Abstract
Lipid A is the bioactive centre of lipopolysaccharide (LPS), and its properties exhibit various endotoxic and biological effects toward host cells. We examined whether Toll-like receptors (TLRs) are mediated by the signals from various synthetic acylated derivatives of d-glucosamine monophosphate. All test synthetic monosaccharide lipid A analogues similar to acylated β-(1-6)-d-glucosamine disaccharide bisphosphates, such as Escherichia coli-type lipid A (compound 506) and its precursor (compound 406), clearly induced nuclear factor (NF)-κB activation in Ba/F3 cells expressing murine TLR4 and its accessory protein MD-2 (Ba/mTLR4/mMD-2), but no induction was found in those expressing murine TLR2 (Ba/mTLR2). Compound 411, the non-reducing sugar moiety of compound 506, exhibited interleukin-8 (IL-8) and tumour necrosis factor-α (TNF-α)-producing activities in human peripheral blood mononuclear cells (PBMC), whereas compound 401, the reducing moiety of compounds 506 and 406, and Gifu lipid A-46 (GLA-46), the non-reducing moiety of compound 406, induced no production of IL-8 and TNF-α, which was similar to the findings for compound 406. Among the synthetic triacylated monosaccharide lipid A analogues, some compounds with three tetradecanoyl (C14) groups or that included a dodecanoyl (C12) group were more active toward murine and human cells than were other analogues with a decanoyl (C10) or hexadecanoyl (C16) group. Furthermore, IL-8 production in PBMC stimulated with the active monosaccharide lipid A analogues as well as compound 506 was clearly inhibited by the monoclonal antibody to human TLR4. These findings suggest that monosaccharide lipid A analogues similar to disaccharide lipid As are capable of activating both murine and human cells through TLR4.
Introduction
Lipopolysaccharide (LPS) is a major component of the outer membrane constituent of Gram-negative bacteria and structurally consists of the O-specific chain, the core oligosaccharide, and lipid A.1 LPS stimulates host cells, and exerts various pathophysiological effects in experimental animals and humans.2 Among its host cells, monocytes/macrophages are important as a primary target for LPS, and LPS-induced activation results in the production of pro-inflammatory cytokines such as tumour necrosis factor-α (TNF-α), and interleukin-1 (IL-1), IL-6, IL-8 and IL-10.3 LPS also induces antigen-independent B-cell mitogenic responses.4
Lipid A represents a lipophilic portion of LPS and its generalized chemical structure consists of a β-(1-6)-linked disaccharide, which carries a glycosidic and a non-glycosidic phosphoryl group, as well as (R)-3-hydroxy fatty acids in ester and amide linkages, some of which are acylated in their 3-hydroxyl group. By using a chemically synthesized compound, hexa-acylated Escherichia coli-type lipid A, designated as compound 506, lipid A has come to be generally regarded as the endotoxic principal and bioactive centre of LPS.5,6 Furthermore, modified and partial lipid A structures have been employed to examine the relationship between those structures and biological activities.3 Among them, compound 406, which is a tetra-acylated biosynthetic precursor of disaccharide lipid A, has been shown to have special properties and to play the role of an LPS agonist toward murine cells as well as of an LPS antagonist toward human cells.7–9 Similarly, some monosaccharide lipid A analogues with structures half of those of complete disaccharide lipid A were demonstrated to act as LPS antagonists to human cells.10
Toll-like receptors (TLRs) have recently been implicated in recognition of pathogen-associated molecular patterns, with at least 10 members identified (TLR1 to TLR10).11–13 Compound 506 activates human and murine cells through TLR4,14–17 while compound 406 was also recognized by TLR4.18,19 Furthermore, compound 406 is able to activate nuclear factor (NF) -κB in human cells but not in murine cells.20 In another study, TLR4 was shown to require an accessory protein, MD-2, to respond efficiently to LPS.21
Some synthetic monosaccharide lipid A analogues are known to activate both human and murine cells, while others activate only murine cells and function as an LPS antagonist to human cells.10,22 In the present study, we attempted to determine which TLR is utilized by monosaccharide lipid A analogues for cellular signalling, to clarify the relationship between the chemical structures of lipid A and TLRs.
Materials and methods
Reagents
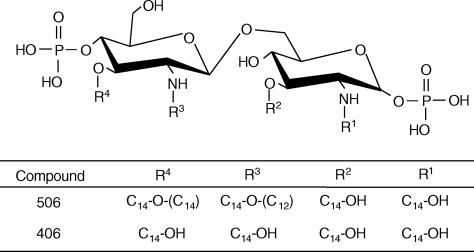
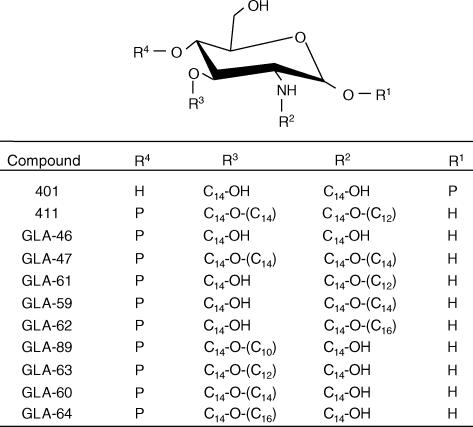
Various monosaccharide lipid A analogues containing Gifu lipid A (GLA) compounds, as well as compound 401 (the reducing moiety of compounds 506 and 406) and compound 411 (the non-reducing moiety of compound 506), E. coli-type lipid A (compound 506), and a biosynthetic precursor of disaccharide lipid A (compound 406) were synthesized chemically as described previously.23–25 The purity of the analogues was confirmed to be very high, without detectable contaminants, by reversed-phase high-performance liquid chromatography analysis, and their chemical structures are indicated in Figs 1 and 2. These synthetic compounds were dissolved at a concentration of 2 mg/ml in a 0·1% (v/v) triethylamine aqueous solution. Staphylococcus aureus peptidoglycan was prepared in our laboratory, as described previously.26 Recombinant human TNF-α was purchased from Dainippon Pharmaceutical (Osaka, Japan). Mouse monoclonal antibodies to human TLR2 (TL2.1) and human TLR4 (HTA125) came from eBioscience (San Diego, CA). Mouse immunoglobulin G2a (IgG2a; Dako, Glostrup, Denmark) was used as an isotype control for TL2.1 and HTA125.
Figure 1.
Chemical structures of synthetic lipid A and its analogue. Abbreviations used: C14-OH, (R)-3-hydroxytetradecanoyl; C14-O-(C12), (R)-3-dodecanoyloxytetradecanoyl; C14-O-(C14), (R)-3-tetradodecanoyloxytetradecanoyl.
Figure 2.
Chemical structures of synthetic acylated glucosamine monophosphates. Abbreviations used: P, PO (OH)2; C14-OH, (R)-3-hydroxytetradecanoyl; C14-O-(C10), (R)-3-dodecanoyloxytetradecanoyl; C14-O-(C12), (R)-3-dodecanoyloxytetradecanoyl; C14-O-(C14), (R)-3-tetradodecanoyloxytetradecanoyl; C14-O-(C16), (R)-3-hexadecanoyloxytetradecanoyl.
Cells
An IL-3-dependent murine Ba/F3 pro-B cell line stably expressing p55Igκ Luc, an NF-κB-dependent luciferase reporter construct (Ba/κB), murine TLR2 and the p55Igκ Luc reporter construct (Ba/mTLR2), and murine TLR4/mD-2 and the p55Igκ Luc reporter construct (Ba/mTLR4/mMD-2) were all kindly provided by Dr Miyake (Division of Infectious Genetics, Department of Microbiology and Immunology, Institute of Medical Science, University of Tokyo, Japan), and maintained in RPMI-1640 (Sigma, St Louis, MO) supplemented with IL-3, 50 μm 2-mercaptoethanol, and 10% fetal bovine serum (FBS; Sigma) in a humidified chamber at 37° in 5% (v/v) CO2.25 Human peripheral blood mononuclear cells (PBMC) were isolated from heparinized venous blood taken from a healthy adult donor, after receiving informed consent, using Histopaque-1077 (Sigma) for gradient centrifugation at 800 g at room temperature for 20 min The isolated PBMC were washed three times with phosphate-buffered saline (Sigma) and suspended in RPMI-1640 medium.
Cytokine production
PBMC (1 × 105 cells) were incubated with or without test specimens in RPMI-1640 medium with 10% FBS at 37° for 24 or 48 hr in 96-well round-bottomed plates (Falcon, Franklin Lakes, NJ). In some experiments, the cells were incubated with or without 10 μg/ml HTA125, TL2.1, or mouse IgG2a at 37° for 30 min before addition of the test specimens. After cultivation, culture supernatants were collected and used for cytokine assays. The culture supernatants were analysed by enzyme-likned immunosorbent assay (ELISA) for secreted IL-8 (Genzyme-Techne, Minneapolis, MN) and TNF-α (eBioscience), and results were determined using a standard curve prepared for each assay.
Luciferase assay
Ba/κB, Ba/mTLR2 and Ba/mTLR4/mMD-2 were inoculated onto 96-well flat-bottomed plates (Falcon) at 1 × 105 cells per 100 μl RPMI-1640 supplemented with 10% FBS and stimulated with the indicated doses of the test specimens at 37°. After 4 hr of incubation, 100 μl Bright-Glo™ luciferase assay reagent (Promega, Madison, WI) was added to each well and luminescence was quantified using a luminometer (Turner Designs Luminometer Model TD-20/20; Promega).
Statistics
Data were analysed by one-way analysis of variance, using the Bonferroni or Dunn method, and the results are presented as the mean ± standard error of the mean (SEM). When an individual experiment is shown, it is representative of at least three independent experiments.
Results
NF-κB activation of Ba/F3 cells in response to various monosaccharide lipid A analogues
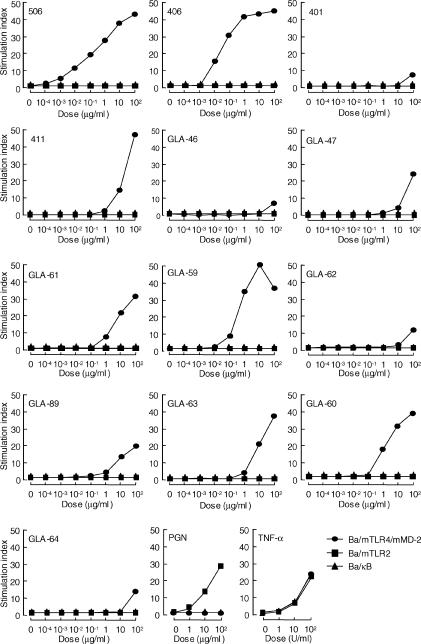
We examined NF-κB activation in Ba/κB, Ba/mTLR2 and Ba/mTLR4/mMD-2 after stimulation with various monosaccharide lipid A analogues, as well as two disaccharide lipid As, compounds 506 and 406, using a luciferase assay. All monosaccharide analogues tested induced NF-κB activation in Ba/mTLR4/mMD-2, whereas there was no response in Ba/mTLR2 and Ba/κB (Fig. 3). Compounds 506 and 406 also exhibited NF-κB activation in Ba/mTLR4/mMD-2 but not in Ba/mTLR2 or Ba/κB.
Figure 3.
NF-κB activation in Ba/F3 cells in response to various synthetic disaccharide and monosaccharide lipid A analogues. Cells were stimulated with the indicated doses of each test specimen in RPMI-1640 supplemented with 10% FBS at 37° for 4 hr. Luciferase activity in the cell lysate was measured. Results are shown as relative luciferase activity, which is a ratio of stimulated activity to non-stimulated activity, in each cell line. Similar results were obtained from three independent experiments. Peptidoglycan (PGN) and TNF-α were used as positive control specimens in this experiment.
Compound 411 (four acyls), 2-N-(R)-3-dodecanoyloxytetradecanoyl-3-O-tetradodecanoyl-d-glucosamine 4-phosphate, which has a structure corresponding to the non-reducing moiety of compound 506, clearly induced NF-κB activation in Ba/mTLR4/mMD-2. GLA-47 (four acyls), in which a tetradecanoyl (C14) group is introduced instead of a dodecanoyl (C12) group and attached to the 2-N-linked 3-hydroxytetradecanoyl (C14-OH) group in compound 411, also showed NF-κB activation (Fig. 3). Furthermore, compound 401 (two acyls), 2-N, 3-O-bis [(R)-3-hydroxytetradecanoyl]-d-glucosamine 1-phosphate, corresponding to the reducing moiety of compounds 506 and 406, induced very weak NF-κB activation. Similar results for GLA-46 (two acyls), 2-N, 3-O-bis [(R)-3-hydroxytetradecanoyl]-d-glucosamine 4-phosphate, with a structure identical to the non-reducing moiety of compound 406, were obtained.
We also examined the effect of the carbon-chain length of 2-N-acyl and 3-O-acyl groups in triacylated monosaccharide lipid A analogues on NF-κB activation in murine Ba/F3 cells. Among these analogues, GLA-59, a C14 group attached to the hydroxyl (OH) group of the 2-N-linked C14-OH group, and GLA-60, a C14 group attached to the OH group of the 3-O-linked C14-OH group, clearly exhibited NF-κB activation in Ba/mTLR4/mMD-2 (Fig. 3). Furthermore, GLA-61 and GLA-63, which were introduced to the C12 groups attached to the OH group of the 2-N- and 3-O-linked C14-OH groups, respectively, demonstrated comparable NF-κB activation. However, GLA-62 and GLA-64, in which a hexadecanoyl (C16) group is introduced to the C14-OH group, and GLA-89, with a decanoyl (C10) group attached to the OH group of the 3-O-linked C14-OH group, induced only weak activities. Peptidoglycan was used as a TLR2 ligand. The addition of TNF-α resulted in almost similar NF-κB activation in these Ba/F3 cells.
Induction of cytokine production in human cells by various monosaccharide lipid A analogues
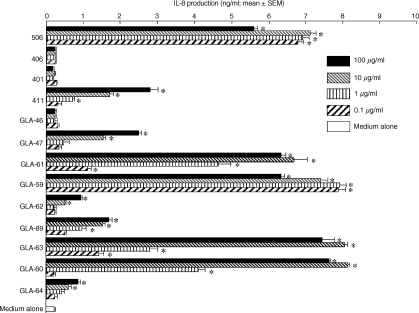
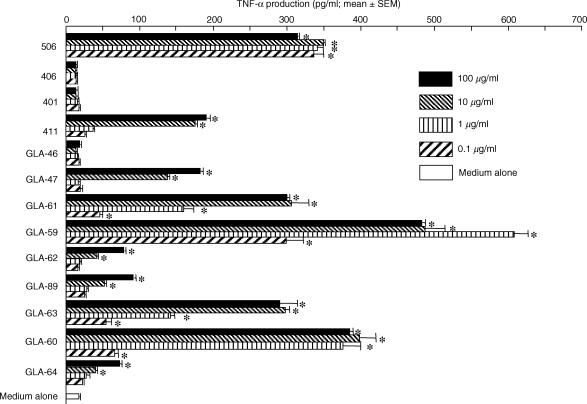
IL-8-producing activities were found in human PBMC after stimulation with various monosaccharide and disaccharide lipid A analogues (Fig. 4). Compound 506, but not compound 406, significantly induced IL-8 production in human PBMC. The non-reducing moiety of compound 506, compound 411, also exhibited a very weak induction of IL-8 production. However, stimulation of these cells with compound 401 and GLA-46 (two acyls), corresponding to the reducing and non-reducing moieties of compound 406, resulted in no induction of IL-8 production in the range of doses from 0·1 to 100 μg/ml. Among the triacylated monosaccharide lipid A analogues, GLA-59 and GLA-60, which have all of the C14 chains, and GLA-61 and GLA-63, with two C14 chains and a C12 chain, were found to be more effective than GLA-62, GLA-64, and GLA-89, with a C16 or C10 chain and two C14 chains. Furthermore, the production patterns of TNF-α were similar to those of IL-8 in PBMC stimulated with these test specimens (Fig. 5).
Figure 4.
IL-8-producing activities in human PBMC by various synthetic disaccharide and monosaccharide lipid A analogues. Cells were stimulated with the indicated doses of each test specimen in RPMI-1640 supplemented with 10% FBS at 37° for 48 hr. After incubation, the supernatants were collected and IL-8 production was determined by ELISA. Experiments were performed at least three times and representative results are shown. Each assay was performed in triplicate wells and data are expressed as the mean ± SEM. Significant differences between groups with and without the test specimens were seen (*P < 0·01).
Figure 5.
TNF-α-producing activities in human PBMC by various synthetic disaccharide and monosaccharide lipid A analogues. Cells were stimulated with the indicated doses of each test specimen in RPMI-1640 supplemented with 10% FBS at 37° for 24 hr. After incubation, the supernatants were collected and TNF-α production was determined by ELISA. Experiments were performed at least three times and representative results are shown. Each assay was performed in triplicate wells and data are expressed as the mean ± SEM. Significant differences between groups with and without the test specimens were seen (*P < 0·01).
Inhibitory effect of mouse monoclonal antibody to human TLR4 on IL-8 production by PBMC
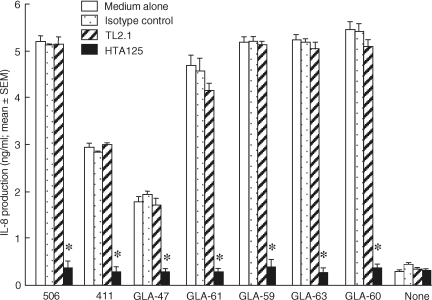
To examine the recognition of human cells in response to active monosaccharide lipid A analogues, PBMC were treated with the mouse monoclonal antibody to human TLR2, TL2.1, the mouse monoclonal antibody to human TLR4, HTA125, or mouse IgG2a (as a control) before the addition of various test specimens. Figure 6 shows that HTA125 significantly inhibited IL-8 production induced by the active monosaccharide lipid A compounds as well as by compound 506, whereas TL2.1 and mouse IgG2a did not. Our results indicate that these monosaccharide lipid A compounds activate human cells through TLR4.
Figure 6.
Effect of mouse monoclonal antibodies to human TLR2 and TLR4 on IL-8-producing activities in PBMC stimulated with synthetic disaccharide and monosaccharide lipid A analogues. Cells were incubated with or without 10 μg/ml of mouse monoclonal antibodies to human TLR2 (TL2.1), to human TLR4 (HTA125), or mouse IgG2a at 37° for 30 min, prior to the addition of compound 506 (10 ng/ml) or various monosaccharide-type lipid A analogues (each 10 μg/ml) supplemented with 10% FBS. After 48 hr of incubation, the supernatants were collected and IL-8 production was determined by ELISA. The experimental protocols were the same as described in the legend to Figure 4. Each assay was carried out in triplicate wells and data are expressed as the mean ± SEM. Significant differences were seen as compared to the medium alone (*P < 0·01).
Discussion
We examined the recognition of human and murine cells in response to monosaccharide lipid A analogues. Ba/mTLR4/mMD-2 responded to all monosaccharide analogues tested, whereas Ba/mTLR2 and Ba/κB did not (Fig. 3). Among these compounds, some analogues activated human PBMC (Figs 4 and 5) and their activities were inhibited by the mouse monoclonal antibody to human TLR4, HTA125 (Fig. 6). These results suggest that the monosaccharide lipid A analogues were recognized by human and murine cells through TLR4. NF-κB activation by Ba/mTLR4/mMD-2 in response to various monosaccharide lipid A analogues was also examined (Fig. 3). These cells definitely responded to compounds 506 and 406 at concentrations over 0·01 μg/ml, however, the monosaccharide lipid A analogues activated the cells at concentrations ranging from 10 to 100 μg/ml. These results suggest that cell activation is related to the numbers of glucosamine and the attached phosphate group.
Compound 411 and GLA-47, with four acyl groups, exhibited weaker activation of both murine and human cells than GLA-59 and GLA-60, with three acyl groups, and the diacylated monosaccharide lipid A analogues, such as compound 401, while GLA-46 induced marginal cell activation (Figs 3–5). Matsuura et al. also found that only triacylated monosaccharide lipid A analogues exhibited human cell activation, whereas diacylated and tetra-acylated monosaccharide lipid A analogues did not.10 They suggested that recognition by these cells was more strictly related to the numbers of acyl groups attached to monosaccharide lipid A analogues.
GLA-59 and GLA-60, carrying three C14 groups, were found to have the ability to activate both murine and human cells (Figs 3–5). On the other hand, the other triacylated monosaccharide lipid A analogues, GLA-89, GLA-62, and GLA-64, with three acyl groups, two of which are C14 and one of which is C10 or C16, exhibited a much weaker ability to induce NF-κB activation in Ba/mTLR4/mMD-2, as well as IL-8 and TNF-α production in human cells than GLA-61 and GLA-63, and GLA-59 and GLA-60, each possessing two C14 and one of C12 or C14. Porphyromonas gingivalis lipid A has a structure different from enterobacterial lipid As, while the presence of fatty acids possessing 16–17 carbon atoms is a unique feature.27 Recently, we found that purified P. gingivalis lipid A and its synthetic compound demonstrated weaker but significant induction of cytokine production through TLR4 when compared with compound 506.25 Those results suggested that the carbon-chain length of the acyl groups in monosaccharide lipid A analogues plays a pivotal role in cell activation through TLR4.
In the present study, compound 411, with a structure corresponding to the non-reducing moiety of compound 506, clearly activated both murine and human cells through TLR4 (Figs 3–5). On the other hand, GLA-46, corresponding to the non-reducing moiety of compound 406, and compound 401, corresponding to the reducing moiety of compounds 506 and 406, induced only weak activation in murine cells and no activation in human cells. A tetra-acylated disaccharide lipid A, compound 406, and tetra-acylated monosaccharide lipid A analogues, compound 411 and GLA-47, activated murine cells (Fig. 3). Furthermore, compound 411 and GLA-47, but not compound 406, activated human PBMC through TLR4 (Figs 4 and 5). Akashi et al. also indicated that compound 406 acted as an agonist toward mouse TLR4/MD-2 but not toward human TLR4/MD-2, while both human and murine MD-2 influenced LPS recognition.20 On the other hand, it has been demonstrated that human TLR recognizes host-specific LPS modification and MD-2 does not mediate differential recognition.28 Therefore, the present results clearly show that monosaccharide lipid A analogues, possessing a variety of acyl groups and with carbon-chain lengths similar to disaccharide lipid As, are capable of activating both human and murine cells through TLR4.
Acknowledgments
This work was supported in part by grants-in-aid for scientific research (B) from the Japan Society for the Promotion of Science (no. 13470390), and for Encouragement of Young Scientists (no. 13771291 and no. 14771014). We thank Mr M. Benton for his critical reading of the manuscript.
Abbreviations
- C10
decanoyl
- C12
dodecanoyl
- C14
tetradecanoyl
- C16
hexadecanoyl
- C14-OH
3-hydroxytetradecanoyl
- IL
interleukin
- LDH
lactate dehydrogenase
- LPS
lipopolysaccharide
- NF
nuclear factor
- OH
hydroxyl
- PBMC
peripheral blood mononuclear cells
- TLRs
Toll-like receptors
- TNF
tumour necrosis factor
References
- 1.Westphal O, Lüderitz O, Galanos C, Mayer H, Rietschel ET. The story of bacterial endotoxin. Adv Immunopharmacol. 1986;3:13–34. [Google Scholar]
- 2.Morrison DC, Ryan JL. Bacterial endotoxins and host immune responses. Adv Immunol. 1979;28:293–450. doi: 10.1016/s0065-2776(08)60802-0. [DOI] [PubMed] [Google Scholar]
- 3.Rietschel ET, Kirikae T, Schade FU, et al. Bacterial endotoxin: molecular relationships of structure to activity and function. FASEB J. 1994;8:217–25. doi: 10.1096/fasebj.8.2.8119492. [DOI] [PubMed] [Google Scholar]
- 4.Severinson E, Larsson EL. Lymphocyte responses to polyclonal T and B cell activators. In: Weir DM, editor. Handbook of Experimental Immunology. Oxford: Blackwell Scientific Publications; 1986. pp. 63.1–63.18. [Google Scholar]
- 5.Kotani S, Takada H, Tsujimoto M, et al. Synthetic lipid A with endotoxic and related biological activities comparable to those of a natural lipid A from an Escherichia coli Re-mutant. Infect Immun. 1985;49:225–37. doi: 10.1128/iai.49.1.225-237.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zähringer U, Lindner B, Rietschel ET. Molecular structure of lipid A, the endotoxic center of bacterial lipopolysaccharides. Adv Carbohydr Chem Biochem. 1994;50:211–76. [PubMed] [Google Scholar]
- 7.Loppnow H, Brade H, Durrbaum I, Dinarello CA, Kusumoto S, Rietschel ET, Flad HD. IL-1 induction-capacity of defined lipopolysaccharide partial structures. J Immunol. 1989;142:3229–38. [PubMed] [Google Scholar]
- 8.Kovach NL, Yee E, Munford RS, Raetz CR, Harlen JM. Lipid IVA inhibits synthesis and release of tumor necrosis factor induced by lipopolysaccharide in human whole blood ex vivo. J Exp Med. 1990;172:77–84. doi: 10.1084/jem.172.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ulmer AJ, Heine H, Feist W, et al. Biological activity of synthetic phosphonooxyethyl analogs of lipid A and lipid A partial structures. Infect Immun. 1992;60:3309–14. doi: 10.1128/iai.60.8.3309-3314.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsuura M, Kiso M, Hasegawa A. Activity of monosaccharide lipid A analogs in human monocytic cells as agonists or antagonists of bacterial lipopolysaccharide. Infect Immun. 1999;67:6286–92. doi: 10.1128/iai.67.12.6286-6292.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chuang TH, Ulevitch RJ. Identification of hTLR10, a novel human Toll-like receptor preferentially expressed in immune cells. Biochem Biophys Acta. 2001;1518:157–61. doi: 10.1016/s0167-4781(00)00289-x. [DOI] [PubMed] [Google Scholar]
- 12.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–45. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 13.Vasselon T, Detmers PA. Toll receptors, a central element in innate immune responses. Infect Immun. 2002;70:1033–41. doi: 10.1128/IAI.70.3.1033-1041.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F. Toll-like receptor 4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem. 1999;274:10689–92. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- 15.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide, evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–52. [PubMed] [Google Scholar]
- 16.Qureshi ST, Larivière L, Leveque G, Clemont S, Moore KJ, Gros P, Malo D. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4) J Exp Med. 1999;189:615–25. doi: 10.1084/jem.189.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poltorak A, Ricciardi-Castagnoli P, Citteria S, Beuter B. Physical contact between lipopolysaccharide and Toll-like receptor 4 revealed by genetic complementation. Proc Natl Acad Sci USA. 2000;97:2163–7. doi: 10.1073/pnas.040565397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poltorak A, He X, Smirnova I, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice, mutations in Tlr4 gene. Science. 1998;282:2085–8. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 19.Lien E, Means TK, Heine H, et al. Toll-like receptor 4 imparts ligand-specific recognition of bacterial lipopolysaccharide. J Clin Invest. 2000;105:497–504. doi: 10.1172/JCI8541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akashi S, Nagai Y, Ogata H, et al. Human MD-2 confers on mouse Toll-like receptor 4 species-specific lipopolysaccharide recognition. Int Immunol. 2001;13:1595–9. doi: 10.1093/intimm/13.12.1595. [DOI] [PubMed] [Google Scholar]
- 21.Shimazu R, Akashi S, Ogata H, Nagai Y, Fukudome K, Miyake K, Kimoto M. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med. 1999;189:1777–82. doi: 10.1084/jem.189.11.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Funatogawa K, Matsuura M, Nakano M, Kiso M, Hasegawa A. Relationship of structure and biological activity of monosaccharide lipid A analogs to induction of nitric oxide production by murine macrophage RAW264.7 cells. Infect Immun. 1998;66:5792–8. doi: 10.1128/iai.66.12.5792-5798.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiso M, Tanaka S, Fujishima Y, Ogawa Y, Hasegawa A. Synthesis of nonreducing-sugar subunit analogs of bacterial lipid A carrying an amide-bound (3R)-3-acyloxytetradecanoyl group. Carbohydr Res. 1987;162:247–56. doi: 10.1016/0008-6215(87)80207-0. [DOI] [PubMed] [Google Scholar]
- 24.Kiso M, Tanaka S, Fujita M, Fujishima Y, Ishida H, Hasegawa A. Synthesis of the optically active 4-O-phosphono-d-glucosamine derivatives related to the nonreducing-sugar subunit of bacterial lipid A. Carbohydr Res. 1987;162:127–40. doi: 10.1016/0008-6215(87)80207-0. [DOI] [PubMed] [Google Scholar]
- 25.Ogawa T, Asai Y, Hashimoto M, Takeuchi O, Kurita T, Yoshikai Y, Miyake K, Uchida H. Cell activation by Porphyromonas gingivalis lipid A molecule through Toll-like receptor 4- and myeloid differentiation factor 88-dependent signaling pathway. Int Immunol. 2002;14:1325–32. doi: 10.1093/intimm/dxf097. [DOI] [PubMed] [Google Scholar]
- 26.Ogawa T, Kotani S, Tsujimoto M, Kusumoto S, Shiba T, Kawata S, Yokogawa K. Contractile effects of bacterial cell walls, their enzymatic digests, and muramyl dipeptides on ileal strips from guinea pigs. Infect Immun. 1982;35:612–19. doi: 10.1128/iai.35.2.612-619.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogawa T. Chemical structure of lipid A from Porphyromonas (Bacteroides) gingivalis lipopolysaccharide. FEBS Lett. 1993;332:197–201. doi: 10.1016/0014-5793(93)80512-s. [DOI] [PubMed] [Google Scholar]
- 28.Hajjar AM, Ernst RK, Tsai JH, Wilson CB, Miller SI. Human Toll-like receptor 4 recognizes host-specific LPS modifications. Nat Immunol. 2002;3:354–9. doi: 10.1038/ni777. [DOI] [PubMed] [Google Scholar]