Abstract
Genetically determined responsiveness to microbial stimuli such as lipopolysaccharide (LPS) may affect the pathophysiology of human sepsis. The D299G mutation in human Toll-like receptor-4 (TLR4) impairs LPS signalling in homozygous and heterozygous individuals. To investigate whether the presence of the TLR4(D299G) mutation may correlate with the development or outcome of sepsis following major visceral surgery the presence of TLR4(D299G) mutation was analysed in 307 Caucasian patients (154 without and 153 with sepsis). Sepsis was caused in 84% of patients by polymicrobial infection. The presence of the mutant TLR4 did not significantly correlate with development or outcome of sepsis. Serum levels of tumour necrosis factor, interleukin (IL)-10, and IL-6 at sepsis onset did not significantly differ between patients carrying wild-type and mutant TLR4. Moreover, studies in a murine model of polymicrobial septic peritonitis demonstrated that TLR4-deficiency did neither influence the systemic cytokine response nor the development of organ injury. The results suggest that the signalling capacity of TLR4 as affected by loss-of-function mutations does not influence human or experimental sepsis caused by polymicrobial infection. Thus, in polymicrobial infection, other innate immune receptors may compensate for TLR4 defects.
Introduction
The innate immune system uses germline encoded receptors to recognize conserved molecular patterns of potentially pathogenic microorganisms. Toll-like receptor-4 (TLR4) has been identified as major signalling receptor for lipopolysaccharide (LPS) in mammals. The TLR4 gene is mutated or deleted in the LPS-resistant mouse strains C3H/HeJ and C57BL10/ScCr and mice rendered TLR4-deficient by gene targeting exhibit greatly diminished LPS responses.1,2 Whereas components of Gram-positive bacteria do not appear to activate TLR4, recent reports suggest that TLR4 may also contribute to the innate immune recognition of respiratory syncytial virus, Mycobacterium tuberculosis, or heat-shock proteins.1,2 Activation of mononuclear phagocytes by LPS results in high production of cytokines that are considered important for the development of inflammatory organ injury and lethal shock in sepsis.3
Sequence analysis of TLR4 in various species revealed that the TLR4 gene is polymorphic and that the extracellular ligand recognition domain is far more variable than the cytoplasmic signalling domain.4 Mutations within the extracellular region of TLR4 were also identified in humans.5 It was shown that the TLR4(D299G) mutation severely impairs LPS responsiveness even when present in a heterozygous state.5 In contrast, the TLR4(T399I) mutation did not affect LPS-stimulated signalling. Similar studies on the human TLR2 gene have identified a polymorphism that appears to affect the immune response against Gram-positive bacteria.6 It therefore appears that mutations of Toll-like receptors occurring in humans may determine the magnitude of the innate immune response against microbial stimuli. Because Toll-like receptors are important regulatory elements in the host response to infection, it is conceivable that mutations altering receptor activity may also influence the development and/or outcome of human sepsis.
The present study addressed the question, whether the presence of the TLR4 loss-of-function mutations may affect the immune response to human or experimental sepsis. We show that the TLR4(D299G) mutation is neither associated with the development nor with the outcome of polymicrobial sepsis in surgical patients. Moreover, serum cytokine levels in patients at sepsis onset as well as systemic cytokines and kidney injury during the course of experimental polymicrobial sepsis in mice were not affected by functional TLR4 mutations.
Materials and methods
Patients
Consecutive sepsis patients (n = 153) treated in our surgical intensive care unit between April 1996 and July 2001 were included in the study. Sepsis occurred after major visceral surgery in all patients. The clinical profiles of the patients enrolled in the study are detailed in Table 1. Patients with acquired or inherited immunodeficiency and patients receiving immunosuppressive therapy were excluded from the study. In accordance with established criteria7 diagnosis of sepsis required confirmation of an infectious process and manifestation of two or more of the following conditions: (1) temperature >38° or <36°; (2) heart rate >90 beats per minute; (3) respiratory rate >20 breaths per minute or PaCO2 < 32 mmHg; and (4) white blood cell count >12000/mm3, <4000/mm3, or >10% immature forms. Because of the low stringency of these criteria, however, patients were only included in the study if sepsis definitions were met for at least three consecutive days. The control group consisted of 154 surgical patients without postoperative sepsis (Table 1). Control patients were chosen to control for age, gender distribution, underlying disease, and surgical procedures. The study received local hospital ethic committee approval. Informed consent was obtained from all patients.
Table 1.
Demographic and clinical data of patients
| Characteristic | Uneventful recovery (n = 154) | Sepsis (n = 153) | P-value |
|---|---|---|---|
| Basic data | |||
| ″Age (mean years) | 60·4 | 62·7 | 0·129 |
| ″Male | 87 (56%) | 98 (64%) | 0·216 |
| ″Female | 67 (44%) | 55 (36%) | |
| Underlying disease | |||
| ″Malignancy | 96 (62%) | 91 (60%) | 0·692 |
| ″Non-malignant disease | 58 (38%) | 62 (40%) | |
| Surgical procedures | |||
| ″Subtotal oesophagectomy | 34 (22%) | 42 (27%) | 0·280 |
| ″Total gastrectomy | 22 (14%) | 23 (15%) | |
| ″Partial pancreatoduodenectomy | 11 (7%) | 5 (3%) | |
| ″Colorectal surgery | 64 (41%) | 53 (35%) | |
| ″Miscellaneous resectional surgery | 23 (15%) | 30 (20%) | |
Data are no. (%) of patients, unless otherwise indicated.
Determination of the TLR4(D299G) mutation
Heparinized whole blood was obtained from the study subjects and genomic DNA was isolated with the QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Genomic DNA was used as template for standard PCR reactions using primers 5′-TCCGATTAGCATACTT AGACTACTACCTCG-3′ (forward) and 5′-AACTTCTGAAAAAGCATTCCCACC-3′ (reverse). The 248 bp amplification product included the missense mutation A (r) G at nucleotide position 896 of human TLR4, which results in the amino acid substitution D299G.5 Presence of the mutant or wildtype TLR4 was determined by nucleotide sequence analysis (GATC-Biotech AG, Konstanz, Germany). Sequences were analyzed by the Chromas Software Version 1.45 (Conor Technelysium, Australia).
Mouse model of septic peritonitis
C3H/HeN mice expressing wild-type TLR4 and TLR4-deficient C3H/HeJ mice were purchased from Harlan Winkelmann (Borchem, Germany). Mice were used at 8–12 weeks of age for all experiments. The colon ascendens stent peritonitis (CASP) procedure used for induction of polymicrobial septic peritonitis was described in detail previously.8 Blood samples were collected 12 hr after CASP surgery.
Serum cytokine determination
Serum cytokine concentrations of sepsis patients were determined by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions (all Biosource Intl., Nivelles, Belgium). Serum levels of tumour necrosis factor (TNF), interleukin (IL)-6, and IL-10 were determined on day 1 of clinical sepsis. Cytokine concentrations in mouse serum samples were also measured by ELISA specific for TNF, IL-10, or IL-6 (all R&D Systems, Minneapolis, MN). The absorbance of the samples was determined on an MRX Microplate Reader (Dynatech, Denkendorf, Germany), with 405 nm as the primary and 630 nm as the reference wave length.
Statistical analysis
Statistical analysis of the data was performed by the Chi square test, the Fisher's exact test, or the Mann–Whitney U-test where appropriate. The level of significance was P < 0·05.
Results
The TLR4(D299G) mutation does not influence outcome and course of sepsis in surgical patients
The missense mutation D299G in the extracellular domain of human TLR4 has been demonstrated to severely impair LPS responses in both homozygous and heterozygous individuals.5 However, it is not known whether this mutation in the TLR4 gene may affect the development or outcome of postoperative sepsis. Because the immune response to LPS is considered important for sepsis pathogenesis3,9,10 we determined the allele frequency and genotype distribution of the TLR4(D299G) mutation in 153 patients developing postoperative sepsis and 154 control surgical patients by DNA sequence analysis of the corresponding region in exon 4 of the TLR4 gene. Control patients were chosen to exhibit similar age, gender distribution, underlying malignant disease, and surgical procedures as compared to sepsis patients therefore allowing to control for these variables. Table 1 shows that sepsis patients and control patients with uneventful postoperative recovery did not differ significantly in any of these clinical parameters. All patients were of Caucasian origin.
The overall allele frequencies in all patients were 585/614 (0·95) for wild-type TLR4 and 29/614 (0·05) for the TLR4(D299G) mutation. Genotype analysis demonstrated that in the entire study population, 278 patients (90·6%) were homozygous for the wild-type allele, 29 patients (9·4%) were heterozygous, and none were homozygous for the mutant allele. The frequency of the mutant TLR4 receptor in our study population is therefore comparable to findings previously reported by other investigations on Caucasian populations.5,11 The results depicted in Table 2 show that the heterozygous genotype occurred in 12·3% (95% CI: 7·3–18·3%) of patients with uneventful recovery and in 6·5% (95% CI: 3·2–12%) of patients developing postoperative sepsis. Importantly the difference in the frequency of the heterozygous genotype between sepsis patients and patients with uneventful postoperative recovery (5·8%; 95% CI: −1·6–13·2%) was not statistically significant (P = 0·123). The results in Table 3 further indicate mutant TLR4 genotype frequencies did not differ significantly between sepsis survivors (5·5%; 95% CI: 1·8–12·1%) and non-survivors (8·1%; 95% CI: 2·7–17·8%; difference: 2·6%; 95% CI: −14·3–19·5%). Significant correlations of the TLR4 genotype distribution were also not observed with clinical parameters such as gender and underlying malignant disease (data not shown).
Table 2.
TLR4 mutations and sepsis occurrence in surgical patients
| TLR4 genotype | Uneventful recovery (n = 154) | Sepsis (n = 153) | P-value |
|---|---|---|---|
| WT/WT (n = 278) | 135 (87·7%) | 143 (93·5%) | 0·123 |
| WT/MUT (n = 29) | 19 (12·3%) | 10 (6·5%) |
WT, TLR4 wild-type; MUT, TLR4(D299G) mutation.
Table 3.
TLR4 mutations and outcome in sepsis patients
| TLR4 genotype | Non-survivors (n = 62) | Survivors (n = 91) | P-value |
|---|---|---|---|
| WT/WT (n = 143) | 57 (91·9%) | 86 (94·5%) | 0·765 |
| WT/MUT (n = 10) | 5 (8·1%) | 5 (5·5%) |
WT, TLR4 wildtype; MUT, TLR4(D299G) mutation.
TLR4 was shown to be essential for the immune response to LPS but not other microbial components.12,13 It may therefore be argued that the association of TLR4 genotype differences with sepsis occurrence may only be evident in infections with contribution of Gram-negative bacteria. However, the data in Table 4 demonstrate that the presence of the mutant TLR4 did not significantly correlate with the type of infection (P = 0·824).
Table 4.
TLR4 genotype distributions and type of infections in sepsis patients
| Sepsis pathogens | WT/WT (n = 143) | WT/MUT (n = 10) | P-value |
|---|---|---|---|
| Mixed Gram-negativeand Gram-positive bacterial | 58 (41%) | 5 (50%) | 0·824 |
| Mixed bacterial and fungal | 62 (43%) | 3 (30%) | |
| Gram-negative bacterial | 15 (10%) | 1 (10%) | |
| Gram-positive bacterial | 8 (6%) | 1 (10%) |
WT, TLR4 wildtype; MUT, TLR4(D299G) mutation.
The TLR4(D299G) mutation does not affect systemic cytokine levels in patients with postoperative sepsis
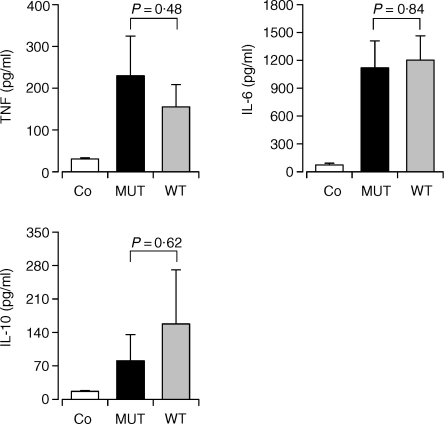
The presence of the TLR4(D299G) mutation even in a heterozygous state was found to be associated with a reduced production of TNF by alveolar macrophages.5 We therefore investigated whether the TLR4(D299G) mutation may influence systemic levels of TNF, IL-6, or IL-10 at the onset of postoperative sepsis. This early time point of analysis was chosen, because triggering of Toll-like receptors by microbial products is considered to represent a critical initial event for the induction of a coordinated innate immune response to infection. Serum cytokine levels were determined in patients carrying the TLR4(D299G) mutation (n = 10) and in patients with wildtype TLR4 (n = 13). Patients with wildtype and mutant TLR4 were matched for sepsis outcome, gender, and underlying malignant disease to control for these variables. The results in Fig. 1 clearly demonstrate that sepsis patients carrying the TLR4(D299G) mutation showed a similar elevation of serum cytokine levels as patients homozygous for the wild-type receptor suggesting that the TLR4(D299G) mutation does not affect the early systemic cytokine response.
Figure 1.
Influence of the TLR4(D299G) mutation on serum cytokine levels in sepsis patients. Cytokine levels were determined in serum samples of sepsis patients homozygous for wild-type TLR4 (WT), sepsis patients heterozygous for the TLR4(D299G) mutation (MUT), and healthy individuals (Co). Serum samples of sepsis patients were obtained on d1 of sepsis. Numbers indicate the results of statistical comparisons between the patient groups for each cytokine.
Cytokine production and organ injury in a murine model of septic peritonitis is independent of functional TLR4
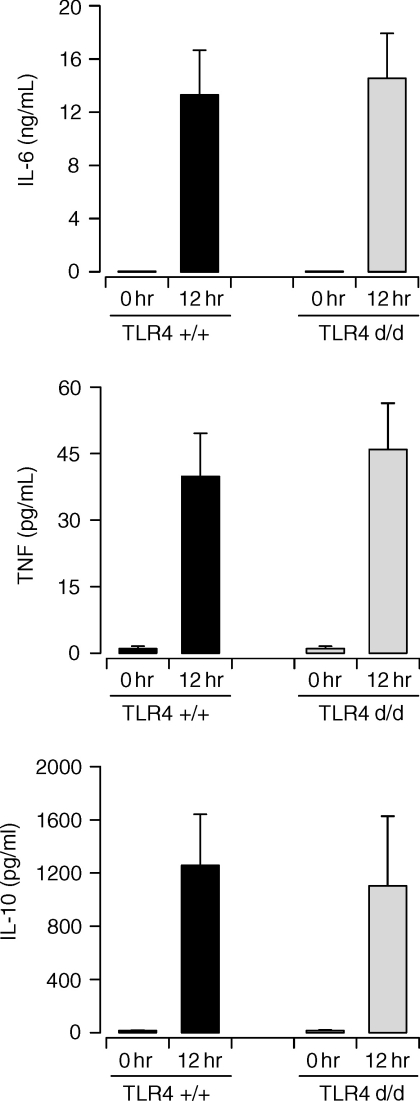
The results obtained with patients suggested that a transdominant loss-of-function mutation of TLR4 does not affect the immune response to postoperative sepsis. To further corroborate these findings the systemic cytokine response during experimental polymicrobial sepsis was examined in TLR4-defective C3H/HeJ as compared to wild-type C3H/HeN mice. Sepsis was induced in mice by the CASP procedure8 resulting in acute septic peritonitis. The results in Fig. 2 clearly show that, similar to our findings with sepsis patients, TLR4-deficiency did not alter the induction of TNF, IL-6, and IL-10 serum levels in septic mice.
Figure 2.
Serum cytokine levels during experimental septic peritonitis are not influenced by TLR4-deficiency. Serum samples were obtained before (0 hr) and 12 hr after CASP surgery of wild-type C3H/HeN (TLR4+/+) and TLR4-deficient C3H/HeJ mice (TLR4d/d). Statistical analysis indicated that for each cytokine serum levels were not significantly different between the two experimental groups (both at 0 hr and 12 hr). The differences between the 0 hr and 12 hr time points were statistically significant in all cases (P < 0·05).
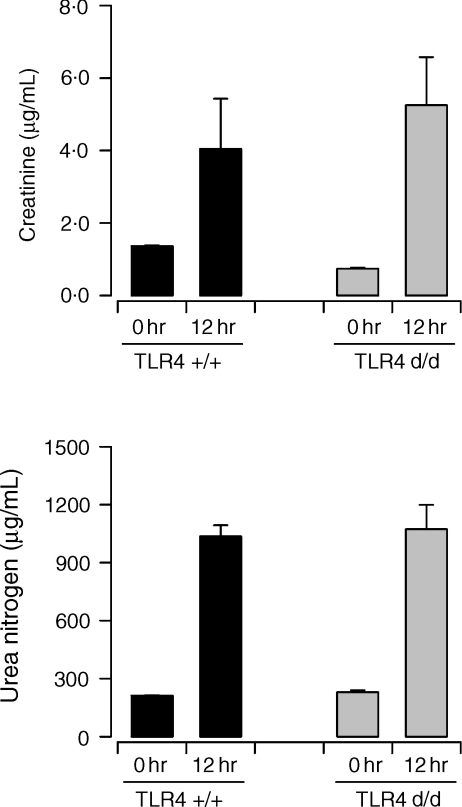
To investigate the potential role of TLR4 in polymicrobial septic peritonitis in more detail, the development of kidney failure was compared in wildtype and TLR4-defective mice. Previously, we have demonstrated that acute kidney injury develops in the CASP model of polymicrobial sepsis.14 The results in Fig. 3 clearly indicate that the absence of a functional TLR4 protein in C3H/HeJ mice did not alter the induction of acute kidney failure as measured by the elevation of serum creatinine and urea nitrogen. These results are consistent with our previous findings showing that survival of mice from septic peritonitis is not influenced by TLR4·15
Figure 3.
Development of acute kidney injury during experimental septic peritonitis is not affected by TLR4-deficiency. Serum samples were obtained before (0 hr) and 12 hr after CASP surgery of wildtype C3H/HeN (TLR4+/+) and TLR4-deficient C3H/HeJ mice (TLR4d/d). Statistical analysis indicated that serum levels of creatinine and urea nitrogen were not significantly different between the two experimental groups (both at 0 hr and 12 hr). The differences between the 0 hr and 12 hr time points were statistically significant in all cases (P < 0·05).
Discussion
Genetically determined differences in the immune reactivity against microbial pathogens and their products may influence the development and outcome of human sepsis. TLRs have been recognized as crucial pattern recognition elements that activate the innate immune response upon encounter of microbes. In humans, the TLR4 mutation D299G was found to attenuate responsiveness to inhaled LPS in heterozygous patients and to abrogate LPS-stimulated inflammatory cytokine production by heterozygote airway epithelial cells and alveolar macrophages.5 Thus, the presence of the TLR4(D299G) mutation on one allele is sufficient to suppress LPS responsiveness. Analysing the frequency of the TLR4(D299G) loss-of-function mutation in patients may therefore help to elucidate a potential contribution of TLR4 to the pathogenesis of human sepsis occurring after major visceral surgery. Our results clearly demonstrate that the presence of the TLR4(D299G) mutation was neither linked to the development nor to the outcome of postoperative sepsis, which was caused in most cases by polymicrobial infection. In addition, serum cytokine levels at sepsis onset did not significantly correlate with the TLR4 genotype of patients.
The low frequency of the TLR4(D299G) mutation in humans represents an inherent problem for elucidating the potential role of TLR4 in infectious diseases by genotyping. Although the patient numbers studied in the present report are comparable to those reported in previous publications5,11,16,17 we nevertheless intended to examine the impact of TLR4 on the immune response to polymicrobial sepsis by an independent approach. Using the murine CASP model we demonstrate that both the systemic cytokine response and the development of acute kidney injury during septic peritonitis were not altered by genetic TLR4-deficiency. Furthermore, we previously found survival of mice in this model to be TLR4-independent.15 Considered together, our studies on human and experimental sepsis therefore suggest that the functional activity of TLR4 may be dispensable for the immune response to polymicrobial sepsis.
Recent reports have indicated that mutations in TLR4 may be associated with a higher incidence of gram-negative infections in intensive care unit patients exhibiting systemic inflammatory response syndrome or septic shock.11,16 In contrast, Read and coworkers have shown that the TLR4(D299G) mutation did not influence susceptibility to or severity of meningococcal disease.17 A possible explanation for these different findings may be provided by the type of microbial pathogens involved suggesting that the functional contribution of TLR4 differs between various types of infection. Thus, it is conceivable that polymicrobial sepsis may expose innate immune cells to numerous different microbial components that activate multiple TLRs thereby rendering individual TLRs dispensable for immune activation. Our observation that about 84% of sepsis cases in the study population were a result of polymicrobial infection is consistent with the possible activation of numerous TLRs in these patients. In accordance with this notion, we have recently shown that in a mouse model of septic peritonitis, even the combined deficiency of both TLR2 and TLR4 did not affect survival.15 Although activation of multiple TLRs may be particularly relevant for polymicrobial infections, this process may also occur during monomicrobial sepsis. For example, microbial components such as LPS, flagellin, and unmethylated CpG-containing DNA may activate TLR4, TLR5, and TLR9 during Gram-negative infection.1,3
Massive activation of mononuclear phagocytes by bacterial components such as endotoxin and subsequent release of large amounts of proinflammatory cytokines are thought to play a crucial role for the induction of septic shock. It has also been suggested that TNF may represent an important mediator of the pathogenic hyperinflammatory response in sepsis.18 Consistent with a role of Toll-like receptors in this process, mice deficient for TLR4 or MyD88 were shown to be resistant against endotoxin-induced septic shock.12,19 Thus, Toll-like receptor signalling may not only contribute to the activation of efficient immune responses against certain pathogens, but, when activated during sepsis may also contribute to hyperinflammation and shock. It is therefore conceivable that loss-of-function mutations of TLRs may not correlate with the clinical course and/or outcome of sepsis, because they may affect both beneficial and detrimental activities.
In summary, our results indicate that the signalling capacity of TLR4 as influenced by loss-of-function mutations does not affect the immune response to human and experimental sepsis caused by polymicrobial infection. Functional defects of TLR4 may therefore be compensated by other members of the TLR family or other classes of innate immune receptors.
Acknowledgments
We thank Felicitas Altmayr, Gabriela Jusek, and Simone Kaiser-Moore for excellent technical assistance and Monika Ries for expert documentation of patient data. This work was supported by the Deutsche Forschungsgemeinschaft through SFB 576 (project A7) and by the Kommission für Klinische Forschung, Klinikum rechts der Isar.
References
- 1.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–80. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 2.Janeway CA, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 3.Beutler B, Poltorak A. Sepsis and evolution of the innate immune response. Crit Care Med. 2001;29:S2–S7. doi: 10.1097/00003246-200107001-00002. [DOI] [PubMed] [Google Scholar]
- 4.Smirnova I, Poltorak A, Chan EKL, McBride C, Beutler B. Phylogenetic variation and polymorphism at the Toll-like receptor 4 locus (TLR4) Genome Biol. 2000;1:research002.1–002.10. doi: 10.1186/gb-2000-1-1-research002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arbour NC, Lorenz E, Schutte BC, et al. TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat Genet. 2000;25:187–91. doi: 10.1038/76048. [DOI] [PubMed] [Google Scholar]
- 6.Lorenz E, Mira J-P, Cornish KL, Arbour NC, Schwartz DA. A novel polymorphism in the Toll-like receptor 2 gene and its potential association with staphylococcal infection. Infect Immun. 2000;68:6398–401. doi: 10.1128/iai.68.11.6398-6401.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992;101:1644–55. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 8.Zantl N, Uebe A, Neumann B, et al. Essential role of γ-interferon in survival of colon ascendens stent peritonitis, a novel murine model of abdominal sepsis. Infect Immun. 1998;66:2300–9. doi: 10.1128/iai.66.5.2300-2309.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bone RC, Grodzin CJ, Balk RA. Sepsis: a new hypothesis for pathogenesis of the disease process. Chest. 1997;112:235–43. doi: 10.1378/chest.112.1.235. [DOI] [PubMed] [Google Scholar]
- 10.Dinarello CA. Cytokines as mediators in the pathogenesis of septic shock. Curr Top Microbiol Immunol. 1996;216:133–65. doi: 10.1007/978-3-642-80186-0_7. [DOI] [PubMed] [Google Scholar]
- 11.Lorenz E, Mira JP, Frees KL, Schwartz DA. Relevance of mutations in the TLR4 receptor in patients with gram-negative septic shock. Arch Intern Med. 2002;162:1028–32. doi: 10.1001/archinte.162.9.1028. [DOI] [PubMed] [Google Scholar]
- 12.Hoshino K, Takeuchi O, Kawai T, et al. Toll-like receptor 4 (TLR4) -deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the lps gene product. J Immunol. 1999;162:3749–52. [PubMed] [Google Scholar]
- 13.Takeuchi O, Hoshino K, Kawai T, et al. Differential roles of TLR2 and TLR4 in recognition of Gram-negative and Gram-positive bacterial cell wall components. Immunity. 1999;11:443–51. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 14.Maier S, Emmanuilidis K, Entleutner M, Zantl N, Werner M, Pfeffer K, Heidecke CD. Massive chemokine transcription in acute renal failure due to polymicrobial sepsis. Shock. 2001;14:187–92. doi: 10.1097/00024382-200014020-00019. [DOI] [PubMed] [Google Scholar]
- 15.Weighardt H, Kaiser-Moore S, Vabulas RM, Kirschning C, Wagner H, Holzmann B. Cutting edge: Myeloid differentiation factor 88 deficiency improves resistance against sepsis caused by polymicrobial infection. J Immunol. 2002;169:2823–7. doi: 10.4049/jimmunol.169.6.2823. [DOI] [PubMed] [Google Scholar]
- 16.Agnese DM, Calvano JE, Hahm SJ, Coyle SM, Calvano SE, Lowry SF. Human Toll-like receptor 4 mutations but not CD14 polymorphisms are associated with an increased risk of gram-negative infections. J Infect Dis. 2002;186:1522–5. doi: 10.1086/344893. [DOI] [PubMed] [Google Scholar]
- 17.Read RC, Pullin J, Gregory S, et al. A functional polymorphism of Toll-like receptor 4 is not associated with likelihood or severity of meningococcal disease. J Infect Dis. 2002;184:640–2. doi: 10.1086/322798. [DOI] [PubMed] [Google Scholar]
- 18.Reinhart K, Karzai W. Anti-tumor necrosis factor therapy in sepsis: update on clinical trials and lessons learned. Crit Care Med. 2001;29:121–5. doi: 10.1097/00003246-200107001-00037. [DOI] [PubMed] [Google Scholar]
- 19.Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11:115–22. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]