Introduction
One of the crucial unanswered questions in the field of T-cell regulation is the origin of CD4+ CD25+ regulatory T cells. Does the thymus produce regulatory T cells as a functionally mature separate lineage, or can some T cells acquire regulatory function in the periphery? While mice live for 2·5 years, humans can live for eight decades and beyond. The constraints on the persistence of human CD4+ CD25+ regulatory T cells will therefore be more extreme as a result of thymic involution early in the human lifespan. A central question is whether the full complement of CD4+ CD25+ regulatory T cells are generated early in life and if so, how do they manage to persist? A consistent observation in both mice and humans is that CD4+ CD25+ T cells in adults have the phenotypic characteristics of a highly differentiated T-cell population. This review seeks to reconcile the observations that CD4+ CD25+ T cells are anergic but highly differentiated. We suggest that it is possible that a subpopulation of CD4+ CD25+ regulatory T cells may arise in the periphery as a consequence of anergy induction in antigen-specific CD4+ T cells that are approaching end-stage differentiation.
CD4+ CD25+ regulatory T cells have a highly differentiated phenotype
Upon repeated stimulation by antigen over time, T cells proliferate and acquire effector functions. There is also a change in expression of surface receptors after stimulation and dynamic alterations of cytokine, homing, adhesion and signalling receptors occur.1–3 Naïve, recently primed and highly differentiated T cells have been distinguished from each other on the differential expression of these molecules.4 The expression of one molecule in particular has been used to discriminate between cells at early and late stages of differentiation. While naïve or recently activated T cells express high levels of CD45RB, highly differentiated CD4+ T cells express low levels of this molecule.5 A consistent observation that has been made by many groups is that CD4+ CD25+ regulatory T cells have a CD45RBlow phenotype in mice and a CD45RO+ CD45RBlow phenotype in humans, indicating that they have experienced many rounds of cell division.6–11
Functional evidence for the advanced differentiation state of CD4+ CD25+ T cells
The expansion of T cells through re-activation cannot occur indefinitely through life and continuous re-activation induces a state of growth arrest known as replicative senescence after a finite number of cell divisions.12–14 This is considered to be a strategy for preventing potentially malignant expansions of cells.12 The reason why replicative senescence occurs is that telomeres, repeating hexameric nucleotide units at the ends of chromosomes, shorten by around 50 base pairs with each cell division.15–17 Telomeres protect against end-to-end fusion of chromosomes and promote their stability.18,19 Although the enzyme telomerase can compensate initially for telomere loss, this enzyme is not induced upon repeated activation of T cells.20–22 Repeated T-cell stimulation therefore leads to a critical point where telomeres are sufficiently reduced and growth arrest as a result of chromosomal aberrations occurs. The key point here is that highly differentiated T cells will have shorter telomeres than those that are naïve or have not experienced extensive cell division.20,23
Human CD4+ CD25+ T cells have short telomeres, which supports the possibility that they are highly differentiated T cells.24 An intriguing possibility is that CD4+ T cells do not differentiate into an end-stage effete population, rather they may give rise to regulatory cells that are very antigen-experienced. This raises the important possibility that old T cells may be useful after all and offers a plausible mechanism by which high concentrations of antigen may actually direct the development of specific regulatory cells. This is a reworking of the venerated concept of high-zone tolerance, but with a new twist.
Human CD4+ CD25+ T cells are susceptible to apoptosis
The anti-apoptotic molecule Bcl-2 plays a central role in the decision of expanded populations of T cells to live or die.3 This molecule is involved in the mitochondrial pathway leading to death and it is down-regulated after T-cell expansion. Highly differentiated T cells are therefore extremely susceptible to apoptosis.5,25,26 However, down-regulation of Bcl-2 is not an irreversible event and certain groups of cytokines can prevent T-cell apoptosis and do so by re-inducing Bcl-2 expression.26,27 A second group of cell surface molecules that are related to the tumour necrosis factor receptor can also trigger T-cell apoptosis if ligated at the time of T-cell activation in the absence of co-stimulation. This phenomenon is known as activation-induced cell death.28 The expression of one of these molecules, CD95 increases as a T cell progressively differentiates.5 Induction of T-cell apoptosis by CD95 ligation can also be prevented by certain cytokines, e.g. interferon-α/β (IFN-α/β)29–31 Highly differentiated T cells, that express low levels of CD45RB also have decreased levels of Bcl-2 but increased levels of CD95.5 However, this does not indicate their imminent death rather that these cells are more dependent on exogenous factors to maintain their survival.26
In humans, freshly isolated CD4+ CD25+ T cells are susceptible to cytokine starvation-induced apoptosis and this is related to low expression of Bcl-2.10 The apoptosis of these cells can be prevented by the addition of interleukin-2 receptor (IL-2R) γ-chain signalling cytokines, such as IL-2 and IL-15, and also IFN-α/β.10 An important observation was that the regulatory activity of these cells was maintained when these cytokines were added.8,10 CD4+ CD25+ T cells express elevated levels of CD95 but are not particularly sensitive to activation-induced cell death.10 These observations suggest that CD4+ CD25+ T cells may depend on the continual presence of exogenous factors to prevent their apoptosis.
Evidence for thymic generation of CD4+ CD25+ T cells
Many studies have shown that CD4+ CD25+ T cells can develop in the thymus. As these have been extensively reviewed elsewhere32–34 we will briefly mention some of the evidence supporting this concept.
CD4+ CD25+ represent 5–10% of CD4+ CD8− thymocytes, in humans, mice and rats.6,35–37 A number of early studies showed that mature CD4+ CD8− thymocytes have the capacity to prevent autoimmunity.38–40 Adoptive transfer experiments showed that transfer of single-positive CD4 thymocytes depleted of CD4+ CD25+ cells produced similar autoimmune diseases in syngeneic T-cell-deficient mice, as do peripheral CD4 T cells. These autoimmune diseases can be prevented by the adoptive transfer of CD25+ thymocytes.6,36,41
Mice thymectomized 3 days after birth develop spontaneous autoimmunity (such as gastritis) which correlates with reduced numbers of immunoregulatory CD25+ T cells present in the peripheral lymphoid pool. Performing the thymectomy earlier (day 0) decreased the likelihood of autoimmunity, probably because of a decreased number of self-reactive T cells. On the other hand, delaying thymectomy until day 7 was also less efficient in inducing autoimmunity, presumably because a sufficient number of regulatory T cells have already emerged into the periphery.42
A number of experiments exclude the possibility that regulatory T cells from the periphery migrate to or recirculate through the thymus, and confirm the thymus as the source of regulatory T cells. CD25+ CD4+ CD8− thymocytes can develop in the fetal thymic organ culture in vitro and emerge in vivo following direct injection of CD4-CD8-thymic precursors into the thymus.6 Furthermore, intrathymic fluorochrome labelling demonstrates that CD4 + CD25 + regulatory T cells leave the thymus to populate the periphery.37
Is thymic production the only way to generate CD4+ CD25+ T regulatory cells?
The strong evidence from animal models showing that CD4+ CD25+ T cells can develop in the thymus does not rule out alternative mechanisms for the generation of regulatory T cells. Mice have a much shorter lifespan than humans and a central question is whether the thymus can replenish the peripheral pool of CD4+ CD25+ T regulatory cells throughout the human lifespan. There are a number of caveats that restrict the possibility of thymic generation as the sole source of CD4+ CD25+ T cells in humans.
As a result of thymic involution the number of CD4+ CD25+ Tregs should decrease over time and yet evidence suggests that this is not the case: therefore thymically derived Tregs must be efficiently maintained in the periphery.
Tregs are anergic and prone to apoptosis and it is not clear how they can be efficiently maintained despite their poor proliferative capacity.
How would regulatory T cells to antigens that are encountered in the periphery develop after thymic involution occurs?
Evidence for the peripheral generation of CD4+ CD25+ T cells in vivo
All the studies that support the peripheral generation of CD4+ CD25+ T cells have been performed in animals. The most convincing data suggesting the peripheral generation of CD4+ CD25+ T cells in vivo comes from the work of Thorstenson and Khoruts43 who adoptively transferred CD4 cells, from OVA-specific DO11.10 T-cell receptor (TCR) transgenic mice on a RAG−/− background, into recipient BALB/c mice. Following low dose tolerance protocols in vivo, intravenous or oral administration of antigen led to the generation of TCR-transgenic CD4+ CD25+ T cells. Since RAG-2-deficient mice do not contain CD4+ CD25+ T cells, this population had to be generated from the CD4+ CD25− cells. These regulatory T cells therefore arise from naïve CD4+ precursors but share many characteristics with naturally occurring CD4+ CD25+ T cells and are able to suppress the responsiveness of naïve T cells in an antigen-specific manner. Zhang and colleagues44 reached the same conclusion. They observed an increase in the number of clonotype-specific CD4+ CD25+ T cells following oral administration of OVA to OVA-TCR transgenic mice. Regulatory T cells expanded in this way are activated and more potent than Tregs present in controls.
As discussed earlier in a classic study Asano and colleagues42 observed that neonatally thymectomized mice lack CD4+ CD25+ T cells. However, although absent at first, CD4+ CD25+ T cells in these mice slowly reappear and reach normal numbers by 3 months. These newly emerging regulatory T cells must arise from the CD4+ CD25− population.
Additional evidence for peripheral induction of Tregs comes from studies with male mice that have been orchiectomized at birth and thymectomized as adults. De novo development of a mature prostate in these mice can be induced by treatment with dihydrotestosterone. Interestingly this newly developed organ does not become the subject of autoimmune attack. In fact, these mice concomitantly develop regulatory T cells that have the capacity to prevent autoimmune prostatitis in the d3Tx models.45 In addition, various tolerance-inducing protocols using monoclonal antibodies against CD4, CD8, or co-stimulatory molecules can also induce regulatory T cells in vivo.46–48
Evidence for peripheral generation of CD4+ CD25+ Regulatory t cells from experiments in vitro
The ability to generate CD4+ CD25+ regulatory T cells from CD4+ CD25− T cells in vitro would lend credence to the fact that they may not be a unique lineage that is derived from the thymus. The repeated stimulation of naïve cord blood cells with immature DC leads to the presence of a population that is suppressive, hyporesponsive and has a similar mechanism of suppression as CD4+ CD25+ cells (contact-dependent, antigen non-specific, antigen-presenting cell-independent, partially overcome by IL-2).49 In addition, treatment with immunosuppressive drugs such as vitamin D3 in combination with mycophelate mofetil leads to the development of CD4+ CD25+ T cells in vivo.50 On the other hand treatment with vitamin D3 and dexamethasone leads to the generation of IL-10-secreting regulatory T cells,50,51 The relationship between these and CD4+ CD25+ T cells is not clear. This brings up a very important point that not all cells that exhibit regulatory function are CD4+ CD25+.32
In humans, it has been shown that clones of antigen-specific CD4+ T cells can be anergised by TCR stimulation without co-stimulation or by peptide presented by T cells to each other (T : T presentation).52–54 This T : T presentation of specific peptide occurs because activated human T cells express major histocompatibility complex (MHC) class II.55,56 Rat T cells also express MHC class II and anergy can be induced in T-cell clones from these animals by T : T presentation.57,58 A fundamentally important observation is that once anergy is induced in human or rat T cells, they acquire the ability to suppress the proliferation of non-anergised cells.24,52,57,59 Furthermore, the phenotype of anergised antigen-specific T cells is virtually identical to that of naturally occurring CD4+ CD25+ regulatory T cells (Table 1). The main point here is that CD4+ CD25+ T cells that are both anergic and suppressive can be generated from responsive, highly differentiated antigen-specific CD4+ CD25− T cells. This indirectly supports the possibility that these cells may be generated in the periphery. It is of note that mouse T cells do not express MHC class II after activation and that peripheral generation of regulatory T cells by this route may not play an important role in this species.
Table 1.
Similarities between T-cell clones and CD4+ CD25+ T cells
| CD4+ CD25+ T cells | CD4+ clones |
|---|---|
| Low CD45RB24 | Low CD45RB5,24 |
| Short telomeres24 | Short telomeres24 |
| Anergic24 | Easily anergised52,59 |
| Apoptosis prone10 | Apoptosis prone5 |
| High CTLA-410 | High CTLA-4* |
| CD25+ | CD25+ |
G. Lombardi, personal communication.
Where do highly differentiated human CD4+ CD25+ T cells come from?
The TCR repertoire of CD4+ CD25+ regulatory T cells in adult humans is as diverse as that of CD4+ CD25− T cells, indicating that they can recognize and suppress many different antigens.24 This, together with their highly differentiated phenotype that is virtually identical to that of memory T cells (CD45RO+, CD45RBlow, short telomeres) suggests that they may be derived from a population that has encountered antigen extensively, not a unique lineage from the thymus. However, CD4+ CD25+ T cells that are anergic and suppressive are also found in human neonates in cord blood but these cells do not have a highly differentiated phenotype.60,61 It is currently not clear if the CD4+ CD25+ cells that are found in adulthood are derived from the neonatal population. If this were the case, these regulatory cells would have to be stable over time and have the capacity to proliferate extensively, to acquire the characteristically short telomeres of this population. Two observations argue against this. First, these cells are susceptible to apoptosis induced by cytokine starvation. Even if their death is prevented by cytokines in vivo, they will be at a continuous disadvantage because of competition with activated responsive T cells that utilize and sequester the same cytokines for their own survival after immune responses.62 Second, CD4+ CD25+ T cells are anergic and even if cytokines like IL-2 can temporarily break anergy, their relatively poor ability to proliferate will impede their persistence through competition with activated and responsive T cells for the same mediators.
Further work is essential to determine the relationship between neonatal and adult CD4+ CD25+ regulatory T cells. The relative expression of unique markers for regulatory T cells, such as FoxP3, may provide important clues to similarities or differences between them.63,64 In view of the multitude of many new types of regulatory T cell that continue to be described,65,66 the possibility that CD4+ CD25+ regulatory T cells in neonates and adults are generated by different mechanisms should not be ignored.
The relationship between anergy, apoptosis and suppression in CD4+ CD25+ regulatory T cells
The induction of anergy by various means in responsive antigen-specific T-cell clones in vitro is associated with extensive activation-induced apoptosis in a large proportion of these cells.59,67 The cells that survive the apoptotic onslaught are anergic to a second round of stimulation with peptide presented by professional antigen-presenting cells.68 The dose of antigen is crucial in the induction of either anergy, suppression, or death of the T cells.69 However, anergy and apoptosis can be dissociated because the addition of anti-CD95 antibody67 and IFN-α/β prevents death but leads to the presence of increased numbers of anergic cells in culture.59 This rescue by IFN-α/β is the only natural means by which CD95-mediated death can be prevented and has been suggested as a potential mechanism that promotes the persistence of anergic and suppressive T cells in vivo.59 Mechanisms that prevent apoptosis may therefore be important both during the induction of anergy in potential T regulatory cells and also for the maintenance of these cells in the periphery.59 It is of note that T cells that have been rendered anergic in vitro can act as suppressor cells both in vitro and in vivo.70 The speculation about differences in how neonatal and adult CD4+ CD25+ T cells arise could be rephrased to how anergy may be induced in immature cells in the thymus and highly differentiated T cells in the periphery. The new focus of the question would then be what prevents deletion of the cells during anergy induction and whether the surviving cells are suppressive.
A model for the peripheral generation of adult CD4+ CD25+ regulatory T cells in humans
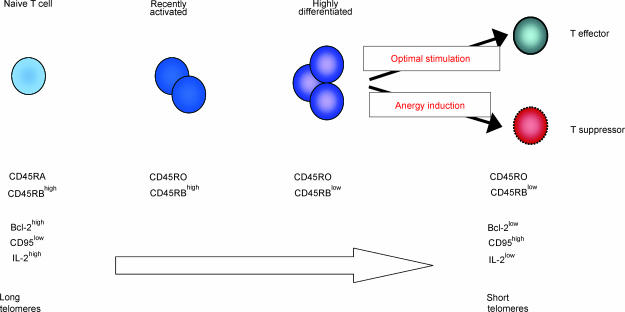
Based on the observations that are highlighted above, the following model for the peripheral generation of CD4+ CD25+ regulatory T cells in adult humans can be proposed (Fig. 1). After antigenic encounter, naïve T cells replicate to a highly differentiated population with the acquisition of the memory phenotype of such cells. Once these cells reach this highly differentiated state, they synthesize insufficient levels of IL-2 for autocrine proliferation and are easily anergised by high doses of antigen that is presented by non-professional antigen-presenting cells.71,72 The deletion of these cells can be prevented by the type-Ir IFNs that enable the survival of an anergic and suppressive CD4+ CD25+ T-cell population.59 This hypothetical mechanism would promote the generation of an anergic/regulatory CD4+ T-cell population that is specific for antigens that are present continuously at high levels in vivo. This would include self- and dietary antigens, and also frequently encountered environmental antigens. This model is supported by a number of observations. These include the fact that memory CD4+ T cells are more susceptible to anergy induction than their naïve counterparts.71,72 Also, highly differentiated antigen-specific CD4+ T cells that are CD45RBlow and have short telomeres can be rendered responsive or anergic/suppressive depending on how the antigen is presented.24,59 In addition, if anergy is induced in these cells, they acquire the phenotype of regulatory CD4+ T cells (CTLA4+, CD25+ G. Lombardi personal communication) and their functional characteristics, including loss of anergy in the presence of IL-2 but reversion to an anergic/suppressive state upon IL-2 removal.52,57,69,73 This model does not preclude the coexistence of other populations of thymically or cytokine-dependent populations of Tregs.
Figure 1.
Model for the generation of CD4+ CD25+ regulatory T cells in the periphery. Naïve CD4+ T cells become primed upon encounter with antigen on antigen-presenting cells, lose CD45RA and acquire CD45RO expression. These cells remain CD45RBhigh during this process and retain the capacity to secrete large amounts of IL-2. They still express Bcl-2 and have long telomeres. Repeated stimulation of the primed cells by frequently encountered antigen leads to generation of highly differentiated CD4+ CD45RO+ CD45RBlow memory/effector T cells. These highly differentiated T cells have short telomeres and are prone to apoptosis, as they express low levels of Bcl-2. They also have a profoundly diminished ability to secrete IL-2, which makes them susceptible to anergy induction. These cells can either remain as effector T cells, or they can become anergised and acquire suppressive capacity.
Conclusion
The generation of apoptosis-prone regulatory cells makes sense for a number of reasons. First, this limits the capacity of an extensively stimulated population for survival and may protect against the development of malignancy. Second, only cells that have experienced extensive encounters with antigen can be induced to become regulatory. Furthermore, generation of regulatory CD4+ T cells by this route of anergy induction has considerable implications for the maintenance of tolerance to self-antigens. These antigens may be presented by many cell types that do not have specialized antigen-presenting cell function and would thus promote the development of anergy and suppressive activity in self-reactive populations. The available data fit this model. We await further evidence to determine if this may be a way to generate CD4+ CD25+ regulatory T cells in humans in vivo.
Acknowledgments
M.V.S. is supported by the BBSRC ERA initiative.
References
- 1.Dutton RW, Bradley LM, Swain SL. T cell memory. Annu Rev Immunol. 1998;16:201–23. doi: 10.1146/annurev.immunol.16.1.201. [DOI] [PubMed] [Google Scholar]
- 2.Sallusto F, Mackay CR, Lanzavecchia A. The role of chemokine receptors in primary, effector and memory immune responses. Annu Rev Immunol. 2000;593:620–45. doi: 10.1146/annurev.immunol.18.1.593. [DOI] [PubMed] [Google Scholar]
- 3.Akbar AN, Salmon M, Savill J, Janossy G. A possible role for bcl-2 in regulating T cell memory-a ‘balancing act’ between cell death and survival. Immunol Today. 1993;14:526–31. doi: 10.1016/0167-5699(93)90181-J. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed R, Gray D. Immunological memory and protective immunity. Understanding their relation. Science. 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 5.Salmon M, Pilling D, Borthwick NJ, Viner N, Janossy G, Bacon PA, Akbar AN. The progressive differentiation of primed T cells is associated with an increasing susceptibility to apoptosis. Eur J Immunol. 1994;24:892–9. doi: 10.1002/eji.1830240417. [DOI] [PubMed] [Google Scholar]
- 6.Itoh M, Takahashi T, Sakaguchi N, Kuniyasu Y, Shimizu J, Otsuka F, Sakaguchi S. Thymus and autoimmunity: production of CD25+ CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J Immunol. 1999;162:5317–26. [PubMed] [Google Scholar]
- 7.Jonuleit H, Schmitt E, Stassen M, Tuettenberg A, Knop J, Enk AH. Identification and functional characterization of human CD4+ CD25+ T cells with regulatory properties isolated from peripheral blood. J Exp Med. 2001;193:1285–94. doi: 10.1084/jem.193.11.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levings MK, Sangregorio R, Roncarolo MG. Human CD25+ CD4+ T regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function. J Exp Med. 2001;193:1295–302. doi: 10.1084/jem.193.11.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25+ CD4+ regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taams L, Smith J, Rustin MH, Salmon M, Poulter LW, Akbar AN. Human anergic/suppressive CD4+ CD25+ T cells: a highly differentiated and apoptosis-prone population. Eur J Immunol. 2001;31:1122–31. doi: 10.1002/1521-4141(200104)31:4<1122::aid-immu1122>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 11.Dieckmann D, Plottner H, Berchtold S, Berger T, Schuler G. Ex vivo isolation and characterization of CD4+ CD25+ T cells with regulatory properties from human blood. J Exp Med. 2001;193:1303–10. doi: 10.1084/jem.193.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Effros RB, Pawelec G. Replicative senescence of T cells: does the Hayflick Limit lead to immune exhaustion? Immunol Today. 1997;18:450–4. doi: 10.1016/s0167-5699(97)01079-7. [DOI] [PubMed] [Google Scholar]
- 13.Pan C, Xue BH, Ellis TM, Peace DJ, Diaz MO. Changes in telomerase activity and telomere length during human T lymphocyte senescence. Exp Cell Res. 1997;231:346–53. doi: 10.1006/excr.1997.3475. [DOI] [PubMed] [Google Scholar]
- 14.Allsopp RC, Chang E, Kashefi-Aazam M, Rogaev EI, Piatyszek MA, Shay J, Harley CB. Telomere shortening is associated with cell division in vitro and in vivo. Exp Cell Res. 1995;220:194–200. doi: 10.1006/excr.1995.1306. [DOI] [PubMed] [Google Scholar]
- 15.Rufer N, Brummendorf TH, Kolvraa S, Bischoff C, Christensen K, Wadsworth L, Schulzer M, Lansdorp PM. Telomere fluorescence measurements in granulocytes and T lymphocyte subsets point to a high turnover of hematopoietic stem cells and memory T cells in early childhood. J Exp Med. 1999;190:157–67. doi: 10.1084/jem.190.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaziri H, Dragowska W, Allsopp RC, Thomas TE, Harley CB, Lansdorp PM. Evidence for a mitotic clock in human hematopoietic stem cells: loss of telomeric DNA with age. Proc Natl Acad Sci USA. 1994;91:9857–60. doi: 10.1073/pnas.91.21.9857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bodnar AG, Kim NW, Effros RB, Chiu CP. Mechanism of telomerase induction during T cell activation. Exp Cell Res. 1996;228:58–64. doi: 10.1006/excr.1996.0299. [DOI] [PubMed] [Google Scholar]
- 18.Levy MZ, Allsopp RC, Futcher AB, Greider CW, Harley CB. Telomere end-replication problem and cell aging. J Mol Biol. 1992;225:951–60. doi: 10.1016/0022-2836(92)90096-3. [DOI] [PubMed] [Google Scholar]
- 19.Rufer N, Dragowska W, Thornbury G, Roosnek E, Lansdorp PM. Telomere length dynamics in human lymphocyte subpopulations measured by flow cytometry. Nat Biotechnol. 1998;16:743–7. doi: 10.1038/nbt0898-743. [DOI] [PubMed] [Google Scholar]
- 20.Weng NP, Levine BL, June CH, Hodes RJ. Human naive and memory T lymphocytes differ in telomeric length and replicative potential. Proc Natl Acad Sci USA. 1995;92:11091–4. doi: 10.1073/pnas.92.24.11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weng NP, Hodes RJ. The role of telomerase expression and telomere length maintenance in human and mouse. J Clin Immunol. 2000;20:257–67. doi: 10.1023/a:1017223602293. [DOI] [PubMed] [Google Scholar]
- 22.Weng NP, Hathcock KS, Hodes RJ. Regulation of telomere length and telomerase in T and B cells: a mechanism for maintaining replicative potential. Immunity. 1998;9:151–7. doi: 10.1016/s1074-7613(00)80597-x. [DOI] [PubMed] [Google Scholar]
- 23.Weng NP, Palmer LD, Levine BL, Lane HC, June CH, Hodes RJ. Tales of tails: regulation of telomere length and telomerase activity during lymphocyte development, differentiation, activation, and aging. Immunol Rev. 1997;160:43–54. doi: 10.1111/j.1600-065x.1997.tb01026.x. [DOI] [PubMed] [Google Scholar]
- 24.Taams LS, Vukmanovic-Stejic M, Smith J, et al. Antigen-specific T cell suppression by human CD4+CD25+ regulatory T cells. Eur J Immunol. 2002;32:1621–30. doi: 10.1002/1521-4141(200206)32:6<1621::AID-IMMU1621>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 25.Akbar AN, Borthwick N, Salmon M, et al. The significance of low bcl-2 expression by CD45RO T cells in normal individuals and patients with acute viral infections. The role of apoptosis in T cell memory. J Exp Med. 1993;178:427–38. doi: 10.1084/jem.178.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akbar AN, Borthwick NJ, Wickremasinghe RG, et al. Interleukin-2 receptor common γ-chain signalling cytokines regulate activated T cell apoptosis in response to growth factor withdrawal: selective induction of anti-apoptotic (bcl-2, bcl-xL), but not pro-apoptotic (bax, bcl-xs) gene expression. Eur J Immunol. 1996;26:294–9. doi: 10.1002/eji.1830260204. [DOI] [PubMed] [Google Scholar]
- 27.Vella AT, Dow S, Potter TA, Kappler J, Marrack P. Cytokine-induced survival of activated T cells in vitro and in vivo. Proc Natl Acad Sci USA. 1998;95:3810–15. doi: 10.1073/pnas.95.7.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lenardo M, Chan KM, Hornung F, McFarland H, Siegel R, Wang J, Zheng L. Mature T lymphocyte apoptosis – immune regulation in a dynamic and unpredictable antigenic environment. Annu Rev Immunol. 1999;17:221–53. doi: 10.1146/annurev.immunol.17.1.221. [DOI] [PubMed] [Google Scholar]
- 29.Pilling D, Akbar AN, Girdlestone J, et al. Interferon β mediates stromal cell rescue of T cells from apoptosis. Eur J Immunol. 1999;29:1041–50. doi: 10.1002/(SICI)1521-4141(199903)29:03<1041::AID-IMMU1041>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 30.Marrack P, Kappler J, Mitchell T. Type I interferons keep activated T cells alive. J Expl Med. 1999;189:521–9. doi: 10.1084/jem.189.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scheel-Toellner D, Pilling D, Akbar AN, Hardie D, Lombardi G, Salmon M, Lord JM. Inhibition of T cell apoptosis by IFN-beta rapidly reverses nuclear translocation of protein kinase C-delta. Eur J Immunol. 1999;29:2603–12. doi: 10.1002/(SICI)1521-4141(199908)29:08<2603::AID-IMMU2603>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 32.Maloy KJ, Powrie F. Regulatory T cells in the control of immune pathology. Nat Immunol. 2001;2:816–22. doi: 10.1038/ni0901-816. [DOI] [PubMed] [Google Scholar]
- 33.Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101:455–8. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 34.Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 35.Stephens LA, Mottet C, Mason D, Powrie F. Human CD4+ CD25+ thymocytes and peripheral T cells have immune suppressive activity in vitro. Eur J Immunol. 2001;31:1247–54. doi: 10.1002/1521-4141(200104)31:4<1247::aid-immu1247>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 36.Stephens LA, Mason D. CD25 is a marker for CD4+ thymocytes that prevent autoimmune diabetes in rats, but peripheral T cells with this function are found in both CD25+ and CD25− subpopulations. J Immunol. 2000;165:3105–10. doi: 10.4049/jimmunol.165.6.3105. [DOI] [PubMed] [Google Scholar]
- 37.Papiernik M, de Moraes ML, Pontoux C, Vasseur F, Penit C. Regulatory CD4 T cells. expression of IL-2R alpha chain, resistance to clonal deletion and IL-2 dependency. Int Immunol. 1998;10:371–8. doi: 10.1093/intimm/10.4.371. [DOI] [PubMed] [Google Scholar]
- 38.Saoudi A, Seddon B, Fowell D, Mason D. The thymus contains a high frequency of cells that prevent autoimmune diabetes on transfer into prediabetic recipients. J Exp Med. 1996;184:2393–8. doi: 10.1084/jem.184.6.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakaguchi S, Takahashi T, Nishizuka Y. Study on cellular events in postthymectomy autoimmune oophoritis in mice. I. Requirement of Lyt-1 effector cells for oocytes damage after adoptive transfer. J Exp Med. 1982;156:1565–76. doi: 10.1084/jem.156.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sakaguchi S, Takahashi T, Nishizuka Y. Study on cellular events in postthymectomy autoimmune oophoritis in mice. I. Requirement of Lyt-1 effector cells for oocytes damage after adoptive transfer. J Exp Med. 1982;156:1577–86. doi: 10.1084/jem.156.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh B, Read S, Asseman C, Malmstrom V, et al. Control of intestinal inflammation by regulatory T cells. Immunol Rev. 2001;182:190–200. doi: 10.1034/j.1600-065x.2001.1820115.x. [DOI] [PubMed] [Google Scholar]
- 42.Asano M, Toda M, Sakaguchi N, Sakaguchi S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J Exp Med. 1996;184:387–96. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thorstenson KM, Khoruts A. Generation of anergic and potentially immunoregulatory CD25+ CD4 T cells in vivo after induction of peripheral tolerance with intravenous or oral antigen. J Immunol. 2001;167:188–95. doi: 10.4049/jimmunol.167.1.188. [DOI] [PubMed] [Google Scholar]
- 44.Zhang X, Izikson L, Liu L, Weiner HL. Activation of CD25+ CD4+ regulatory T cells by oral antigen administration. J Immunol. 2001;167:4245–53. doi: 10.4049/jimmunol.167.8.4245. [DOI] [PubMed] [Google Scholar]
- 45.Taguchi O, Kontani K, Ikeda H, Kezuka T, Takeuchi M, Takahashi T, Takahashi T. Tissue-specific suppressor T cells involved in self-tolerance are activated extrathymically by self-antigens. Immunology. 1994;82:365–9. [PMC free article] [PubMed] [Google Scholar]
- 46.Chen ZK, Cobbold SP, Waldmann H, Metcalfe S. Amplification of natural regulatory immune mechanisms for transplantation tolerance. Transplantation. 1996;62:1200–6. doi: 10.1097/00007890-199611150-00002. [DOI] [PubMed] [Google Scholar]
- 47.Waldmann H, Cobbold S. Regulating the immune response to transplants. A role for CD4+ regulatory cells? Immunity. 2001;14:399–406. doi: 10.1016/s1074-7613(01)00120-0. [DOI] [PubMed] [Google Scholar]
- 48.Hall BM, Fava L, Chen J, Plain KM, Boyd RA, Spicer ST, Berger MF. Anti-CD4 monoclonal antibody-induced tolerance to MHC-incompatible cardiac allografts maintained by CD4+ suppressor T cells that are not dependent upon IL-4. J Immunol. 1998;161:5147–56. [PubMed] [Google Scholar]
- 49.Jonuleit H, Schmitt E, Schuler G, Knop J, Enk AH. Induction of interleukin 10-producing, nonproliferating CD4+ T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med. 2000;192:1213–22. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gregori S, Casorati M, Amuchastegui S, Smiroldo S, Davalli AM, Adorini L. Regulatory T cells induced by 1 alpha,25-dihydroxyvitamin D3 and mycophenolate mofetil treatment mediate transplantation tolerance. J Immunol. 2001;167:1945–53. doi: 10.4049/jimmunol.167.4.1945. [DOI] [PubMed] [Google Scholar]
- 51.Barrat FJ, Cua DJ, Boonstra A, et al. In vitro generation of interleukin 10-producing regulatory CD4+ T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J Exp Med. 2002;195:603–16. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lombardi G, Sidhu S, Batchelor R, Lechler R. Anergic T cells as suppressor cells in vitro. Science. 1994;264:1587–9. doi: 10.1126/science.8202711. [DOI] [PubMed] [Google Scholar]
- 53.Lombardi G, Hargreaves R, Sidhu S, et al. Antigen presentation by T cells inhibits IL-2 production and induces IL-4 release due to altered cognate signals. J Immunol. 1996;156:2769–75. [PubMed] [Google Scholar]
- 54.Lamb JR, Skidmore BJ, Green N, Chiller JM, Feldmann M. Induction of tolerance in influenza virus-immune T lymphocyte clones with synthetic peptides of influenza hemagglutinin. J Exp Med. 1983;157:1434–47. doi: 10.1084/jem.157.5.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ko HS, Fu SM, Winchester RJ, Yu DT, Kunkel HG. Ia determinants on stimulated human T lymphocytes. Occurrence on mitogen- and antigen-activated T cells. J Exp Med. 1979;150:246–55. doi: 10.1084/jem.150.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.LaSalle JM, Tolentino PJ, Freeman GJ, Nadler LM, Hafler DA. Early signaling defects in human T, cells anergized by T, cell presentation of autoantigen. J Exp Med. 1992;176:177–86. doi: 10.1084/jem.176.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taams LS, van Eden W, Wauben MH. Antigen presentation by T cells versus professional antigen-presenting cells (APC): differential consequences for T cell activation and subsequent T cell–APC interactions. Eur J Immunol. 1999;29:1543–50. doi: 10.1002/(SICI)1521-4141(199905)29:05<1543::AID-IMMU1543>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 58.Broeren CP, Wauben MH, Lucassen MA, Van Meurs M, Van Kooten PJ, Boog CJ, Claassen E, van Eden W. Activated rat T cells synthesize and express functional major histocompatibility class II antigens. Immunology. 1995;84:193–201. [PMC free article] [PubMed] [Google Scholar]
- 59.Lombardi G, Dunne PJ, Scheel-Toellner D, et al. Type 1 IFN maintains the survival of anergic CD4+ T cells. J Immunol. 2000;165:3782–9. doi: 10.4049/jimmunol.165.7.3782. [DOI] [PubMed] [Google Scholar]
- 60.Ng WF, Duggan PJ, Ponchel F, Matarese G, Lombardi G, Edwards AD, Isaacs JD, Lechler RI. Human CD4+ CD25+ cells: a naturally occurring population of regulatory T cells. Blood. 2001;98:2736–44. doi: 10.1182/blood.v98.9.2736. [DOI] [PubMed] [Google Scholar]
- 61.Wing K, Ekmark A, Karlsson H, Rudin A, Suri-Payer E. Characterization of human CD25+ CD4+ T cells in thymus, cord and adult blood. Immunology. 2002;106:190–9. doi: 10.1046/j.1365-2567.2002.01412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Almeida AR, Legrand N, Papiernik M, Freitas AA. Homeostasis of peripheral CD4+ T cells: IL-2R alpha and IL-2 shape a population of regulatory cells that controls CD4+ T cell numbers. J Immunol. 2002;169:4850–60. doi: 10.4049/jimmunol.169.9.4850. [DOI] [PubMed] [Google Scholar]
- 63.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+ CD25+ regulatory T cells. NatImmunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 64.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 65.Bach JF. Regulatory T cells under scrutiny. Nat Rev Immunol. 2003;3:189–98. doi: 10.1038/nri1026. [DOI] [PubMed] [Google Scholar]
- 66.Hayday A, Tigelaar R. Immunoregulation in the tissues by γδ T cells. Nat Rev Immunol. 2003;3:233–42. doi: 10.1038/nri1030. [DOI] [PubMed] [Google Scholar]
- 67.Hargreaves RG, Borthwick NJ, Montani MG, Piccolella E, Carmichael P, Lechler RI, Akbar AN, Lombardi G. Dissociation of T cell anergy from apoptosis by blockade of Fas/Apo-1 (CD95) signaling. J Immunol. 1997;158:3099–107. [PubMed] [Google Scholar]
- 68.Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–54. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 69.Taams LS, van Eden W, Wauben MH. Dose-dependent induction of distinct anergic phenotypes: multiple levels of T cell anergy. J Immunol. 1999;162:1974–81. [PubMed] [Google Scholar]
- 70.Chai JG, Bartok I, Chandler P, Vendetti S, Antoniou A, Dyson J, Lechler R. Anergic T cells act as suppressor cells in vitro and in vivo. Eur J Immunol. 1999;29:686–92. doi: 10.1002/(SICI)1521-4141(199902)29:02<686::AID-IMMU686>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 71.Hayashi RJ, Loh DY, Kanagawa O, Wang F. Differences between responses of naive and activated T cells to anergy induction. J Immunol. 1998;160:33–8. [PubMed] [Google Scholar]
- 72.Marelli-Berg FM, Weetman A, Frasca L, Deacock SJ, Imami N, Lombardi G, Lechler RI. Antigen presentation by epithelial cells induces anergic immunoregulatory CD45RO+ T cells and deletion of CD45RA+ T cells. J Immunol. 1997;159:5853–61. [PubMed] [Google Scholar]
- 73.Takahashi T, Kuniyasu Y, Toda M, Sakaguchi N, Itoh M, Iwata M, Shimizu J, Sakaguchi S. Immunologic self-tolerance maintained by CD25+ CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. 1998;10:1969–80. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]