Abstract
It is critical, both for the host and for the long-term benefit of the bacteria that colonize the gut, that bacterial overgrowth with subsequent bacterial translocation, which may lead to sepsis and death of the host, be avoided. Secretory IgA (sIgA) is known to be a key factor in this process, agglutinating bacteria and preventing their translocation in a process termed ‘immune exclusion’. To determine whether human sIgA might facilitate the growth of normal enteric bacteria under some conditions, the growth of human enteric bacteria on cultured, fixed human epithelial cells was evaluated in the presence of sIgA or various other proteins. Human sIgA was found to facilitate biofilm formation by normal human gut flora and by Escherichia coli on cultured human epithelial cell surfaces under conditions in which non-adherent bacteria were repeatedly washed away. In addition, the presence of sIgA resulted in a 64% increase in adherence of E. coli to live cultured epithelial cells over a 45-min period. Mucin, another defence factor thought to play a key role in immune exclusion, was found to facilitate biofilm formation by E. coli. Our findings suggest that sIgA may contribute to biofilm formation in the gut.
Introduction
The interaction of secretory IgA (sIgA) with normal gut bacteria is considered to be a protective one.1,2 The classic paradigm is that sIgA agglutinates bacteria, thereby mediating clearance of those bacteria from the gut3 and preventing invasion of the body by the bacteria4 in a process known as ‘immune exclusion.’ However, some evidence suggests that sIgA might actually benefit bacterial growth inside the intestine.5 For example, decreased expression of sIgA receptors by gut bacteria in IgA-deficient individuals compared to bacteria in normal individuals suggests that there may be selection pressure in the gut for bacteria that bind sIgA.6 In addition, increased expression of sIgA receptors by gut bacteria in infants fed mothers milk compared to infants not fed mother's milk7 is also consistent with the idea that binding of sIgA by gut bacteria may benefit those bacteria. The observation that normal flora elicit sIgA production and subsequently colonize the gut in the presence of the sIgA8,9 is consistent with the idea that sIgA may act in a promicrobial fashion inside the gut.
Much of the current view regarding the role of sIgA in gut immunity is based on studies involving the adherence of bacteria to cultured epithelial cells in the presence or absence of sIgA.3 In those studies, bacteria were agglutinated by sIgA prior to brief incubations with epithelial cells. In this study, the effect of sIgA on the adhesion of enteric bacteria to epithelial cells was determined under conditions in which bacteria were not agglutinated prior to incubation with the epithelial cells. In addition, growth of enteric bacteria on cultured, fixed epithelial cells was monitored for a period of hours in the presence and absence of sIgA and mucin. The potential implications of this study for new models of gut immunity are discussed.
Materials and methods
Materials
Minimum essential Eagle's medium, mucin (Type III from porcine stomach) and human haemoglobin were obtained from Sigma Chemical Co. (St Louis, MO). Bovine serum albumin (BSA) Fraction V, heat-shock lyophilysate was obtained from Roche Diagnostics Corp. (Indianapolis, IN). Human IgG (Immune Globulin Intravenous Gammagard S/D) was purchased from Baxter Healthcare Corp. (Glendale, CA). The mucin, IgG, BSA and haemoglobin were dissolved into phosphate-buffered saline (PBS) and dialysed against two changes of PBS followed by a final dialysis against minimum essential Eagle's medium. The dialysed IgG, BSA and haemoglobin were sterile-filtered through a 0·2-μm filter prior to use. The dialysed mucin preparation was sterilized by boiling for 15 min prior to use. Sodium pyruvate, HEPES, and non-essential amino acids were obtained from GibcoBRL (Grand Island, NY). Igase was purchased from Mo Bi Tec (Marco Island, FL). Solvable™ was purchased from Perkin Elmer Life Sciences (Boston, MA). Biotinylated, succinylated wheat germ agglutinin (succ-WGA) was obtained from Vector Laboratories, Inc. (Burlingame, CA). CaCo2 cells, an immortal line of human gut epithelial cells (cell number HTB-37) were obtained from the American Type Culture Collection (Rockville, MD). Nunc NunclonD tissue culture tubes (110 × 16 mm), Costar six-well cell-culture plates, Becton Dickinson PET track-etched membrane (310-μm pore size) cell-culture inserts for six-well plates and Becton Dickinson 96-well tissue-culture plates were obtained from VWR Scientific (Morrisville, NC). The Escherichia coli used was a non-pathogenic clinical isolate obtained at the Duke University Medical Center. Uniformly labelled [14C]glucose was obtained from Amersham (Piscataway, NJ) Unneeded human milk from anonymous donors was obtained from the Duke University Medical Center Pediatric Intensive Care Unit.
The sIgA was purified from human milk using thiophilic adsorption chromatography10 or by size exclusion chromatography on a 2·5-cm (internal diameter) by 90-cm (length) Sepharose CL-4B column. Purified sIgA was stored in 10% glycerol and flash frozen until use. Purification on the Sepharose column resulted in a greater loss of IgA2 than IgA1, since the IgA2 eluted later, resulting in contamination of part of the IgA2 fraction with other milk proteins. The IgA preparation was determined to contain approximately 85% IgA1 and 15% IgA2 based on two independent assessments. First, 84% of the IgA preparation was cleaved with Igase (specific for IgA1). Second, quantitative analysis of the elution profile from the Sepharose column, which contains the IgA1 peak followed by the IgA2 peak (partially resolved), was conducted. The absorbance profile at 280 nm was fitted to Gaussian distributions and the areas under the curve were calculated using the program grams/32, version 5·10 (Galactic Industries Corp., Salem, NH).) Based on this analysis, our preparations contained 80–85% IgA1, and 50–60% of the IgA2 was discarded during the preparation.
Before use, purified sIgA was thawed and dialysed against two changes of PBS followed by a final dialysis against minimum essential Eagle's medium. The sIgA was then sterilized by filtration through a 0·2-μm filter. Mucin preparations were dialysed in the same fashion, and were sterilized by boiling for 20 min prior to use.
Enteric bacteria
Enteric bacteria were obtained by inoculating minimal medium containing sodium pyruvate, HEPES, and non-essential amino acids with faecal material obtained from normal human donors and culturing the bacteria overnight at 37° under anaerobic conditions. Minimal medium was used to ensure that the bacteria did not take up antigens that might be present in more complex broth mixtures. The bacteria cultured in this manner were found to be almost entirely E. coli by the Duke University Clinical Microbiology Laboratory. Some Bacteroides species (obligate anaerobes) were also identified, although they were less abundant.
Biofilm growth
Human gut epithelial (CaCo2) cells were cultured in 110 × 16 mm NunclonD tissue culture tubes. After the cells became confluent, they were fixed with 0·1% glutaraldehyde for 10 min. In some experiments, cells were blocked with 10% BSA for 1 hr to confirm that results were not due to free aldehyde groups remaining after fixation. Next, to initiate bacterial growth in the tubes, the fixed cells were incubated for 6 hr under anaerobic conditions with minimal medium (1·5 ml) that was inoculated with human gut bacteria. Following this incubation, the medium was slowly drained from the tube by gently inverting the tube, and fresh medium containing 25 nCi/ml 14C was then slowly added with or without sIgA or other proteins as indicated. During the next 2·5 days, the medium was changed four times at 6–18-hr intervals. For each change, tubes were gently washed three times with PBS by inverting the tube and slowly adding fresh solution each time or by gently removing and adding solution using a 16-gauge, 5·25′′ Angiocath. After the third wash with the buffered saline, the saline was slowly drained and 1·5 ml minimal medium with appropriate protein was gently added.
Bacteria in biofilms were quantified by measuring incorporation of 14C into the biofilm. For this purpose, bacteria were removed from the fixed epithelial cell surfaces by vortexing at maximum speed for 2 min. Removal of biofilms by this method was confirmed by visual inspection. The suspended bacterial cells were washed three times with PBS, resuspended in 50 μl deionized water, dissolved in 500 μl Solvable™, and diluted into 10 ml scintillation fluid (Packard Hionic-Fluor™). The amount of 14C was then quantified using a Wallac 1409 liquid scintillation counter. Using a serial dilution of 14C-labelled bacteria, this method was found to be linear (r2 > 0·99) over greater than a thousand-fold range [(from 85 000 (d.p.m.) per minute disintegrations to 40 d.p.m.]. Standard errors for all measurements were calculated using graphpad prism version 3·00 for Windows (GraphPad Software; San Diego, CA).
Treatment of biofilms with Igase
Cells treated with Igase were cultured in an identical fashion to cells cultured in the presence of sIgA, except that 2·0 μg/ml Igase was added during the final 7 hr of incubation. Igase is specific for IgA1 and specifically cleaved 84% of the sIgA in our preparations as determined by sodium dodecyl sulphate–polyacrylamide gel electrophoresis followed by densitometric scanning of Western blots stained for α-chain.
Binding of sIgA and biotinylated, succinylated-wheat germ agglutinin (succ-WGA) to human epithelial cells
Removal of cell surface carbohydrate by bacteria was assessed using biotinylated, succinylated wheat germ agglutinin (succ-WGA), a lectin that recognizes the structure GlcNAcβ1–4GlcNAc. This carbohydrate is expressed at the base of complex N-linked mannose structures and is thus protected from lectin binding on intact cells. Following removal of mannose and other terminal saccharides, this structure is exposed. The binding of biotinylated succ-WGA and sIgA to cultured human epithelial cells was determined using enzyme-linked assays (ELA). Human epithelial cells were cultured in 96-well plates and, after becoming confluent, were fixed with 0·1% glutaraldehyde for 10 min. Plates were then incubated overnight at 37° with bacteria and then washed vigorously 20 times with PBS to remove almost all adherent bacteria. Following these washes, biotinylated succ-WGA (4 μg/ml in a 0·1% gelatin solution) or sIgA (20 μg/ml in PBS) was added and incubated at 22° for 3 hr. Wells were next washed three times with PBS and then alkaline phosphatase-conjugated avidin (for detection of lectin) or alkaline phosphatase-conjugated goat anti-human α-chain (for detection of sIgA) was added. After a 1-hr incubation, the wells were washed four times with PBS, and 100 μl/well of a developing solution consisting of p-nitropheyl phospate in a 100-mm diethanolamine buffer was added. The absorbance at 405 nm (A405) was determined using an EL 340 Bio Kinetics Reader (Bio-Tek Instruments, Winooski, VT). The absorbance in wells incubated without biotinylated succ-WGA or sIgA was taken as the ‘background’ and subtracted from the total.
Effect of sIgA on bacterial adherence to live cells
Human gut epithelial (CaCo2) cells were cultured on cell culture inserts in Costar six-well plates until they were confluent. Medium containing E. coli with or without 0·5 mg/ml sIgA was added to each insert and incubated for 45 min at 37°. Cells were washed by submerging the inserts in PBS and washing the cell surface three times with 5 ml medium each time. The cells remained submerged in PBS for the entire duration of the wash to facilitate gentle washing. The greater density of the medium compared to the PBS facilitated the washing. Experiments were conducted in triplicate. For these experiments, the number of adherent bacteria was quantified by diluting the bacteria, plating them on agar, and counting the number of plaque-forming units following an overnight incubation. Using this method, we could not be sure that the number of bacteria in the presence of sIgA was underestimated because of aggregation of the bacteria. However, the use of 14C-labelling was prohibited by substantial uptake of the isotope by the live epithelial cells, even in the absence of bacteria.
Results
The sIgA-mediated adherent growth of human enteric bacteria on cultured, fixed human epithelial cells
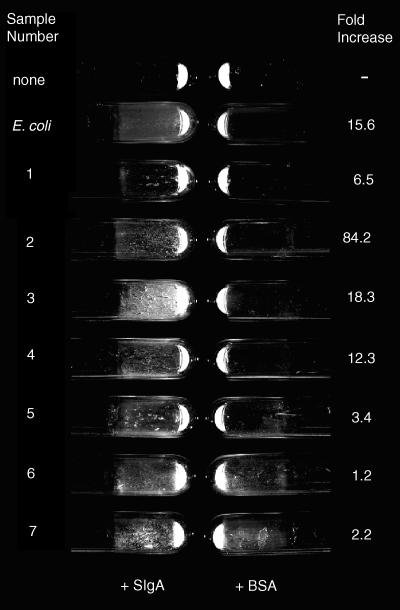
Direct evidence that human sIgA might facilitate the growth of human enteric bacteria and of bacteria (mixed species) cultured from stool samples from all individuals tested is shown in Table 1. The presence of 0·5 mg/ml sIgA resulted in a 15·6-fold increase in the growth of adherent E. coli compared to the control containing 0·5 mg/ml BSA (Table 1). Bacteria were grown on glutaraldehyde-fixed human epithelial (CaCo2) cells under conditions in which the growth medium was changed four times (with three saline washes during each change) over a period of 2 days.
Table 1.
Adherent growth of Escherichia coli and bacteria cultured from human faecal samples in the presence of human sIgA or BSA (mean ± SEM)
| Sample number | Growth (+ sIgA) d.p.m. | Growth (+ BSA) d.p.m. | Growth increase with sIgA |
|---|---|---|---|
| E. coli | 7933·4 ± 23·8 | 508·4 ± 152·4 | 15·6-fold |
| 1 | 2047·3 ± 18·5 | 315·75 ± 22·7 | 6·5-fold |
| 2 | 8407·7 ± 1231·3 | 99·8 ± 47·1 | 84·2-fold |
| 3 | 4603·7 ± 26·3 | 251·55 ± 14·8 | 18·3-fold |
| 4 | 1660·8 ± 186·8 | 134·5 ± 14·8 | 12·3-fold |
| 5 | 2916·4 ± 34·3 | 847·85 ± 39·9 | 3·4-fold |
| 6 | 3078·8 ± 239·3 | 2623·65 ± 184·8 | 1·2-fold |
| 7 | 3941·6 ± 95·5 | 1752·0 ± 130·9 | 2·2-fold |
Glucose-rich medium was inoculated with E. coli or with faecal samples from healthy volunteers (samples 1 to 7) and incubated at 37° overnight. The adherent growth of these bacteria in the presence of 0·5 mg/ml BSA or 0·5 mg/ml human sIgA was then assayed. For sample 2, the sIgA and BSA concentrations were increased to 2·5 mg/ml, since biofilm formation was not visible with 0·5 mg/ml human sIgA. (There was, however, a greater than 14-fold increase in adherent growth of sample 2 in the presence of 0·5 mg/ml human sIgA compared to 0·5 mg/ml BSA.) Bacteria were grown on glutaraldehyde-fixed human epithelial (CaCo2) cells under conditions in which the growth medium was changed four times (with three saline washes during each change) over a period of 2 days. Bacterial growth was quantified by incorporation of uniformly labelled [14C]glucose, the experiment was run in duplicate, and the standard errors are shown.
Bacterial biofilms (plaques) were evident with the naked eye (Fig. 1) on fixed human epithelial cells when bacteria were grown in the presence of sIgA to a greater extent than in controls containing BSA, suggesting that the biofilm formation was indeed mediated by sIgA. Of note is the observation that bacteria from at least two human faecal samples (samples 6 and 7, Table 1, Fig. 1) formed appreciable biofilms even in the absence of sIgA. However, adherent bacterial growth was still greater in the presence of sIgA than in controls (Table 1). This result was reproduced when biofilms were grown in a different orientation (with culture tubes horizontal rather than vertical), indicating the robustness of the biofilm growth in the absence of sIgA by bacteria in some but not all samples.
Figure 1.
Biofilms on cultured human epithelial cells formed by E. coli or by human faecal bacteria obtained from seven healthy volunteers. The sample identifications on the left refer to either the bacterial species (E. coli) or to the number of the donor. The ‘fold increase’ on the right is the ratio of bacterial growth in the presence of sIgA over the growth in the presence of BSA. Biofilms were grown with the tubes in a vertical position so that the biofilms formed on the side of the tube. Culture tubes were washed three times with saline and the tubes were emptied of all liquid before the photograph was taken. The quantitative bacteria growth is shown in Table 1 and the conditions of the experiment are as described for Table 1. Representative tubes are shown in the photograph.
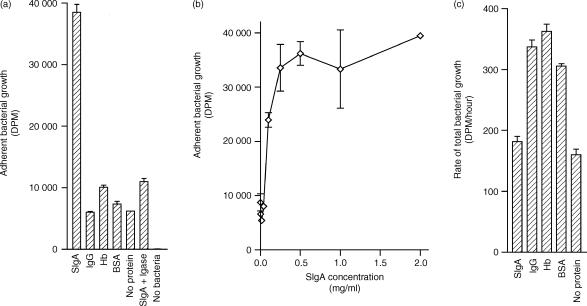
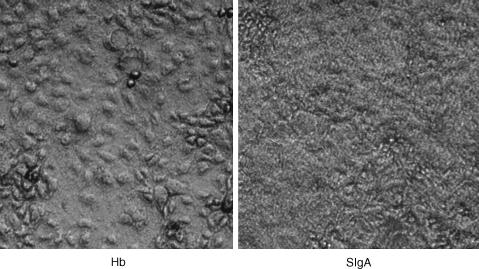
One human faecal sample was selected at random for further study (sample 4 from Table 1). Other proteins, including BSA, human IgG and human haemoglobin, or the absence of protein did not enhance adherent growth to the same extent as did sIgA (Fig. 2a). Exposure of adherent bacteria to Igase, an enzyme that cleaved 84% of the sIgA in our preparations, eliminated the increased adherent growth from the epithelial cell surface, indicating that the promicrobial effect was indeed the result of sIgA. Increased adherence of bacteria in the presence of sIgA was observed at sIgA concentrations of 0·25 mg/ml and above (Fig. 2b). The increased adherent growth of bacteria from sample 4 (Table 2) in the presence of sIgA was readily observed using a light microscope (Fig. 3).
Figure 2.
Effect of sIgA on bacterial growth. Bacteria were grown in the presence of uniformly labelled [14C]glucose on cultured human epithelial cells and the uptake of 14C was used as a measure of bacterial growth. (a) Bacterial growth was measured in the presence of 0·5 mg/ml of various proteins following four changes of growth medium with three saline washes between each change. (b) Using the same washing procedure, adherent bacterial growth was measured as a function of sIgA concentration. (c) The total (non-adherent + adherent) growth rate of enteric bacteria in the presence of 0·5 mg/ml of various proteins was measured. In this case, bacteria were grown in 15-ml conical tubes (no epithelial cells), vortexed for 2 min to dislodge adherent growth, and quantified by 14C-incorporation. All experiments were run in duplicate, and the standard errors are shown. Results comparing sIgA and BSA are representative of eight experiments.
Figure 3.
A biofilm as viewed under the light microscope. Phase contrast microscopy revealed the formation of bacterial plaques covering fixed, cultured epithelial cells in the presence of 0·5 mg/ml sIgA (right panel) but not 0·5 mg/ml of other proteins, including hamoglobin (Hb, left panel). In the left panel, the gross topology of the epithelial cells is evident, but is obscured by the bacterial plaque in the right panel.
Increased adherent growth in the presence of sIgA is not the result of an accelerated growth rate of bacteria in the presence of sIgA, since bacteria grown without medium changes and washes grew the same or even better in the absence of protein or in the presence of albumin, IgG, or haemoglobin than in the presence of sIgA (Fig. 2c). This observation is consistent with previous observations.11
Mucin-mediated adherent growth of E. coli on cultured, fixed human epithelial cells
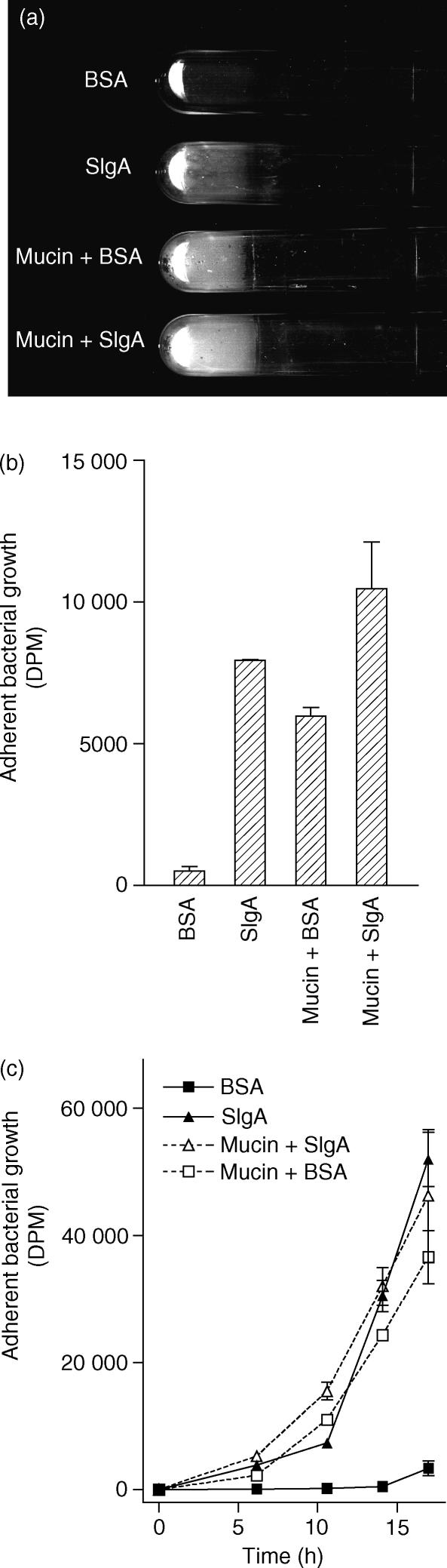
Mucus, like sIgA, is present in high abundance in the gut and is thought to be an important factor in immune exclusion. The presence of 5% porcine stomach mucin, like the presence of sIgA, facilitated the growth of biofilms by E. coli on fixed, cultured human epithelial cells. (Fig. 4). The biomass of bacteria in biofilms formed by E. coli in the presence of 50 mg/ml mucin was similar to that formed in the presence of 0·5 mg/ml sIgA. Biofilms were also formed by E. coli in the presence of 50 mg/ml mucin and 0·5 mg/ml sIgA in combination (Fig. 4). Additional experiments are needed to determine whether sIgA and mucin act synergistically in facilitating biofilm formation.
Figure 4.
Biofilms on cultured human epithelial cells formed by E. coli in the presence of 0·5 mg/ml sIgA, 50 mg/ml mucin, or both 0·5 mg/ml sIgA and 50 mg/ml mucin. (a,b) Biofilms were grown for 2·5 days as described in the Materials and methods section with the tubes in a vertical position so that the biofilms formed on the side of the tube. Tubes were washed three times with saline and the tubes were emptied of all liquid before the photograph was taken. Representative tubes are shown in (a), and the amount of adherent growth as quantified by 14C-incorporation is shown in (b). The experiment was reproducible. (c) The rate of adherent bacterial growth is shown. Cultured human epithelial cells were incubated with E. coli in the position described above. The ‘standard protocol’, which entails removal of non-adherent growth four times over a period of 2·5 days (see Materials and Methods section), was not utilized. For this experiment, the frequency with which non-adherent bacteria were removed and fresh media were added to cultures was increased as described in the text. All experiments were run in duplicate, and the standard errors are shown.
The rate of biofilm growth was evaluated. For this experiment, the ‘standard protocol’, which entails removal of non-adherent growth four times over a period of 2·5 days (see Materials and Methods section), was not utilized. Rather, non-adherent growth was removed and fresh medium was added at 6, 11 and 14 hr following initiation of the experiment. At each of these time-points, and at 17 hr, some cultures were terminated and the growth of adherent bacteria was quantified by 14C incorporation. As shown in Fig. 4(c), increased adherent growth in the presence of sIgA, mucin, or sIgA and mucin was evident by 6 hr. By 11 hr, the biofilms were clearly visible to the naked eye.
Increased binding of sIgA to human epithelial cells following incubation with human enteric bacteria
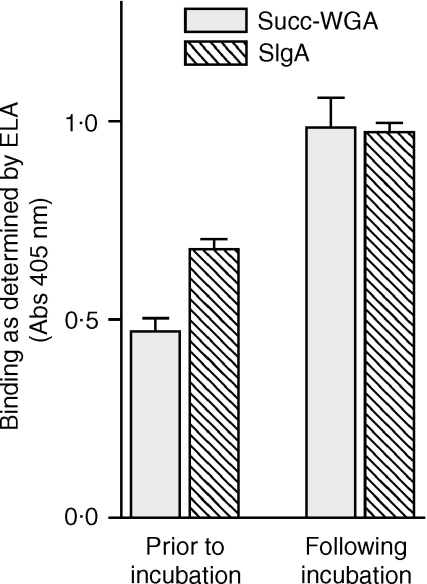
It has been postulated that the binding of sIgA to enteric bacteria may block epithelial receptors on the bacteria, thus preventing bacterial adhesion to epithelial cells.12 The basis of this idea is that sIgA and epithelial cells share carbohydrate structures that are recognized by bacterial adhesins.12 However, in a process facilitated by the host, bacteria are known to remove cell surface carbohydrates from gut epithelium in vivo.13 Further, natural antibodies are known to bind to autologous cells following removal of their cell surface carbohydrates. 14,15 Thus, it might be postulated that sIgA actually facilitates the adhesion of bacterial cells to epithelial cells under certain conditions. Removal of cell surface carbohydrate by bacteria was assessed using succ-WGA, a lectin that recognizes a structure (GlcNAcβ1–4GlcNAc) that is exposed following removal of terminal saccharides. Binding of succ-WGA to cultured epithelial cells more than doubled following exposure of the cells to enteric bacteria based on an ELA (Fig. 5). Prior to the lectin-binding assay, cells were washed extensively (20 saline washes) to remove almost all adherent bacteria. This result indicates that structures underlying complex saccharides are exposed by bacteria and suggests that cell surface carbohydrates are removed by the bacteria.
Figure 5.
Exposure of ‘neoepitopes’ on cultured human epithelial cells following incubation with human enteric bacteria. The binding of succinylated wheat germ agglutinin (succ-WGA) and sIgA were evaluated using ELA as described in the Materials and Methods section. The binding of succ-WGA was used to evaluate the exposure of saccharides normally buried on the cell surface, as described in the text. Twelve wells were used for each condition, and the standard errors are shown. The experiment shown is representative of three experiments performed.
To determine whether binding of sIgA increased following exposure of the cultured epithelial cells to bacteria, the binding of sIgA to epithelial cells was evaluated by enzyme-linked immunosorbent assay before and after incubation of the cells with enteric bacteria (sample 4 from Table 1). Cells were extensively washed (20 washes with saline) following incubation with bacteria to remove most of the adherent bacteria. There was a greater than 40% increase in the binding of sIgA to the cells (Fig. 5) consistent with the idea that removal of cell surface carbohydrate from epithelial cells results in an increase in the binding of sIgA.
The sIgA-mediated increase in adherence of E. coli to live human epithelial cells
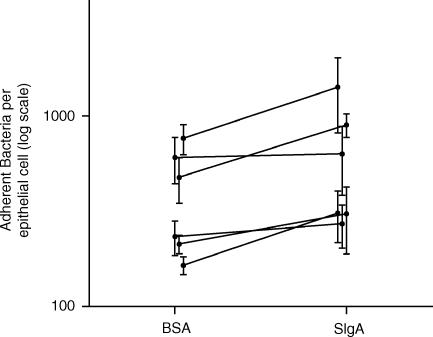
Because live human epithelial cells provide a more realistic model of the gut than do fixed cells, experiments were conducted using live, rather than fixed, epithelial cells. As described in the Materials and Methods section, live human epithelial cells were cultured in porous inserts so that they could receive nutrients from their basal side while bacteria were present on the apical side of the cells. Under these conditions, human epithelial cells could be co-cultured with E. coli for up to 1 hr before detaching from the tissue culture insert. Thus, live human epithelial cells were co-cultured with E. coli in the presence of 0·5 mg/ml BSA or 0·5 mg/ml sIgA for 45 min and then gently washed as described above. In six trials, the presence of 0·5 mg/ml sIgA resulted in a 17–88% increase in adherence of E. coli to live human epithelial cells (Fig. 6), with a mean increase of 64 ± 12% (mean ± SE, P = 0·003 using a t-test). Thus, the presence of sIgA can mediate adherence and/or adherent growth to live human epithelial cells in vitro. However, it remains to be determined to what extent intact biofilms might be formed by bacteria on live human epithelial cells in vivo.
Figure 6.
Adherence of E. coli to live human epithelial cells in the presence or absence of sIgA. The number of bacteria adherent to live epithelial cells was determined in the presence of 0·5 mg/ml sIgA or 0·5 mg/ml BSA. Six trials were conducted, with the results of a given trial represented by two points connected by a solid line in the graph. Each trial was conducted in triplicate and the standard errors are shown. In all trials, the number of adherent bacteria in the presence of sIgA was greater than the number adherent in the presence of BSA. However, there was considerable variability in the number of adherent bacteria between trials, perhaps because of day-to-day variability in the cultured epithelial cells. The number of bacteria was determined by measuring the number of plaque forming units.
Discussion
The general consensus in the field of immunology and gut immunity has been that the primary function of antibodies present in the gut is to prevent the translocation of micro-organisms from the gut lumen into other parts of the body (immune exclusion). The idea that sIgA-mediated biofilm formation may be an important component of immune exclusion is worthy of consideration, given the data presented herein. Another possibility is also worthy of consideration: Unlike antibodies in blood, antibodies in the gut are present in an environment that must necessarily support the growth of the normal flora, since that flora has a number of beneficial effects to the host.16,17 Thus, if indeed biofilms are a necessary part of the gut ecology as is thought,18–22 the hypothetical role of sIgA in biofilm formation in the gut may actually be promicrobial. This raises the possibility that, by mediating biofilm formation, sIgA may have a greater diversity of function than previously known, facilitating microbial growth (‘immune inclusion’) while at the same time preventing migration of the same microbes from the bowel (‘immune exclusion’).
Although no in vitro system can accurately reproduce the mammalian gut, the in vitro data presented in this paper show that, at least under some conditions, components of the gut immune system can be promicrobial. This observation is in contrast to other reports showing that, under in vitro conditions considerably different than those used by us, components of the immune system can prevent adherence of bacteria. The finding that sIgA and mucins can be promicrobial provides an impetus for further consideration of the role of immunity in the gut. The finding that biofilms are present in the normal gut provides further impetus for additional research in this area. Future areas of investigation include the potential roles of bacterial adhesins, bacterial exopolymers, secretory component and antibody specificity in the sIgA-mediated biofilm formation.
The mechanism(s) underlying the sIgA-mediated formation of biofilms on cultured gut epithelial cells are of interest. Bacterial agglutinins are known to increase the adhesion of bacteria to a variety of surfaces,23 the initial step in biofilm formation. Thus, sIgA, which agglutinated all of the bacteria used in our experiments, probably facilitates adhesion of bacteria to the gut epithelium. In addition, the rapid breakdown of sIgA-mediated biofilms by Igase (Fig. 2a) suggests that agglutination of bacteria by sIgA is important in the maintenance of biofilm structure. Finally, the inability of human IgG, which binds to enteric bacteria but does not agglutinate them, to mediate biofilm formation (Fig. 2a) provides further support for the importance of agglutination in aIgA-mediated biofilm formation. If indeed bacterial agglutination is a prerequisite for biofilm formation, then it is perhaps not surprising that mucin, which binds a variety of bacterial species,24–26 facilitates biofilm formation.
Whether bacterial adhesins produced by bacteria in biofilms adhere directly to epithelial cells or whether they are anchored to epithelial cells indirectly by such molecules as sIgA and mucins is a matter for future study. However, several observations are consistent with the idea that bacteria may be indirectly anchored by host molecules. First, the present study indicates that sIgA binding to epithelial cells increases following removal of cell surface carbohydrates by enteric bacteria. Thus, it is reasonable to postulate that sIgA is capable of simultaneous binding to epithelium and micro-organisms. This idea is supported by the observations that sIgA (1) is equipped with carbohydrates that are recognized by bacterial receptors27 and (2) contains a secretory component that can also bind to certain bacteria.28 A second indication that bacteria may be indirectly anchored by host molecules is that mucins, which were found to facilitate biofilm formation in vitro, are anchored to gut epithelium29 and bind to a variety of enteric bacteria.24–26 Finally, studies suggest that sIgA, which binds to enteric bacteria, also binds to the adherent mucus layer of the respiratory tract in an interaction that is mediated by the secretory component.30 Although it remains unknown whether such interactions take place in the gut, the studies do suggest that there are a number of potential ways in which bacteria may be indirectly anchored to the epithelium.
Given that the turnover of the colonic gut epithelium is about 3 days,31,32 the rapid (within 6 hr) sIgA-mediated formation of biofilms in vitro is consistent with the idea that sIga-mediated biofilms might form in vivo. However, the rate of formation of biofilms in the gut remains unknown and the expected equilibrium between biofilm growth and shedding is uncharacterized. Further, the fraction of the epithelial surface normally covered by biofilms remains unknown. Addressing these issues may prove challenging given the technical limitations imposed by the sensitivity of the epithelial lining to fixatives33 and the sensitivity of bacterial extracellular matrices to fixatives.29,34 However, it is hoped that this work will stimulate further investigation of the possibility that sIgA might act in a promicrobial fashion in the gut, facilitating sessile growth of the normal microbial flora.
Acknowledgments
We thank Sarah C. Hensel for expert assistance in preparation of this manuscript. This work was supported in part by the Fannie E. Rippel Foundation and by grant P30 DK34987 from the National Institutes of Health.
Abbreviations
- BSA
bovine serum albumin
- PBS
phosphate-buffered saline
- sIgA
secretory IgA
- succ-WGA
succinylated wheat germ agglutinin
References
- 1.Biancone L, Monteleone I, Del Vecchio Blanco G, Vavassori P, Pallone F. Resident bacterial flora and immune system. Digest Liver Dis. 2002;34:S37–43. doi: 10.1016/s1590-8658(02)80162-1. [DOI] [PubMed] [Google Scholar]
- 2.Schiffrin EJ, Blum S. Interactions between the microbiota and the intestinal mucosa. Eur J Clin Nutr. 2002;56:S60–4. doi: 10.1038/sj.ejcn.1601489. [DOI] [PubMed] [Google Scholar]
- 3.Williams RC, Gibbons RJ. Inhibition of bacterial adherence by secretory immunoglobulin A. a mechanism of antigen disposal. Science. 1972;177:697–9. doi: 10.1126/science.177.4050.697. [DOI] [PubMed] [Google Scholar]
- 4.Brandtzaeg P. Development and basic mechanisms of human gut immunity. Nutr Rev. 1998;56:S5–18. doi: 10.1111/j.1753-4887.1998.tb01645.x. [DOI] [PubMed] [Google Scholar]
- 5.Mestecky J, Russell MW, Elson CO. Intestinal IgA: novel views on its function in the defence of the largest mucosal surface. Gut. 1999;44:2–5. doi: 10.1136/gut.44.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friman V, Adlerberth I, Connell H, Svanborg C, Hanson LA, Wold AE. Decreased expression of mannose-specific adhesins by Escherichia coli in the colonic microflora of immunoglobulin A-deficient individuals. Infect Immun. 1996;64:2794–8. doi: 10.1128/iai.64.7.2794-2798.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wold AE, Adlerberth I. Breast feeding and the intestinal microflora of the infant – implications for protection against infectious diseases. Adv Exp Med Biol. 2000;478:77–93. doi: 10.1007/0-306-46830-1_7. [DOI] [PubMed] [Google Scholar]
- 8.Shroff KE, Cebra JJ. Development of mucosal humoral immune responses in germ-free (GF) mice. Adv Exp Med Biol. 1995;371A:441–6. doi: 10.1007/978-1-4615-1941-6_92. [DOI] [PubMed] [Google Scholar]
- 9.Shroff KE, Meslin K, Cebra JJ. Commensal enteric bacteria engender a self-limiting humoral mucosal immune response while permanently colonizing the gut. Infect Immun. 1995;63:3904–13. doi: 10.1128/iai.63.10.3904-3913.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hutchens TW, Magnuson JS, Yip TT. Secretory IgA, IgG, and IgM immunoglobulins isolated simultaneously from colostral whey by selective thiophilic adsorption. J Immunol Methods. 1990;128:89–99. doi: 10.1016/0022-1759(90)90467-a. [DOI] [PubMed] [Google Scholar]
- 11.Brandtzaeg P, Fjellanger I, Gjeruldsen ST. Adsorption of immunolgobulin A onto oral bacteria in vivo. J Bacteriol. 1968;96:242–9. doi: 10.1128/jb.96.1.242-249.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wold AE, Mestecky J, Tomana M, Kobata A, Ohbayashi H, Endo T, Eden CS. Secretory immunoglobulin A carries oligosaccharide receptors for Escherichia coli type 1 fimbrial lectin. Infect Immun. 1990;58:3073–7. doi: 10.1128/iai.58.9.3073-3077.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bry L, Falk PG, Midtvedt T, Gordon JI. A model of host–microbial interactions in an open mammalian ecosystem [see comments] Science. 1996;273:1380–3. doi: 10.1126/science.273.5280.1380. [DOI] [PubMed] [Google Scholar]
- 14.Grant HW, Hadley GP, Adhikari M, Fernandes-Costa F. T-cryptantigen (TCA) activation in surgical neonates: a hidden problem. Pediatr Surg Int. 1998;14:204–7. doi: 10.1007/s003830050488. [DOI] [PubMed] [Google Scholar]
- 15.Novak RW. The pathobiology of red cell cryptantigen exposure. Pediatr Pathol. 1990;10:867–75. doi: 10.3109/15513819009064722. [DOI] [PubMed] [Google Scholar]
- 16.MacDonald TT, Pettersson S. Bacterial regulation of intestinal immune responses. Inflamm Bowel Dis. 2000;6:116–22. doi: 10.1097/00054725-200005000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Harp JA, Chen W, Harmsen AG. Resistance of severe combined immunodeficient mice to infection with Cryptosporidium parvum: the importance of intestinal microflora. Infect Immun. 1992;60:3509–12. doi: 10.1128/iai.60.9.3509-3512.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Macfarlane S, McBain AJ, Macfarlane GT. Consequences of biofilm and sessile growth in the large intestine. Adv Dental Res. 1997;11:59–68. doi: 10.1177/08959374970110011801. [DOI] [PubMed] [Google Scholar]
- 19.Cassels FJ, Wolf MK. Colonization factors of diarrheagenic E. coli and their intestinal receptors. J Industr Microbiol. 1995;15:214–26. doi: 10.1007/BF01569828. [DOI] [PubMed] [Google Scholar]
- 20.Banwell JG, Howard R, Kabir I, Costerton JW. Bacterial overgrowth by indigenous microflora in the phytohemagglutinin-fed rat. Can J Microbiol. 1988;34:1009–13. doi: 10.1139/m88-177. [DOI] [PubMed] [Google Scholar]
- 21.Banwell JG, Howard R, Cooper D, Costerton JW. Intestinal microbial flora after feeding phytohemagglutinin lectins (Phaseolus vulgaris) to rats. Appl Environ Microbiol. 1985;50:68–80. doi: 10.1128/aem.50.1.68-80.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292:1115–18. doi: 10.1126/science.1058709. [DOI] [PubMed] [Google Scholar]
- 23.Liljemark WF, Bloomquist CG, Germaine GR. Effect of bacterial aggregation on the adherence of oral streptococci to hydroxyapatite. Infect Immun. 1981;31:935–41. doi: 10.1128/iai.31.3.935-941.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slomiany BL, Murty VL, Piotrowski J, Slomiany A. Salivary mucins in oral mucosal defense. Gen Pharmacol. 1996;27:761–71. doi: 10.1016/0306-3623(95)02050-0. [DOI] [PubMed] [Google Scholar]
- 25.Szoke I, Pascu C, Nagy E, Ljung A, Wadstrom T. Binding of extracellular matrix proteins to the surface of anaerobic bacteria. J Med Microbiol. 1996;45:338–43. doi: 10.1099/00222615-45-5-338. [DOI] [PubMed] [Google Scholar]
- 26.Piotrowski J, Murty VL, Czajkowski A, Slomiany A, Yotsumoto F, Majka J, Slomiany BL. Association of salivary bacterial aggregating activity with sulfomucin. Biochem Mol Biol Int. 1994;32:713–21. [PubMed] [Google Scholar]
- 27.Moshier A, Reddy MS, Scannapieco FA. Role of type 1 fimbriae in the adhesion of Escherichia coli to salivary mucin and secretory immunoglobulin A. Current Microbiol. 1996;33:200–8. doi: 10.1007/s002849900100. [DOI] [PubMed] [Google Scholar]
- 28.Giugliano LG, Ribeiro ST, Vainstein MH, Ulhoa CJ. Free secretory component and lactoferrin of human milk inhibit the adhesion of enterotoxigenic Escherichia coli. J Med Microbiol. 1995;42:3–9. doi: 10.1099/00222615-42-1-3. [DOI] [PubMed] [Google Scholar]
- 29.Allen A, Pearson JP. The gastrointestinal adherent mucous gel barrier. Meth Mol Biol. 2000;125:57–64. doi: 10.1385/1-59259-048-9:057. [DOI] [PubMed] [Google Scholar]
- 30.Phalipon A, Cardona A, Kraehenbuhl JP, Edelman L, Sansonetti PJ, Corthesy B. Secretory component: a new role in secretory IgA-mediated immune exclusion in vivo. Immunity. 2002;17:107–15. doi: 10.1016/s1074-7613(02)00341-2. [DOI] [PubMed] [Google Scholar]
- 31.Lipkin M, Bell B, Sherlock P. Cell proliferation kinetics in the gastrointestinal tract of man. I. Cell renewal in colon and rectum. J Clin Invest. 1963;42:767–76. doi: 10.1172/JCI104769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deschner EE, Raicht RF. Kinetic and morphologic alterations in the colon of a patient with multiple polyposis. Cancer. 1981;47:2440–5. doi: 10.1002/1097-0142(19810515)47:10<2440::aid-cncr2820471022>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 33.Luft JH. The structure and properties of the cell surface coat. Int Rev Cytol. 1976;45:291–382. doi: 10.1016/s0074-7696(08)60081-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fassel TA, Edmiston CE., Jr Bacterial biofilms: strategies for preparing glycocalyx for electron microscopy. Meth Enzymol. 1999;310:194–203. doi: 10.1016/s0076-6879(99)10017-x. [DOI] [PubMed] [Google Scholar]