Abstract
Thrombolytic agents, used to restore blood flow to ischaemic tissues, activate several enzymatic systems with pro-inflammatory effects, thus potentially contributing to the pathogenesis of ischaemia–reperfusion injury. Platelet-activating factor (PAF), a phospholipid mediator of inflammation, has been implicated in the pathogenesis of this process. We previously showed that the infusion of streptokinase (SK) induces the intravascular release of PAF in patients with acute myocardial infarction (AMI), and that cultured human endothelial cells (EC) synthesized PAF in response to SK and plasmin (PLN). In the present study, we investigated the role of the membrane attack complex (MAC) of complement in the PLN-induced synthesis of PAF. In vivo, we showed a correlation between the levels of soluble terminal complement components (sC5b-9) and the concentrations of PAF detected in blood of patients with AMI infused with SK. In vitro both EC and polymorphonuclear neutrophils (PMN), incubated in the presence of PLN and normal human serum, showed an intense staining for the MAC neoepitope, while no staining was detected when they were incubated with PLN in the presence of heat-inactivated normal human serum. Moreover, the insertion of MAC on EC and PMN plasmamembrane elicited the synthesis of PAF. In conclusion, our results elucidate the mechanisms involved in PAF production during the activation of the fibrinolytic system, showing a role for complement products in this setting. The release of PAF may increase the inflammatory response, thus limiting the beneficial effects of thrombolytic therapy. Moreover, it may have a pathogenic role in other pathological conditions, such as transplant rejection, tumoral angiogenesis, and septic shock, where fibrinolysis is activated.
Introduction
The activation of the fibrinolytic system is involved in the pathogenesis of several pathological conditions, including transplant rejection, tumoral angiogenesis, and septic shock.1 On the other hand, the use of fibrinolytic agents represents the therapy of choice for the treatment of many thrombotic diseases, such as acute myocardial infarction (AMI).2 Thrombolytic agents, besides their well-known action on coagulation, directly activate other enzymatic systems with pro-inflammatory effects, thus potentially contributing to the pathogenesis of ischaemia–reperfusion injury.3,4 For instance, both streptokinase (SK) and recombinant tissue-type plasminogen activator (rt-PA) activate the classic pathway of the complement system.5 Complement, by promoting endothelial activation and neutrophil-endothelium adhesion, has a determinant role in sustaining the acute inflammatory response, thus contributing to the extension of tissue damage.6 In AMI, in particular, the activation of complement system and its involvement in inducing irreversible myocardial cellular damage have been well documented: (a) in experimental studies, the inhibition of complement activation by soluble complement receptor type 1 reduces the extent of irreversible myocardial cellular damage;7,8 (b) in vivo, deposition of complement components in the myocardium of patients with AMI has been shown;9 and (c) complement activation has been shown in the plasma of patients with AMI.10,11 We have previously shown that SK infusion induces the intravascular release of platelet-activating factor (PAF) in patients with AMI.12 In addition, we found that plasmin (PLN) triggers the synthesis of PAF from endothelial cells (EC).12 PAF, a phospholipid mediator of inflammation13 may exert direct cardiovascular effects14 or promote the activation of EC, polymorphonuclear neutrophils (PMN), monocytes, and platelets.13
In the present study, we tested the hypothesis that the membrane attack complex (MAC) of complement may contribute to induce the synthesis of PAF observed in patients with AMI treated with thrombolytic agents. Therefore, we evaluated: (a) the correlation between the levels of soluble terminal complement components (sC5b-9) and the concentrations of PAF detected in blood of patients with AMI infused with SK; (b) the ability of PLN to induce the insertion of the MAC of complement in the plasma membrane of human EC and PMN; (c) the ability of MAC to induce the synthesis of PAF by human EC and PMN.
Materials and methods
Materials
SK (Streptase) was obtained from Behringwerke AG (Marburg, Germany). Human PLN, purified complement proteins (C5-C9), chloramine-T, and methionine were provided by Sigma Chemical Co (St Louis, MO). Culture media were obtained from Flow Laboratories (McLean, VA). Cobra venom factor (CVF) and anti-sC5b-9 monoclonal antibody (clone aE11), specific for the neo-epitope of C9 expressed after complement activation, have been provided by Quidel (San Diego, CA). Synthetic C16 PAF (1-hexadecyl-2-acetyl-sn-glyceryl-3-phosphorylcholine) was obtained from Bachem Feinchemikalien (Bubendorf, Switzerland). WEB 2170, a specific PAF-receptor antagonist15 was from Boehringer Ingelheim (Germany). C3b-coated Baker's yeast particles (BYS), used as substrate for phagocytosis, were prepared as previously described.16
Patients
A subset of the AMI patients enrolled in a previous study12 was used in the present investigation. Enrolling, treatment allocation criteria, and clinical procedures were previously detailed.12 Group A consisted of seven patients who were treated with SK (1·5 mU in 100 ml saline infused i.v. over 60 min), Group B consisted of seven patients given a control infusion of saline over 60 min.12 Similar conventional drugs were given to patients of both groups; in particular, none of the patients received heparin before the protocol for blood withdrawal was completed. The clinical characteristics of the patients are depicted in Table 1. The Killip scale was used to classify patients with AMI depending on clinical manifestations of heart failure on admission.17 Peripheral venous blood samples were obtained from each patient before and 30, 60, 90, 120, and 180 min after the beginning of infusion to measure the intravascular levels of PAF and of sC5b-9. sC5b-9 was measured on plasma aliquots stored at −80° by enzyme-linked immunosorbent assay (ELISA; Quidel). The α2-antiplasmin was assayed with the chromogenic substrate S-2251.18
Table 1.
Clinical characteristics of the two patient groups
| Group A | Group B | |
|---|---|---|
| (SK-treated subjects) | (Control subjects) | |
| Women/men | 2/5 | 2/5 |
| Age (years) | 55·14 ± 2·71 | 59·14 ± 4·18 |
| Infarct location, n | ||
| Anterior | 4/7 | 4/7 |
| Inferior | 3/7 | 3/7 |
| Infarct related artery, n | ||
| LAD | 6/7 | 4/7 |
| LCx | 2/7 | 1/7 |
| RCA | 2/7 | 5/7 |
| Killip scale >2, n | 0/7 | 0/7 |
| Myocardial enzymes (peak) | ||
| CPK, U/L | 3342 ± 760 | 2991 ± 1008 |
| CPK-MB, U/L | 257 ± 154 | 300 ± 237 |
| AST, U/L | 297 ± 194 | 315 ± 250 |
LAD, left anterior descending coronary artery; LCx, left circumflex coronary artery; and RCA, right coronary artery. The χ2 test or Student's t-test was applied where appropriate. No indication is given where the statistics were not significant.
The study was approved by the local Ethical Committee. All the patients gave informed consent.
Cell preparations
EC were obtained by treating human umbilical cord veins with collagenase, cultured and characterized as previously described.19 Experiments were performed on subcultures at the second or third passage. For experimental purpose, EC were grown to confluence in 3·5 cm-diameter wells of cluster plates, washed and incubated in medium 199 without NaHCO3 containing 0·25% bovine serum albumin, 20 mmol/l HEPES (pH 7·4), and, where appropriate, 15% normal human serum, then added of PLN, thrombin, or CVF, or exposed to the assembly of the MAC of complement. In selected experiments, EC were simultaneously exposed to PLN and MAC in order to evaluate whether an additive or synergic effect occurred.
Human PMN were prepared from healthy donors by differential centrifugation and gelatin sedimentation, followed by osmotic shock as previously described.16
Indirect immunofluorescence on human umbilical vein EC and PMN
In order to show the assembly of MAC induced by PLN on EC and PMN membranes, cells were incubated with PLN (1 U/ml) in the presence of 15% normal human serum as a source for complement components. As positive control, CVF (6·14 U/ml), an activator of the alternative pathway of complement, was used. As negative control, cells were incubated with PLN and CVF in the presence of 15% heat-inactivated human serum, or with 15% normal human serum in the absence of complement activators. Control experiments included incubation of cells with non-immune isotypic control antibodies or the omission of primary antibodies followed by the appropriate labelled secondary antibodies.
The formation of the MAC was verified by indirect immunofluorescence using a monoclonal antibody directed against the MAC neoantigen (clone aE11, Quidel).
Immunofluorescence on purified PMN or cultured EC was performed as previously described.20 Briefly, cells stimulated as detailed above were fixed in 3·5% paraformaldehyde containing 2% sucrose for 15 min at room temperature and washed in phosphate-buffered saline (PBS). Cells were then incubated with the monoclonal antibody directed against the MAC neoantigen (10 µg/ml) followed by fluoroscein isothiocyanate (FITC)-conjugated anti-mouse immunoglobulin G (IgG).
MAC expression on EC was analysed semiquantitatively by measuring fluorescence intensity by digital image analysis (Windows MicroImage, ver. 3.4 CASTI Imaging) on images obtained using a low light video camera (Leica DC100) as previously described.21 The background fluorescence was subtracted by digital image analysis. The results were expressed as relative fluorescence intensity on a scale from 0 (fluorescence of background of tissue) to 255 (fluorescence of standard filter).
Assembly of sublytic concentrations of MAC using purified complement components, C5b-9
The assembly of MAC at sublytic concentrations was accomplished by the method described in detail by Kilgore et al.22 A C5b-like activation product was generated by oxidation with chloramine-T:23 briefly, 10 µg of C5 was incubated in vernal-buffered saline in the presence of 10 µl of 0·32 mmol/l chloramine-T for 10 min at room temperature and the reaction stopped by adding 10 µl of 1 mmol/l methionine. To form the modified C5b-like C5C6 complex (C5C6*), 20 µg of purified C6 in 300 µl of serum-free medium was incubated (24 hr at 37°) with the chloramine-T-treated C5. Assembly of the MAC on EC and PMN was initiated by a 15-min preincubation (37°) with 0·5 µg/ml of the modified C5C6* activation product and C7 (10 µg/ml).22,23 The cells were then washed with serum-free medium to remove excess C5C6* activation product and C7. The complement components C8 and C9 (10 µg/ml) were then added to the cells.
As control, cells were subsequently incubated in the presence of the C5b-like C5C6 complex (C5C6*) and C7, then of C8, but C9 was omitted. To rule out the possibility that C9 itself, or a contaminant (i.e. lipopolysaccharides) in the C9, may trigger the synthesis of PAF, cells were incubated in the presence of C5C6*, C8 and C9, and C7 was omitted.
Alterations of membrane integrity and cell death were assessed by measuring the release of the intracellular enzyme lactate dehydrogenase (LDH) in the supernatants using an ELISA kit purchased from Sigma. In preliminary experiments, increasing concentrations of the C5b-like C5C6 complex (C5C6*) in the presence of fixed concentrations of C7, C8, and C9 were tested for their ability to induce lactate dehydrogenase (LDH) release. The concentration of C5C6* of 0·5 µg/ml was chosen for all the subsequent experiments since it did not induce a significant release of LDH (106·3 ± 2·5% of control unstimulated EC); on the contrary LDH was released when the concentration of C5C6* was increased to 1·0 µg/ml (112·8 ± 3·6% of control unstimulated EC).
Purification, quantification, and characterization of PAF
The methods used to purify and quantify PAF were previously described in detail.16,19 Briefly, blood samples and cell pellets were submitted to total lipid extraction and the extracted lipids were fractionated by thin-layer chromatography with 65 : 36 : 6 (vol/vol/vol) chloroform : methanol : water as a solvent, followed by high-performance liquid chromatography (HPLC).19,24 PAF concentration was measured by aggregation of washed rabbit platelets, using a calibration curve with synthetic PAF for each series of assays.19,24 PAF-like bioactivity was characterized on the basis: (1) of its physicochemical properties (insensitivity to phospholipase A2 or base-catalysed methanolysis and resistance to phospholipase A1 or treatment with weak acids and bases);19,24 (2) of the inhibitory effect of the specific PAF-receptor antagonist WEB 2170;15 and (3) of the spectra obtained by HPLC-tandem mass spectrometry (HPLC-MS/MS).25
Statistical analysis
All data are expressed as mean ± SEM. Students's t-test for paired data of χ2 test were used, where appropriate, for statistical comparison between the groups. One-way anova with Newman–Keul's multicomparison test was performed where indicated. Correlation between PAF and complement levels was assessed with linear regression analysis. The statistical analysis was done using GraphPad Prism®, version 3.02 (GraphPad Software Inc., San Diego, CA).
Results
In vivo studies
The clinical characteristics of the patients included in the present study are resumed in Table 1. No significant difference was detected between the two study groups for sex, age, haemodynamic variables, infarct location, drugs used, ejection fraction (calculated from the contrast ventriculogram at the time of cardiac catheterization). A significant reduction in plasma α2-antiplasmin levels (81·4% ± 3·6%; P < 0·01) was observed in patients of group A.
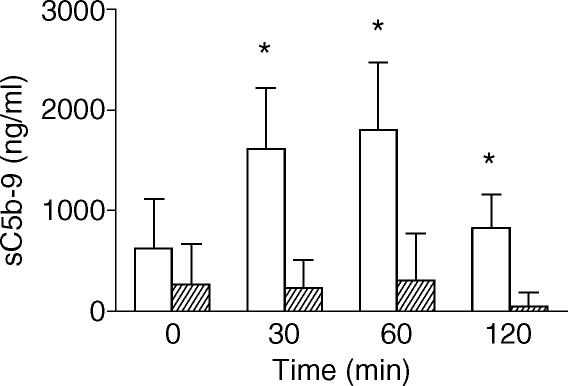
sC5b-9 levels in patients of group A before the beginning of the thrombolytic treatment were not significantly different from those measured in patients of group B (Fig. 1). Upon SK infusion, sC5b-9 levels increased in patients of group A, with a peak at 30 and 60 min, while remained unchanged in patients of group B (Fig. 1). As expected, SK caused a transient but evident decrease in white blood cell number 30 min after the beginning of the infusion (14·83% ± 3·97% of basal values; P < 0·05). No significant decrease in white blood cell count was observed in patients of Group B.
Figure 1.
Plasma sC5b-9 levels (ng/ml) in patients with AMI treated (open columns) or not treated (shaded columns) with SK. Data are expressed as mean ± SEM. *P < 0·05 versus SK-untreated AMI patients.
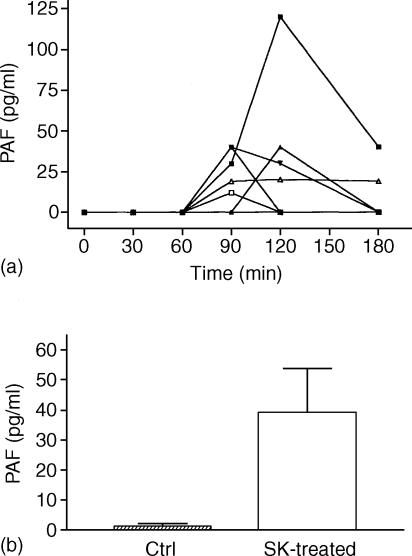
PAF bioactivity was detectable in peripheral blood samples of six out of seven patients in group A (Fig. 2). Among these, five were positive at 90 min, four at 120 min, and two at 180 min after the beginning of SK infusion. In contrast, PAF was not detectable at any time in blood samples of patients of group B.
Figure 2.
(a) Individual concentrations of PAF detected in the circulation of the seven patients with AMI treated with SK (group A). PAF concentration was determined by bioassay on washed rabbit platelets as described in ‘Materials and Methods’. (b) PAF levels detected in blood of patients with AMI treated (open columns) or not treated (Ctrl; shaded columns) with SK. Data are expressed as mean ± SEM.
The individual intravascular levels of PAF significantly correlated with the peak levels of sC5b-9, measured at 60 min after the beginning of SK infusion (r = 0·3084; P < 0·01). This result led us to hypothesize a causative link between complement activation induced by SK and/or PLN and intravascular release of PAF.
In vitro studies
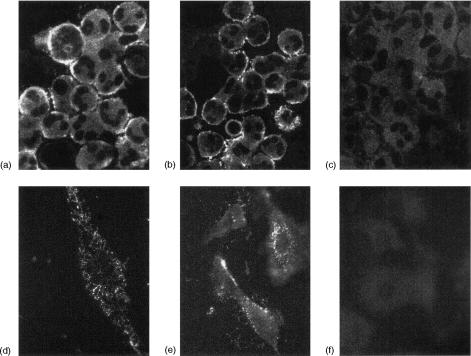
We first evaluated the ability of PLN, in the presence of 15% normal human serum as a source for complement components, to induce the insertion of MAC in the plasma membrane. Both EC and PMN, incubated in the presence of PLN (1 U/ml) and 15% normal human serum, showed an intense surface staining for the MAC neoepitope (Fig. 3b, e), with a fine punctate diffuse distribution. The intensity and pattern of staining were similar to that observed after incubation of the cells with CVF, an activator of the alternative pathway of complement, in the presence of 15% normal human serum (Fig. 3a, d). When EC and PMN were incubated with PLN (1 U/ml) in the presence of 15% heat-inactivated human serum, or with 15% normal human serum in the absence of complement activators, no staining was observed (Fig. 3c, f). Control experiments, in which the cells were incubated with the non-immune isotypic control antibody or with the appropriate labeled secondary antibody without the primary antibody, were always negative (data not shown). The semiquantitative analysis showed that PLN incubation induced a 3·25 ± 0·34-fold increase in MAC expression on EC, comparable to that observed upon stimulation with CVF (3·51 ± 0·28).
Figure 3.
Expression of MAC neoepitope on PMN and EC. Upper panel: PMN were stimulated with CVF 6·14 U/ml for 5 min (a) or PLN 1 U/ml for 15 min in the presence of 15% normal human serum (b), or, as control, of 15% heat-inactivated human serum (c). Lower panel: EC were stimulated with CVF 6·14 U/ml for 5 min (d) or PLN 1 U/ml for 15 min in the presence of 15% normal human serum (e), or, as control, of 15% heat-inactivated human serum (f).
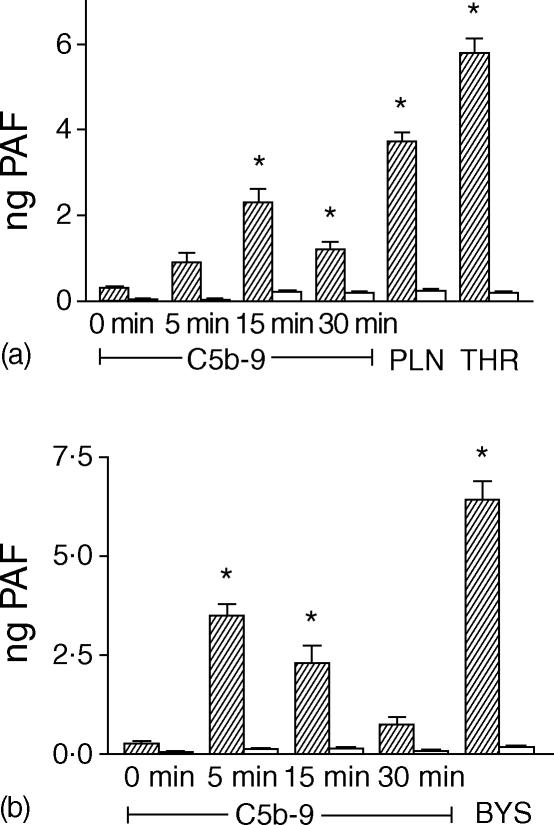
As normal human serum contains large amounts of plasma acetylhydrolase, the main catabolic enzyme for PAF, the effect of complement activation on the induction of PAF synthesis from EC and PMN was studied in the absence of serum. Therefore, the assembly of sublytic concentrations of MAC (C5b-9) in the plasma membrane was obtained using purified complement components, as previously described.22 In preliminary experiments we tested any single complement component obtained from Sigma for its ability to induce the synthesis of PAF from EC and/or PMN: no effect was observed. On the contrary, human cultured EC synthesized PAF in response to the insertion of MAC (C5b-9) at sublytic concentrations in the plasma membrane: time-course experiments showed that the synthesis of PAF peaked at 15 min and was transient, persisting only up to 30 min (Fig. 4a). In these experimental conditions, PAF remained entirely associated with the cells since it was extracted from the cell pellet and it was not detectable in the cell-free supernatant. As control, cells were subsequently incubated in the presence of the C5b-like C5C6 complex (C5C6*) and C7, then of C8, but C9 was omitted, or incubated in the presence of C5C6*, C8 and C9, and C7 was omitted. In these experimental conditions, the synthesis of PAF from EC was never observed.
Figure 4.
(a) Synthesis of PAF by EC in response to the insertion of MAC (C5b-9) at sublytic concentrations for various periods of time (shaded columns). As negative control, EC were subsequently incubated in the presence of the C5b-like C5C6 complex (C5C6*) and C7, then of C8, but not C9, for various periods of time (open columns). As positive control, EC were incubated with PLN (1 U/ml for 15 min) or thrombin (THR) (2 U/ml for 5 min) in the absence of serum. PAF was expressed as ng of PAF associated with 2·5 × 106 cells. Data are from a representative experiment, with triplicate points. anova with Newman–Keul's multicomparison test was performed (*P < 0·05 versus control). (b) Synthesis of PAF by PMN in response to the insertion of MAC (C5b-9) at sublytic concentrations for various periods of time (shaded columns). As negative control, PMN were subsequently incubated in the presence of the C5b-like C5C6 complex (C5C6*) and C7, then of C8, but not C9, for various periods of time (open columns). As positive control, PMN were stimulated with C3b-coated Baker's yeast particles (BYS) for 20 min in the absence of serum. PAF was expressed as ng of PAF associated with 2·5 × 106 cells. Data are from a representative experiment, with triplicate points. anova with Newman–Keul's multicomparison test was performed (*P < 0·05 versus control).
Moreover, no additive neither synergic effect was observed on PAF synthesis when EC were exposed to PLN and MAC (data not shown).
MAC insertion also stimulated the synthesis of PAF by human PMN; the kinetic was even more rapid than for EC, peaking at 5 min and lasting no longer than 15 min (Fig. 4b). When C7 or C9 were omitted from the incubation medium, we did not observe synthesis of PAF.
Discussion
The results of the present study elucidate the role of complement in SK-induced intravascular release of PAF in patients with AMI. We have previously shown that SK infusion induces the intravascular release of PAF in patients with AMI and that SK and PLN directly stimulate the synthesis of PAF by cultured human EC.12 In the present study, we observed a correlation between the peak values of the soluble terminal complement components sC5b-9 and the amounts of PAF detected in the blood of patients with AMI treated with SK. This data led us to evaluate whether a causative link between the full activation of the complement cascade and the release of PAF into the circulation. The in vitro experiments show that PLN induced, in the presence of normal human serum as a source for complement, the insertion of MAC in the plasma membrane of EC and PMN. MAC assembly in turn stimulated the synthesis of PAF from EC and PMN. PLN is known to be generated into the circulation of patients with AMI treated with SK.26 The concentrations of PLN required for the in vitro complement activation and MAC insertion in the present experimental setting were compatible with those measured in vivo.26 The present results suggest that, besides the direct effect of SK and PLN on EC, the assembly of MAC induced by PLN may represent an additional mechanism for PAF synthesis by EC and PMN. It is well known that the interaction of MAC with EC can result in a number of pro-inflammatory actions and it triggers changes similar to those induced by cytokines. MAC induces, within minutes, the formation of intracellular gaps,27 the expression of P-selectin28 and the activation of proteinases that cleave and release heparan sulphate proteoglycan from EC surfaces.29 In addition, MAC stimulates, over a period of hours, the up-regulation of tissue factor, cyclooxygenase-2 and chemokines.22,30–32 In this study, we provide the first direct evidence that the assembly of MAC is able to induce the synthesis of PAF from EC and PMN. Our results confirm and extend previous observations by Kilgore et al.33 who showed that the assembly of MAC on confluent human EC induced a concentration- and time-dependent increase in neutrophil adhesion. Although our results suggest a link between the insertion of MAC triggered by PLN and the release of PAF into the circulation, the correlation between the peak values of sC5b-9 and the amounts of PAF detected in the blood of patients with AMI treated with SK is rather week. Therefore, it seems reasonable to hypothesize that other complement products, such as C3a and C5a, could contribute in vivo to induce the synthesis of PAF. For instance C5a, the most potent chemotactic anaphylatoxin, is able to induce the synthesis of PAF in vitro by neutrophils34,35 and eosinophils.36
PAF is a phospholipid mediator of inflammation with diverse and potent physiological effects, which belongs to a family of biologically active, structurally related alkyl phosphoglycerides.13,14 Experimental studies indicate that PAF has a critical role in the development of myocardial ischaemia–reperfusion injury,14 a condition in which the recruitment of PMN is critical.3 In the patients with AMI treated with thrombolytic agents, PAF synthesis and release may limit the beneficial effects of thrombolytic therapy by promoting platelet- and leucocyte-adhesion and activation on the EC surface, as well as transmigration into ischaemic tissue.14 Indeed, blockade of PAF receptors abrogated hypotension and platelet activation observed in rabbits treated with SK and rt-PA.37 Moreover, PAF was shown to act as a mediator of the rapid and transient adhesion of the PMN to the endothelium induced by several stimuli such as thrombin, histamine, elastase38 and PLN itself.24 In blood PAF half-life is very short, as it is rapidly inactivated by a specific PAF acetylhydrolase.39 Therefore, it is detectable only when production overcomes its catabolism. The observation that PAF synthesized upon PLN and MAC stimulation by EC and PMN remains entirely cell-associated in vitro suggests that PAF bioactivity detected in the circulation of patients with AMI treated with SK derives from circulating leucocytes. Previous studies in experimental models indicate that cell-associated PAF acts as cell-to-cell and intracellular mediator.13,14
In addition to myocardial ischaemia–reperfusion injury, a similar interplay between the fibrinolytic and the complement systems may occur in other pathological conditions, for instance transplant rejection, tumoral angiogenesis, and septic shock.1 The elucidation of the molecular mechanism underlying the biological events observed in these clinical settings may lead to the development of new promising therapeutic approaches.
Acknowledgments
This work was supported by the CNR targeted project Biotechnology (to G.C.), MIUR Cofin 2001 (to G.C.), MIUR Cofin 2002 (to G.M.), and Fondi Ateneo 60% (to G.C. and G.M.).
References
- 1.Sidelmann JJ, Gram J, Jespersen J, et al. Fibrin clot formation and lysis: basic mechanisms. Semin Thromb Hemost. 2000;26:605–18. doi: 10.1055/s-2000-13216. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong PW, Collen D. Fibrinolysis for acute myocardial infarction. Current status and new horizons for pharmacological reperfusion, part 1. Circulation. 2001;103:2862–6. doi: 10.1161/01.cir.103.24.2987. [DOI] [PubMed] [Google Scholar]
- 3.Hansen PR. Role of neutrophils in myocardial ischemia and reperfusion. Circulation. 1995;91:1872–85. doi: 10.1161/01.cir.91.6.1872. [DOI] [PubMed] [Google Scholar]
- 4.Winn RK, Ramamoorthy C, Vedder NB, et al. Leukocyte–endothelial cell interactions in ischemia-reperfusion injury. Ann N Y Acad Sci. 1997;832:311–21. doi: 10.1111/j.1749-6632.1997.tb46259.x. [DOI] [PubMed] [Google Scholar]
- 5.Agostoni A, Gardinali M, Frangi D, et al. Activation of complement and kinin systems after thrombolytic therapy in patients with acute myocardial infarction. A comparison between streptokinase and recombinant tissue-type plasminogen activator. Circulation. 1994;90:2666–70. doi: 10.1161/01.cir.90.6.2666. [DOI] [PubMed] [Google Scholar]
- 6.Monsinjon T, Richard V, Fontaine M. Complement and its implications in cardiac ischemia/reperfusion: strategies to inhibit complement. Fundam Clin Pharmacol. 2001;15:293–306. doi: 10.1046/j.1472-8206.2001.00040.x. [DOI] [PubMed] [Google Scholar]
- 7.Shandelya S, Kuppusamy P, Herskowitz A, et al. Soluble complement receptor type 1 inhibits the complement pathway and prevents contractile failure in the postischemic heart: evidence that complement activation is required for neutrophil-mediated reperfusion injury. Circulation. 1993;88:2812–26. doi: 10.1161/01.cir.88.6.2812. [DOI] [PubMed] [Google Scholar]
- 8.Homeister JW, Satoh PS, Kilgore KS, et al. Soluble complement receptor type 1 prevents human complement-mediated damage of the rabbit isolated heart. J Immunol. 1993;150:1055–64. [PubMed] [Google Scholar]
- 9.Schafer HJ, Mathey D, Hugo F, et al. Deposition of the terminal C5b-9 complement complex in infarcted area of human myocardium. J Immunol. 1986;137:1945–9. [PubMed] [Google Scholar]
- 10.Yasuda M, Takeuchi K, Hiruma M, et al. The complement system in ischemic heart disease. Circulation. 1990;81:156–63. doi: 10.1161/01.cir.81.1.156. [DOI] [PubMed] [Google Scholar]
- 11.Langlois PF, Gawryl MS. Detection of the terminal complement complex in patient plasma following acute myocardial infarction. Atherosclerosis. 1988;70:95–105. doi: 10.1016/0021-9150(88)90103-7. [DOI] [PubMed] [Google Scholar]
- 12.Montrucchio G, Bergerone S, Bussolino F, et al. Streptokinase induces intravascular release of platelet-activating factor in patients with acute myocardial infarction and stimulates its synthesis by cultured human endothelial cells. Circulation. 1993;88:1476–83. doi: 10.1161/01.cir.88.4.1476. [DOI] [PubMed] [Google Scholar]
- 13.Prescott SM, Zimmerman GA, Stafforini DM, McIntyre GA. Platelet-activating factor and related lipid mediators. Annu Rev Biochem. 2000;69:419–45. doi: 10.1146/annurev.biochem.69.1.419. [DOI] [PubMed] [Google Scholar]
- 14.Montrucchio G, Alloatti G, Camussi G. The role of platelet-activating factor in cardiovascular pathophysiology. Physiol Rev. 2000;80:1669–99. doi: 10.1152/physrev.2000.80.4.1669. [DOI] [PubMed] [Google Scholar]
- 15.Heuer HO, Casals-Stenzel J, Muacevic G, et al. Pharmacologic activity of bepafant (WEB2170), a new and selective tetrazepinoic antagonist of platelet-activating factor. J Pharmacol Exp Ther. 1990;225:962–8. [PubMed] [Google Scholar]
- 16.Camussi G, Tetta C, Bussolino F, et al. Synthesis and release of platelet-activating factor is inhibited by plasma alpha1-proteinase inhibitor or by alpha1-antichymotrypsin and is stimulated by proteinases. J Exp Med. 1988;168:1293–306. doi: 10.1084/jem.168.4.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Killip T, Kimball JT. Treatment of myocardial infarction in a coronary care unit. A two year experience with 250 patients. Am J Cardiol. 1967;20:457–64. doi: 10.1016/0002-9149(67)90023-9. [DOI] [PubMed] [Google Scholar]
- 18.Teger-Nilsson AC, Friberger P, Gyzander E. Determination of a new rapid plasma inhibitor in human blood by means of a plasmin specific tripeptide substrate. J Clin Laboratory Invest. 1977;37:403–9. [PubMed] [Google Scholar]
- 19.Camussi G, Aglietta M, Malavasi F, et al. The release of platelet-activating factor from human endothelial cells in culture. J Immunol. 1983;131:2397–403. [PubMed] [Google Scholar]
- 20.Camussi G, Brentjens JR, Noble B, Kerjaschki D, Malavasi F, Roholt OA, Farquhar MG, Andres G. Antibody-induced redistribution of Heymann antigen on the surface of cultured glomerular visceral epithelial cells: possible role in the pathogenesis of Heymann glomerulonephritis. J Immunol. 1985;135:2409–16. [PubMed] [Google Scholar]
- 21.Doublier S, Ruotsalainen V, Salvidio G, et al. Nephrin redistribution on podocytes is a potential mechanism for proteinuria in patients with primary acquired nephrotic syndrome. Am J Pathol. 2001;158:1723–31. doi: 10.1016/S0002-9440(10)64128-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kilgore KS, Flory CM, Miller BF, et al. The membrane attack complex of complement induces interleukin-8 and monocyte chemoattractant protein-1 secretion from human umbilical vein endothelial cells. Am J Pathol. 1996;149:953–61. [PMC free article] [PubMed] [Google Scholar]
- 23.Vogt W, Zimmermann B, Hesse D, et al. Activation of the fifth component of human complement, C5, without cleavage, by methionine oxidizing agents. Mol Immunol. 1992;29:251–6. doi: 10.1016/0161-5890(92)90106-8. [DOI] [PubMed] [Google Scholar]
- 24.Montrucchio G, Lupia E, De Martino A, et al. Plasmin promotes an endothelium-dependent adhesion of neutrophils. Involvement of platelet-activating factor and P-Selectin. Circulation. 1996;93:2152–60. doi: 10.1161/01.cir.93.12.2152. [DOI] [PubMed] [Google Scholar]
- 25.Savu SR, Silvestro L, Sorgel F, et al. Determination of 1-O-acyl-2-acetyl-sn-glyceryl-3-phosphorylcholine, platelet-activating factor and related phospholipids in biological samples by high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr B Biomed Appl. 1996;628:35–45. doi: 10.1016/0378-4347(96)00070-9. [DOI] [PubMed] [Google Scholar]
- 26.Mentzer RL, Budzynski AZ, Sherry S. High-dose, brief duration intravenous infusion of streptokinase in acute myocardial infarction: description of effects in the circulation. Am J Cardiol. 1986;57:1220–6. doi: 10.1016/0002-9149(86)90192-x. [DOI] [PubMed] [Google Scholar]
- 27.Saadi S, Platt JL. Transient perturbation of endothelial integrity induced by antibodies and complement. J Exp Med. 1995;181:21–31. doi: 10.1084/jem.181.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foreman KE, Vaporciyan AA, Bonish BK, et al. C5a-induced expression of P-selectin in endothelial cells. J Clin Invest. 1994;94:1147–55. doi: 10.1172/JCI117430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ihrcke NS, Platt JL. Shedding of heparan sulfate proteoglycans by stimulated EC. evidence for proteolysis of cell surface molecules. J Cell Physiol. 1996;168:625–37. doi: 10.1002/(SICI)1097-4652(199609)168:3<625::AID-JCP15>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 30.Saadi S, Holzknecht RA, Patte CP, et al. Complement-mediated regulation of tissue factor activity in endothelium. J Exp Med. 1995;182:1807–14. doi: 10.1084/jem.182.6.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bustos M, Coffman TM, Saadi S, et al. Modulation of eicosanoid metabolism in EC in a xenograft model: role of cyclooxygenase-2. J Clin Invest. 1997;100:1150–8. doi: 10.1172/JCI119626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Selvan RS, Kapadia HB, Platt JL. Complement-induced expression of chemokine genes in endothelium: regulation by IL-1-dependent and -independent mechanisms. J Immunol. 1998;161:4388–95. [PubMed] [Google Scholar]
- 33.Kilgore KS, Ward PA, Warren JS. Neutrophil adhesion to human endothelial cells is induced by the membrane attack complex. the roles of P-selectin and platelet-activating factor. Inflammation. 1998;22:583–98. doi: 10.1023/a:1022362413939. [DOI] [PubMed] [Google Scholar]
- 34.Dahinden CA, Kurimoto Y, Wirthmuller U. Growth factors, lipid mediators and effector cells. J Lipid Med. 1990;2:S129–S136. [PubMed] [Google Scholar]
- 35.Stahl GL, Morse DS, Martin SL. Eicosanoid production from porcine neutrophils and platelets: differential production with various agonists. Am J Physiol. 1997;272:C1821–C1828. doi: 10.1152/ajpcell.1997.272.6.C1821. [DOI] [PubMed] [Google Scholar]
- 36.Lee T, Lenihan DJ, Malone B, Roddy LL, Wasserman SI. Increased biosynthesis of platelet-activating factor in activated human eosinophils. J Biol Chem. 1984;259:5526–30. [PubMed] [Google Scholar]
- 37.Montrucchio G, Alloatti G, Mariano F, et al. Role of platelet-activating factor in hypotension and platelet activation induced by infusion of thrombolytic agents in rabbits. Circ Res. 1993;72:658–70. doi: 10.1161/01.res.72.3.658. [DOI] [PubMed] [Google Scholar]
- 38.Zimmermann GA, Prescott SM, McIntyre TM. Endothelial cell interactions with granulocytes: tethering and signaling molecules. Immunol Today. 1992;13:93–100. doi: 10.1016/0167-5699(92)90149-2. [DOI] [PubMed] [Google Scholar]
- 39.Arai H, Koizumi H, Aoki J, Inoue K. Platelet-activating factor acetylhydrolase (PAH-AH) J Biochem. 2002;131:635–40. doi: 10.1093/oxfordjournals.jbchem.a003145. [DOI] [PubMed] [Google Scholar]