Abstract
Little is known about the homeostatic mechanisms by which the levels of peripheral lymphocytes are maintained. The survival of naïve T cells in vivo must be maintained by some factors that have not been characterized in an in vitro culture system. In this study, we established a culture system of stromal cells derived from murine lymph nodes and investigated the action of the stromal cells in supporting the survival of resting T cells in vitro. Most of the T cells cocultured with the stromal cells did not die, and the supernatant of cultured stromal cells increase the viability of T cells. This T-cell survival-supporting activity was maintained for more than 7 days. Although interleukin (IL)-4, IL-6, IL-7, and interferon-β also rescued peripheral T cells from spontaneous cell death, medium-soluble and heat-sensitive factor(s) derived from the stromal cells supported the survival of T cells more effectively and for a longer time than did these cytokines. T cells maintained in the culture system with the stromal cells appeared to remain in a resting G0/G1 state and did not show remarkable DNA synthesis. From these results, it is presumed that some soluble factor(s) other than the tested cytokines that have been identified as supporting T-cell survival are produced from lymph node stromal cells. These factor(s) play an important role in maintenance of resting T cells.
Introduction
Maintenance of the homeostasis of T cells that control all the immunocompetent cells is important for the achievement of proper immune response. The number of peripheral T cells is strictly regulated by the balance of cell death and cell proliferation. In a condition in which T cells do not encounter large amount of antigens, the T cells do not proliferate and are maintained in a resting state. At the same time, the resting T cells do not die and keep alive to maintain the stable number.1 In contrast, T cells rapidly undergo apoptosis and die in an in vitro culture when the cells are cultured in usual media supplemented with fetal bovine serum.2,3 This fact suggests that some factors that are necessary for T cells to stay alive are not provided in usual in vitro culture systems.
Many studies have focused on the mechanisms by which T cells live, and multiple factors are responsible for T-cell survival.1,4–10 Candidates of such survival factors include cell surface-expressed molecules and secreted soluble factors. In the immune system, many soluble factors called cytokines work for T-cell survival.7–12 One strong candidate, interleukin-7 (IL-7), which is important in the proliferation and development of immature T cells, has also been shown to support the survival of mature T cells.8–10 Other soluble factors, such as interferon-β (IFN-β), IL-4, and IL-6, have also been shown to have such supporting activity. It is still unknown, however, which one of these factors is necessary and efficient for the survival of resting T cells in vivo.
In this study, T cells from mouse lymph nodes, cocultured with the lymph node-derived stromal cells (LNS), survived for 7 days without a significant decrease in number of live cells. This finding indicates that stromal cells derived from murine lymph nodes produce factors necessary for T-cell survival. We investigated the characteristics of the LNS cells and the mechanism by which the survival of T cells was maintained.
Materials and methods
Establishment and preparation of LNS cells in culture
Lymph node cells were obtained from swollen lymph nodes of IL-2Rβ−/− mice and re-suspended at 106 cells/ml in RPMI-1640 medium supplemented with 10% fetal bovine serum, 5 × 10−5 m 2-mercaptoethanol, 100 µg/ml penicillin, and 100 µg/ml streptomycin. The re-suspended cells were cultured in six-well tissue culture plates at 5 ml/well for 7 days at 37°, and then the non-adherent cells were washed away. The remaining adherent cells containing LNS cells were cultured for a further 3 days. Then the stromal cells detached from the plates were collected, re-suspended at 5 × 105 cells/ml, and were set up in 24-well plates at 106 cells/well.
Preparation of stromal-conditioned medium (SCM)
SCM was prepared from the culture supernatant of confluent LNS cells. In brief, the LNS cells in 6-well plates were washed with phosphate-buffered saline (PBS) to remove exogenous serum products. The LNS cells were incubated in serum free RPMI medium at 37° for 3 days, and the supernatant was recovered, filtered and stored at −80° until use.
Isolation of T cells
Lymphocytes were obtained from lymph nodes of 6–8-week-old C57BL/6 mice. T cells were isolated from the lymphocytes by using nylon wool columns as previously reported.13 The percentage of T cells after this preparation was always more than 80%.
Cell culture system and assay for cell survival rate
T cells (106/ml) were cultured in the 24-well plates containing the confluent LNS cells described above or were incubated in presence or absence of SCM, IL-4, IL-6, IL-7 or IFN-β at 37° for various time periods. In another experiment, T cells were cultured in a diffusion chamber (Falcon, Becton Dickinson Laboratory Ware, Franklin Lakes, NJ) to avoid direct contact with LNS cells in 24-well plates. T cells were harvested from culture plates and re-suspended in 0·5 ml of PBS containing 10 µg/ml propidium iodide (PI). The percentages of dead and live cells were determined using a FACS Calibur™ (Becton Dickinson).
Antibodies and recombinant cytokines
Recombinant murine IL-4, IL-7, IFN-α/β, and anti-mouse IL-4, anti-mouse IL-6, and anti-mouse IL-7 antibodies were purchased from R & D systems, Inc. (Minneapolis, MN). Anti-IFN-α/β, anti-BCL-2 and anti-BCL-XL (polyclonal) antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). fluoroscein isothiocyanate (FITC)-conjugated anti-CD4 (clone H 129.19) and phycoerythrin (PE)-conjugated anti-CD8 (clone 53-6.7) antibodies were obtained from Sigma-Aldrich (St Louis, MO). FITC-conjugated anti-mouse CD69 monoclonal antibody (mAb; clone H1.2F3), PE-conjugated anti-CD62L mAb (clone MEL14), PE-conjugated anti-CD45RB mAb (clone 16 A), FITC-conjugated anti-CD44 mAb (clone IM7), FITC-conjugated anti-CD11a mAb (clone M17/4), PE-conjugated anti-CD11b mAb (clone M1/70), biotin-conjugated anti-CD11c mAb (clone HL3), PE-conjugated anti-CD40 mAb (clone 3/23), biotin-conjugated anti-CD54 mAb (clone 3E2), FITC-conjugated anti-CD86 mAb (clone GL1), and PE-conjugated anti-I-Ab mAb (clone AF6-120.1) were obtained from BD-PharMingen (San Diego, CA). Biotin-conjugated antibodies were visualized by secondary staining with streptavidin-conjugated RED670 (Gibco BRL-Invitrogen, Carlsbad, CA).
Enzyme-linked immunosorbent assay (ELISA)
ELISA to detect IL-4 and IL-6 in SCM was performed using mouse IL-4 ELISA set (BD-PharMingen) and mouse IL-6 ELISA set (BD-PharMingen), respectively. ELISA assay for IL-7 was performed using the above-mentioned ELISA set with recombinant murine IL-7, anti-mouse IL-7 antibody (R & D systems, Minneapolis, MN) and biotynilated anti-mouse IL-7 antibody (BD-PharMingen).
Cell proliferation assay
Proliferation of T cells was evaluated by measuring [3H]thymidine (TdR, 925 GBq/mm, Amersham Corp., Arlington Heights, IL) incorporation. In brief, T cells were pulsed with [3H]TdR (37 kBq/well) for an additional 4 hr, and [3H]TdR uptakes were determined by liquid scintillation counting. All experiments were performed in triplicate.
Cell cycle analysis
Approximately 106 T cells were harvested and re-suspended in 200 µl PBS. Then the cells were rapidly fixed in 1 ml of 70% ice-cold ethanol. After centrifugation, the cells were re-suspended in 1 ml PBS containing 10 µg/ml PI and 100 µg/ml ribo-nuclease A and then incubated at 37° for 30 min. Fluorescence (FL-2) was recorded using a FACS Calibur™ flow cytometer (Becton Dickinson).
Analysis of Bcl-2 and Bcl-XL proteins
For immunoblot analysis, cell lysates were prepared from cells using a buffer containing 125 mm Tris-HCL (pH 6·8), 4% sodium dodecyl sulphate (SDS), 10% 2-ME and 20% glycerol, and the amount of protein for each lane was normalized (50 µg/lane). The lysates were subjected to SDS–polyacrylamide gel electrophoresis (PAGE) on 10% gel and electrotransferred to nitrocellulose membranes as previously described.14 Subsequently, each membrane was incubated with anti-Bcl-2 or anti-Bcl-XL antibody and then with horseradish peroxidase (HRP)-labelled secondary goat anti-rabbit immunoglobulin G (IgG) antibody (Tago, Burlingame, CA). The protein bands were visualized using Western blot chemiluminescence reagent (Dupont-NEN, Boston, MA) according to the instructions of the manufacturer. The molecular sizes of the developed proteins were determined in comparison with those of prestained proteins (New England Biolabs Inc., Beverly, MA).
Expression of cytokines
Total cellular RNA was isolated from stromal cells using RNAzol-B (Cinna/Biotex Laboratories, Houston, TX) according to the manufacturer's instructions. Single-stranded cDNA was synthesized by reverse transcriptase (RT) with random hexamer oligonucleotides as primers, and the desired cDNA was amplified by polymerase chain reaction (PCR) using a TaKaRa RNA LA PCR kit (TaKaRa Biomedicals, Kyoto, Japan). Primers (sense and antisense) for the amplification of each cytokine cDNA were as follows: β-actin sense, TGG AAT CCT GTG GCA TCC ATG AAA C; β-actin antisense, TAA AAC GCA GCT CAG TAA CAG TCC G; IL-1α sense, CTC TAG AGC ACC ATG CTA CAG AC; IL-1α antisense, TGG AAT CCA GGG GAA ACA CTG; IL-2 sense, TGA TGG ACC TAC AGG AGC TCC TGA G; IL-2 antisense, GAG TCA AAT CCA GAA CAT GCC GCA G; IL-3 sense, GAA GTG GAT CCT GAG GAC AGA TAC G; IL-3 antisense, GAC CAT GGG CCA TGA GGA ACA TTC; IL-4 sense, CGA AGA ACA CCA CAG AGA GTG AGC T; IL-4 antisense, GAC TCA TTC ATG GTG CAG CTT ATC G; IL-5 sense, CTC TAG TAA GCC CAC TTC TA; IL-5 antisense, TGA TAC ATG AAT AAC ATC CC; IL-6 sense, TGG AGT CAC AGA AGG AGT GGC TAA G; IL-6 antisense, TCT GAC CAC AGA AGG AGT GGC TAA G; IL-7 sense, AAA TGC AGC TGA CTG CTG GC; IL-7 antisense, TCT CCA GTC TAA AAC AGG AC; IL-10 sense, TAC CTG GTA GAA GTG ATG CC; IL-10 antisense, CAT CAT GTA TGC TTC TAT GC; tumour necrosis factor-α (TNF-α) sense, GGC AGG TCT ACT TTG GAG TCA TTG C; TNF-α antisense, ACA TTC GAG GCT CCA GTG AAT TCG G; lymphotoxin (LT)-α sense, TGG CTG GGA ACA GGG GAA GGT TGA C; LT-α antisense, CGT GCT TTC TTC TAG AAC CCC TTG G. The conditions for PCR were 1 min at 94°, 2 min at 55° and 3 min at 72° for 30 cycles. The efficiency of reverse transcription and the amount of RNA used in RT–PCR were verified by detection of the murine β-actin mRNA. The PCR products were analysed on a 2% TAE agarose gel and visualized by ethidium bromide staining.
Results
LNS cells support the survival of resting T cells in vitro
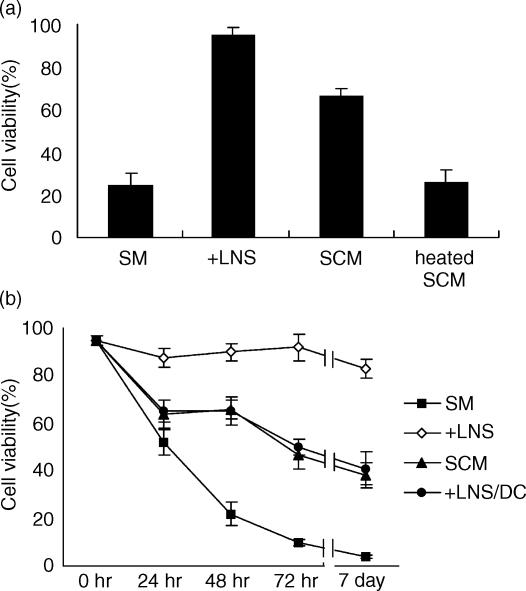
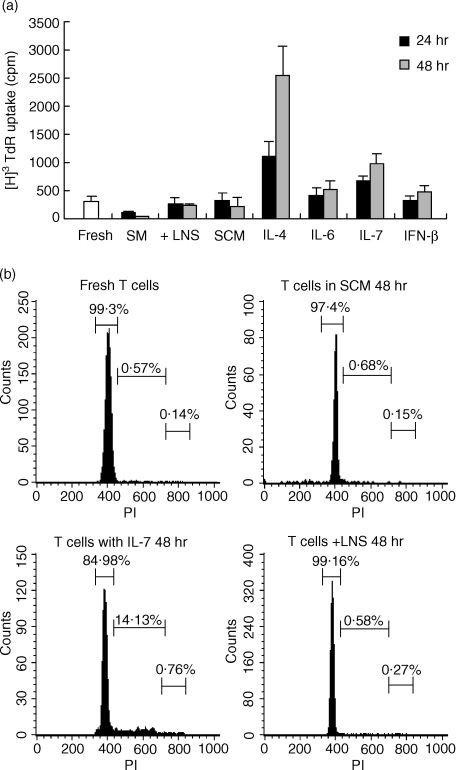
In vitro LNS cells were cocultured with T lymphocytes as described in Materials and Methods. In all of the experiments, the indicator T cells were not treated with antibodies to purify naïve T cells because preliminary experiments showed that such treatment to eliminate the minor population did not greatly affect the overall viability and eventually worsened the viability of T cells (data not shown). When fresh T cells were cultured without stromal cells in the usual medium containing fetal bovine serum, spontaneous cell death occurred and viability decreased to approximately 20% after 48 hr of culture (Fig. 1a). In contrast, when T cells were cocultured with the LNS cells, more than 90% of the T cells were still alive after 48 hr of culture. The conditioned medium prepared from the SCM also increased the survival rate of T cells. This activity was removed by heating the supernatant at 95° for 5 min, indicating that the supporting activity was mediated by heat-sensitive material(s). The time course study of T-cell survival clearly demonstrated that the supporting activity of stromal cells was striking during the initial 24 hr and continued for more than 7 days (Fig. 1b). The effect of the conditioned medium was not remarkable during the initial 24 hr of culture, but significant viability-supporting activity was observed for the subsequent days. Survival of T cells cultured with the LNS cells but separated by the diffusion chamber to prevent direct contact with the LNS cells was very similar to that of the cells cultured in conditioned media, indicating that factor(s) produced from the LNS cells was soluble and diffused in the media.
Figure 1.
Viability of T cells after in vitro culture with the LNS cells or in the conditioned medium. (a) T cells obtained from lymph nodes of normal C57BL/6 mice were cultured for 48 hr with confluent LNS cells (+ LNS) in a simple medium (SM), in a stromal-conditioned medium containing 50% of the culture supernatant of LNS cells (SCM), or in a medium containing 50% of the LNS cells culture supernatant treated at 95° for 5 min (heated SCM). The viability of T cells was determined by PI staining and FACS analysis as described in Materials and Methods. Data are means ± SE from three independent experiments. (b) T cells were cultured with confluent LNS cells (+ LNS) in a simple medium (SM), in a 50% SCM, or with the LNS cells separated by a diffusion chamber (+ LNS/DC) and harvested after indicated periods. Data of cell viability are presented as means ± SE from three independent experiments.
Characteristics of stromal cells from the lymph nodes of IL-2Rβ−/− mice
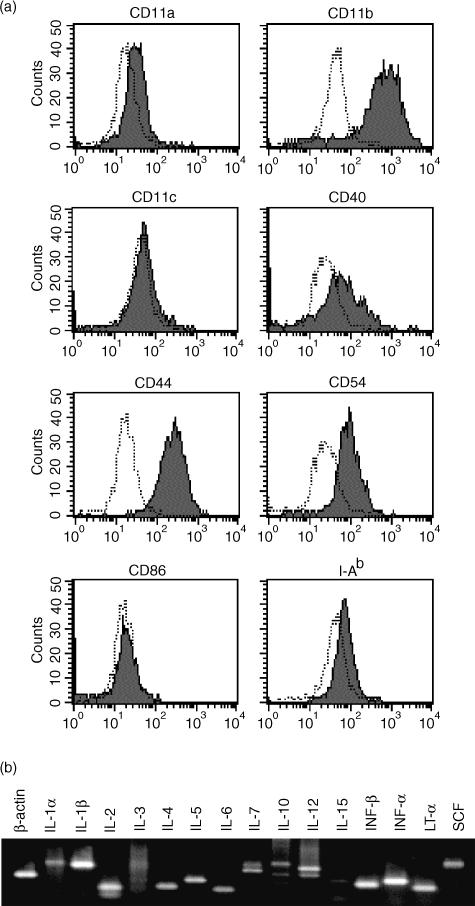
Molecules expressed on cell surfaces relate to adhesion via cell–cell interactions and may be important factors for supporting cell survival. We examined the expression of such cell surface molecules in the LNS cells obtained from IL-2Rβ−/− mice by flow cytometry. Figure 2 shows the expression profiles of CD11a-c, CD40, CD44, CD54, CD86 and class II major histocompatibility complex (MHC) (I-Ab). The LNS cells expressed high levels of CD11b and CD44, moderate levels of CD11c and CD54, and very low levels of CD11a and I-Ab but did not express CD40 and CD86. The LNS cells were negative for CD4 and CD8 expressions (data not shown). The high expression level of CD11b (Mac-1) suggested that most of the LNS cells were in the lineage of macrophages. The high expression level of CD11b but rather low level or negative expression of CD86 (B7-2) and class II MHC indicated that the major population was not dendritic cells. Yet the cells were still heterogeneous and may have contained several different cell lineages including macrophages and dendritic cells. Although we tried to establish clones of the LNS cells, such clonally expanded cells always lost the function to support T-cell survival (data not shown). In this study therefore all the assays were performed within 2 weeks after the LNS cells had been prepared.
Figure 2.
Expression of cell surface molecules in lymph node-derived stromal cells. (a) Stromal cells derived from lymph nodes of IL-2Rβ−/− mice were stained with fluorescence-conjugated antibodies and were analysed by flow cytometry. Shaded peaks and open peaks with dotted lines indicate the fluorescence intensities of cells stained with a non-specific control antibody labelled with the same fluorescent reagents. (b) RNA was extracted from stromal cells derived from lymph nodes of IL-2Rβ−/− mice, and RT–PCR was performed using primers to detect the expression of indicated cytokines.
We also examined the expression of cytokines from these LNS cells by RT–PCR analyses (Fig. 2b) and found that the cells expressed mRNA of various cytokines. The expressions of IL-1β, IL-12, IFN-β and TNF-α were clearly demonstrated.
T-cell survival-supporting effects of related cytokines compared with the effects of LNS cells-derived factors
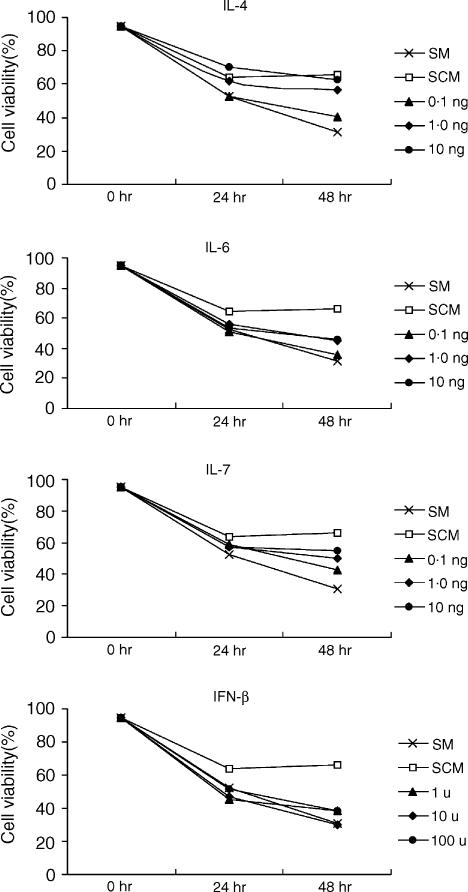
The effect of various cytokines on the prevention of death of T cells in vitro was examined. T cells isolated from lymph nodes of C57BL/6 mice were cultured with various concentrations of IL-4, IL-6, IL-7, and IFN-β for 24 or 48 hr, and the percentages of living cells were compared with those of live cells cultured in the SCM containing 50% of the culture supernatant of the LNS cells. As shown in Fig. 3, all of the cytokines tested had activity to support T-cell survival in a dose-dependent manner. This supporting activity was different for each cytokine: at the highest concentration tested (10 ng/ml or 100 U/ml), the activity was strongest for IL-4, followed by those for IL-7 and IL-6, and INF-β showed the weakest activity. Although every cytokine tested showed some degree of activity to support T-cell survival, only the highest concentration of IL-4 showed an activity equivalent to that of SCM, and no other cytokines were able to perform survival-supporting activity to the same level as SCM (Fig. 3).
Figure 3.
Effects of known cytokines to support the survival of T cells. T cells isolated from lymph nodes of C57BL/6 mice were cultured with various concentrations (as indicated in the figure, per ml) of IL-4, IL-6, IL-7, and IFN-β for 24 or 48 hr, and cell viability was determined. The data of cells cultured in the stromal-conditioned medium (SCM) containing 50% of culture supernatant of the LNS cells were added in each panel to compare the effect of each cytokine with that of SCM. Three different experiments were performed, and the average values are shown.
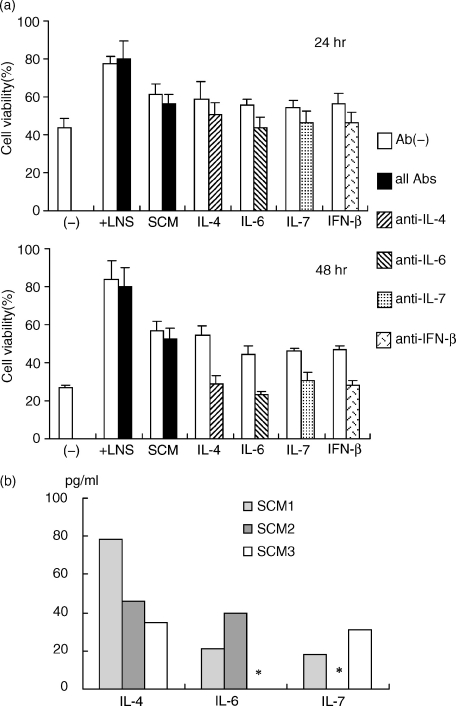
Next, we used neutralizing antibodies to various cytokines added to the T-cell coculture system to test the roles of known cytokines in the LNS cells. As shown in Fig. 4(a), all of the antibodies used showed clear neutralizing activity for the tested cytokines in supporting T-cell survival and decreased the viability of T cells to a level equal to that of T cells cultured without cytokines. A cocktail of all of the antibodies (anti-IL-4, -6, -7, and IFN-β) did not affect the supporting activity of the LNS cells and SCM (Fig. 4a). This result strongly suggested that none of the cytokines tested were essential factors contributing to the action of LNS cells. Furthermore, IL-4, IL-6 and IL-7 levels in the supernatant of the LNS culture were less than 0·1 ng/ml, which was not enough to support the survival of T cells (Fig. 3) but was 10 times less than that suppressed by the specific neutralizing antibody (Fig. 4b).
Figure 4.
Blocking of the activity to support T-cell survival with neutralizing antibodies against various cytokines. (a) T cells were cultured with 1·0 ng/ml (IL-4, IL-6 and IL-7) or 10 U/ml (IFN-β) of cytokines with or without indicated neutralizing antibodies (5 µg/ml) for 24 or 48 hr, and cell viability was determined. T cells were also cocultured with the confluent layer of LN-derived stromal cells (+ LNS) or cultured in SCM with or without a cocktail of all antibodies (5 µg/ml each) for 24 or 48 hr, and cell viability was determined. Three experiments were performed, and the data are means ± SE. (b) IL-4, IL-6, and IL-7 levels in SCM were measured by ELISA. Three independently prepared SCM were analysed. *Results below the reliability level in the ELISA system we used (<15·6 pg/ml).
Characteristics of T cells supported by LNS cells compared with those of T cells supported by known cytokines
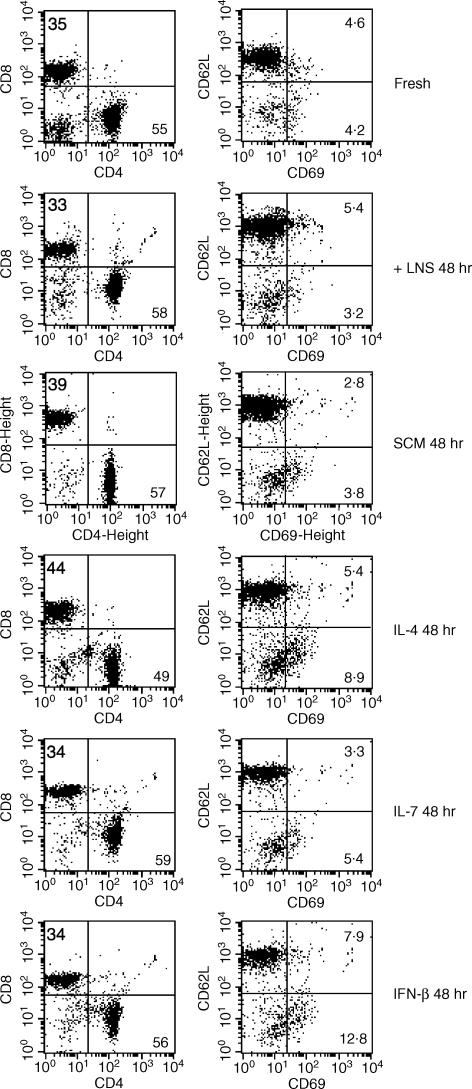
We next analysed whether T cells cocultured with LNS cells or cultured in SCM had changed their characteristics or not. As shown in Fig. 5, the CD4/CD8 ratio was unchanged after 48 hr of culture with LNS cells or in SCM, whereas the CD4/CD8 ratio was slightly decreased after culture with IL-4. When the activation state was checked by the expression of CD69, most of the fresh T cells were negative for CD69 expression and less than 10% of fresh T cells were CD69-positive. This activation state was unchanged after 48 hr of culture with LNS cells or in SCM (Fig. 5). In contrast, the percentage of CD69+ cells was increased after culture with IFN-β. We also examined the expression of CD62L (Fig. 5), CD44 and CD45RB (data not shown) to check the rate of memory-type T cells, and T cells that had been cultured with LNS cells or in SCM for 48 hr showed unchanged expressions of these markers. Thus, it was confirmed that T cells cultured with the LNS cells in our coculture system or cultured in stromal-conditioned medium containing 50% of the culture supernatant of LNS cells were maintained with the character of naïve T cells.
Figure 5.
Cell surface markers of T cells cultured with LNS cells. Freshly isolated T cells and T cells cocultured with LNS cells, cultured in SCM or cultured with recombinant IL-4 (1 ng/ml), IL-7 (2 ng/ml), or IFN-β (100 U/ml) for 48 hr were stained with fluorescence-conjugated antibodies and analysed by flow cytometry. Numbers in each panel indicates the percentages of cells in a quadrant where the numbers are.
Next, we examined whether T cells cultured with the LNS cells, in SCM or with cytokines remain in a resting state or proliferate. First, DNA synthesis was measured for T cells cocultured with LNS cells, or cultured in SCM or with a cytokine (Fig. 6a). A significant increase in DNA synthesis was observed in T cells cultured with IL-4 or IL-7, whereas DNA synthesis in T cells cocultured with LNS cells or cultured in SCM was the same level as that in fresh T cells. DNA synthesis in T cells cultured with IL-6 or IFN-β was also not significantly higher than that in fresh T cells.
Figure 6.
DNA synthesis and cell cycle analysis of T cells cocultured with lymph node-derived stromal (LNS) cells, cultured in SCM or cultured with a cytokine. (a) T cells isolated from C57BL/6 lymph nodes were cultured in a simple medium (SM), cocultured with LNS cells, cultured in SCM or cultured with the indicated recombinant cytokine. Concentrations of cytokines were 10 ng/ml for IL-4, IL-6 and IL-7 and 100 U/ml for IFN-β. After 24 or 48 hr of culture, cells were pulsed with [3H]thymidine, and its uptake was measured as described in Materials and Methods. Three independent experiments were performed, and data are presented as means ± SE. (b) Fresh T cells, T cells cultured with IL-7 (10 ng/ml) for 48 hr, T cells cultured in SCM, and T cells cultured with LNS cells for 48 hr were prepared, and the cell cycles were analysed as described in Materials and Methods. Markers and numbers in each panel represent the areas of G0/G1, S, and G2/M phases and the percentages of cells in the area, respectively.
We also performed cell-cycle analysis of T cells cocultured with LNS cells or cultured in SCM in comparison with those cultured with IL-7 (Fig. 6b). The cell cycle analysis revealed that most of the fresh T cells (>99%) were in G0/G1 phase and that only a very small percentage (∼0·6%) was in S phase. T cells cocultured with LNS cells or cultured in SCM for 48 hr showed a similar pattern of cell cycle to that of fresh T cells, whereas T cells cultured with IL-7 for 48 hr showed a different pattern, i.e. an increased percentage of S-phase cells (∼14%) compared with that of fresh cells and cells cocultured with LNS cells (∼0·6%) or cultured in SCM (∼0·7%).
Expression of Bcl-2 and Bcl-XL in surviving T cells was maintained by coculture with LNS cells
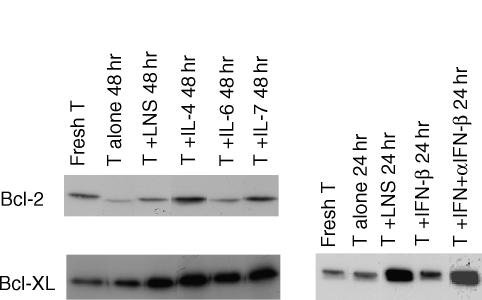
To define the role of Bcl-2 family proteins in survival of resting T cells, we examined the expression of Bcl-2 and Bcl-XL in T cells cocultured with LNS cells or cultured with recombinant cytokines. When the T cells were cultured in a simple medium containing no special factors to support T-cell survival, most of the cells died (see Fig. 1) and the T cells that survived showed reduced expression of Bcl-2 and Bcl-XL (Fig. 7). When the T cells were cultured with LNS cells, most of the T cells survived, with maintenance of Bcl-2 and Bcl-XL expression (Fig. 7). IL-4, IL-6 and IL-7, which had been shown to possess activity supporting survival of T cells to some extent (Fig. 3), were found to increase the expression of Bcl-XL. IFN-β had very weak activity to induce Bcl-XL expression compared with that of LNS cells or other cytokines. The neutralizing antibody against IFN-β did not reduce the maintenance of Bcl-XL expression by LNS cells, indicating that IFN-β was not the factor responsible for the effect of LNS cells on Bcl-XL expression.
Figure 7.
Expression of Bcl family proteins in T cells cultured with LNS cells. Fresh T cells or T cells cultured with LNS cells or with a recombinant cytokine (IL-4, IL-6, and IL-7, 10 ng/ml) in the absence of supporting cells and cytokines (T alone) for 48 hr, were collected and analysed for their expression of Bcl-2 and Bcl-XL by immunoblotting as described in Materials and Methods. In the right panel, T cells were cultured with IFN-β (100 U/ml) for 24 hr with or without a neutralizing antibody and analysed for Bcl-XL expression.
Discussion
We established murine LNS cells that had activity to support the survival of cocultured T cells for more than 7 days (see Fig. 1). Generally, stromal cells can be obtained from some lymphoid tissue/organs such as bone marrow, spleen or lymph nodes.15–17 The interaction between stromal cells and lymphocytes is thought to be important in lymphocyte functioning, homing, proliferation, and survival.18 The character of stromal cells may determine the type of lymphocytes that remain in a local area to interact with the stromal cells. As lymph nodes are much richer in T cells than are the spleen, the characterization of LNS cells is important for the analysis of T cells. LNS cells are usually obtained from human patients with metastatic lymph node tumours.19,20 In this study, we established for the first time an in vitro culture of lymph node-derived stromal cells from mice bearing no tumours, and we identified the activity of these cells that support the survival of peripheral resting T cells. The lymph nodes of IL-2Rβ-deficient mice, from which we could establish the LNS cells in culture, were swollen and contained many activated T cells.21 The stromal cells in such IL-2Rβ−/− mice may interact with activated T cells and increase their numbers in vivo. This may have enabled the establishment of stromal cells in culture from murine lymph nodes. The established LNS cells in vitro promoted T-cell survival but did not activate them (Fig. 5). The LNS cells we established could be maintained in vitro without any loss of their T-cell survival/supporting activity for 2–3 weeks. However, culture of the LNS cells for more than 1 month resulted in a decrease in their survival-supporting activity (data not shown). The LNS cells we used were a heterogeneous cell population, and the functional cells might be lost in culture for a longer time. In order to establish LNS cell clones, technical improvement may be needed to maintain such functional cells in culture for a longer time.
T cells rapidly die in vitro when they are not stimulated. Studies to identify the factor responsible for the survival of T cells and to clarify the mechanism by which T cells survive have shown that there are many different factors responsible for T-cell survival. It seems that such factors include cell surface-expressed molecules and soluble factors. The LNS cells we established also seem to promote T-cell survival with various factors, including both soluble factors and cell–cell interacting molecules. A conditioned medium prepared from the culture supernatant of LNS cells showed clear T-cell survival/supporting activity (Fig. 1), but the activity of the conditioned medium never reached the level of that of LNS cells, indicating that cell–cell interaction is needed for full supporting activity to T-cell survival. The survival activity mediated by cell–cell interaction may be important for short-term maintenance of T-cell survival, while a soluble factor(s) may work for long-term survival (Fig. 1b). In the current understanding for T-cell survival factors working with cell–cell attachment, the interaction between MHC and the T-cell receptor (TCR) is essential for T cells, not only for survival but also for development.5–7,22,23 This MHC–TCR interaction is also thought to work in our system using the LNS cells because the LNS cells were derived from IL-2Rβ-deficient mice of C57BL/6 genetic background and we used T cells from C57BL/6 mice. In our experimental system, however, the affinity of the MHC and the TCR do not seem to be essential because BALB/c T cells that hat not been positively selected for H-2b showed similar survival after coculture with the C57BL/6-derived LNS cells (data not shown). Although it is difficult to estimate how much the T-cell survival is dependent on MHC–TCR interaction, the cell–cell contacting factor(s) must be participating an important role for survival of naïve T cells.
So what is the soluble factor produced by the LNS cells? So far, some cytokines have been shown to work for T-cell survival. For immature T cells and activated T cells in a proliferating state, the factors essential for survival are rather well understood. For instance, they are IL-7 for immature T cells and IL-2 or IL-15 for activated T cells.8–10,24–26 In contrast, the factors essential for survival of resting T cells are not clear. IL-7 seems to be the most likely factor that also works for resting T cells.1 Our analysis, however, showed that IL-7 caused DNA synthesis and cell cycle movement in mature T cells, which is different from the action of our LNS cells (Figs 6 and 7). Type I IFN was also shown to work for T-cell survival, but mainly for survival of activated T cells or anergic T cells and for maintenance of memory-type T cells.27–30 In our study, IFN-β did not effectively increase the survival of naïve T cells. The activity of IFN-β was very weak compared with that of LNS cells and induced an increase in memory-type T cells (Fig. 5). Our result showing an increase in memory-type T cells after culture with IFN-β supports the idea that type I IFN works for induction and maintenance of memory-type T cells. Although it is not clear whether anti-apoptotic Bcl proteins are induced in activated T cells by type I IFN,29,30 our results indicate that type I IFN is not essential for the survival of naïve T cells and that other factors that maintain Bcl-2 and Bcl-XL are important. The LNS cells supported T-cell survival but had no effect on either the activation/memory phenotype or for cell proliferation, indicating that the action of LNS cells was close to the physiological state. None of IL-4, IL-6, IL-7, and IFN-β are indispensable factors for the activity of LNS cells because the addition of neutralizing antibodies (anti-IL-4, anti-IL-6, anti-IL-7, and anti-IFN-β, all together) had no inhibitory effect at all (Fig. 5).
Many factors, including chemokines, are now thought to be candidates for lymphocyte survival factors. SDF-1 (stromal cell-derived factor-1), a chemokine, has been reported to support the survival of resting CD4+ T cells and that of B lymphoma cells.31,32 These findings suggest that SDF-1 coordinately works with other cytokines and for some different cell lineages. Future studies are needed to identify the factor(s) essential for the survival of resting T cells without activation/proliferation. The LNS cells we have established may become a strong tool to identify this factor.
Acknowledgments
This work was supported by grants from the Ministry of Science, Education, Sports and Culture of Japan and from the Naito Memorial Science Foundation.
References
- 1.Marrack P, Bender J, Hildeman D, et al. Homeostasis of αβ TCR+ T cells. Nat Immunol. 2000;1:107–11. doi: 10.1038/77778. [DOI] [PubMed] [Google Scholar]
- 2.Perandones CE, Illera VA, Peckham D, Stunz LL, Ashman RF. Regulation of apoptosis in vitro in mature murine spleen T cells. J Immunol. 1993;151:3521–9. [PubMed] [Google Scholar]
- 3.Teague TK, Marrack P, Kappler JW, Vella AT. IL-6 rescues resting mouse T cells from apoptosis. J Immunol. 1997;158:5791–6. [PubMed] [Google Scholar]
- 4.Boise LH, Thompson CB. Hierarchical control of lymphocyte survival. Science. 1996;274:67–8. doi: 10.1126/science.274.5284.67. [DOI] [PubMed] [Google Scholar]
- 5.Revy P, Sospedra M, Barbour B, Trautmann A. Functional antigen-independent synapses formed between T cells and dendritic cells. Nat Immunol. 2001;2:925–31. doi: 10.1038/ni713. [DOI] [PubMed] [Google Scholar]
- 6.Kondo T, Cortese I, Markovic-Plese S, Wandinger KP, Carter C, Brown M, Leitman S, Martin R. Dendritic cells signal T cells in the absence of exogenous antigen. Nat Immunol. 2001;2:932–8. doi: 10.1038/ni711. [DOI] [PubMed] [Google Scholar]
- 7.Boursalian TE, Bottomly K. Survival of naive CD4 T cells: roles of restricting versus selecting MHC class II and cytokine milieu. J Immunol. 1999;162:3795–801. [PubMed] [Google Scholar]
- 8.Vella A, Teague TK, Ihle J, Kappler J, Marrack P. Interleukin 4 (IL-4) or IL-7 prevents the death of resting T cells. stat6 is probably not required for the effect of IL-4. J Exp Med. 1997;186:325–30. doi: 10.1084/jem.186.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hassan J, Reen DJ. IL-7 promotes the survival and maturation but not differentiation of human post-thymic CD4+ T cells. Eur J Immunol. 1997;28:3057–65. doi: 10.1002/(SICI)1521-4141(199810)28:10<3057::AID-IMMU3057>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 10.Tsuda K, Toda M, Kim G, et al. Survival-promoting activity of IL-7 on IL-2-dependent cytotoxic T lymphocyte clones: resultant induction of G1 arrest. J Immunol Meth. 2000;236:37–51. doi: 10.1016/s0022-1759(99)00235-5. [DOI] [PubMed] [Google Scholar]
- 11.Ku CC, Murakami M, Sakamoto A, Kappler J, Marrack P. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science. 2000;288:675–8. doi: 10.1126/science.288.5466.675. [DOI] [PubMed] [Google Scholar]
- 12.Teague TK, Schaefer BC, Hildeman D, Bender J, Mitchell T, Kappler JW, Marrack P. Activation-induced inhibition of interleukin 6-mediated T cell survival and signal transducer and activator of transcription 1 signaling. J Exp Med. 2000;191:915–26. doi: 10.1084/jem.191.6.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki H, Zhou YW, Kato M, Mak TW, Nakashima I. Normal regulatory α/β T cells effectively eliminate abnormally activated T cells lacking the interleukin 2 receptor βin vivo. J Exp Med. 1999;190:1561–72. doi: 10.1084/jem.190.11.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu J, Suzuki H, Zhou YW, et al. Cepharanthine activates caspases and induces apoptosis in Jurkat and K562 human leukemia cell lines. J Cell Biochem. 2001;82:200–14. doi: 10.1002/jcb.1155. [DOI] [PubMed] [Google Scholar]
- 15.Ni K, O'Neill H. Spleen stromal cells support haemopoiesis and in vitro growth of dendritic cells from bone marrow. Br J Haematol. 1999;105:58–67. [PubMed] [Google Scholar]
- 16.Castro A, Bono MR, Simon V, Vargas L, Rosemblatt M. Spleen-derived stromal cells. Adhesion molecules expression and lymphocyte adhesion to reticular cells. Eur J Cell Biol. 1997;74:321–8. [PubMed] [Google Scholar]
- 17.Miau LH, Jan YW, Shen BJ, Tsai WH, Lee HS, Lee SC. Identification of a novel variant hepatocyte growth factor secreted by spleen-derived stromal cells. Biochem Biophys Res Commun. 1996;223:487–91. doi: 10.1006/bbrc.1996.0921. [DOI] [PubMed] [Google Scholar]
- 18.Rouse RV, Reichert RA, Gallatin WM, Weissman IL, Butcher BC. Localization of lymphocyte subpopulations in peripheral lymphoid organs: directed lymphocyte migration and segregation into specific microenvironments. Am J Anat. 1984;170:391–405. doi: 10.1002/aja.1001700313. [DOI] [PubMed] [Google Scholar]
- 19.Kataoka S, Naito M, Fujita N, Ishii H, Ishii S, Yamori T, Nakajima M, Tsuruo T. Control of apoptosis and growth of malignant T lymphoma cells by lymph node stromal cells. Exp Cell Res. 1993;207:271–6. doi: 10.1006/excr.1993.1193. [DOI] [PubMed] [Google Scholar]
- 20.Tsuda H, Nishimura H, Sawada T, Takatsuki K. The roles of lymph node stromal cells in proliferation of lymphoid leukaemia cells. Br J Cancer. 1990;61:362–4. doi: 10.1038/bjc.1990.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suzuki H, Kundig TM, Furlonger C, et al. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor β. Science. 1995;268:1472–6. doi: 10.1126/science.7770771. [DOI] [PubMed] [Google Scholar]
- 22.Brocker T. Survival of mature CD4 T lymphocytes is dependent on major histocompatibility complex class II-expressing dendritic cells. J Exp Med. 1997;186:1223–32. doi: 10.1084/jem.186.8.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirberg J, Berns A, von Boehmer H. Peripheral T cell survival requires continual ligation of the T cell receptor to major histocompatibility complex-encoded molecules. J Exp Med. 1997;186:1269–75. doi: 10.1084/jem.186.8.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dooms H, Desmedt M, Vancaeneghem S, Rottiers P, Goossens V, Fiers W, Grooten J. Quiescence-inducing and antiapoptotic activities of IL-15 enhance secondary CD4+ T cell responsiveness to antigen. J Immunol. 1998;161:2141–50. [PubMed] [Google Scholar]
- 25.Thomis DC, Berg LJ. Peripheral expression of Jak3 is required to maintain T lymphocyte function. J Exp Med. 1997;185:197–206. doi: 10.1084/jem.185.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanchot C, Lemonnier FA, Perarnau B, Freitas AA, Rocha B. Differential requirements for survival and proliferation of CD8 naive or memory T cells. Science. 1997;276:2057–62. doi: 10.1126/science.276.5321.2057. [DOI] [PubMed] [Google Scholar]
- 27.Chang L, Crowston JG, Sabin CA, Khaw PT, Akbar AN. Human tenon's fibroblast-produced IFNβ and the prevention of T-cell apoptosis. Invest Ophthalmol Vis Sci. 2001;42:1531–8. [PubMed] [Google Scholar]
- 28.Lombardi G, Dunne PJ, Scheel-Toellner D, et al. Type 1 IFN maintains the survival of anergic CD4+ T cells. J Immunol. 2000;165:3782–9. doi: 10.4049/jimmunol.165.7.3782. [DOI] [PubMed] [Google Scholar]
- 29.Pilling D, Akbar AN, Girdlestone J, et al. Interferon-β mediates stromal cell rescue of T cells from apoptosis. Eur J Immunol. 1999;29:1041–50. doi: 10.1002/(SICI)1521-4141(199903)29:03<1041::AID-IMMU1041>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 30.Marrack P, Kappler J, Mitchell T. Type I interferons keep activated T cells alive. J Exp Med. 1999;189:521–30. doi: 10.1084/jem.189.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suzuki Y, Rahman M, Mitsuya H. Diverse transcriptional response of CD4+ T cells to stromal cell-derived factor (SDF)-1: cell survival promotion and priming effects of SDF-1 on CD4+ T cells. J Immunol. 2001;167:3064–73. doi: 10.4049/jimmunol.167.6.3064. [DOI] [PubMed] [Google Scholar]
- 32.Arai J, Yasukawa M, Yakushijin Y, Miyazaki T, Fujita S. Stromal cells in lymph nodes attract B-lymphoma cells via production of stromal cell-derived factor-1. Eur J Haematol. 2000;64:323–32. doi: 10.1034/j.1600-0609.2000.90147.x. [DOI] [PubMed] [Google Scholar]