Abstract
Activation of complement is a biological function of human C-reactive protein (hCRP), whereas rat CRP (rCRP) has been claimed to be unable to activate complement. As important biological functions of proteins are probably conserved among species, we re-evaluated, using various ligands, the capability of rCRP to activate complement. The activation of complement by hCRP and rCRP was investigated in solid- and fluid-phase systems. In the solid-phase system, purified CRP was fixed to enzyme-linked immunosorbent assay (ELISA) plates and incubated with human or rat recalcified plasma. Dose-dependent binding of human and rat C3 and C4 was observed to human and rat CRP, respectively. In the fluid-phase system, recalcified rat plasma, which contains about 500 mg/l of CRP, or human plasma supplemented with hCRP, were incubated with lyso-phosphatidylcholine. A dose-dependent activation of complement was observed upon incubation with this ligand, as reflected by the generation of activated C4 as well as of CRP–complement complexes. This activation was, in both cases, inhibited by preincubation of plasma with p-aminophosphorylcholine, a specific inhibitor of the interaction of CRP with its ligands, or by chelation of calcium ions. We conclude that rat CRP, similarly to human CRP, can activate autologous complement. These results support the notion that opsonization of ligands with complement is an important biological function of CRP.
Introduction
C-Reactive protein (CRP) is a pentraxin found in most vertebrates (e.g. mice, rats and humans) and in invertebrates such as the horseshoe crab (Limulus polyphemus).1–3 Human CRP (hCRP), discovered because it binds to pneumoccocal C-polysaccharide (CPS),4 is an acute-phase protein, of which plasma levels can increase up to 1000-fold following tissue damage or infection. CRP also binds to phosphorylcholine (PCh), which amongst others is found in membrane phospholipids. Among the effector functions exerted by CRP upon binding to ligands, is activation of complement. This activation proceeds via the classical pathway and occurs in vitro,2,5 as well as in vivo.6 Opsonization of its ligands with complement fragments is probably an important function of CRP.
In rats, CRP (rCRP) is not a typical acute-phase protein.7 However, in contrast to humans, rats have much higher plasma CRP concentrations under basal conditions, i.e. about 300–500 mg/l, which is 100 times higher than the concentration in humans.8 Remarkably, in contrast to hCRP, rCRP has been reported to be unable to activate complement,9,10 despite the fact that hCRP and rCRP share 70% amino acid homology.11 Complement activation by rCRP has only been studied using the C-polysaccharide of Streptococcus pneumoniae.9 Hence, it is possible that rCRP can activate complement upon binding to other ligands.
We recently developed assays for complexes of CRP and activated complement fragments, and showed that these complexes specifically reflect CRP-mediated complement activation.6 Moreover, significant levels of CRP–complement have been found in plasma samples, indicating that hCRP can activate complement in vivo. In the present study we investigated the binding of rat complement fragments to solid-phase bound CRP. In addition, we developed an assay for complexes between rat C3 and rCRP, and used this assay to re-evaluate the capability of rCRP to activate the complement system. Our results indicate that rCRP can activate autologous complement and support the hypothesis that activation of complement upon binding to a suitable ligand is an important biological function of this pentraxin.
Materials and methods
Materials
Cyanogen bromide (CNBr)-activated Sepharose 4B and Sephadex® G25 Fine were obtained from Amersham Pharmacia Biotech AB (Uppsala, Sweden). PCh, the calcium salt of PCh chloride, and 1-α-lyso-phosphatidylcholine (lyso-PC) were obtained from Sigma Chemical Co. (St Louis, MO). CPS was obtained from Statens Serum Institut (Copenhagen, Denmark). The molecular weight (MW) protein standard, 10-kDa protein ladder (10 000–200 000 MW) was from GibcoBRL, Life Technologies, Inc. (Gathersburg, MD). Streptavidin coupled to polymerized horseradish peroxidase (poly-HRP) was obtained from the Business Unit Immune Reagents of our institute (Sanquin). Streptavidin coupled to monomeric peroxidase (strept-PO) was purchased from Amersham Pharmacia Biotech. L-C-biotin-N-hydroxysuccimide ester was from Pierce Chemical Co. (Rockford, IL).
Plasma samples
Recalcified normal rat plasma (NRP) was prepared from rat blood collected in 10 mm EDTA. The blood was centrifuged for 10 min at 1300 g. The supernatant was then supplemented with CaCl2 to a final concentration of 12 mm. After incubation for 20 min at 37°, the fibrin clot was removed by centrifugation at 1300 g for 15 min at 4°. Finally, the recalcified plasma was stored in aliquots at −70° until required for use in activation experiments. Recalcified normal human plasma (NHP) was obtained in a similar manner.
Antibodies and proteins
Rabbit serum containing polyclonal antibodies against hCRP (KH61) was obtained from the Business Unit Immune Reagents of our institute. Mouse monoclonal antibodies (mAbs) against human C3d or C4d were as previously described.12 Notably, these antibodies also react with C3b and C3bi, and C4b and C4bi, respectively. Antibodies were purified using affinity chromatography on protein G–Sepharose (Pharmacia Fine chemicals, Uppsala, Sweden) according to the manufacturer's instructions, and biotinylated using standard procedures. The mouse mAb against rat C3/C3b/bi has been described previously.13 Human C1q was purified as described previously14 and biotinylated according to the instructions provided by Pierce. Aggregated human immunoglobulin G (AHG) was prepared as described previously.15
Purification of rat and human CRP
rCRP was purified from rat plasma collected in 10 mm EDTA from healthy Wistar rats. Ascites fluid, therapeutically collected from carcinoma patients, was used as a source for human CRP. For the purification of rat and human CRP, 4 vol of recalcified plasma, or ascites, were mixed with 1 vol of 5× binding buffer [2·5 m NaCl, 50 mm CaCl2, 10 mm MgCl2, 50 mm Veronal buffer, 0·5% (wt/vol) Tween-20, 0·5% sodium azide (all final concentrations) pH 7·4] and applied onto PCh bound to Sepharose. The PCh–Sepharose had been equilibrated with binding buffer [0·5 m NaCl, 10 mm CaCl2, 2 mm MgCl2, 10 mm Veronal buffer, 0·1% (wt/vol) Tween-20, 0·02% (wt/vol) sodium azide]. After incubation overnight at 4°, the gel was washed with binding buffer until the absorbance at 280 nm was < 0·04. Bound proteins were then eluted with phosphate-buffered saline, pH 7·4 (PBS), containing 10 mm EDTA. The collected fractions were dialysed against PBS and stored at 4°, until analysed using sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE; see below).
Preparation of rabbit antibodies against rCRP
rCRP was eluted from PCh–Sepharose using a PCh gradient (0–2 mm), as described previously.16 The fractions containing rCRP were pooled and subjected to preparative electrophoresis. Proteins were separated by SDS–PAGE (4–12% gel) and blotted onto polyvinylidene difluoride (PVDF) membrane, 0·45-µm pore size (Millipore Corporation, Bedford, MA). Two bands of 24 000 and 50 000 MW were excised, eluted as described previously,17 and used as immunogens.
Rabbits were immunized by intramuscular injection of 50 µg of purified rCRP emulsified in Freund's complete adjuvant (Difco Laboratories, Amsterdam, the Netherlands), followed by three booster injections of 50 µg of purified rCRP in Freund's incomplete adjuvant. Affinity chromatography was used to purify specific antibodies from the rabbit antisera. Four milligrams of rCRP was coupled to CNBr-activated Sepharose 4B, according to the manufacturer's instructions. The gel was then incubated with the antiserum, washed and layered on top of a Sephadex® G25 Fine gel-filtration column equilibrated in PBS. Bound antibodies were then eluted with 0·2 m glycine, pH 2·8. To remove contaminating antibodies, the affinity-purified anti-rCRP antibodies were subsequently absorbed with CRP-depleted rat plasma coupled to CNBr-activated Sepharose beads. CRP-depleted rat plasma was prepared by absorbing recalcified rat plasma onto PCh–Sepharose in the presence of 0·5 m NaCl.
One milligram of purified rabbit anti-rat CRP was biotinylated, using standard procedures, and tested for specificity in immunoblotting. NRP, or CRP-depleted rat plasma was electrophoresed on SDS polyacrylamide gels under non-reducing conditions, as described above. The proteins were then transferred onto PVDF membranes, 0·2-µm pore size (Novex, San Diego, CA).18 The membranes were then incubated for 1 hr in PBS containing 4% (vol/vol) cow's milk and subsequently for 90 min with biotinylated anti-rCRP, diluted 1 : 500 in PBS containing 0·4% (vol/vol) of milk and 0·1% (wt/vol) Tween-20 (J. T. Baker, Deventer, Holland). After washing in the same buffer, the membranes were incubated for 1 hr with strept-PO (diluted 1 : 500 also in the same buffer) and washed. Peroxidase was visualized by staining using the enhanced chemiluminescence (ECL) technique (Amersham-Pharmacia).
General procedure for enzyme-linked immunosorbent assays (ELISAs)
Nunc, Maxisorp plates (Roskilde, Denmark) were incubated overnight at room temperature with protein (antibodies or CRP) diluted in 0·1-m carbonate/bicarbonate buffer, pH 9·6. The final volume of this and the other steps was 100 µl. After washing the plates twice with PBS, residual binding sites were blocked for 1 hr at room temperature with PBS containing 2% (vol/vol) cow's milk. The plates were then washed with PBS containing 0·02% (wt/vol) Tween-20 (PBS-T). Samples to be tested were then incubated in the indicated buffer for 1 hr at room temperature. Plates were then washed with PBS-T and incubated with strept-PO, diluted 1 : 1000, or with poly-HRP, diluted 1 : 10 000, in PBS containing 2% (vol/vol) milk to block non-specific binding. Finally, plates were washed and the peroxidase activity was visualized by incubation with 3,3′,5,5′,-tetra-methyl-benzidine (TMB), 100 µg/ml in 0·11 m sodium acetate, pH 5·5, containing 0·003% (vol/vol) H2O2. The reaction was stopped after 10 min by the addition of 2 m H2SO4, and the absorbance at 450 nm was measured using a Titertek Multiscan plate reader (Flow Laboratories, McLean, VA).
ELISA for rCRP
Purified rabbit antibodies against rCRP (1 µg/ml) were used as catching antibodies. The samples to be tested were serially diluted in PBS containing 0·1% (wt/vol) Tween 20 and 0·2% (w/v) gelatin (PTG). Biotinylated anti-rCRP were diluted in ELISA-ultraperformance buffer supplemented with 10 mm EDTA. Plates were developed with strept-PO. Purified rCRP was used as a standard.
ELISA for activated rat C4
Activation of rat C4 was detected as described previously.19 Results were expressed as percentage of the maximal amount of activated C4 generated in rat serum by incubation with purified C1s (Calbiochem, La Jolla, CA). As the assay does not discriminate between C4b, C4bi or C4c, the activation product detected was referred to as C4b/c.
ELISA for rat CRP–C3 complexes
An in-house standard was prepared by incubating NRP with lyso-PC at 800 µg/ml, for 1 hr at 37°, and absorbing the mixture onto PCh–Sepharose for 3 hr at 4°. The Sepharose beads were washed extensively with veronal buffer containing 0·65 m NaCl, 20 mm CaCl2, 4 mm MgCl2 and 0·1% (wt/vol) Tween-20. CRP and CRP–complement complexes were then eluted from the Sepharose beads with PTG-25 mm EDTA, overnight at 4°. Samples to be tested were also absorbed onto PCh–Sepharose, using a similar procedure, except that they were not incubated with lyso-PC. The eluates of samples were stored at −70° until required for testing.
In the ELISA, rat C3 mAb was used as catching antibody. The samples to be tested, i.e. the eluates from the PCh–Sepharose (see above), were diluted in PTG containing 25 mm EDTA. Binding of CRP–C3 complexes was visualized with biotinylated rabbit anti-rCRP, as described above for the ELISA for rCRP. Bound anti-CRP was measured using poly-HRP.
Binding of biotinylated human C1q to solid-phase rCRP or hCRP
Rat or human CRP was incubated overnight in 0·1 m carbonate/bicarbonate buffer, pH 9·6, in ELISA plates. Incubation volumes were 100 µl. After blocking residual binding sites with 0·1% (wt/vol) bovine serum albumin (BSA) in PBS, the plates were washed with 50 mm NaCl, 20 mm Tris (pH 7·4), 10 mm CaCl2 and 0·1% Tween-20, and incubated for 1 hr at room temperature with biotinylated C1q diluted to 0·0625–50 µg/ml in the same buffer. After five washes with Tris buffer, the plates were incubated with poly-HRP diluted 1–10 000 in PBS containing 2% milk, for 20 min at room temperature. Finally, peroxidase activity was visualized and measured as described above.
Binding of rat C1q to solid-phase rCRP or hCRP
Different concentrations (0·25–10 µg/ml) of rCRP or hCRP were coated onto ELISA plates as described above. The plates were then washed twice with 50 mm NaCl, 20 mm Tris (pH 7·4), 10 mm CaCl2, 0·1% (wt/vol) Tween-20, and incubated with 0·1% (wt/vol) BSA in PBS to block residual binding sites. After one wash, serial dilutions of NRP in Tris (pH 7·4) containing 0·1% Tween-20, were incubated in the plates for 1 hr at room temperature. As a control, inactivated rat plasma (30 min, 56°) was also tested. Finally, the plates were incubated with rabbit anti-rat C1q in Tris–Tween buffer. Bound rabbit antibodies were detected by a subsequent incubation with poly-HRP, as described above.
Deposition of rat or human C3 on solid-phase rCRP or hCRP
To assess the complement-activating capability of CRP bound to a solid phase, ELISA plates were coated with 10 µg/ml of rat or human CRP, as described above. After this and the other incubations the plates were washed with PBS containing 0·1% (wt/vol) Tween-20. Residual binding sites on the plates were blocked by incubation for 1 hr at room temperature with BSA (0·1%, wt/vol) in PBS. After five washes, the plates were incubated for 1 hr at 37° with recalcified NHP or NRP appropriately diluted in veronal-buffered saline containing 5 mm CaCl2 and 1 mm MgCl2, pH 7·4 (VB2+), containing 0·1% (wt/vol) Tween-20. The deposition of rat or human C3b/c and C4b/c was detected by incubation, for 1 hr at room temperature, with biotinylated antibodies against rat or human C4b/c or C3b/c, respectively. Finally, the plates were incubated with strept-PO (diluted 1 : 1000) for 30 min at room temperature. The peroxidase activity was visualized with TMB, as described above.
Complement activation by hCRP in the fluid phase
Recalcified NHP (50 µl) (1 vol) was incubated for 1 hr at 37° with 1 vol of rat or human CRP (4, 20 or 50 µg/ml) in VB2+, 1 vol of VB2+ containing 800 µg/ml of lyso-PC, and 1 vol of VB2+. CRP (1 vol) preincubated for 1 hr with PCh (20 mm in 1 vol of VB) was used as a negative control. Then, 10-µl samples of the incubation mixtures were tested for complement-activation products in the ELISA for human C4b/c and C3b/c, respectively, as described by Wolbink et al.6
Complement activation by rCRP in the fluid phase
To study activation of the autologous complement system by native rCRP, 1 vol of recalcified NRP (containing 492 µg/ml CRP) was added to 1 vol of VB2+ containing different concentrations (0·1–0·8 mg/ml) of lyso-PC, and 2 vol of VB2+. The mixtures were then incubated for 1 hr at 37°. As a control, a similar procedure was performed, except that VB2+ containing 1 m NaCl, rather than VB2+, was used. As another negative control, NRP (1 vol) was incubated with 1 vol of lyso-PC, in the presence of PCh (20 mm in 1 vol of VB2+). The mixtures were then tested for C4b/c generation and for the presence of rCRP–C3 complexes. The effect of CPS on CRP-mediated complement activation in rat plasma was tested in a similar manner. To assess the specificity of complement–CRP complexes for CRP-induced complement activation, NRP was also incubated with AHG (1·25 mg/ml, final concentration) and tested for CRP–C3 complexes and generation of C4b/c.
Results
Purification and characterization of CRPs
Rat and human CRP were purified by affinity chromatography using PCh–, as described above in the Materials and methods. The apparent MW of both hCRP and rCRP was estimated on SDS–PAGE (data not shown). rCRP migrated under non-reducing conditions as two bands of 24 000 and 50 000 MW, whereas hCRP migrated as one band of 21 000 MW. Under reducing conditions, rCRP migrated as one band of 27 000 MW and hCRP as one band of 23 000 MW. The observed migration patterns fitted well with published data.7,9,16
Specificity of anti-rCRP
Rabbits were injected with rCRP purified by preparative SDS–PAGE, as described above in the Materials and methods. Specific antibodies were purified using affinity chromatography with CRP–Sepharose and absorbed onto CRP-depleted NRP coupled to Sepharose. The absorbed fraction was then biotinylated according to standard procedures. The specificity of the rabbit polyclonal anti-rCRP, purified in this way, was then analysed by Western blot. The antibodies reacted with 24 000-MW, 50 000-MW and high-MW bands of rCRP in the NRP. Similar bands were observed when purified CRP was tested (not shown). The high-MW bands correspond well with those observed by others in purified rCRP preparations.9 Depletion of NRP for CRP by adsorption onto PCh–Sepharose, completely abolished the reactivity of anti-rCRP with the depleted plasma. Hence, we concluded that the rabbit polyclonal antibodies against rCRP were specific, reacting with rCRP monomers and dimers. The specificity of the anti-rCRP was further supported by the observation that in double immunodiffusion, the antiserum was found to be completely identical to an antiserum against rat CRP, kindly provided by Dr M. B. Pepys (data not shown).
ELISA for rCRP
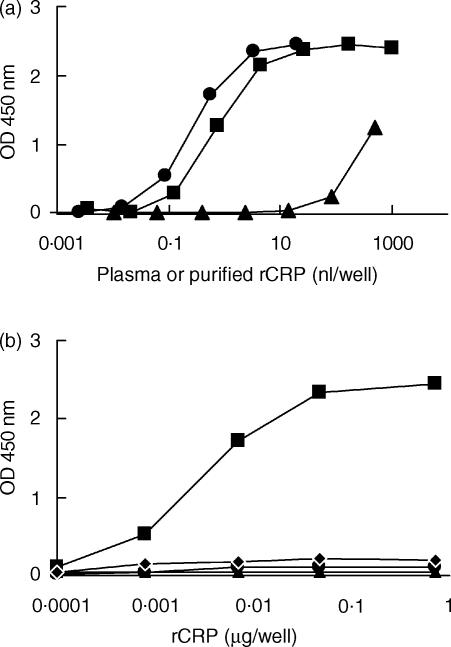
Using the polyclonal antibodies described above, an ELISA for rCRP was developed. The assay appeared to have a sensitivity of about 5 ng/ml rCRP, as assessed using purified rCRP. Figure 1(a) shows the dose–response curves of NRP and NRP depleted for CRP. Absorption onto PCh–Sepharose caused a shift of the dose–response curves of about 1000-fold, indicating that about 0·1% of rCRP was not removed by this procedure. The substitution of the catching anti-rCRP by rabbit immunoglobulin G (IgG) completely abrogated the response of purified rCRP or NRP in the ELISA, as did substitution of the biotinylated anti-rCRP with biotinylated normal rabbit IgG (Fig. 1b). These results demonstrated the specificity of the ELISA. The intra-assay coefficient of variation was less than 11% and that of the interassay coefficient was less than 16%.
Figure 1.
Enzyme-linked immunosorbent assay (ELISA) for rat C-reactive protein (rCRP). (a) Dose–response curves of normal rat plasma (▪), depleted rat plasma (▴), or purified CRP (•). (b) A control experiment in which the catching anti-rCRP or the detecting biotinylated anti-rCRP was replaced with normal rabbit immunoglobulin G (IgG) (▴) or biotinylated normal rabbit IgG (•), or both (⋄). Purified rat CRP was tested in each of these ELISAs. Results obtained with the normal ELISA are also shown (▪).
Complement activation by solid-phase CRP
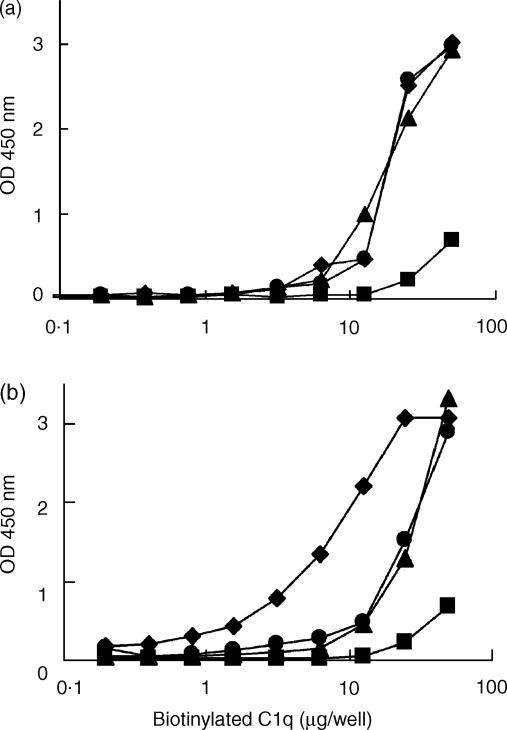
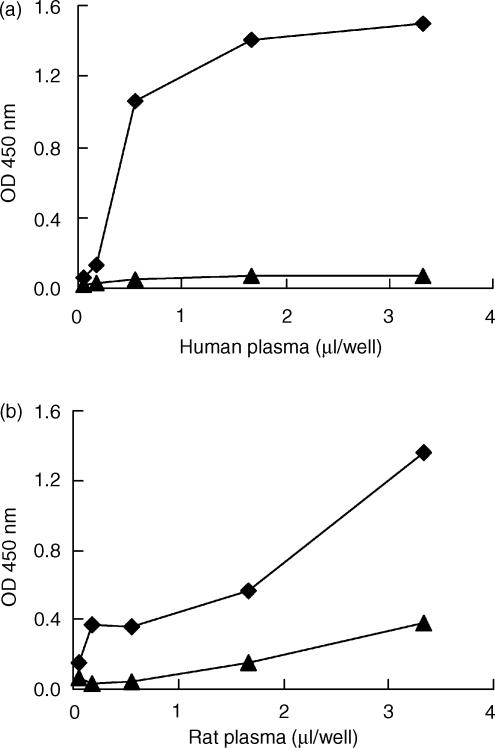
Rat and human CRP bound biotinylated human C1q. This binding was dose dependent for both C1q and CRP (Fig. 2). Purified rat C1q was not available. Binding of rat C1q to rCRP was tested in a different way, by incubation of CRP-coated plates with different dilutions of NRP. C1q binding was then assessed with biotinylated rabbit anti-rC1q. In this assay, rC1q appeared to bind to rCRP (see Fig. 3). Incubation of the rat plasma for 30 min at 56° completely abolished the binding of C1q (data not shown). Next, we investigated the deposition of rat or human C3b onto either rCRP or hCRP fixed onto the solid phase. Upon incubation with NRP or NHP, and using biotinylated mAbs against human C3b/c or rat C3b/c, deposition of activated complement factors on rCRP or hCRP was observed (Fig. 4). This deposition was completely abolished in the presence of 20 mm EDTA, indicating that deposition was caused by an activation process and not by non-specific binding of activated complement components to the CRP-coated plates.
Figure 2.
Binding of human C1q to human (a) or rat (b) C-reactive protein (CRP) fixed onto an enzyme-linked immunosorbent assay (ELISA) plate. CRP, at different concentrations (10 µg/ml, ⋄; 1 µg/ml, *; 0·5 µg/ml, ▴; or buffer, •), was coated onto ELISA plates. The coated plates were incubated with different dilutions (0·0625 to 50 µg/ml) of biotinylated human C1q. After incubation with streptavidin-polymerized horseradish peroxidase, the peroxidase activity was visualized with 3,3′,5,5′,-tetra-methyl-benzidine (TMB).
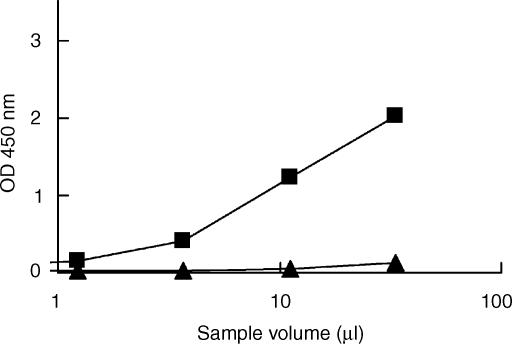
Figure 3.
Binding of rat C1q to rat C-reactive protein (rCRP). Different concentrations of rCRP (10 µg/ml, ⋄; 1·25 µg/ml, ▪; or 0·08 µg/ml, *) or buffer (•) were coated onto enzyme-linked immunosorbent assay (ELISA) plates and incubated with rat plasma. Bound C1q was detected with rabbit anti-rat C1q.
Figure 4.
Fixation of human or rat C3b on human or rat C-reactive protein (CRP) onto an enzyme-linked immunosorbent assay (ELISA) plate. ELISA plates were coated with human (a) or rat (b) CRP at 10 µg/ml (⋄), or with buffer (▴), and incubated with increasing volumes (0 to 3·33 µl) of human or rat plasma. Then, C3b/c fixation on CRP was detected with specific monoclonal antibody (mAb), as described in the Materials and methods. This figure is representative of three experiments, with similar results obtained on each occasion.
Complement activation by fluid-phase CRP
The incubation of recalcified NHP with 50 µg/ml of rCRP or hCRP in the presence of lyso-PC (400 µg/ml) yielded maximal generation of human C3b/c and C4b/c, as measured by ELISA. This generation was blocked when hCRP or rCRP was preincubated with 20 mm PCh. Incubation of recalcified NHP with lyso-PC alone did not generate a significant amount of activated complement factors. These results thus demonstrate that rCRP is able to activate complement in human plasma. To assess whether it can also activate endogenous complement in rat plasma, we incubated rat plasma with different concentrations of lyso-PC. As rat plasma contains approximately 0·5 mg/ml of CRP, we did not add purified CRP to this plasma. As described in Table 1, a dose-dependent activation of C4 was observed upon addition of lyso-PC to rat plasma. This activation was abrogated in the presence of high salt or PCh, a low-molecular-weight ligand for CRP, confirming that the observed activation was caused by CRP.
Table 1.
C-reactive protein (CRP)-dependent activation of complement in rat plasma in vitro
| Activator (mg/ml) | CRP–C3 (U/ml) | C4b/c (% NRA) |
|---|---|---|
| lyso-PC | ||
| ″0·8 | 693·0 (±32) | 15·2 (±0·4) |
| ″0·4 | 473·0 (±52) | 11·2 (±4·4) |
| ″0·2 | 276·0 (±40) | 10·2 (±2·2) |
| ″0·1 | 168·0 (±7) | 9·4 (±2·8) |
| ″lyso-PC 0·8 + PCh | 31·0 (±0·54) | 3·7 (±0·2) |
| ″lyso-PC 0·8 + NaCl | 10·7 (±0·56) | 2·3 (±0·9) |
| CPS | ||
| ″0·250 | 93·9 (±3·2) | 13·2 (±4) |
| ″0·125 | 126·0 (±11) | 14·5 (±4·5) |
| ″CPS 0·125 + PCh | 30·9 (±0·6) | 5·1 (±2·4) |
| ″CPS 0·125 + NaCl | ND | 2·1 (±0·5) |
| Controls | ||
| ″Buffer (60 min, 37°) | 52·0 (±8) | 8·6 (±0·3) |
| ″Buffer (on ice) | 3·0 (±0·22) | 1·2 (±0·8) |
| ″AHG (1·25 mg/ml) | 9·0 (±0·44) | 43·3 (±0·4) |
| ″Trypsin (0·25 mg/ml) | 19·0 (±3) | 13·0 (±4·6) |
Normal rat plasma was incubated with lyso-phosphatidylcholine (lyso-PC) or pneumococcal C-polysaccharide (CPS), as described in the Materials and methods. The concentration of C4b/c and CRP–C3 complexes was then measured. The results represent the mean ± standard error of the mean (SEM) of three separate experiments. Generation of C4b/c and of complexes by either activator was inhibited by 10 mm EDTA (data not shown), 20 mm phosphorylcholine (PCh), or 1 m NaCl (NaCl).
AHG, aggregated human IgG; ND, not determined.
We have previously demonstrated that activation of human complement by hCRP results in the generation of measurable amounts of complexes between CRP and activated C3 or C4.6 To further substantiate activation of rat complement by rCRP, we developed an ELISA for the quantification of rat complement–CRP complexes in plasma. When NRP, incubated with 800 µg/ml of lyso-PC, was tested in this assay, a significant dose–response was observed, whereas no complexes were detected in NRP (Fig. 5). Substitution of the capture antibody (anti-rat CRP) or the detecting antibody with an irrelevant mAb or biotinylated normal rabbit IgG, respectively, abolished the response observed in the ELISA (results not shown). As shown in Table 1, the generation of CRP–C3 complexes in rat plasma by lyso-PC was dose dependent and inhibited in the presence of 1 m NaCl (which prevents binding of C1q to CRP) or PCh.
Figure 5.
Enzyme-linked immunosorbent assay (ELISA) for rat C-reactive protein (CRP)–C3 complexes. Phosphorylcholine (PCh)–Sepharose eluates of normal rat plasma (▴) or rat plasma incubated with lyso-phosphatidylcholine (lyso-PC) (▪) were prepared as described in the Materials and methods, and tested in the ELISA.
When CPS was used to activate rat complement via the CRP, results similar to those obtained with lyso-PC were observed, although somewhat fewer complexes were generated (Table 1). In contrast, no CRP–C3 complexes were generated when rat plasma was incubated with aggregated IgG, although this activator induced a marked activation of C4 (Table 1). Similarly, incubation of rat plasma with trypsin also did not generate CRP–C3 complexes.
Discussion
The ability of ligand-bound CRP to activate the complement system seems to be a conserved function of this protein.2,6,9,20–22 A remarkable exception, however, is rat CRP, which, based on studies with CPS as a ligand, was found to be unable to activate complement.9,10 In the present study we demonstrate, using various approaches and various ligands, that rCRP can activate complement.
We isolated rCRP using PCh coupled to Sepharose. SDS–PAGE revealed a migration pattern similar to that described in the literature.7,9,16 The apparently higher MW of the monomeric subunit under reducing conditions has also been described previously and is suggested to be a result of the existence of an intrachain disulphide bridge.7,9,23 Hence, we concluded that the protein purified from rat plasma met the criteria previously described for rCRP.
Human C1q binds only to aggregated or complexed CRP to initiate complement activation, and does not bind to monomeric CRP.24,25 In our experiments, both rat and human CRP were aggregated onto ELISA plates. This method of using protein-coated surfaces for studying complement fixation has been used previously, for example, in studies of complement activation by IgG or immunoglobulin M (IgM), defensins or C3-binding glycoprotein.26–29 rCRP fixed onto an ELISA plate bound human C1q, in agreement with observations by Baltz et al.,23 suggesting that it can activate human complement. rCRP on the ELISA plate also fixed rat C1q, as did C3 upon incubation with recalcified rat plasma; notably, fixation was not observed in the presence of EDTA. Thus, we concluded that rCRP fixed onto an ELISA plate can activate the rat complement system.
It could be argued that the affinity purification of rCRP, or the fixation of this protein onto an ELISA plate, induces conformational changes leading to complement-activating properties that do not reflect the changes induced by binding to natural ligands. Therefore, we also studied activation of complement by native CRP interacting with ligands in rat plasma. The CRP baseline level in rats is about 0·5 g/l.8,30–32 Hence, we studied activation of complement via CRP by incubating NRP with different CRP ligands, without additional CRP supplementation. Addition of lyso-PC or CPS induced the generation of C4b/c in rat plasma, which was inhibited by EDTA and also by p-amino-phenylphosphorylcholine, a monovalent ligand for CRP that prevents its aggregation on multivalent ligands. These results strongly suggested that native CRP in rat plasma could activate complement upon incubation with appropriate ligands.
In previous studies we demonstrated that the formation of human CRP–complement complexes, i.e. complexes of CRP and activated C4 or C3, specifically reflects CRP-mediated complement activation.6 Moreover, these complexes were detected in several human diseases where CRP presumably is involved in the activation of complement.6,33–35 We developed a similar ELISA for the quantification of rat CRP–C3 complexes. Control experiments with non-specific polyclonal rabbit IgG and an irrelevant mAb of the same subclass as the anti-C3 mAb, showed the specificity of the ELISA for rCRP–C3 complexes. Using lyso-PC and CPS as ligands, a significant generation of rCRP–C3 complexes was observed in rat plasma. These complexes were not generated during incubation of rat plasma with aggregated IgG, although this complement activator induced significant C4 activation (see Table 1). CRP–C3 complexes were not formed in rat plasma in the presence of high salt concentration, free p-aminophenylphosphorylcholine or EDTA, which are features typical for CRP-dependent complement activation.6,33,34 Thus, taken together, the results indicated that rat CRP–C3 complexes specifically reflect CRP-mediated activation and are not the result of an innocent bystander phenomenon.
Griselli et al.,10 although postulating that rCRP is unable to activate autologous complement, describe the deposition of rat C3 and rCRP in the ischemic myocardium in a rat acute myocardial infarction model, in agreement with a role for rCRP in the activation of the autologous complement system in vivo. Currently, we are investigating complement activation by CRP in various rat models. Our preliminary results show increases in the amount of CRP–C3 complexes in models for hind-limb ischemia reperfusion, liver ischemia reperfusion and bacterial meningitis (data not shown). These findings together suggest that rCRP can also activate autologous complement in vivo. The discrepancy between our findings and those of deBeer et al., who reported that rCRP is unable to activate complement,9 is presumably a result of the use of different methods to assess complement activation in plasma. Whereas we used a sensitive assay for a specific activation product, i.e. CRP–C3 complexes, in combination with other assays, deBeer et al. used crossed immunoelectrophoresis, which is less sensitive than our ELISAs.
In conclusion, using various approaches and different ligands we show that rat CRP can activate autologous complement. Hence, this property of the protein seems to be widely distributed among animal species. Therefore, we suggest that complement activation upon binding to its ligands is an important function of CRP.
Acknowledgments
The Landsteiner Blood Transfusion Research Foundation financially supported this study (grant no. LSBR 9903) together with the Netherlands Organization for Research (NOW) (grant no. 901-12-095).
Abbreviations
- AHG
aggregated human IgG
- CPS
pneumococcocal C-polysaccharide
- CRP
C-reactive protein
- hCRP
human C-reactive protein
- NHP
normal human plasma
- NRP
normal rat plasma
- PC
phosphatidylcholine
- PCh
phosphorylcholine
- PBS
phosphate-buffered saline, pH 7·4
- PBS-T
PBS containing 0·02% (wt/vol) Tween-20
- PTG
PBS-T containing 0·2% (wt/vol) gelatin
- poly-HRP
streptavidin-polymerized horseradish peroxidase
- rCRP
rat C-reactive protein
- strept-PO
streptavidin coupled to monomeric peroxidase
- VB2+
veronal-buffered saline containing 5 mm CaCl2 and 1 mm MgCl2, pH 7·4
References
- 1.Etlinger HM, Coe JE. Complement activation by female protein, the hamster homologue of human C-reactive protein. Int Arch Allergy Appl Immunol. 1986;81:189–91. doi: 10.1159/000234132. [DOI] [PubMed] [Google Scholar]
- 2.Nakanishi Y, Kodama H, Murai T, Mikami T, Izawa H. Activation of rainbow trout complement by C-reactive protein. Am J Vet Res. 1991;52:397–401. [PubMed] [Google Scholar]
- 3.Shrive AK, Metcalfe AM, Cartwright JR, Greenhough TJ. C-reactive protein and SAP-like pentraxin are both present in Limulus polyphemus haemolymph: crystal structure of Limulus SAP. J Mol Biol. 1999;290:997–1008. doi: 10.1006/jmbi.1999.2956. [DOI] [PubMed] [Google Scholar]
- 4.Tillet WS, Francis T. Serological reactions in pneumonia with a non protein somatic fraction of pneumococcus. J Exp Med. 1930;52:561–71. doi: 10.1084/jem.52.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siegel J, Rent R, Gewurz H. Interactions of C-reactive protein with the complement system. I. Protamine-induced consumption of complement in acute phase sera. J Exp Med. 1974;140:631–47. doi: 10.1084/jem.140.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolbink GJ, Brouwer MC, Buysmann S, ten Berge IJ, Hack CE. CRP-mediated activation of complement in vivo: assessment by measuring circulating complement–C-reactive protein complexes. J Immunol. 1996;157:473–9. [PubMed] [Google Scholar]
- 7.Rassouli M, Sambasivam H, Azadi P, Dell A, Morris HR, Nagpurkar A, Mookerjea S, Murray RK. Derivation of the amino acid sequence of rat C-reactive protein from cDNA cloning with additional studies on the nature of its dimeric component. J Biol Chem. 1992;267:2947–54. [PubMed] [Google Scholar]
- 8.Nunomura W, Takakuwa Y, Higashi T. Changes in serum concentration and mRNA level of rat C-reactive protein. Biochim Biophys Acta. 1994;1227:74–8. doi: 10.1016/0925-4439(94)90109-0. [DOI] [PubMed] [Google Scholar]
- 9.de Beer FC, Baltz ML, Munn EA, Feinstein A, Taylor J, Bruton C, Clamp JR, Pepys MB. Isolation and characterization of C-reactive protein and serum amyloid P component in the rat. Immunology. 1982;45:55–70. [PMC free article] [PubMed] [Google Scholar]
- 10.Griselli M, Herbert J, Hutchinson WL, Taylor KM, Sohail M, Krausz T, Pepys MB. C-reactive protein and complement are important mediators of tissue damage in acute myocardial infarction. J Exp Med. 1999;190:1733–40. doi: 10.1084/jem.190.12.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor JA, Bruton CJ, Anderson JK, Mole JE, de Beer FC, Baltz ML, Pepys MB. Amino acid sequence homology between rat and human C-reactive protein. Biochem J. 1984;221:903–6. doi: 10.1042/bj2210903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolbink GJ, Bollen J, Baars JW, ten Berge RJ, Swaak AJ, Paardekooper J, Hack CE. Application of a monoclonal antibody against a neoepitope on activated C4 in an ELISA for the quantification of complement activation via the classical pathway. J Immunol Methods. 1993;163:67–76. doi: 10.1016/0022-1759(93)90240-8. [DOI] [PubMed] [Google Scholar]
- 13.van den Berg TK, Dopp EA, Daha MR, Kraal G, Dijkstra CD. Selective inhibition of immune complex trapping by follicular dendritic cells with monoclonal antibodies against rat C3. Eur J Immunol. 1992;22:957–62. doi: 10.1002/eji.1830220412. [DOI] [PubMed] [Google Scholar]
- 14.van den Berg RH, Faber-Krol M, van Es LA, Daha MR. Regulation of the function of the first component of complement by human C1q receptor. Eur J Immunol. 1995;25:2206–10. doi: 10.1002/eji.1830250814. [DOI] [PubMed] [Google Scholar]
- 15.van Dam AP, Hack CE. Formation of C3–IgG complexes in serum by aggregated IgG and by non-immunoglobulin activators of complement. Immunology. 1987;61:105–10. [PMC free article] [PubMed] [Google Scholar]
- 16.Pruden DJ, Connolly KM, Stecher VJ. Single-step purification of rat C-reactive protein and generation of monospecific C-reactive protein antibody. J Chromatogr. 1988;437:399–410. doi: 10.1016/s0021-9673(00)90413-8. [DOI] [PubMed] [Google Scholar]
- 17.Szewczyk B, Summers DF. Efficient elution of purified proteins from polyvinylidene difluoride membranes (Immobilon) after transfer from SDS–PAGE and their use as immunogens. Mol Biotechnol. 1994;2:129–34. doi: 10.1007/BF02824805. [DOI] [PubMed] [Google Scholar]
- 18.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bos IG, van Mierlo GJ, Bleeker WK, Rigter GM, te VH, Dickneite G, Hack CE. The potentiation of human C1-inhibitor by dextran sulphate is transient in vivo: studies in a rat model. Int Immunopharmacol. 2001;1::1583–95. doi: 10.1016/s1567-5769(01)00073-x. [DOI] [PubMed] [Google Scholar]
- 20.Kushner IMHK. Studies of acute phase protein. II. Localization of Cx-reactive protein in heart in induced myocardial infarction in rabbit. J Clin Invest. 1963;42:286–92. doi: 10.1172/JCI104715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kushner IMHK. Studies of acute phase protein. I. An immunohistochemical method for the localization of Cx-reactive protein in rabbits. Association with necrosis in local inflammatory lesions. J Exp Med. 1961;114:961–73. doi: 10.1084/jem.114.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gershov D, Kim S, Brot N, Elkon KB. C-Reactive protein binds to apoptotic cells, protects the cells from assembly of the terminal complement components, and sustains an antiinflammatory innate immune response: implications for systemic autoimmunity. J Exp Med. 2000;192:1353–64. doi: 10.1084/jem.192.9.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baltz ML, de Beer FC, Feinstein A, et al. Phylogenetic aspects of C-reactive protein and related proteins. Ann N Y Acad Sci. 1982;389:49–75. doi: 10.1111/j.1749-6632.1982.tb22125.x. [DOI] [PubMed] [Google Scholar]
- 24.Jiang HX, Siegel JN, Gewurz H. Binding and complement activation by C-reactive protein via the collagen-like region of C1q and inhibition of these reactions by monoclonal antibodies to C-reactive protein and C1q. J Immunol. 1991;146:2324–30. [PubMed] [Google Scholar]
- 25.Thompson D, Pepys MB, Wood SP. The physiological structure of human C-reactive protein and its complex with phosphocholine. Structure Fold Des. 1999;7:169–77. doi: 10.1016/S0969-2126(99)80023-9. [DOI] [PubMed] [Google Scholar]
- 26.Prohaszka Z, Nemet K, Csermely P, Hudecz F, Mezo G, Fust G. Defensins purified from human granulocytes bind C1q and activate the classical complement pathway like the transmembrane glycoprotein gp41 of HIV-1. Mol Immunol. 1997;34:809–16. doi: 10.1016/s0161-5890(97)00097-7. [DOI] [PubMed] [Google Scholar]
- 27.Rieben R, Roos A, Muizert Y, Tinguely C, Gerritsen AF, Daha MR. Immunoglobulin M-enriched human intravenous immunoglobulin prevents complement activation in vitro and in vivo in a rat model of acute inflammation. Blood. 1999;93:942–51. [PubMed] [Google Scholar]
- 28.Wettero J, Bengtsson T, Tengvall P. C1q-independent activation of neutrophils by immunoglobulin M-coated surfaces. Biomed Mater Res. 2001;57:550–8. doi: 10.1002/1097-4636(20011215)57:4<550::aid-jbm1201>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 29.Stanilova SA, Slavov ES. Comparative study of circulating immune complexes quantity detection by three assays – CIF-ELISA, C1q-ELISA and anti-C3 ELISA. J Immunol Methods. 2001;253:13–21. doi: 10.1016/s0022-1759(01)00370-2. [DOI] [PubMed] [Google Scholar]
- 30.de Beer FC, Soutar AK, Baltz ML, Trayner IM, Feinstein A, Pepys MB. Low density lipoprotein and very low density lipoprotein are selectively bound by aggregated C-reactive protein. J Exp Med. 1982;156:230–42. doi: 10.1084/jem.156.1.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rowe IF, Soutar AK, Trayner IM, et al. Rabbit and rat C-reactive proteins bind apolipoprotein B-containing lipoproteins. J Exp Med. 1984;159:604–16. doi: 10.1084/jem.159.2.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nunomura W. C-reactive protein in rat: in development, pregnancy and effect of sex hormones. Comp Biochem Physiol A. 1990;96:489–93. doi: 10.1016/0300-9629(90)90667-h. [DOI] [PubMed] [Google Scholar]
- 33.Bruins P, te VH, Eerenberg-Belmer AJ, Yazdanbakhsh AP, de Beaumont EM, Eijsman L, Trouwborst A, Hack CE. Heparin–protamine complexes and C-reactive protein induce activation of the classical complement pathway: studies in patients undergoing cardiac surgery and in vitro. Thromb Haemost. 2000;84:237–43. [PubMed] [Google Scholar]
- 34.Molenaar ET, Voskuyl AE, Familian A, van Mierlo GJ, Dijkmans BA, Hack CE. Complement activation in patients with rheumatoid arthritis mediated in part by C-reactive protein. Arthritis Rheum. 2001;44:997–1002. doi: 10.1002/1529-0131(200105)44:5<997::AID-ANR178>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 35.Wolbink GJ, Bossink AW, Groeneveld AB, de Groot MC, Thijs LG, Hack CE. Complement activation in patients with sepsis is in part mediated by C-reactive protein. J Infect Dis. 1998;177:81–7. doi: 10.1086/513803. [DOI] [PubMed] [Google Scholar]