Abstract
There is increasing evidence that primitive progenitors migrate from the bone marrow (BM) via the peripheral circulation to tissue sites where they undergo in situ differentiation to provide a continued source of effector cells, such as eosinophils, during an allergic inflammatory response. To study mechanisms of progenitor cell mobilization in allergic reactions, we investigated fluctuations in the expression of the eotaxin receptor, CC chemokine receptor 3 (CCR3), on CD34+ cells from stable asthmatics following allergen (i.e. antigen) challenge. BM aspirates were taken from seven early responder (ER) and 10 dual responder (DR) asthmatics who, following antigen challenge developed only an early bronchoconstrictor response and an early and late- bronchoconstrictor response, respectively. Expression of CCR3 was detected on primitive (CD34+ cells) and eosinophil-lineage committed progenitors (CD34+ interleukin-5 receptor alpha-subunit+ cells) by flow cytometry and confirmed by co-localization of CCR3 messenger RNA to CD34 immunopositive cells using in situ hybridization. When preantigen levels were compared to 24-hr postantigen levels, significant increases in BM CD34+ CCR3+ cells were detected in DR, who also developed a significant sputum and blood eosinophilia and increased methacholine airway responsiveness. In contrast, a significant attenuation of BM CD34+ CCR3+ cells was observed in ER. In a dose-dependent manner eotaxin, but not interleukin (IL)-5, stimulated CD34+ progenitor cell migration in vitro. This migrational response to eotaxin was abrogated by anti-CCR3 monoclonal antibody and primed by preincubation with IL-5. We propose that fluctuations in CCR3 expression on human BM CD34+ cells may facilitate chemokine-mediated progenitor cell mobilization to the peripheral circulation and the resultant development of pulmonary eosinophilia, a cardinal feature of asthma.
Introduction
There is a considerable body of evidence that increased production of eosinophil-lineage committed progenitors within the bone marrow is associated with the onset and maintenance of upper and lower airway eosinophilic inflammation in response to allergen exposure in atopic subjects.1,2 We have shown that increased numbers of haemopoietic progenitors (CD34+ cells) and CD34+ cells expressing the membrane-bound isoform of interleukin-5 receptor α-subunit (IL-5Rα) are found in the bone marrow of atopic asthmatics, compared with chronic bronchitic and normal subjects.3,4 To establish that differential airway responses to allergen are reflected by different progenitor cell responses in the bone marrow, two groups of asthmatic subjects were examined based on their airway responses to allergen: early responders (ER), who only develop an early bronchoconstrictor response; and dual responders (DR), who, following allergen inhalation, develop both an early and a late bronchoconstrictor response. Significant increases in bone marrow CD34+ IL-5Rα+ cells and IL-5-responsive eosinophil/basophil colony-forming units (Eo/Baso-CFU) were only detected in DR who also developed delayed airway hyperresponsiveness and airway eosinophilia 24 hr postallergen.5,6 Similar antigen-driven increases in CD34+ IL-5Rα+ cell numbers, and resultant enhancement of IL-5-dependent eosinophilopoiesis, have become evident in the bone marrow following nasal allergen challenge in various mouse models of eosinophilic airway inflammation.7,8
Although bone marrow haemopoietic events may trigger the increased production and ultimate egress of mature eosinophils from the bone marrow, there is increasing evidence that primitive and lineage-committed progenitors migrate to mucosal tissue sites, where local haemopoietic events may contribute to the increased recruitment of effector cells. In animal studies, using bromodeoxy uridine (BrdU) to label actively proliferating cells undergoing DNA synthesis within the bone marrow, increases in the numbers of BrdU+ cells in the bronchoalveolar lavage fluid 24-hr postallergen are interpreted to reflect the ingress of newly divided myeloid cells in the airways in response to antigen challenge.9,10 In human studies, CD34 immunopositive cells were extracted from human nasal polyp and nasal explant tissue and shown to undergo IL-5-driven proliferation and differentiation into Eo/Baso-CFU in vitro and ex vivo,11,12 confirming the presence of true blast cells in mucosal tissue. Increased numbers of CD34+ IL-5Rα+ cells have also been detected in induced sputum13 and bronchial biopsy tissue14 from atopic asthmatics compared with normals. In the latter study, CD34+ IL-5Rα+ cell numbers correlated with asthma severity, as judged by airway calibre, suggesting that control of progenitor cell trafficking may be a prerequisite for reduction of eosinophilic inflammation in the airway in asthma. Little, however, is known about the factors that orchestrate the mobilization of progenitors during an allergic inflammatory response.
Intravenous infusion of eotaxin has been shown to stimulate the rapid egress of eosinophil progenitor cells from the bone marrow sinuses of guinea-pigs into the peripheral circulation.15 In human studies, intravenous infusion of IL-5 stimulated an increase in the numbers of circulating progenitor cells and of eosinophils expressing CCR3, proposing an essential interplay between IL-5 and eotaxin in mobilization of pro-inflammatory cells from the bone marrow.16 In the present study we have investigated the hypothesis that ligation of the CC chemokine receptor 3 (CCR3)17 may stimulate the migration of progenitor cells during an allergic inflammatory response. We have studied fluctuations in CCR3 expression on CD34+ progenitors in asthmatic subjects in response to allergen challenge and investigated the function of this receptor on bone marrow progenitors in the context of allergen-induced development of an airway eosinophilic response.
Materials and methods
Materials
Materials were obtained as follows: Percoll from Pharmacia (Uppsala, Sweden); McCoys 5A, Iscove's modified Dulbecco's phosphate-buffered saline (PBS) and fetal calf serum (FCS) from Gibco (Burlington, Ont., Canada); methylcellulose, bovine serum albumin (BSA) grade V, heparin, sodium azide and paraformaldehyde from Sigma Chemical Co. (Mississauga, Ont., Canada); May–Grunwald–Giemsa stain from BDH (Mississauga, Ont., Canada), Diff-Quik stain from American Scientific Products (McGaw Park, IL) and dithiothreitol (Sputolysin) from Calbiochem (San Diego, CA). Stromal cell derived factor-1 (SDF-1), eotaxin and IL-5 were purchased from R & D Systems (Minneapolis, MN).
Antibodies
Phycoerythrin (PE)-conjugated immunoglobulin G1 (IgG1) CD34 (HPCA-2), fluorescein isothiocyanate (FITC)-conjugated IgG1 CD45 (anti-HLE1), PE-conjugated isotype-control antibody (i.e. anti-IgG1–PE) and streptavidin-conjugated peridinin chlorophyll protein (PerCp) were purchased from Becton-Dickinson (Mississauga, Ont., Canada). Non-neutralizing monoclonal antibodies (mAbs) directed against the α-subunits of IL-5R (IL-5Rα; α16) were a kind gift from Dr Jan Tavernier (University of Ghent, Belgium). Anti-CCR3 mAb (MAB155) and IgG2a isotype control were purchased from R & D Systems. Cytokine receptor antibodies were biotinylated as previously described.5
Subjects
Seventeen non-smokers with mild, stable asthma (seven ER and 10 DR) were studied (Table 1). The asthmatic subjects were classified into two groups based on their airway responses to allergen:
Table 1.
Subject characteristics and allergen-induced airway responses
| Early responder asthmatics | Dual responder asthmatics | |
|---|---|---|
| Age | 24 (20–30) | 23 (21–50) |
| Sex | 3 M : 7 F | 2 M : 5 F |
| % fall in FEV1 | ||
| ″EAR | 31 (43.9–22.1) | 27.4 (51.3–17.9) |
| ″LAR | 6.1 (9.7–2.9) | 16.6 (37.1–12.5) |
| Methacholine PC20 | ||
| ″Pre-allergen | 5.2 (11.3–1.0) | 4.2 (11.4–0.1) |
| ″Post-allergen | 5.8 (11.7–0.3) | 2.5†,** (17.3–0.1) |
Values are presented as medians with ranges except for PC20 values which are geometric means with ranges.
P < 0.005 for within group comparison of pre-allergen versus 24 hr post-allergen log PC20 values and
P < 0.05 for between group comparisons of pre-allergen versus 24 hr post-allergen log PC20 values. Dual responder, but not the early responder, asthmatics developed a significant increase in methacholine airway responsiveness 24 hr post-allergen P < 0.005). In addition, there was a significant difference between the two groups in the allergen-induced shift in log PC20 values (P < 0.05).
M, male; F, female.
ER, who only developed an early fall (of >15% from baseline) in the forced expiratory volume in 1 second (FEV1); and
DR, who, following allergen inhalation, developed both an early and a late bronchoconstrictor response (defined as a fall in FEV1 of >15% and >12% from baseline, respectively).
The early and late bronchoconstrictor responses were taken to be the maximal percentage fall in FEV1 within 2 hr after allergen inhalation and between 3 and 7 hr after allergen inhalation, respectively. The definitions of early and late asthmatic response were established before the study, and the subjects were characterized as ER and DR by their airway responses to a screening allergen inhalation challenge. All subjects had a baseline FEV1 of >70% of the predicted normal on all study days, none had had a respiratory tract infection for at least 4 weeks prior to entering the study and required only infrequent use of inhaled α2-agonist.18 This study was approved by the Research Advisory Board of McMaster University Health Sciences Corporation, and each subject gave written, informed consent.
Study design
Subjects attended the laboratory on three occasions, as follows:
Visit 1. One week prior to allergen challenge to document full medical history, and to undergo skin-prick test sensitivity testing to allergen extracts, spirometry, methacholine-inhalation test and induction of sputum to assess baseline airway inflammation.
Visit 2. Allergen-challenge procedure. Prior to allergen challenge, a bone marrow aspirate was collected and spirometry measurements were taken hourly for 7 hr postallergen inhalation to follow the allergen-induced bronchoconstrictor response. Blood samples were taken pre- and 5 hr postallergen challenge.
Visit 3. Blood, sputum and bone marrow aspirates were collected 24 hr postallergen challenge. Spirometry measurements and methacholine inhalation challenge were also performed to assess the changes in airway calibre and hyperresponsiveness.
Allergen-inhalation challenge and methacholine-inhalation challenge
Allergen challenge was performed as described by O'Byrne et al.,19 and the concentration of the allergen extract for inhalation was determined from a formula using skin-prick test and methacholine PC20 results (PC20 = provocative concentration of methacholine causing a 20% fall in FEV1).20 The starting concentration of allergen was chosen to be three doubling doses below that predicted to cause a 20% fall in FEV1. Doubling incremental concentrations of allergen were inhaled at 10-min intervals until a decrease of ≥15% occurred in the FEV1 from baseline.
Methacholine inhalation was performed by the tidal breathing method, as described by Cockcroft et al.21 Subjects inhaled normal saline and then incremental doubling concentrations of methacholine phosphate from a Wright nebulizer for 2 min. The test was terminated when a fall in FEV1 of 20% of the postsaline value occurred, and the PC20 was calculated.
Indices of inflammation: sputum, blood and bone marrow differential cell counts
Sputum was induced by inhalation of hypertonic saline and processed according to the method of Pizzichini et al.22 Sputum cell plugs were processed using 0·1% dithiothreitol and Dulbecco's PBS. Cytospins were prepared from the pelleted cells and differential counts were performed on Diff-Quik-stained slides. Means of duplicate slides were obtained (500 cells counted/slide) and expressed as absolute counts (104 cells/ml). Venous blood was collected into EDTA-treated tubes. Total cell counts were performed using a Neubauer haemocytometer and differential cell counts were made from blood smears stained by Diff-Quik. Similarly, differential cell counts were made from smears of whole bone marrow stained by Diff-Quik. Differential cell counts were obtained from the mean of two slides (1000 cells counted/slide) and cell populations were expressed as the absolute counts (104 cells/ml) for sputum and blood and as percentage values for bone marrow samples.
Isolation and immunofluorescence staining of bone marrow and blood progenitors
For progenitor cell enumeration, heparinized (1000 U/ml) samples of bone marrow (5 ml) were aspirated from the iliac crest, and venous blood (20 ml) was collected. From each sample, low-density mononuclear cells (MNC) were isolated by sedimentation on Percoll density gradients (specific gravity 1·077), as previously described.23 Monocytes were depleted from the MNC fraction by incubation in plastic flasks for 2 hr at 37°. Non-adherent MNC (NAMNC; containing progenitor cells and lymphocytes) were first stained with saturating amounts of biotin-conjugated anti-IL-5Rα, anti-CCR3 or the isotype-control antibody (determined in preliminary studies) in a final volume of 100 µl of ice-cold fluorescence-activated cell sorter (FACS) staining buffer (PBS plus 0·1% NaN3, 2·5% each of mouse serum and human serum) for 30 min at 4°. The cells were then washed with 3 ml of PBS + 0·1% sodium azide, resuspended in FACS staining buffer and stained with streptavidin-conjugated PerCp together with saturating concentrations of FITC–CD45 IgG1 (anti-HLE1) and PE–CD34 IgG1 (HPCA-2) for 30 min at 4°. Lysis buffer (Becton Dickinson Canada) was then added and the cells incubated for 5 min, after which they were washed twice with 3 ml of PBS + 0·1% sodium azide and finally fixed in 500 µl of PBS + 1% paraformaldehyde. Cells were refrigerated until acquired by flow cytometry.
Flow cytometry and gating strategy
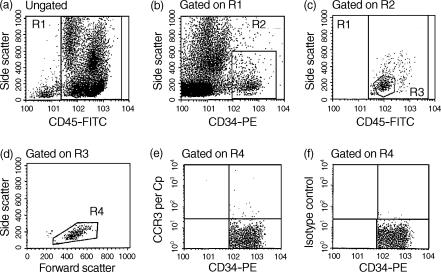
The stained NAMNC were analysed using a FACScan flow cytometer equipped with an argon ion laser [Becton Dickinson Instrument Systems (BDIS)] using the cellquest programme (BDIS). Progenitor cells were identified based on their unique cell size, granularity and immunofluorescence characteristics.5 Off-line analysis was performed using the pc lysis software supplied by BDIS. True CD34+ blast cells were identified as cells with CD34high/CD45dull staining and low side scatter (Fig. 1a, A–D). Within the true CD34+ population, specific staining of PerCp-linked cytokine/chemokine receptor mAbs (Fig. 1a, E) or control antibody (Fig. 1a, F) was detected, and data were collected as numbers of cells at the 99% confidence limit (i.e. relative to a marker set to include only 1% of cells stained with control antibody).
Figure 1.
Detection of CC chemokine receptor 3 (CCR3) expression on bone marrow CD34+ cells from an atopic asthmatics by multigating flow cytometry. Plots (a)–(d) represent staining of bone marrow low-density non-adherent mononuclear cells with CD45–fluorescein isothiocyanate (FITC)/CD34–phycoerythrin (PE). Details of the gating strategy are described in the Materials and methods, and have been published.5 Events in region R4 (true progenitor cells) were further analysed for staining with peridinin chlorophyll protein (PerCp)-linked CCR3 monoclonal antibody (mAb) (e) or isotype control (f). Data were collected as the number of positive cells at the 99% confidence limit (i.e. relative to a marker set to include only 1% of cells stained with control antibody).
In situ hybridization and immunohistochemistry
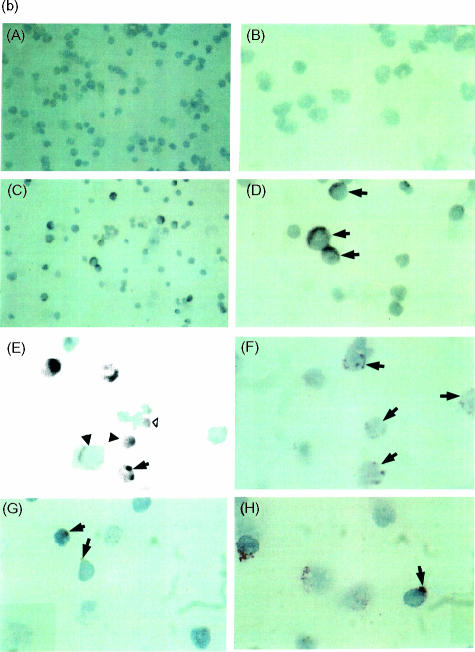
To confirm the cell-surface expression of CCR3 on CD34+ progenitor cells detected by flow cytometry, we co-localized CCR3 messenger RNA to CD34 immunopositive cells by sequential non-radioactive in situ hybridization (ISH) and immunocytochemistry (ICC), as previously described.24 Briefly, a population of unstimulated CD34+ cells was enriched from a bone marrow aspirate by positive selection using magnetic antibody cell sorting (MACS) (purity determined by flow cytometry to be >85%), and cytospin preparations were prepared as previously described.5 Peripheral blood eosinophils, purified by negative selection using CD16-coated MACS beads as previously described,25 were used as a positive control for detection of CCR3 mRNA. The cell phenotypes were first identified by ICC using an alkaline phosphatase–anti-alkaline phosphatase (APAAP) technique and phenotype-specific murine anti-human mAb (CD34; Dako, High Wycombe, UK) (EG2+ Pharmacia). After developing with Fast Red for CD34+ immunostaining, slides were hybridized with 200 ng of digoxigenin-labelled riboprobes for CCR3 (antisense and sense) in hybridization buffer at 50° overnight, then washed in 2 × saline sodium citrate (SSC), 1 × SSC and 0·5 SSC, respectively; unhybridized probe was removed by digestion with RNAse A.24 After blocking with 2% normal sheep serum, the slides were incubated with sheep anti-digoxigenin Fab fragment-conjugated alkaline phosphatase (1 : 200 dilution; Boehringer Mannheim, Mannheim, Germany) at room temperature for 3 hr. The signals were developed with BCIP/NBT (X-phosphate-5-bromo-4-chloro-3-indoly phosphate/nitro-blue tetrazolium). Positive cells expressing phenotypic markers (red colour), mRNA for CCR3 (dark blue colour), or both (mixed colours), were counted (Fig. 2b). A minimum of 200 cells were counted per slide.
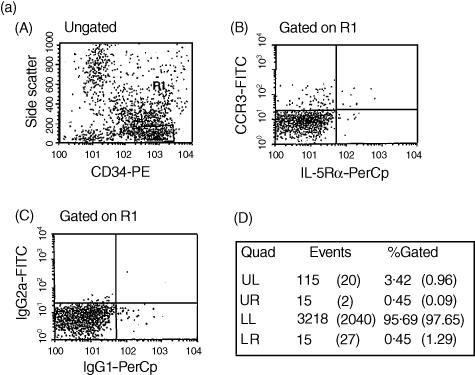
Figure 2.
(a) Detection of CC chemokine receptor 3 (CCR3) and interleukin-5 receptor alpha-subunit (IL-5Rα) co-expression on CD34+ cells by flow cytometry. An enriched population of CD34+ cells (purity >85%) from bone marrow non-adherent mononuclear cells (NAMNC) was collected by a magnetic cell separation technique. Cells were stained with CD34–phycoerythrin (PE), anti-CCR3–fluorescein isothiocyanate (FITC) and anti-IL-5Rα–peridinin chlorophyll protein (PerCp). (A) CD34-positive staining events were gated (gate R1); (B) cells within R1 were assessed for co-expression of both CCR3 and IL-5Rα; (C) a 99·9% confidence limit was set for this rare cell type (>0·1% staining with isotype-control antibodies); (D) region statistics show that of the total number of CD34+ cells counted, 3·42% were CD34+ CCR3+, 0·45% were CD34+ IL-5Rα+ and 0·45% were CD34+ CCR3+ IL-5Rα+ (isotype-control values are expressed in parenthesis). The proportion of CD34+ CCR3+ IL-5Rα+ cells represented 11% of the CD34+ CCR3+ cells and 50% of the CD34+ IL-5Rα+ cells in this sample. (b) Co-localization of mRNA for CCR3 to CD34+ cells using sequential immunocytochemistry (ICC) and in situ hybridization (ISH) on cytospins of bone marrow-derived CD34+ cells from an atopic asthmatic subject. (A)–(D) Staining with Dig-labelled CCR3 sense (A, B) and antisense (C, D) riboprobes, at low (magnification × 400; a and c) and high (magnification × 1000; b and d) power. Some of the CCR3 mRNA+ cells (dark blue) are indicated (→). (E) A combination of ICC and ISH in eosinophils (positive control cells) was performed. Single EG2+ stained red is indicated (▵) and single ISH+ (CCR3 mRNA) are dark blue (▴). Double positive cells are dark red (→). (F) Cytospins of purified bone marrow CD34+ cell ICC showing CD34+ cells (→). (G) Single ISH of CCR3 mRNA+ cells (→). (H) Double ICC/ISH of CD34+/CCR3+ cells (→).
Transmigration assay
The migrational response of progenitors in vitro was assessed using transwell chambers (24-well cell clusters with 5-µm pore polycarbonate filters (Costar, Boston, MA), as previously described with minor modifications.26 An enriched population of bone marrow CD34+ cells (purity >85%), isolated from atopic asthmatic subjects by MACS,3 were loaded into each filtered transwell insert (1 × 105/100 µl). These transwell inserts were then placed in a larger well containing 600-µl dilutions of the chemoattractant in serum-free medium, previously incubated for 15 min at 37° in 5% CO2. After 4 hr, the filtered transwell insert was carefully removed and the cells in the bottom chamber (representing migrated cells) were aspirated. These cells were resuspended in FACS staining buffer and stained with PE-conjugated CD34 mAb and FITC-conjugated CD45 mAb, as described above. To obtain absolute values of migratory cells, flow cytometric counts for each sample were obtained during a constant predetermined time period. Data for the enumeration of CD34+ cells are expressed as the percentage of total numbers of CD45+ cells counted in the bottom chamber.
Colony-forming assay
Bone marrow-derived NAMNC were isolated, as described above, and cultured in duplicate in 0·9% methylcellulose at 0·25 × 106 cells per 35 × 10-mm tissue culture dish (Falcon Plastics, Oxnard, CA) in Iscove's modified Dulbecco's medium containing 20% FCS, 1% penicillin-streptomycin and 5 × 10−5 m 2-mercaptoethanol in the presence of various cytokine growth factors, including IL-5 and eotaxin. Cultures were incubated for 14 days at 37° and 5% CO2 and then enumerated by light microscopy. Eo/Baso-CFUs were identified by their distinct morphology, previously described as tight granulated, compact round refractile cell aggregates.23
Statistical analysis
The data are presented as arithmetic mean ± standard error of the mean (SEM), except PC20 values and absolute numbers of CD34+ progenitor cells, which were logarithmically transformed and are expressed as geometric mean and standard error of geometric mean (%SEM). For statistical analyses of within-group comparisons between pre- and postallergen challenge time-points, a paired Student's t-test (two-tailed) was performed. Student's non-paired t-tests (two-tailed) were performed for all between-group comparisons. Changes in blood and sputum differentials at several time-points after allergen, and data from the transmigration and priming assays, were assessed using repeated measures analysis of variance (rm anova; Statistica version 5·1, Tulsa, OK). Significance was accepted at the 95% confidence level.
Results
Allergen-induced changes in airway physiology
Allergen-induced changes in airway responses (bronchoconstrictor and methacholine PC20) for all asthmatic subjects in this study are summarized in Table 1. Compared with preallergen levels, a significant increase in methacholine airway responsiveness 24 hr after inhaled allergen was detected in DR only (P < 0·005). In addition, there was a significant difference between ER and DR in the allergen-induced shift in log PC20 values (P < 0·05) (Table 1).
Allergen-induced changes in sputum, blood and bone marrow eosinophils
Allergen-induced changes in sputum, blood and bone marrow eosinophil numbers are summarized in Table 2. A significant increase in the absolute number of sputum eosinophils was found in both ER and DR when preallergen levels were compared with those at 7- and 24-hr postallergen inhalation challenge. However, the magnitude of the allergen-induced increase in sputum eosinophil numbers at 7 and at 24 hr was significantly greater in DR compared with ER (P < 0·05) (Table 2). In the peripheral blood there was a significant increase in eosinophil numbers at 24 hr postallergen challenge compared to preallergen levels in DR only (P < 0·005). In contrast, no significant change in bone marrow eosinophil numbers was detected in either ER or DR when 24-hr postallergen levels were compared with preallergen levels (Table 2).
Table 2.
Allergen-induced changes in sputum, peripheral blood and bone marrow eosinophils
| Early responder asthmatics | Dual responder asthmatics | |
|---|---|---|
| Sputum | (n = 7) | (n = 10) |
| ″Pre-allergen | 8 ± 1 | 21 ± 4 |
| ″7 hr post-allergen | 54 ± 21** | 147 ± 19**,† |
| ″24 hr post-allergen | 32 ± 12** | 80 ± 8**,† |
| Peripheral blood | (n = 7) | (n = 10) |
| ″Pre-allergen | 33 ± 7 | 25 ± 3 |
| ″24 hr post-allergen | 34 ± 8 | 42 ± 4** |
| Bone marrow | (n = 6) | (n = 5) |
| ″Pre-allergen | 2.9 ± 0.8% | 3.8 ± 1.6% |
| ″24 hr post-allergen | 2.5 ± 0.6% | 3.5 ± 1.4% |
Data in the sputum and blood are presented as cells/104 per ml and as a percentage of total WBC in the bone marrow. In sputum, significant increases from baseline in eosinophils were detected at 7 hr and 24 hr post-allergen in both early- and dual- responder asthmatics. However, the allergen-induced shift at 7 hr and 24 hr was greater in dual- compared with early- responder asthmatics. A significant increase in blood eosinophil numbers was detected 24 hr post-allergen inhalation challenge in dual responder asthmatics only. There were no significant allergen-induced changes in bone marrow eosinophils in either group of asthmatic subjects when pre-allergen were compared with 24 hr post-allergen levels
P < 0.005 for within group analysis
P < 0.05 for between group analysis.
CCR3 expression on progenitor cells
CCR3 expression was detected on bone marrow CD34+ progenitor cells from atopic asthmatic subjects, as determined by flow cytometry (Fig. 1). In addition, to investigate the expression of CCR3 on eosinophil-lineage committed progenitors, we performed triple staining on an enriched population of bone marrow CD34+ cells (purity >85%). As shown in Fig. 2, expression of CCR3 was detected on CD34+ IL-5Rα+ cells. In this experiment, the number of CD34+ CCR3+ IL-5Rα+ cells represented 11% of the CD34+ CCR3+ cells and 50% of the CD34+ IL-5Rα+ cells. Similar findings were obtained in three separate experiments, indicating that CCR3 is expressed by both primitive and lineage-committed progenitor cells.
Allergen-induced changes in the phenotype of progenitor cells from bone marrow and peripheral blood
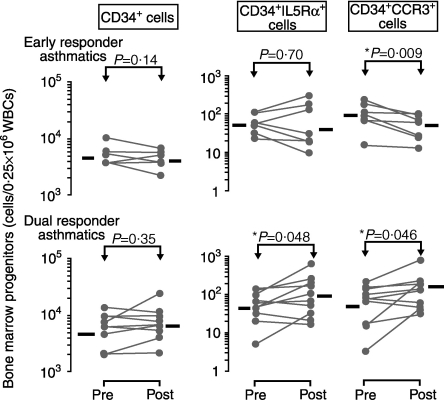
In accordance with previously published findings, no significant change in the total number of bone marrow CD34+ cells was found when preallergen levels were compared with 24-hr postallergen levels in either group of asthmatics [DR: 4898 (%SEM 720) to 5623 (%SEM 910); ER: 5129 (%SEM 628) to 4169 (%SEM 510) cells/0·25 × 106 white blood cells (WBC)] (Fig. 3).5 A significant increase in the absolute number of CD34+ IL-5Rα+ cells when preallergen levels were compared with 24-hr postallergen levels in bone marrow samples from DR [46 (%SEM 9) to 83 (%SEM 18) cells/0·25 × 106 WBC; P < 0·05], but not in ER [55 (%SEM 9) to 48 (%SEM 26) cells/0·25 × 106 WBC], was also detected (Fig. 3).
Figure 3.
Allergen-induced changes in CD34+ cells and CD34+ cells co-expressing interleukin-5 receptor alpha-subunit (IL-5Rα) and CC chemokine receptor 3 (CCR3) in bone marrow aspirates taken from early responder (n = 7) and dual-responder (n = 10) asthmatics. Significant increases in both CD34+ IL-5Rα+ and CD34+ CCR3+ cell numbers were detected in dual responders 24 hr postallergen. In contrast, a significant attenuation in CD34+ CCR3+ cell number was detected in early responders. Horizontal bars represent the geometric mean of each data set. (For statistical significance, a Student's paired t-test was performed on log-transformed data.) WBCs, white blood cells.
In enumerating the expression of the eotaxin receptor, CCR3, on bone marrow CD34+ cells, we found similar levels of expression at baseline for both groups of asthmatic subjects (DR: 47 (%SEM 12) versus ER: 79 (%SEM 18) cells/0·25 × 106 WBC). When preallergen levels were compared with 24-hr postallergen levels, a significant increase in the absolute numbers of bone marrow CD34+ CCR3+ cells was detected in DR [47 (%SEM 12) to 100 (%SEM 19) cells/0·25 × 106 WBC, P < 0·05] (Fig. 3). In contrast, a significant decrease in the absolute number of CD34+ CCR3+ cells was detected in bone marrow samples from ER, 24 hr postallergen [79 (%SEM 18) to 38 (%SEM 8) cells/0·25 × 106 WBC, P < 0·01].
In blood samples, when preallergen levels were compared with 24-hr postallergen levels, there were no significant changes in the total numbers of progenitor cells or the phenotype of CD34+ cells in both groups of asthmatics (Table 3). However, in comparing pre-allergen blood levels of both CD34+ IL-5Rα+ and CD34+ CCR3+ cells, significantly greater numbers of both cell types were detected in DR, compared with ER, asthmatics (Table 3).
Table 3.
Summary of FACS data from peripheral blood
| Early responder asthmatics (n = 7) | Dual responder asthmatics (n = 10) | |
|---|---|---|
| CD34+ | ||
| ″Pre-allergen | 741 (%77) | 832 (%109) |
| ″24 hr post-allergen | 776 (%108) | 1068 (%89) |
| CD34+IL5Rα+ | ||
| ″Pre-allergen | 7 (%3) | 25 (%8)* |
| ″24 hr post-allergen | 15 (%6) | 18 (%6) |
| CD34+CCR3+ | ||
| ″Pre-allergen | 6 (%3) | 19 (%2)* |
| ″24 hr post-allergen | 5 (%2) | 17 (%6) |
Data are presented as cells/106 WBC and expressed as geometric mean and standard error of geometric mean (%SEM). Significantly higher baseline (pre-allergen) levels of CD34+ IL5Rα+ and CD34+CCR3+ cells were detected in the blood of dual responder compared with early responder asthmatics. However, when pre-allergen were compared with 24 hr post-allergen levels, no significant change in numbers of blood CD34+IL5Rα+ and CD34+CCR3+ cells were detected in either group of asthmatics.
P < 0.05 for between group comparisons of baseline levels of progenitor cells.
In situ hybridization and immunohistochemistry
Co-localization experiments using simultaneous in situ hybridization and immunocytochemistry were performed to confirm that CD34+ progenitor cells express mRNA for CCR3. Non-radioactive in situ hybridization showed that 56·9% of bone marrow CD34-immunopositive cells expressed CCR3 mRNA. A representative example from an atopic subject is shown in Fig. 2(b).
Transmigration assay
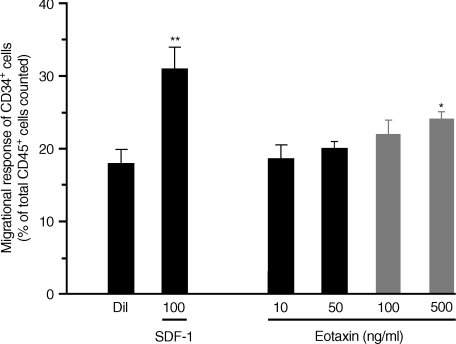
In order to assess the functional role of CCR3 expression on CD34+ cells from atopic asthmatic subjects (n = 4), an in vitro migrational assay was used (Fig. 4). The CXC chemokine, SDF-1 (optimal concentration 100 ng/ml), previously described as a potent progenitor cell chemoattractant, was included in this assay as a positive control.26 Compared with diluent, a significant (but weak) dose-dependent migratory response was detected for bone marrow CD34+ cells stimulated by eotaxin, which was optimal at 500 ng/ml (diluent: 18 ± 2% versus eotaxin (500 ng/ml): 24 ± 1% of CD45+ cells in the bottom well, n = 6, P < 0·05) (Fig. 4). Eotaxin (500 ng/ml) stimulated a migratory response of 4 ± 2·5% CD34+ cells and this was comparable with the level of CCR3 expression detected on an enriched population of bone marrow CD34+ cells by flow cytometry (CCR3 expression on 3·42% of CD34+ cells, Fig. 2).
Figure 4.
Transwell migrational assay of bone marrow CD34+ cells, in vitro. Compared with diluent control, stromal cell derived factor-1 (SDF-1) and eotaxin stimulated a significant dose-dependent migrational response of CD34+ cells, which was optimal at 100 ng/ml and 500 ng/ml, respectively (*P < 0·05; **P < 0·01). The eotaxin-, but not SDF-1-stimulated progenitor cell migratory response was completely attenuated in the presence of anti-CC chemokine receptor 3 (CCR3). Data are expressed as mean ± standard error of the mean (SEM) of six separate experiments performed in duplicate.
In the presence of a neutralizing CCR3 mAb (25 µg/ml), eotaxin-stimulated, but not SDF-1-stimulated, CD34+ cell migration was completely abrogated (Fig. 4). In three separate experiments, there was no appreciable migratory response of progenitor cells stimulated by IL-5 over a wide concentration range (5–500 ng/ml) compared with diluent control.
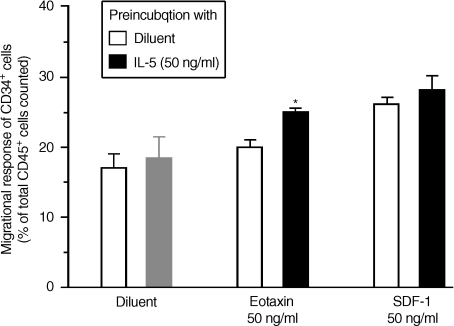
In priming experiments where an enriched population of bone marrow CD34+ cells were preincubated with IL-5 (50 ng/ml) for 1 hr at 37°, we found a significant increase in the subsequent migratory responses of CD34+ cells to suboptimal concentration of eotaxin (50 ng/ml, P < 0·05) (Fig. 5). In contrast, preincubation with IL-5 did not stimulate the migrational response of progenitor cells to suboptimal doses of SDF-1 (50 ng/ml). The priming effect of IL-5 on the migrational responses of eotaxin-mediated progenitor cells was not enhanced further by longer incubation (either 4 or 24 hr) with CD34+ cells.
Figure 5.
Interleukin-5 (IL-5) priming of bone marrow CD34+ cell migratory responses in vitro. Preincubation with IL-5 (50 ng/ml) for 1 hr at 37° significantly increased the migratory response of CD34+ to a suboptimal dose of eotaxin (50 ng/ml, *P < 0·05) but not suboptimal doses of SDF-1 (50 ng/ml) or diluent. Data are expressed as mean ± standard error of the mean (SEM) of six separate experiments performed in duplicate.
Colony-forming assay
In 14-day methycellulose cultures of bone marrow NAMNC, eotaxin, over a wide concentration range (1–500 ng/ml) did not stimulate the significant growth of either Eo/Baso or granulocyte–macrophage (GM) colonies compared with diluent control (Table 4). In addition, in cultures of NAMNC with optimal concentrations of IL-5 (1 ng/ml), the addition of eotaxin did not further increase the number of Eo/B-CFU detected.
Table 4.
Hemopoietic activity of eotaxin
| Eo/Baso-CFU | GM-CFU | |
|---|---|---|
| Diluent | 0 | 0 |
| Eotaxin (1–500 ng/ml) | 0 | 0 |
| IL-5 (1 ng/ml) | 12.5 ± 2** | 0 |
| IL-5 (1 ng/ml) + Eotaxin (1 ng/ml) | 12.0 ± 1** | 0 |
| IL-5 (1 ng/ml) + Eotaxin (10 ng/ml) | 11.5 ± 3** | 0 |
| IL-5 (1 ng/ml) + Eotaxin (100 ng/ml) | 12.0 ± 2** | 0 |
Data are presented as Eo/Baso-CFU or GM-CFU detected in 14 day methycellulose colonies for 0.25×106 BM-derived NAMNC plated as previously described 6. Eotaxin (1–500 ng/ml) did not stimulate the growth of either Eo/Baso-CFU or GM-CFU. In addition, Eotaxin did not enhance the numbers of Eo/Baso-CFU grown in the presence of previously determined optimal concentrations of either IL-5 (1 ng/ml).
P < 0.01 for comparisons between diluent and cytokine growth factor-stimulated colonies.
Discussion
The findings from this study confirm that:
the CC chemokine receptor, CCR3, is expressed on human primitive (CD34+) and eosinophil lineage-committed (CD34+ IL-5Rα+) progenitors;
the number of bone marrow CD34+ CCR3+ cells is significantly increased in DR asthmatics (who characteristically develop a late-bronchoconstrictor response, sputum and blood eosinophilia and increased methacholine airway responsiveness 24 hr postallergen inhalation);
a significant attenuation in the number of CD34+ CCR3+ cells was observed in ER asthmatics who do not develop a blood eosinophilia and airway hyperresponsiveness following allergen inhalation;
using an in vitro transwell migration assay to assess the function of CCR3 on progenitor cells, eotaxin stimulates a migratory response by CD34+ cells; and
preincubation with IL-5, which itself does not stimulate CD34+ cell migration, significantly primed the migratory response of CD34+ cells to eotaxin.
Together, these findings suggest that following allergen-inhalation challenge in asthmatics, upregulation of CCR3 expression on CD34+ cells may facilitate chemokine-mediated egress of eosinophil-lineage committed progenitors from the bone marrow to the blood, thereby promoting the development of an eosinophilic response, a distinctive feature of allergic inflammation.
Eotaxin, first described as a potent eosinophil-selective chemotactic activity, was identified by microsequencing as a CC chemokine27–29 and shown to mediate its effect through ligation of a specific receptor, CCR3.30–32 Although several ligands for CCR3 have been described, including regulated on activation, normal, T-cell expressed, and secreted (RANTES), monocyte chemotactic peptide (MCP)- 2, -3 and -4, it is clear that only eotaxin and other, less potent, eosinophil-selective chemoattractants (eotaxin-2 and eotaxin-3) mediate their agonist effects mainly through ligation of CCR3.33 To date, the expression of CCR3 has been shown to be restricted to mature eosinophils and, to a lesser extent, to basophils, some T helper 2 (Th2) cells, mast cells34 and dendritic cells.35 Although the expression of CCR3 mRNA has been co-localized to mature and immature eosinophils in bone marrow samples, the expression of CCR3 on CD34+ progenitor cells in asthmatic subjects has not been reported to date.36 Studies on the sequence of expression of CCR3 on eosinophils have described this receptor as a late differentiation marker.17,37 However, these studies were performed on transformed leukaemic cell lines, including HL-60 clone 15 cells and AML14 cells, which may sometimes provide different results from findings with freshly isolated cells. Using flow cytometry and limiting-dilution assays to assess eotaxin-stimulated cell proliferation, the expression of CCR3 has been reported on primitive progenitor cells from murine bone marrow.38 We confirm the expression of CCR3 on human bone marrow and blood CD34+ progenitor cells, at the protein level by flow cytometry and at the mRNA level by sequential immunocytochemistry and in situ hybridization (Fig. 2b). We have previously shown that CD34+ cells can express membrane-bound IL-5Rα, and suggested that this may be the phenotype of the earliest eosinophil lineage-committed progenitor cell.5 As we have, in the present study, shown for the first time that CCR3 is co-expressed on a subset of CD34+ IL-5Rα+ cells (Fig. 2), we propose that this may be the phenotype of an eosinophil lineage-committed progenitor cell that has the potential to respond to a chemokine signal and egress from the bone marrow sinus into the peripheral circulation during an allergic inflammatory response. This may account for our findings that increased numbers of both CD34+ CCR3+ and CD34+ IL-5Rα+ cells were detected in the blood of DR, compared with ER, asthmatics (Table 3).
Eosinophils arise from CD34+ pluripotent stem cells that differentiate and mature under the influence of lineage-specific growth factors such as IL-5.39 An important step in the selective recruitment of eosinophils to sites of allergic inflammation is the mobilization of mature and immature eosinophil pools from the bone marrow sinuses into the peripheral circulation. This traffic has been shown to be mediated by IL-5, acting systemically, and eotaxin, acting locally, at the site of inflammation.40–42 Although in some animal models eotaxin has been shown to have myeloproliferative properties,38,43 we were unable to detect any colony-forming activity of eotaxin on bone marrow CD34+ cells, either alone or in combination with stem cell factor or IL-5 in 14-day methylcellulose cultures (Table 4). It has been shown that in guinea-pigs eotaxin acts systemically to activate the bone marrow and stimulate mobilization of both mature eosinophils and eosinophil progenitors: using an in situ perfusion system of guinea-pig femoral bone marrow, eotaxin infusion into the arterial supply stimulated not only a rapid selective release of eosinophils into the draining vein but the release of eosinophil colony-forming progenitor cells as well.15 In contrast, infusion of IL-5 stimulated the delayed and sustained release of mature eosinophils, but not of eosinophil progenitors. In the present study, we used an in vitro transwell migration assay to investigate the role of the CCR3 receptor on human CD34+ progenitor cells. We showed that ligation of the CCR3 receptor resulted in a migratory response by bone marrow CD34+ cells to eotaxin and that the proportion of progenitors that migrated to eotaxin was comparable with the level of CCR3 expression detected on enriched CD34+ cells (Fig. 4). The migrational response elicited by eotaxin was modest compared with that elicited by SDF-1, previously described as a potent progenitor cell chemoattractant.26 Our findings with eotaxin are in contrast to results reported by Auiti et al. who showed a negligible chemotactic effect of eotaxin on CD34+ cells isolated from normal donors.26 This disparity is further supported by our recent findings that CD34+ cells from atopic asthmatics have a significantly greater migrational response to eotaxin than progenitor cells from normal subjects.44 Based on our findings that IL-5 can prime a CD34+ cell migratory response to eotaxin (Fig. 5), it is probable that elevated levels of IL-5 in atopic asthmatics compared with normal subjects may cause in vivo priming, resulting in the observed migratory responsiveness of CD34+ cells from atopic asthmatics to eotaxin.45
In the present study, we have shown that although no changes in the total numbers of CD34+ cells were detected in the bone marrow, the number of CD34+ CCR3+ and CD34+ IL-5Rα+ cells were significantly increased in DR 24 hr postallergen (Fig. 3). We propose that whereas upregulation of IL-5Rα on CD34+ cells within the bone marrow may favour increased eosinophilopoiesis within that microenvironment, the upregulation of CCR3 on CD34+ cells may favour increased traffic of progenitor cells from the bone marrow to the peripheral circulation in the presence of a positive concentration gradient of chemokines, such as eotaxin. The inability to detect similar significant changes in the blood at 24 hr may reflect the speed of homing of the progenitors or the dilutional effect of the blood (Table 3). Moreover, the kinetics of change in the progenitor cell population may be different within the blood compared to the bone marrow compartment; this is the subject of an ongoing study.
In long-term culture studies, it has been shown that IL-5 upregulates CCR3 expression on differentiating eosinophils within 3–7 days.37,46 Relevant to this we have recently reported that mRNA levels of IL-5 are significantly increased within the bone marrow of DR compared with ER asthmatics 24 hr postallergen challenge.47 It is therefore probable that local increases in IL-5 alone or in combination with other pro-inflammatory factors within the bone marrow may upregulate CCR3 expression on CD34+ progenitor cells from DR asthmatics. Conversely, our findings of significantly lower levels of blood-derived CD34+ CCR3+ and CD34+ IL-5Rα+ cells in ER compared with DR asthmatics (Table 3), and downregulation of CCR3 expression on CD34+ cells from ER asthmatics in response to allergen challenge (Fig. 3), may reflect an active means of preventing progenitor-cell mobilization from the bone marrow sinuses. Studies have shown that eotaxin treatment of differentiated acute myeloid leukemia (AML) cells results in marked downregulation of CCR3 expression for at least 18 hr,17 and that IL-3 causes a significant downregulation of CCR3 expression on eosinophils.48 Whether increased local generation of eotaxin, or possibly IL-3, within the bone marrow of ER compared with DR asthmatics causes the ligand-mediated internalization of CCR3 and thus the observed significant attenuation of CD34+ CCR3+ cell numbers postallergen, still remains to be investigated.
Under normal conditions of haemopoiesis, the bone marrow acts as a site for the turnover and traffic of mature leucocytes to the peripheral circulation. However, in inflammatory conditions, such as atopic asthma, in addition to the increased release of mature eosinophils, increased egress of primitive and eosinophil-lineage committed progenitor cells occurs. Although the precise mechanisms which trigger progenitor cell traffic remain to be fully elucidated, this study provides evidence to support the hypothesis that, through upregulation of CCR3 in vivo, increased responsiveness to an eotaxin gradient may result in the mobilization of eosinophil progenitors from the bone marrow to the peripheral circulation during an allergic inflammatory response. Our results also point out the likelihood that the effects of both IL-5 and eotaxin may have to be abrogated if eosinophil inflammation is to be fully controlled.
Acknowledgments
The authors gratefully acknowledge financial support from the Canadian Institutes for Health Research and the Wellcome Trust Foundation, UK. We are grateful to Lorna Wood for her role in recruitment of patients for this study, Tracey Rerecich for sputum processing and Qiu Meng for technical support in performing in situ hybridization. Roma Sehmi is a recipient of a Centennial Research Fellowship from the Canadian Institutes for Health Research.
Abbreviations
- APAAP
alkaline phosphatase–anti-alkaline phosphastase
- BrdU
bromodeoxy uridine
- CCR3
CC chemokine receptor 3
- CFU
colony-forming units
- DR
dual responders
- ER
early responders
- FACS
fluorescence activated cell sorter
- FCS
fetal calf serum
- FEV1
forced expiratory volume in 1 second
- FITC
fluorescein isothiocyanate
- ICC
immunocytochemistry
- IL-5Rα
interleukin-5 receptor alpha-subunit
- ISH
in situ hybridization
- mAb
monoclonal antibody
- MACS
magnetic antibody cell sorting
- MNC
mononuclear cells
- NAMNC
non-adherent mononuclear cells
- PE
phycoerythrin
- PerCp
peridinin chlorophyll protein
- SDF-1
stromal cell derived factor-1
- SSC
orthogonal or side light scatter
References
- 1.Sehmi R, Denburg JA. Differentiation of human eosinophils: role in allergic inflammation. In: Marone G, editor. Human Eosinophils Biological and Clinical Aspects. 76. Vol. 2. Basel: Karger; 2000. pp. 29–44. [PubMed] [Google Scholar]
- 2.Cyr MM, Denburg JA. Systemic aspects of allergic disease: the role of the bone marrow. Curr Opin Immunol. 2001;13:727–32. doi: 10.1016/s0952-7915(01)00286-2. [DOI] [PubMed] [Google Scholar]
- 3.Sehmi R, Howie K, Sutherland DR, Schragge W, O'Byrne PM, Denburg JA. Increased levels of CD34+ hemopoietic progenitor cells in atopic subjects. Am J Respir Cell Mol Biol. 1996;15:645–54. doi: 10.1165/ajrcmb.15.5.8918371. [DOI] [PubMed] [Google Scholar]
- 4.Sehmi R, Howie K, Rerecich T, Watson R, Foley R, O'Byrne PM, Denburg JA. Increased numbers of eosinophil progenitor cells (CD34+ IL5Rα+) in the bone marrow of atopic asthmatic subjects. J Allergy Clin Immunol. 2000;105:S172. [Google Scholar]
- 5.Sehmi R, Wood LJ, Watson RM, Foley R, Hamid Q, O'Byrne PM, Denburg JA. Allergen-induced increases in IL-5 receptor α-subunit expression on bone marrow-derived CD34+ cells from asthmatic subjects: a novel marker of progenitor cell commitment towards eosinophilic differentiation. J Clin Invest. 1997;100:2466–75. doi: 10.1172/JCI119789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wood LJ, Inman MD, Watson RM, Foley R, Denburg JA, O'Byrne PM. Changes in bone marrow inflammatory cell progenitors after inhaled allergen in asthmatic subjects. Am J Respir Crit Care Med. 1998;157:99–105. doi: 10.1164/ajrccm.157.1.9704125. [DOI] [PubMed] [Google Scholar]
- 7.Inman MD, Ellis R, Wattie R, Denburg JA, O'Byrne PM. Allergen-induced increases in airway responsiveness, airway eosinophilia and bone marrow eosinophil progenitors in mice. Am J Respir Crit Care Med. 1999;21:473–9. doi: 10.1165/ajrcmb.21.4.3622. [DOI] [PubMed] [Google Scholar]
- 8.Saito H, Matsumoto K, Denburg AE, et al. Pathogenesis of murine experimental allergic rhinitis: a study of local and systemic consequences of IL-5 deficiency. J Immunol. 2002;168:3017–23. doi: 10.4049/jimmunol.168.6.3017. [DOI] [PubMed] [Google Scholar]
- 9.Wood LJ, Inman MD, Denburg JA, O'Byrne PM. Allergen challenge increases cell traffic between bone marrow and lung. Am J Respir Cell Mol Biol. 1998;18:759–67. doi: 10.1165/ajrcmb.18.6.3006. [DOI] [PubMed] [Google Scholar]
- 10.Tomaki M, Zhao LL, Lundahl J, Sjöstrand M, Jordana M, Lindén A, O'Byrne PM, Lötvall J. Eosinophilopoiesis in a murine model of allergic airway eosinophilia: involvement of bone marrow IL-5 and IL-5 receptor α. J Immunol. 2000;165:4040–50. doi: 10.4049/jimmunol.165.7.4040. [DOI] [PubMed] [Google Scholar]
- 11.Kim YK, Uno M, Hamilos DL, Beck L, Bochner B, Schleimer R, Denburg JA. Immunolocalization of CD34 in nasal polyposis. Effect of topical corticosteroids. Am J Respir Cell Mol Biol. 1999;20:388–97. doi: 10.1165/ajrcmb.20.3.3060. [DOI] [PubMed] [Google Scholar]
- 12.Cameron L, Christodoulopoulos P, Lavigne F, et al. Evidence of local eosinophil differentiation within allergic nasal mucosa: inhibition with soluble IL-5 receptor. J Immunol. 2000;164:1538–45. doi: 10.4049/jimmunol.164.3.1538. [DOI] [PubMed] [Google Scholar]
- 13.Efthimiadis A, O'Byrne PM, Hargreave FE, Sehmi R. Induced sputum: elevated CD34+ progenitor cells in asthmatics. Am J Respir Crit Care Med. 2002;165:A482. [Google Scholar]
- 14.Robinson DS, Damia R, Zeibecoglou K, Molet S, North J, Yamada T, Kay AB, Hamid Q. CD34+/Interleukin-5Rα messenger RNA+ cells in the bronchial mucosa in asthma: potential airway eosinophil progenitors. Am J Respir Cell Mol Biol. 1999;20:9–13. doi: 10.1165/ajrcmb.20.1.3449. [DOI] [PubMed] [Google Scholar]
- 15.Palframan RT, Collins PD, Williams TJ, Rankin SM. Eotaxin induces a rapid release of eosinophils and their progenitors from the bone marrow. Blood. 1998;91:2240–8. [PubMed] [Google Scholar]
- 16.Stirling RG, van Rensen ELJ, Barnes PJ, Fan Chung K. Interleukin-5 induces CD34+ eosinophil progenitor mobilization and eosinophil CCR3 expression in asthma. Am J Respir Crit Care Med. 2001;164:1403–9. doi: 10.1164/ajrccm.164.8.2010002. [DOI] [PubMed] [Google Scholar]
- 17.Zimmermann N, Daugherty BL, Stark JM, Rothenberg ME. Molecular analysis of CCR-3 events in eosinophilic cells. J Immunol. 2000;164:1055–64. doi: 10.4049/jimmunol.164.2.1055. [DOI] [PubMed] [Google Scholar]
- 18.Morris JF, Koski A, Johnson LC. Spirometer standards for healthy non-smoking adults. Am Rev Respir Dis. 1997;103:57–67. doi: 10.1164/arrd.1971.103.1.57. [DOI] [PubMed] [Google Scholar]
- 19.O'Byrne PM, Dolovich J, Hargreave FE. Late asthmatic response. Am Rev Respir Dis. 1987;136:740–56. doi: 10.1164/ajrccm/136.3.740. [DOI] [PubMed] [Google Scholar]
- 20.Cockcroft DW, Murdock KY, Kirby J, Hargreave FE. Prediction of airway responsiveness to allergen from skin sensitivity to allergen and airway responsiveness to histamine. Am Rev Respir Dis. 1987;135:264–7. doi: 10.1164/arrd.1987.135.1.264. [DOI] [PubMed] [Google Scholar]
- 21.Cockcroft DW. Measurement of airway responsiveness to inhaled histamine or methacholine; method of continuous aerosol generation and tidal breathing inhalation. In: Hargreave FE, Woolcock AJ, editors. Airway Responsiveness: Measurement and Interpretation. Mississauga: Astra Pharmaceutical, Canada, Ltd; 1985. pp. 22–8. [Google Scholar]
- 22.Pizzichini E, Pizzichini MMM, Efthimiadis A, et al. Indices of airway inflammation in induced sputum. reproducibility and validity of cell and fluid-phase measurements. Am J Respir Crit Care Med. 1996;154:308–17. doi: 10.1164/ajrccm.154.2.8756799. [DOI] [PubMed] [Google Scholar]
- 23.Gibson PG, Manning PJ, O'Byrne PM, Girgis-Gabardo A, Dolovich J, Denburg JA, Hargreave FE. Allergen-induced asthmatic responses. Relationship between increases in airway responsiveness and increases in circulating eosinophils, basophils and their progenitors. Am Rev Respir Dis. 1991;143:331–5. doi: 10.1164/ajrccm/143.2.331. [DOI] [PubMed] [Google Scholar]
- 24.Ying S, Robinson DS, Meng Q, et al. Enhanced expression of eotaxin and CCR3 mRNA and protein in atopic asthma. Association with airway hyperresponsiveness and predominant co-localization of eotaxin mRNA to bronchial epithelial and endothelial cells. Eur J Immunol. 1997;27:3507–16. doi: 10.1002/eji.1830271252. [DOI] [PubMed] [Google Scholar]
- 25.Sehmi R, Cromwell O, Wardlaw AJ, Moqbel R, Kay AB. Interleukin-8 is a chemoattractant for eosinophils purified from subjects with a blood eosinophilia but not from normal healthy subjects. Clin Exp Immunol. 1993;23:1027–36. doi: 10.1111/j.1365-2222.1993.tb00295.x. [DOI] [PubMed] [Google Scholar]
- 26.Aiuti A, Webb IJ, Bleul C, Springer TJ. The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J Exp Med. 1997;185:111–20. doi: 10.1084/jem.185.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jose PJ, Griffith-Johnson DA, Collins PD, et al. Eotaxin: a potent eosinophil chemoattractant cytokine detected in a guinea pig model of allergic airways inflammation. J Exp Med. 1994;179:881–7. doi: 10.1084/jem.179.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jose PJ, Adcock IM, Griffith-Johnson DA, Berkman N, Wells TNC, Williams TJ, Power CA. Eotaxin: cloning of an eosinophil chemoattractant cytokine and increased mRNA expression in allergen challenged guinea-pig lungs. Biochem Biophys Res Commun. 1994;205:788–94. doi: 10.1006/bbrc.1994.2734. [DOI] [PubMed] [Google Scholar]
- 29.Ponath P, Qin S, Ringler I, et al. Cloning of the human eosinophil chemoattractant eotaxin: expression, receptor binding and functional properties provide a mechanism for the selective recruitment of eosinophils. J Clin Invest. 1996;97:604–12. doi: 10.1172/JCI118456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kitaura M, Nakajima T, Imai S, Harada C, Combadiere C, Tiffany L, Murphy PM, Yoshie O. Molecular cloning of human eotaxin, an eosinophil-selective CC chemokine and identification of a specific eosinophil eotain receptor, CC chemokine receptor 3. J Biol Chem. 1996;271:7725. doi: 10.1074/jbc.271.13.7725. [DOI] [PubMed] [Google Scholar]
- 31.Ponath P, Qin S, Post TW, et al. Molecular cloning and characterization of a human eotaxin receptor expressed selectively on eosinophils. J Exp Med. 1996;183:2437–48. doi: 10.1084/jem.183.6.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daugherty BL, Siciliano SL, Demartino JA, Malkowitz L, Sirotina A, Springer MS. Cloning, expression and characterization of the human eosinophil eotaxin receptor. J Exp Med. 1996;183:2349–54. doi: 10.1084/jem.183.5.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nickel R, Beck LA, Stellato C, Schleimer RP. Chemokines and allergic disease. J Allergy Clin Immunol. 1999;104:723–42. doi: 10.1016/s0091-6749(99)70281-2. [DOI] [PubMed] [Google Scholar]
- 34.Gutierrez-Ramos J-C, Lloyd C, Gonzalo JA. Eotaxin: from an eosinophilic chemokine to a major regulator of allergic inflammation. Immunol Today. 1999;20:500–4. doi: 10.1016/s0167-5699(99)01522-4. [DOI] [PubMed] [Google Scholar]
- 35.Beaulieu S, Du Robbiani DFX, Rodriguez E, et al. Expression of a functional eotaxin (CC chemokine ligand 11) receptor CCR3 by human dendritic cells. J Immunol. 2002;169:2925–36. doi: 10.4049/jimmunol.169.6.2925. [DOI] [PubMed] [Google Scholar]
- 36.Zeibecoglou K, Ying S, Yamada T, et al. Increased mature and immature CCR3 messenger RNA+ eosinophils in bone marrow from patients with atopic asthma compared with atopic and non-atopic control subjects. J Allergy Clin Immunol. 1999;103:99–106. doi: 10.1016/s0091-6749(99)70532-4. [DOI] [PubMed] [Google Scholar]
- 37.Tiffany HL, Alkhatib G, Combadiere C, Berger EA, Murphy PM. CC chemokine receptors 1 and 3 are differentially regulated by IL-5 during maturation of eosinophilic HL-60 cells. J Immunol. 1998;160:1385–92. [PubMed] [Google Scholar]
- 38.Peled A, Gonzalo JA, Lloyd C, Gutierrez-Ramos J-C. The chemotactic cytokine eotaxin acts as a granulocyte–macrophage colony-stimulating factor during lung inflammation. Blood. 1998;91:1909–16. [PubMed] [Google Scholar]
- 39.Sanderson CJ, Warren DJ, Strath M. Identification of a lymphokine that stimulates eosinophilic differentiation in vitro. Its relationship to interleukin 3 and functional properties of eosinophils produced in cultures. J Exp Med. 1985;162:60–74. doi: 10.1084/jem.162.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Collins PD, Marleau S, Griffith-Johnson DA, Jose PJ, Williams TJ. Co-operation between interleukin-5 and the chemokine eotaxin to induce eosinophil accumulation, in vivo. J Exp Med. 1995;182:1169–74. doi: 10.1084/jem.182.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mould AW, Matthaei KI, Young IA, Foster PS. Relationship between interleukin-5 and eotaxin in regulating blood and tissue eosinophilia in mice. J Clin Invest. 1997;99:1064–71. doi: 10.1172/JCI119234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li D, Wang D, Griffith-Johnson DA, Wells TNC, Williams TJ, Jose PJ, Jeffery PK. Eotaxin protein and gene expression in guinea-pig lungs. constitutive expression and upregulation after allergen challenge. Eur Respir J. 1997;10:1946–54. doi: 10.1183/09031936.97.10091946. [DOI] [PubMed] [Google Scholar]
- 43.Quackenbush EJ, Wershil BK, Aguirre V, Gutierrez-Ramos J-C. Eotaxin mediated myelopoiesis and mast cell development from embryonic hematopoietic progenitors. Blood. 1998;92:1887–97. [PubMed] [Google Scholar]
- 44.Dorman S, Post J, Foley R, O'Byrne PM, Sehmi R. Chemokine receptors on bone marrow CD34+ cells from atopic asthmatics compared with normal subjects: evidence for a dissociation between levels of receptor expression and migrational responsiveness of progenitors. Am J Respir Crit Care Med. 2003;167 [Abstract A642] [Google Scholar]
- 45.Sehmi R, Wardlaw AJ, Cromwell O, Kurihara K, Waltmann P, Kay AB. Interleukin-5 selectively enhances the chemotactic responsiveness of eosinophils obtained from normal but not eosinophilic subjects. Blood. 1992;79:2952–9. [PubMed] [Google Scholar]
- 46.Robinson DS, North J, Zeibecoglou K, et al. Eosinophil development and bone marrow and tissue eosinophilia in atopic asthma. Int Arch Allergy Appl Immunol. 1999;118:98–100. doi: 10.1159/000024039. [DOI] [PubMed] [Google Scholar]
- 47.Wood LJ, Sehmi R, Dorman S, et al. Allergen-induced increases in bone marrow T-lymphocytes and interleukin-5 expression in asthmatic subjects. Am J Respir Crit Care Med. 2002;166:883–9. doi: 10.1164/rccm.2108015. [DOI] [PubMed] [Google Scholar]
- 48.Dulkys Y, Kluthe C, Buschermohle T, Barg I, Knob S, Kapp A, Proudfoot A, Elsner J. IL-3 induces down-regulation of CCR3 protein and mRNA in human eosinophils. J Immunol. 2001;167:3443–53. doi: 10.4049/jimmunol.167.6.3443. [DOI] [PubMed] [Google Scholar]