [PubMed] [Google Scholar]
- 41.Gerosa F, Paganin C, Peritt D, Paiola F, Scupoli MT, Aste-Amezaga M, Frank I, Trinchieri G. Interleukin-12 primes human CD4 and CD8 T cell clones for high production of both interferon-γ and interleukin-10. J Exp Med. 1996;183:2559–69. doi: 10.1084/jem.183.6.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahonen CL, Gibson SJ, Smith RM, Pederson LK, Lindh JM, Tomai MA, Vasilakos JP. Dendritic cell maturation and subsequent enhanced T-cell stimulation induced with the novel synthetic immune response modifier R-848. Cell Immunol. 1999;197:62–72. doi: 10.1006/cimm.1999.1555. [DOI] [PubMed] [Google Scholar]
- 43.Foss DL, Zilliox MJ, Murtaugh MP. Differential regulation of macrophage interleukin-1 (IL-1), IL-12, and CD80–CD86 by two bacterial toxins. Infect Immun. 1999;67:5275–81. doi: 10.1128/iai.67.10.5275-5281.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aste-Amezaga M, Ma X, Sartori A, Trinchieri G. Molecular mechanisms of the induction of IL-12 and its inhibition by IL-10. J Immunol. 1998;160:5936–44. [PubMed] [Google Scholar]
- 45.Panunto-Castelo A, Souza MA, Rogue-Barreira MC, Silva JS. KM (±), a lectin from Artocarpus integrifolia, induces IL-12 p40 production by macrophages and switches from type 2 to type 1 cell-mediated immunity against Leishmania major antigens, resulting in BALB/c mice resistant to infection. Glycobiology. 2001;11:1035–42. doi: 10.1093/glycob/11.12.1035. [DOI] [PubMed] [Google Scholar]
- 46.Asnagli H, Murphy KM. Stability and commitment in T helper cell development. Curr Opin Immunol. 2001;13:242–7. doi: 10.1016/s0952-7915(00)00210-7. [DOI] [PubMed] [Google Scholar]
- 47.Jeannin P, Delneste Y, Seveso M, Life P, Bonnefoy JY. IL-12 synergizes with IL-2 and other stimuli in inducing IL-10 production by human T cells. J Immunol. 1996;156:3159–65. [PubMed] [Google Scholar]
- 48.Windhagen A, Anderson DE, Carrizosa A, Williams RE, Hafler DA. IL-12 induces human T cells secreting IL-10 with IFN-γ. J Immunol. 1996;157:1127–31. [PubMed] [Google Scholar]