Abstract
Immunomodulators such as cyclosporin A (CsA) and SAR943 (32-deoxorapamycin) inhibit single allergen-induced allergic inflammation such as eosinophilic and lymphocytic infiltration and mRNA expression for interleukin (IL)-4 and IL-5. We examined the effects of CsA and SAR943, administered orally, on asthmatic responses in a rat model of chronic allergic inflammation. Sensitized Brown-Norway (BN) rats were exposed to ovalbumin (OVA) aerosol every third day on six occasions. CsA (5 mg/kg/day), SAR943 (2·5 mg/kg/day) or vehicle (Neoral™) was administered orally, once a day, from days 10 to 21 (a total of 12 doses). We measured eosinophilic and T-cell inflammation in the airways, proliferation of airway cells by incorporation of bromodeoxyuridine (BrdU) and bronchial responsiveness to acetylcholine. CsA had no effects, while SAR943 inhibited airway smooth muscle (ASM, P < 0·05) and epithelial cell (P < 0·01) BrdU incorporation, and the number of CD4+ T cells (P < 0·05), without effects on BHR. ASM thickness was not significantly increased following chronic allergen exposure. Therefore, CsA and SAR943 have no effect on chronic eosinophilic inflammation, while SAR943, but not CsA, had a small effect on the proliferation of ASM and epithelium.
Introduction
Asthma is a chronic inflammatory airway disease characterized by spontaneous airflow limitation and non-specific bronchial hyperresponsiveness (BHR). Changes to the structure of the airways, in particular alterations to the airway smooth muscle (ASM), may contribute to the irreversible airflow obstruction that is often observed in asthma.1 Increased numbers of T cells expressing T-helper (Th) type 2 cytokines, such as interleukin (IL)-4 and IL-5, have been identified in the airways of asthmatics.2,3 Th2 cells may orchestrate asthmatic inflammation by increasing specific immunoglobulin E (IgE) levels through an action of IL-4 on B cells and by increasing the terminal differentiation and activation of eosinophils through the release of IL-5.4,5 Th2 cells may modulate the development of chronic structural changes in the airways. For example, over-expression of Th2 cytokines (such as IL-13) in the airways of mice leads to features of airway wall remodelling, including ASM hyperplasia,6,7 and depletion of activated T cells and the elimination of T-cell-specific cytokine production affects the development of pathological changes in airway structure and function.8
T-cell immunosuppressants such as cyclosporin A (CsA) have a limited role in the treatment of asthma. CsA has been shown to improve lung function and decrease oral corticosteroid requirements in chronic severe asthmatics and to inhibit the late-phase response following allergen challenge in mild atopic asthmatics.9–11 At high doses, CsA inhibits BHR and airway wall remodelling after chronic antigen challenge in sensitized cats.12 CsA may act by suppressing calcineurin, which is important in the signal-transduction pathways necessary for the expression of many cytokines, including IL-2, IL-3, IL-4, IL-5 and granulocyte–macrophage colony-stimulating factor (GM-CSF), and thereby inhibit T-cell activation and proliferation.13 Rapamycin, another T-cell immunomodulator, acts at a late stage in T-cell activation, and inhibits the proliferation of T cells, whilst CsA is insensitive at this stage of activation.14 Rapamycin also has direct effects in inhibiting the proliferation of other cell types, such as epidermal cells and keratinocytes,15,16 and therefore such immunomodulators may also act directly on structural cells. Because rapamycin exhibits adverse physicochemical properties, its formulation and administration in an appropriate therapeutic form have been difficult. SAR943 (32-deoxorapamycin), a novel rapamycin derivative with immunosuppressive properties, has greater chemical stability in galenical formulation.17 Fujitani & Trifilieff have reported on the anti-inflammatory properties of SAR943 in a murine model of single allergen challenge;18 they also demonstrated that SAR943 inhibited ASM cell proliferation in vitro.18 These results contrast with the lack of effect of SAR943 on the allergic inflammation and BHR induced by single allergen exposure in sensitized rats.19 We have previously investigated the differential effects of CsA and SAR94317 on chronic allergen-induced airway asthmatic and proliferative responses in the Brown-Norway (BN) rat model of chronic inflammation. We examined the hypothesis that inhibition of T-cell activation and proliferation may result in a reduction of structural cell proliferation and remodelling. In the present study we focused on the effects of these T-cell immunomodulators on ASM and epithelial cell DNA synthesis, ASM thickening and chronic eosinophilic inflammation following repeated allergen exposure in BN rats actively sensitized to ovalbumin (OVA).
Materials and methods
Sensitization and challenges
Pathogen-free, male BN rats weighing 220–250 g (Harlan, Bicester, UK) were sensitized on days 1, 2 and 3 using intraperitoneal (i.p.) injections of 1 mg/kg OVA prepared in 1 ml of 0·9% sterile saline containing 100 mg Al(OH)3 as adjuvant. On days 6, 9, 12, 15, 18 and 21 after the start of sensitization, animals were exposed to either saline or 1% OVA aerosol for 20 min.
Study design
Four groups were studied:
Sensitized, vehicle-treated and repeatedly exposed to saline aerosol (saline group; n = 8). The rats of this group received vehicle (Neoral®; Novartis, Horsham, UK), 1 ml orally, once a day, from day 10 to day 21 of the procedure (a total of 12 doses).
Sensitized, vehicle-treated and repeatedly exposed to OVA (OVA group; n = 8). The procedures were the same as described above for the saline group, except that the aerosol was 1% OVA.
Sensitized, CsA treated and OVA exposed (CsA group; n = 8). The procedures were the same as described above for the saline group. Rats received 5 mg/kg CsA once a day, from days 10 to 21, administered by gavage 2 hr prior to antigen exposure on days 12, 15, 18 and 21 (60 mg/kg in total).
Sensitized, SAR943 treated and OVA exposed (SAR group; n = 8): The procedures were the same as described above for the saline group. Rats received 2·5 mg/kg SAR943 once a day, from days 10 to 21, administered by gavage 2 hr prior to antigen exposure on days 12, 15, 18 and 21 (30 mg/kg in total).
All rats were studied 18–24 hr after exposure to either 1% OVA or 0·9% NaCl aerosol.
Bromodeoxyuridine dosing
5-Bromo-2′-deoxyuridine (BrdU; Sigma Chemicals, Poole, UK) was dissolved in dimethylsulphoxide (DMSO) and diluted with sterile water, giving a final concentration of DMSO of <7%. Rats were injected i.p., with 50 mg/kg BrdU in 1 ml of solution, immediately following the allergen challenges on days 12, 15, 18 and 21, and received a second dose 8 hr later (a total of eight injections).
Measurement of bronchial responsiveness to acetylcholine
Bronchial responsiveness was measured 18–24 hr after the final allergen challenge, as previously described.20 Briefly, rats were anaesthetized, a tracheostomy was performed and lung resistance measured by the method of von Neergard & Wirz21 using an in-house developed software program (LabVIEW 2; National Instruments, Austin, TX). Increasing half-log10 concentrations of acetylcholine were administered by inhalation for 45 breaths and the lung resistance was measured. The concentration of acetylcholine required to increase baseline resistance by 200% (PC200) was determined by linear interpolation of log concentration–lung resistance curves.
Tissue collection
Rats were killed using an i.p. overdose of sodium pentobarbitone (500 mg/kg). The lungs were rapidly removed and insufflated with OCT Tissue Tek™ mounting medium (Raymond A. Lamb, London, UK) diluted 1 : 1 with phosphate-buffered saline (PBS). Regions of the left and right lung lobes were mounted on cork blocks with the main bronchi uppermost, snap-frozen in melting isopentane and stored at −25°.
BrdU and α-smooth muscle actin immunohistochemistry
Detailed methods have previously been described.22 Briefly, for the detection of cells undergoing DNA synthesis, cryostat sections were incubated with a primary anti-BrdU monoclonal antibody (mAb) solution (clone BU-1; Amersham International, Bucks., UK). After labelling with a biotinylated rat-adsorbed antiserum to mouse immunoglobulin G (IgG) (Vector Laboratories, Peterborough, UK), BrdU-positive cells were visualized using 3,3-diaminobenzidinetetrachloride solution (Sigma) with glucose oxidase-nickel enhancement to give a black end-product.23 For the visualization of α-smooth muscle actin, cryostat sections were incubated with a primary anti α-smooth-muscle actin mAb (clone 1A4; Sigma). After labelling with a biotinylated rat-adsorbed antiserum to mouse IgG (Vector Laboratories) the α-smooth-muscle actin staining was visualized using the alkaline phosphatase/anti-alkaline phosphatase method. Nuclei that were not immunoreactive for BrdU were counterstained by application of the fluorescent DNA ligand, 4,6-diamidino-2-phenylindole hydrochloride (DAPI) (Sigma), and mounted under glass coverslips.
Eosinophil major basic protein (MBP) and T-cell immunohistochemistry
Detailed methods have previously been described.20,22 Briefly, for the detection of eosinophils, we used an IgG1 mAb against human MBP, clone BMK-13 (Monosan, Uden, the Netherlands). The cryostat sections were incubated with BMK-13. After labelling with a biotinylated horse anti-mouse secondary mAb, positively stained cells were visualized using the alkaline phosphatase/anti-alkaline phosphatase method. For staining CD2+, CD4+ or CD8+ T lymphocytes in tissue sections, the sections were incubated with mouse anti-rat CD2, CD4 or CD8 mAb (pan T-cell markers; PharMingen, Cambridge Bioscience, Cambridge, UK). After labelling with a biotinylated goat anti-mouse secondary mAb, positively stained cells were visualized using the alkaline phosphatase/anti-alkaline phosphatase method. All sections were counterstained with Harris Hematoxylin (BDH, Dorset, UK) and mounted in Glycergel (DAKO, Ely, UK). Cellular influx around the five largest airways in each lung section was assessed as the number of positively stained cells in the bronchial submucosa and expressed per mm of basement membrane. MBP-positive cells were enumerated in the lung parenchyma.
Quantification of DNA synthesis and ASM area
Quantification of images was performed as previously described.20,22 Briefly, DNA synthesis in ASM cells was measured as the number of BrdU-immunoreactive nuclei divided by the total number of nuclei (BrdU + DAPI nuclei) within the α-smooth muscle actin-defined immunoreactive area. Epithelial cell DNA synthesis was measured as the number of BrdU-positive cells per unit length of the epithelium defined by a basement membrane mask. ASM thickness was measured as the total α-smooth muscle actin-immunoreactive area around each airway, expressed per unit length of basement membrane. The five largest airways from a single lung section were used to calculate the DNA synthesis and ASM thickness indices from each treatment group.22
Analysis of data
Mean indices were statistically analysed after logarithmic transformation by one-way analysis of variance, followed by t-tests with Bonferroni correction, which were used to evaluate significant differences between groups. Values are expressed as means (95% confidence intervals), with P-values of <0·05 considered significant.
Results
Bronchial responsiveness to acetylcholine
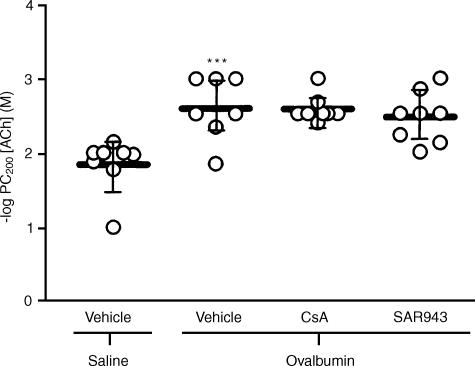
Neither CsA nor SAR943 altered body weight and there were no significant differences in the baseline lung resistance (RL) values following saline challenge in the five experimental groups (data not shown). There was a significant increase in bronchial responsiveness of the sensitized, repeated allergen-exposed and vehicle-treated rats (−log PC200 (M): 2.60; 2.26–2.93. Mean; 95% confidence intervals) compared with sensitized, repeated saline-exposed and vehicle-treated rats (−log PC200 (M): 1.85; 1.55–2.15; P < 0.001). Neither CsA (−log PC200 (M): 2.59; 2.43–2.74) nor SAR943 (−log PC200 (M): 2.48; 2.19–2.76) altered BHR (Fig. 1).
Figure 1.
Bronchial responsiveness to acetylcholine in sensitized rats exposed repeatedly to saline or ovalbumin: There was a greater than half-log order increase in the mean -log concentration of acetylcholine required to increase baseline resistance by 200% (PC200 value) in rats repeatedly exposed to allergen compared with the saline-exposed group (***P <0·001). Neither cyclosporin A (CsA) nor SAR943 attenuated the -log PC200 values compared with the vehicle-treated rats. Data are expressed as mean-log PC200 values, with bars representing 95% confidence intervals.
ASM and epithelial BrdU indices
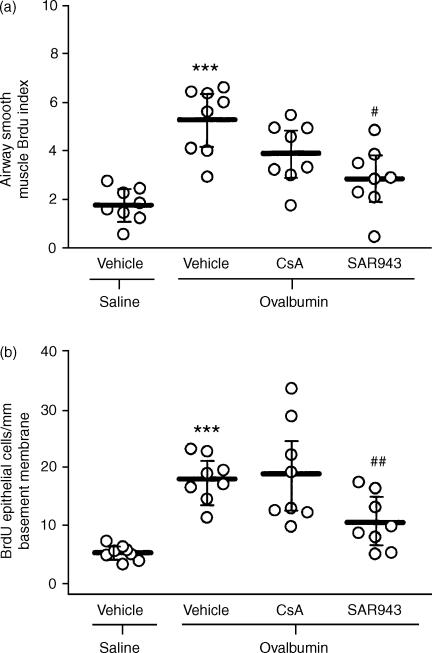
There was a significant increase in the ASM cell BrdU index in the sensitized, allergen-exposed and vehicle-treated group (5·24%; 4·08–6·39) compared with the sensitized, saline-exposed and vehicle-treated group (1·77%; 1·18–2·37, P < 0·001). CsA had no significant effect (3·87%; 2·82–4·92), and SAR943 only a partial effect (2·82%; 1·74–3·90, P < 0·05; Fig. 2a). Repeated allergen exposure also caused a significant increase in the number of BrdU-positive cells in the epithelium (17·9/mm basement membrane; 14·5–21·2) compared with the sensitized, saline-exposed and vehicle-treated controls (5·1; 4·1–6·2; P < 0·001). CsA had no effect (18·8, 11·5–26·1), whereas SAR943 inhibited BrdU uptake (10·3; 6·4–14·3, P < 0·01; Fig. 2b).
Figure 2.
Effects of cyclosporin A (CsA) and SAR943 on airway smooth muscle (ASM) cell (a) and epithelial cell (b) bromodeoxyuridine (BrdU) incorporation following repeated allergen challenge. (a) There was an approximate three-fold increase in the mean BrdU index of the ASM cells of repeated allergen-exposed compared with repeated saline-exposed rats (***P < 0·001). SAR943, but not CsA, caused significant attenuation of the ASM BrdU index compared with vehicle-treated rats (#P < 0·05). (b) There was a greater than two-fold increase in epithelial cell BrdU incorporation after repeated allergen challenge (***P < 0·001 compared with repeated saline-exposed rats). SAR943, but not CsA, caused significant attenuation of epithelial BrdU incorporation compared with vehicle-treated rats (##P < 0·01). Horizontal bars represent the mean values for each group, with the bars representing the 95% confidence intervals.
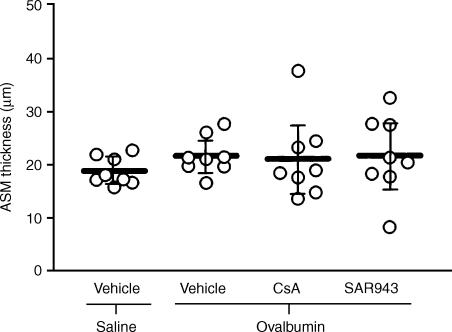
ASM thickness
The ASM of the sensitized, allergen-exposed and vehicle-treated group had a greater mean thickness, of 21·6 µm (18·5–24·6), compared with the 18·7 µm (16·4–20·9) of the sensitized, saline-exposed and vehicle-treated rats, but the difference was not significant. Neither CsA or SAR943 had any effect (Fig. 3).
Figure 3.
Airway smooth muscle (ASM) thickness after repeated allergen exposure. Following repeated exposure to allergen there was no significant increase in ASM area per unit length of basement membrane compared with rats exposed repeatedly to saline. Both cyclosporin A (CsA) and SAR943 were without significant effect. Data are expressed as mean values, with bars representing 95% confidence intervals.
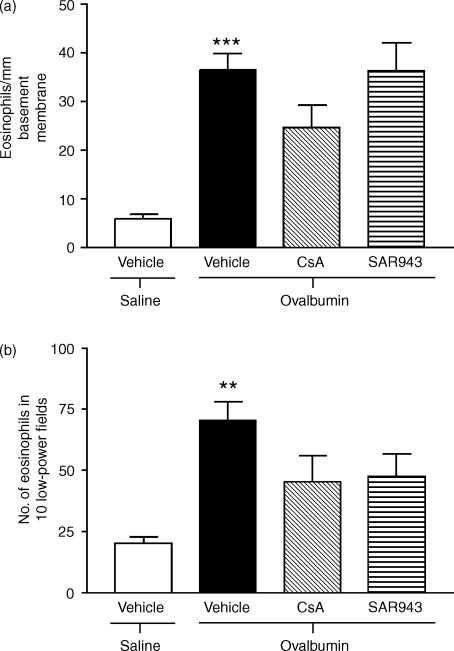
Eosinophil recruitment to the lungs
Repeated exposure to allergen caused a sixfold increase in the number of MBP-positive eosinophils around the airways of sensitized, allergen-exposed and vehicle-treated rats (36·6 cells/mm of basement membrane; 28·9–44·2) compared with the sensitized, saline-exposed and vehicle-treated group (6·0 cells/mm of basement membrane; 3·9–8·2; P < 0·001). Neither CsA (24·61 cells/mm of basement membrane; 13·43–35·80) nor SAR943 (36·32 cells/mm of basement membrane; 23·09–49·54) attenuated the number of eosinophils (Fig. 4a). Similar results were observed in the parenchyma (Fig. 4b).
Figure 4.
Effects of cyclosporin A (CsA) and SAR943 on eosinophil major basic protein (MBP)-positive eosinophil recruitment to the airways (a) and parenchyma (b) following repeated allergen challenge. (a) There was a significant increase in the number of MBP-positive eosinophils around the airways following repeated allergen exposure of rats compared with repeated saline exposure (***P < 0·001). Neither CsA nor SAR943 attenuated eosinophil recruitment to the airways as compared with the vehicle-treated group. (b) There was also a significant increase in MBP-positive eosinophil recruitment into the parenchyma of repeatedly allergen-exposed, compared with repeatedly saline-exposed, rats (**P < 0·01). Neither CsA nor SAR943 altered eosinophil recruitment compared with vehicle-treated rats. Data are expressed as mean values ± standard error of the mean (SEM).
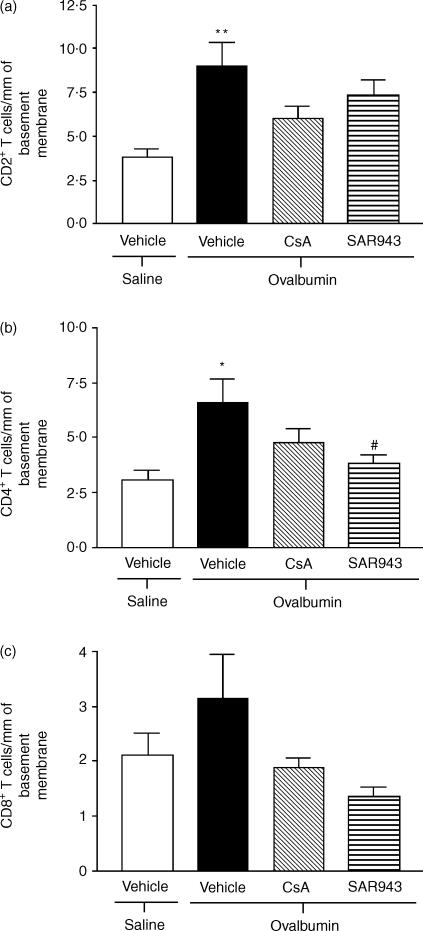
T-cell counts
CD2+, CD4+ and CD8+ T-cell counts were increased following chronic allergen exposure. CsA had no significant effect on these cell counts, but SAR943 caused a significant reduction in the number of CD4+ T cells (Fig. 5).
Figure 5.
Effects of cyclosporin A (CsA) and SAR943 on CD2+ (a), CD4+ (b) and CD8+ (c) T-cell recruitment to the airways following repeated challenge with allergen. There was a significant increase in the number of (a) CD2+ (**P < 0·01) T cells and (b) CD4+ (*P < 0·05) T cells around the airways following repeated allergen exposure of rats compared with repeated saline exposure. Neither CsA nor SAR943 attenuated the recruitment of CD2+ T cells to the airways as compared with the vehicle-treated group (a). SAR943, but not CsA, attenuated the recruitment of CD4+ T cells to the airways, as compared with the vehicle-treated group (#P < 0·05) (b). Neither CsA nor SAR943 altered CD8+ T-cell recruitment compared with vehicle-treated rats. Data are expressed as mean values ± standard error of the mean (SEM).
Discussion
Following repeated allergen exposure of sensitized BN rats, significant increases were detected in both ASM and epithelial-cell DNA synthesis (as measured by BrdU incorporation), as previously reported.20,22 Treatment of sensitized and repeatedly allergen-exposed rats with a derivative of rapamycin, SAR943, significantly attenuated ASM and epithelial-cell DNA synthesis, which are features of airway remodelling. However, no change in ASM thickness was observed in the present study following repeated exposure to allergen. CsA was without effect on ASM and epithelial-cell DNA synthesis.
Both CsA and SAR943 had no effect on tissue eosinophilic infiltration, while SAR943 reduced the number of CD4+ T cells. Similar results were previously obtained in our laboratory with CsA.24,25 Because SAR943 concomitantly reduced CD4+ T-cell numbers and decreased ASM proliferation, one may postulate that T cells may be linked to ASM hyperplasia as they can induce ASM cell proliferation.26
The dose of CsA employed in our study was effective in inhibiting both alloantibody and cellular immunity responses in various models in the rat.27–30 Our data are similar to those reported previously regarding the effect of CsA in single-allergen exposure of sensitized BN rats,24,25 where no inhibition of allergen-induced BHR by CsA was detected, although there was a significant reduction in eosinophils, CD4+ T cells and CD8+ T cells in the airways submucosa.24 In the present study, CsA caused a trend towards a reduction of eosinophils, T cells and BrdU incorporation into the muscle, but this was not significant. Padrid et al. showed a reduction in eosinophilic cellular inflammation and in the thickness of ASM layers in chronic antigen-challenged cats using a higher dose of CsA.12 It is not clear why there should be a difference in the responsiveness to CsA between these two different chronic models. One possibility is that the differential effects of CsA on cytokine expression may determine the overall response. Therefore, in our previous study of single allergen exposure, we found that CsA inhibited the expression of Th2 cytokines (such as IL-2, IL-4 and IL-5) in the lung, in addition to the Th1 cytokine, interferon-γ (IFN-γ).25
Rapamycin is a macrolide antibiotic and a relatively novel immunosuppressant used in organ transplantation with a 10- to 100-fold stronger potency than CsA in preventing acute rejection of vascularized allograft in animal models.31,32 In the present study we used a derivative of rapamycin – SAR493 or 32-deoxorapamycin – which has a similar range of action as rapamycin.17 Rapamycin inhibited growth factor-dependent proliferation of both lymphoid and non-lymphoid cells, including vascular smooth-muscle cells33 and rat renal mesangial cells, which display functional and morphological features that are similar to those of ASM cells.34,35 When compared with CsA, rapamycin was much more effective against platelet-derived growth factor-induced vascular smooth-muscle cell proliferation36 and CsA exerted minimal effects on proliferation of keratinocytes at doses achieved in vivo in humans, while rapamycin was effective.37 Rapamycin has been shown to inhibit proliferating cell nuclear antigen exposure and blocks the cell cycle in the G1 phase of human keratinocyte stem cells.16 A direct effect of SAR943 on ASM proliferation has been reported recently.19 Therefore, in the current study, SAR943 may have had direct inhibitory effects on ASM cell proliferation, but CsA had no effect.
The expression of BHR may be dependent not only on factors affecting muscle phenotype and behaviour, but also on non-muscle components of the allergic response, such as release of mediators that could augment bronchial responsiveness directly without changing muscle phenotype and behaviour. In our model, we have previously shown that both cysteinyl-leukotrienes and endothelin contribute to airway smooth-muscle proliferation, but only cysteinyl-leukotrienes to BHR.20,38 Fujitani & Trifilieff have recently reported that SAR943, when administered intranasally in mice, inhibited allergen-induced increase in inflammatory cells infiltrating the lungs, epithelial cell proliferation, mucus hypersecretion and associated BHR.19 It is possible that the lack of greater effect in our model may be related to the more chronic exposure protocol we used or, less likely, to species differences. However, we did find inhibition of ASM proliferation.
In summary, we have shown that the T-cell inhibitor, CsA, and the rapamycin derivative, SAR943, did not inhibit BHR and airway eosinophilic inflammation. However, SAR943 was effective in inhibiting both ASM and epithelial cell DNA incorporation. These studies indicate that rapamycin and its derivatives may have direct antiproliferative effects on structural cells following allergen challenge, irrespective of their T-cell inhibitory effects. The relationship of BHR with ASM is probably very complex.
Acknowledgments
This study was partly supported by a Wellcome Trust grant.
Abbreviations
- ASM
airway smooth muscle
- BHR
bronchial hyperresponsiveness
- BrdU
bromodeoxyuridine
- CsA
cyclosporin A
- OVA
ovalbumin
- GM-CSF
granulocyte–macrophage colony-stimulating factor
- IgE
immunoglobulin E
- IL
interleukin
- Th
T helper
References
- 1.James AL, Pare PD, Hogg JC. The mechanics of airway narrowing in asthma. Am Rev Res Dis. 1989;139:242–6. doi: 10.1164/ajrccm/139.1.242. [DOI] [PubMed] [Google Scholar]
- 2.Hamid Q, Azzawi M, Sun Y, Moqbel R, Wardlaw AJ, Corrigan CJ, Bradley B, Durham SR, Collins JV, Jeffery PK, Quint DJ, Kay AB. Expression of mRNA for interleukin-5 in mucosal bronchial biopsies from asthma. J Clin Invest. 1991;87:1541–9. doi: 10.1172/JCI115166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robinson DS, Hamid Q, Bentley AM, Ying S, Kay AB, Durham SR. Activation of CD4+ T cells and increased IL-4, IL-5 and GM-CSF mRNA positive cells in bronchoalveolar lavage fluid (BAL) 24 hours after allergen inhalation challenge of atopic asthmatic patients. J Allergy Clin Immunol. 1993;92:313–24. doi: 10.1016/0091-6749(93)90175-f. [DOI] [PubMed] [Google Scholar]
- 4.Favre C, Saeland S, Caux C, Duvert V, De Vries JE. Interleukin-4 has basophilic and eosinophilic cell growth-promoting activity on cord blood cells. Blood. 1990;75:67–73. [PubMed] [Google Scholar]
- 5.Swain SL, Weinberg AD, English M, Huston G. IL-4 directs the development of Th2-like helper effectors. J Immunol. 1990;145:3796–806. [PubMed] [Google Scholar]
- 6.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–61. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 7.Zhu Z, Lee CG, Zheng T, et al. Airway inflammation and remodeling in asthma. Lessons from interleukin 11 and interleukin 13 transgenic mice. Am J Respir Crit Care Med. 2001;164:S67–S70. doi: 10.1164/ajrccm.164.supplement_2.2106070. [DOI] [PubMed] [Google Scholar]
- 8.Gavett SH, Chen X, Finkelman F, Wills-Karp M. Depletion of murine CD4 T lymphocytes prevents antigen-induced airway hyperreactivity and pulmonary eosinophilia. Am J Respir Cell Mol Biol. 1994;10:587–93. doi: 10.1165/ajrcmb.10.6.8003337. [DOI] [PubMed] [Google Scholar]
- 9.Alexander AG, Barnes NC, Kay AB. Cyclosporin A in corticosteroid-dependent chronic severe asthma. Lancet. 1992;339:324–7. doi: 10.1016/0140-6736(92)91646-p. [DOI] [PubMed] [Google Scholar]
- 10.Fukuda T, Asakawa J, Motojima S, Makino S. Cyclosporine A reduces T lymphocyte activity and improves airway hyperresponsiveness in corticosteroid-dependent chronic severe asthma. Ann Allergy Asthma Immunol. 1995;75:65–72. [PubMed] [Google Scholar]
- 11.Khan LN, Kon ON, Macfarlane AJ, Meng Q, Ying S, Barnes NC, Kay AB. Attenuation of the allergen-induced late asthmatic reaction by cyclosporin A is associated with inhibition of bronchial eosinophils, interleukin-5, granulocyte macrophage colony-stimulating factor, and eotaxin. Am J Respir Crit Care Med. 2000;162:1377–82. doi: 10.1164/ajrccm.162.4.9911117. [DOI] [PubMed] [Google Scholar]
- 12.Padrid PA, Cozzi P, Leff AR. Cyclosporine A inhibits airway reactivity and remodeling after chronic antigen challenge in cats. Am J Respir Crit Care Med. 1996;154:1812–8. doi: 10.1164/ajrccm.154.6.8970375. [DOI] [PubMed] [Google Scholar]
- 13.Sigal NH, Dumont FJ. Cyclosporin A, FK-506, and rapamycin: pharmacologic probes of lymphocyte signal transduction. Annu Rev Immunol. 1992;10:519–60. doi: 10.1146/annurev.iy.10.040192.002511. [DOI] [PubMed] [Google Scholar]
- 14.Dumont FJ, Staruch MJ, Koprak SL, Melino MR, Sigal NH. Distinct mechanisms of suppression of murine T cell activation by the related macrolides FK-506 and rapamycin. J Immunol. 1990;144:251–8. [PubMed] [Google Scholar]
- 15.Javier AF, Bata-Csorgo Z, Ellis CN, Kang S, Voorhees JJ, Cooper KD. Rapamycin (sirolimus) inhibits proliferating cell nuclear antigen expression and blocks cell cycle in the G1 phase in human keratinocyte stem cells. J Clin Invest. 1997;99:2094–9. doi: 10.1172/JCI119382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duncan JI. Differential inhibition of cutaneous T-cell-mediated reactions and epidermal cell proliferation by cyclosporin A, FK-506, and rapamycin. J Invest Dermatol. 1994;102:84–8. doi: 10.1111/1523-1747.ep12371737. [DOI] [PubMed] [Google Scholar]
- 17.Sedrani R, Cottens S, Kallen J, Schuler W. Chemical modification of rapamycin: the discovery of SDZ RAD. Transplant Proc. 1998;30:2192–4. doi: 10.1016/s0041-1345(98)00587-9. [DOI] [PubMed] [Google Scholar]
- 18.Fujitani Y, Trifilieff A. In vivo and in vitro effects of SAR 943, a rapamycin analogue, on airway inflammation and remodeling. Am J Respir Crit Care Med. 2003;167:193–8. doi: 10.1164/rccm.200205-455OC. [DOI] [PubMed] [Google Scholar]
- 19.Huang TJ, Eynott P, Salmon M, Nicklin PL, Chung KF. Effect of topical immunomodulators on acute allergic inflammation and bronchial hyperresponsiveness in sensitised rats. Eur J Pharmacol. 2002;437:187–94. doi: 10.1016/s0014-2999(02)01295-5. [DOI] [PubMed] [Google Scholar]
- 20.Salmon M, Liu YC, Ma KJC, Rousell J, Huang TJ, Hisada T, Nicklin PL, Chung KF. Contribution of upregulated airway endothelin-1 expression to airway smooth muscle and epithelial cell DNA synthesis after repeated allergen exposure of sensitised Brown-Norway rats. Am J Respir Cell Mol Biol. 2000;23:618–25. doi: 10.1165/ajrcmb.23.5.3909. [DOI] [PubMed] [Google Scholar]
- 21.Von Neergaard K, Werz K. Über eine methode zur messung der lungenelastizität am lebenden menschen, isbesondere beim emphysem. Z Klin Med. 1927;105:35–50. [Google Scholar]
- 22.Salmon M, Walsh DA, Koto H, Barnes PJ, Chung KF. Repeated allergen exposure induces airway smooth muscle and epithelial cell DNA synthesis and airway remodeling in sensitised Brown-Norway rats. Eur Respir J. 1999;14:633–41. doi: 10.1034/j.1399-3003.1999.14c25.x. [DOI] [PubMed] [Google Scholar]
- 23.Shu S, Ju G, Fan L. The glucose oxidase-DAB-nickel method in peroxidase histochemistry of the central nervous system. Neurosci Lett. 1988;85:169–71. doi: 10.1016/0304-3940(88)90346-1. [DOI] [PubMed] [Google Scholar]
- 24.Elwood W, Lotvall J, Barnes PJ, Chung KF. Effects of cyclosporin A and dexamethasone on allergen-induced bronchial hyperresponsiveness and inflammatory cell. Am Rev Respir Dis. 1992;145:1289–94. doi: 10.1164/ajrccm/145.6.1289. [DOI] [PubMed] [Google Scholar]
- 25.Huang TJ, Newton R, Haddad EB, Chung KF. Differential regulation of cytokine expression after allergen exposure of sensitized rats by cyclosporin A and corticosteroids: relationship to bronchial hyperresponsiveness. J Allergy Clin Immunol. 1999;104:644–52. doi: 10.1016/s0091-6749(99)70337-4. [DOI] [PubMed] [Google Scholar]
- 26.Lazaar AL, Albelda SM, Pilewski JM, Brennan B, Pure E, Panettieri RA., Jr T lymphocytes adhere to airway smooth muscle cells via integrins and CD44 and induce smooth muscle cell DNA synthesis. J Exp Med. 1994;180:807–16. doi: 10.1084/jem.180.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cunningham C, Power DA, Stewart KN, Catto GR. The influence of cyclosporin A on alloantibody responses in inbred rats: provisional evidence for a serum factor with anti-idiotypic activity. Clin Exp Immunol. 1988;72:130–5. [PMC free article] [PubMed] [Google Scholar]
- 28.Carriere V, Auriault C, Dessaint JP, Capron A. Differential effect of cyclosporin A (CyA) on IgE response: role of CyA-induced suppressor cells. Immunol Lett. 1987;16:145–9. doi: 10.1016/0165-2478(87)90122-2. [DOI] [PubMed] [Google Scholar]
- 29.Mason DW, Morris PJ. Inhibition of the accumulation, in rat kidney allografts, of specific- but not nonspecific-cytotoxic cells by cyclosporine. Transplantation. 1984;37:46–51. doi: 10.1097/00007890-198401000-00014. [DOI] [PubMed] [Google Scholar]
- 30.Ryffel B, Feurer C, Heuberger B, Borel JF. Immunosuppressive effect of cyclosporin A in two lymphocyte transfer models in rats: comparison of in vivo and in vitro treatment. Immunobiology. 1982;163:470–83. doi: 10.1016/S0171-2985(82)80061-2. [DOI] [PubMed] [Google Scholar]
- 31.Calne RY, Collier DS, Lim S, Pollard SG, Samaan A, White DJ, Thiru S. Rapamycin for immunosuppression in organ allografting. Lancet. 1989;2:227. doi: 10.1016/s0140-6736(89)90417-0. [DOI] [PubMed] [Google Scholar]
- 32.Chen HF, Wu JP, Luo HY, Daloze PM. The immunosuppressive effect of rapamycin on pancreaticoduodenal transplants in the rat. Transplant Proc. 1991;23:2239–40. [PubMed] [Google Scholar]
- 33.Cao W, Mohacsi P, Shorthouse R, Pratt R, Morris RE. Effects of rapamycin on growth factor-stimulated vascular smooth muscle cell DNA synthesis. Inhibition of basic fibroblast growth factor and platelet-derived growth factor action and antagonism of rapamycin by FK506. Transplantation. 1995;59:390–5. [PubMed] [Google Scholar]
- 34.Johnson RJ, Iida H, Alpers CE, Majesky MW, Schwartz SM, Pritzi P, Gordon K, Gown AM. Expression of smooth muscle cell phenotype by rat mesangial cells in immune complex nephritis. Alpha-smooth muscle actin is a marker of mesangial cell proliferation. J Clin Invest. 1991;87:847–58. doi: 10.1172/JCI115089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang W, Chan YH, Lee W, Chan L. Effect of rapamycin and FK506 on mesangial cell proliferation. Transplant Proc. 2001;33:1036–7. doi: 10.1016/s0041-1345(00)02320-4. [DOI] [PubMed] [Google Scholar]
- 36.Moon JI, Kim YS, Kim MS, Kim EH, Kim HJ, Kim SI, Park K. Effect of cyclosporine, mycophenolic acid, and rapamycin on the proliferation of rat aortic vascular smooth muscle cells: in vitro study. Transplant Proc. 1999;32:2026–7. doi: 10.1016/s0041-1345(00)01542-6. [DOI] [PubMed] [Google Scholar]
- 37.Taylor RS, Cooper KD, Headington JT, Ho VC, Ellis CN, Voorhees JJ. Cyclosporine therapy for severe atopic dermatitis. J Am Acad Dermatol. 1989;21:580–3. doi: 10.1016/s0190-9622(89)80236-1. [DOI] [PubMed] [Google Scholar]
- 38.Salmon M, Walsh DA, Huang TJ, Barnes PJ, Leonard TB, Hay DW, Chung KF. Involvement of cysteinyl leukotrienes in airway smooth muscle cell DNA synthesis after repeated allergen exposure in sensitized Brown Norway rats. Br J Pharm. 1999;127:1151–8. doi: 10.1038/sj.bjp.0702669. [DOI] [PMC free article] [PubMed] [Google Scholar]