Abstract
Using a high throughput gene microarray technology that detects ∼22 000 genes, we found that arginase I was the most significantly up-regulated gene in the murine spinal cord during experimental autoimmune encephalomyelitis (EAE). By Northern blot and arginase enzyme assay, we detected high levels of arginase I mRNA and protein, respectively, in the spinal cord of EAE mice, but not in the spinal cord of normal mice or mice that had recovered from EAE. In vitro, both microglia and astrocytes produced arginase and nitric oxide synthase, two enzymes that are involved in arginine metabolism. To explore the roles of arginase in EAE, we injected the arginase inhibitor amino-6-boronohexanoic acid (ABH) into mice during the inductive and effector phases of the disease. Compared with mice that received vehicle control, mice treated with ABH developed milder EAE with delayed onset, reduced disease score and expedited recovery. Spleen mononuclear cells from ABH-treated mice produced more nitric oxide and secreted less interferon-γ and tumour necrosis factor-α as compared to control mice. These results indicate that arginase plays important roles in autoimmune inflammation in the central nervous system.
Introduction
Experimental autoimmune encephalomyelitis (EAE) is an inflammatory disease of the central nervous system (CNS) that has long been used as an animal model for human multiple sclerosis (MS). EAE can be induced in susceptible strains of animals by immunization with myelin antigens such as myelin basic protein (MBP), proteolipid protein (PLP), or myelin oligodendrocyte glycoprotein (MOG).1,2 Although it is well recognized that development of MS or EAE requires the expression of a large number of genes, the exact number and identities of these genes are unknown. Recently, we have performed genome-wide profiling of spinal cords during EAE using the Affymetrix gene microarray U74 system.3 Of ∼22 000 genes examined, arginase I was found to be the most significantly up-regulated gene in the spinal cord during EAE. The goal of this study is to explore the potential roles of arginase in EAE.
Arginase (l-arginine ureahydrolase, or amidinohydrolase) is a hydrolytic enzyme responsible for converting arginine to ornithine and urea.4 It plays a fundamental role in nitrogen metabolism, and is widely distributed in the biosphere. In higher organisms, two forms of arginase exist which are designated as arginase I (Arg I) and arginase II (Arg II). Arg I is expressed primarily in the liver and is a critical component of the urea cycle. By contrast, Arg II is expressed in many extrahepatic tissues, such as brain, spinal cord, kidney, small intestine and mammary gland. Although Arg I and Arg II share similar enzyme activities, they are encoded by different genes, and have different pI, immunological reactivity, and subcellular locations.4
In addition to participating in the urea cycle, recent studies suggest that arginase also plays important roles in nitric oxide (NO) synthesis, polyamine metabolism, proline and glutamate synthesis, and γ-aminobutyric acid (GABA) formation. Nitric oxide synthase (NOS) also uses arginine as substrate, and competes with arginase for arginine. Therefore, arginase can also exert its biological function through regulating NO production. We report here that arginase plays an important role in a T-cell mediated autoimmune disease in the CNS.
Materials and methods
Mice
C57BL/6 (B6) mice, 6–7 weeks of age, were purchased from Jackson Laboratory (Bar Harbor, ME), and housed in the University of Pennsylvania Animal Care Facilities under pathogen-free conditions. All procedures used were preapproved by the Institutional Animal Care and Use Committee.
Reagents
Mouse MOG 38–50 peptide was synthesized using Fmos solid phase methods and purified through HPLC by Research Genetics (Huntsville, AL). Pertussis toxin was purchased from List Biological Laboratories (Campbell, CA). Triton-X 100, MnCl2, l-Arginine, H2SO4, H3PO4,α-isonitrosopropiophenone, and urea were all purchased from Sigma (St. Louis, MO). Purified rat anti-mouse interleukin (IL)-4, IL-10 and interferon-γ (IFN-γ) monoclonal antibodies (mAb) were purchased from PharMingen (San Diego, CA).
Induction and clinical evaluation of EAE5,6
For the induction of EAE, mice received (1) a subcutaneous injection on flanks of 300 µg MOG38-50 peptide in 0·1 ml phosphate-buffered saline (PBS) emulsified in an equal volume of complete Freund's adjuvant containing 5 mg/ml of mycobacterium tuberculosis H37RA (Difco, St. Louis, MO), and (2) an intravenous injection of 100 ng pertussis toxin in 0·1 ml PBS. A second injection of pertussis toxin (100 ng per mouse) was given 48 hr later. Mice were examined daily for signs of EAE and scored as follows: 0 – no disease; 1 – tail paralysis; 2 – hind limb weakness; 3 – hind limb paralysis; 4 – hind limb plus forelimb paralysis; 5 – moribund or dead.
Probe preparation, gene chip hybridization and gene chip data analysis
Purified total RNA was used to synthesize double strand cDNA with a SuperScript kit (Life Technologies, Inc., La Jolla, CA), using oligo-dT primers incorporating a T7 RNA polymerase promoter (Genset, La Jolla, CA).3 Biotin-labelled cRNAs were then generated from the cDNA by in vitro transcription with T7 RNA polymerase (Enzo kit, Enzo Diagnostics, Farmingdale, NY). Subsequent hybridization, washing and staining with Affymetrix murine U74 chips were carried out following the manufacturer's instructions (Affymetrix, Inc., Santa Clara, CA). All experiments were repeated at least once to ensure the reproducibility of the results. Analysis of DNA microarray data was carried out using GeneChip Analysis Suite 4.0 software (Affymetrix, Inc.). Both ‘Per Chip’ and ‘Per Gene’ normalizations were performed to compensate for potential differences in the quantity and composition of the mRNAs prepared from different samples. The fold change for each gene during EAE was calculated as follows: Fold change = relative gene expression levels of the sample/relative gene expression levels of the spinal cord of naïve mice.
Northern blot
Total RNA was extracted from various tissues using the phenol/chloroform-sarcosyl extraction/LiCl precipitation method. Purified RNA, 10 µg per sample, was separated by electrophoresis on a 1·5% denaturing agarose gel. The fractionated RNA was then transferred onto a Hybond™ N+ membrane (Amersham Pharmacia Biotech, Inc., Piscataway, NJ), and blotted with 32P-labelled full-length Arg I or GAPDH cDNA probe using the Amersham rediprime™ kit.
Measurement of arginase activity
Arginase enzyme activity was measured as previously described,7 with slight modifications. Briefly, 50 µl tissue homogenates or 106 cells were mixed with 100 µl of 0·1% Triton X-100. After 30 min of incubation on a shaker, 100 µl of 25 mm Tris-HCl and 20 µl of 10 mm MnCl2 were added to the sample. The arginase was then activated by heating the sample for 10 min at 56°, and arginine hydrolysis was conducted by incubating the sample with 100 µl of 0·5 m l-arginine (pH 9·7) at 37° for 60–120 min. The reaction was stopped with 900 µl of H2SO4 (96%)/H3PO4 (85%)/H2O (1/3/7, v/v/v), and the sample was mixed with 40 µl of 9% isonitrosopropiophenone (dissolved in 100% ethanol). After heating at 95° for 30 min, urea concentration in the sample was determined by spectrometry at 540 nm. One unit of enzyme activity is defined as the amount of enzyme that catalyses the formation of 1 µmol of urea in 1 min.
Microglia and astrocyte culture
Microglia and astrocytes were prepared from newborn mice as previously described.8 In brief, mixed glial cells were first cultured in complete Dulbecco's modified Eagle's minimal essential medium. After 8–10 days, microglia growing on the surface of adherent astrocytes were harvested by shaking for 1·5–2 hr at 260 r.p.m. Culture flasks were then shaken at 100 r.p.m. for an additional 24 hr to remove contaminating oligodendrocytes, and astrocytes were detached with 0·25% trypsin–ethylenediaminetetraacetic acid. The purity of microglial cells and astrocytes was about 96% and 94%, respectively.
Cytokine assay9,10
Splenocytes were cultured at 1·5 × 106 cells/well in 0·2 ml of complete medium with or without 5–25 µg/ml of MOG38-50 peptide. Culture supernatants were collected 40 h later and cytokine concentrations were determined by sandwich enzyme-linked immunosorbent assay using mAbs specific for corresponding cytokines according to the manufacturer's instructions (PharMingen).
Measurement of nitrite
NO was determined by measuring the end product nitrite, using a method based on the Griess reaction. Briefly, aliquots of culture supernatant (100 μl) were mixed with 100 μl of Griess reagent at room temperature for 10 min. Absorbance was measured at 540 nm in an automated microplate reader. The concentration of nitrite was determined by reference to a standard curve of sodium nitrite. Culture medium was used as the blank.
Statistical analysis
Arginase activity, cytokine concentration, and nitrite concentration were analysed by analysis of variance (anova). Disease scores were analysed by Mann–Whitney test.
Results
Arginase is the most significantly up-regulated gene in the spinal cord of mice with EAE
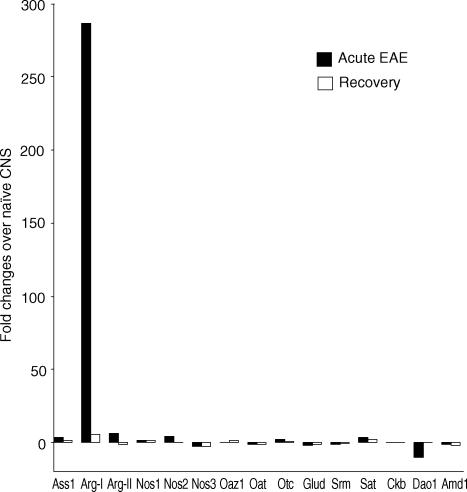
To determine the effect of inflammation on global gene expression in the CNS, we first immunized C57BL/6 mice with self-MOG peptide and killed them during the acute phase of EAE or during disease recovery. The acute phase of EAE is defined as the first EAE attack in mice leading to a clinical score of 3 or 4 (see Materials and methods for disease score criteria). Disease recovery is defined as a decrease of 2 graduations in clinical score for 5 or more consecutive days. Non-immunized naïve mice were used as normal controls. Total RNA was isolated from the spinal cords and analysed using the Affymetrix U74A and U74B chips as described in Materials and Methods. Two independent experiments were performed to ensure the reproducibility of the results. Genes whose expression levels were reproduced in samples in both experiments (which accounted for >98% of the total genes tested) were subjected to a hierarchical clustering analysis to separate genes into clusters based on the similarity of expression across samples. This led to the identification of several clusters of genes whose expression was up-regulated in the spinal cord of mice with EAE. One of these clusters contained Arg I whose expression in the spinal cord was increased by 287-fold during EAE. This places Arg I as the most significantly up-regulated gene of all the ∼22 000 genes examined. Figure 1 illustrates the relative expression levels of Arg I and its related genes involved in arginine metabolism and urea cycle. Although most genes were not significantly affected by EAE, Arg I, Arg II and NOS 2 were up-regulated whereas d-amino acid oxidase down-regulated in the spinal cord of mice with EAE.
Figure 1.
Effect of EAE on gene expression in the spinal cord as determined by microarray analysis. C57BL/6 mice, three mice per group, were immunized with MOG peptide and sacrificed during the acute phase of EAE (filled square) or during disease recovery (open square) as described in Methods. Total RNA was isolated and analyzed using the Affymetrix U74A and U74B gene chips. Fold changes in gene expression were calculated using spinal cords of naïve mice as controls. Only genes involved in arginine metabolism and urea cycle are shown. Results are pooled from two independent experiments. Ass1, argininosuccinate synthetase 1; Arg-I, arginase I; Arg-II, arginase II; Nos1, nitric oxide synthase 1; Nos2, nitric oxide synthase 2; Nos3, nitric oxide synthase 3; Oaz1, ornithine decarboxylase antizyme; Oat, ornithine aminotransferase; Otc, ornithine transcarbamylase; Glud, glutamate dehydrogenase; Srm, spermidine synthase; Sat, spermidine/spermine N1-acetyl transferase; Ckb, creatine kinase, brain; Dao1, d-amino acid oxidase; Amd1, S-adenosylmethionine decarboxylase 1.
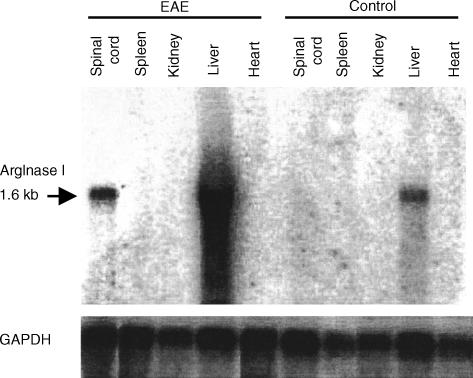
To confirm the microarray results, we also performed Northern blot analysis for Arg I. As shown in Fig. 2, a single 1·6 kb Arg I mRNA was detected only in the liver of normal mice. By contrast, in mice with acute EAE, a high level of Arg I mRNA was also detected in the spinal cord whereas the level of Arg I mRNA in the liver was markedly increased. However, none of the other organs tested expressed detectable amounts of Arg I. Thus, during EAE, Arg I mRNA was specifically up-regulated in the spinal cord.
Figure 2.
Arginase I gene expression in normal and EAE mice as determined by Northern blot. Mice were treated as described in Figure 1 and sacrificed either before immunization (control) or after the disease onset (EAE). Total RNA was isolated from various organs and subjected to Northern blot analysis for Arg I and GAPDH as described in Materials and Methods. Results are representative of two experiments.
Arginase enzyme activity was dramatically up-regulated in the spinal cord during EAE
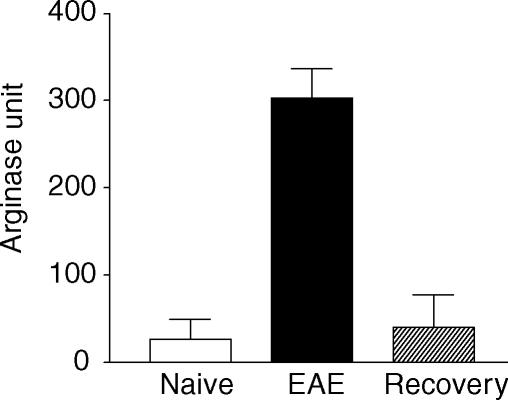
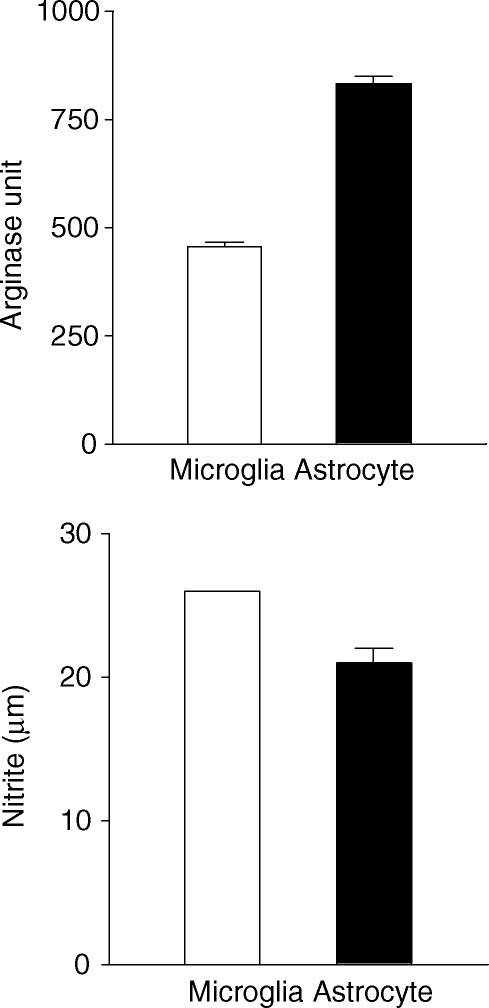
Increased gene transcription does not always lead to higher levels of protein expression. To examine whether the up-regulation of arginase mRNA in EAE translates to increased arginase enzyme expression, we compared arginase enzyme expression in the spinal cord of mice with or without EAE. As shown in Fig. 3, the spinal cord of naïve mice contained very low levels of arginase activity (27·3 ± 22·2 units). This was increased by more than 10-fold in mice with acute EAE (303·6 ± 32·7 units). Importantly, arginase activity returned to normal levels in mice that had recovered from EAE (40·0 ± 37·5 units). Thus, arginase activity in the spinal cord correlates with the severity of EAE. Arginase can be produced by several cell types including hepatocytes, lymphoid cells and myeloid cells. To determine whether cells of the CNS are also capable of producing arginase, we isolated microglia and astrocytes from CNS and tested them in vitro. As shown in Fig. 4, significant arginase activities were detected in both microglia and astrocyte cultures. Additionally, both microglia and astrocytes produced significant amounts of NO in the culture (Fig. 4), suggesting that arginine can also be converted to NO and urea in glial cells.
Figure 3.
Arginase activity in the spinal cord as determined by enzyme assay. Whole spinal cord homogenates of individual mouse were prepared in 2 ml PBS from naïve mice, mice with acute EAE (disease score = 4), and mice that had recovered from EAE. Arginase activity of 50 µl homogenates was determined as described in Materials and Methods. Results shown are mean + SD of arginase unit from each group with three mice per group. Data are representative of two experiments.
Figure 4.
Both microglia and astrocytes are capable of producing arginase and NO synthase in the culture. Microglia and astrocytes, 106 cells each, were isolated, cultured and tested for arginase and NOS activities as described in Methods. Data presented are mean + SD of nitrite (for NOS) and arginase unit (for arginase) from triplicate cultures. Results are representative of two experiments.
Arginase inhibition ameliorates EAE
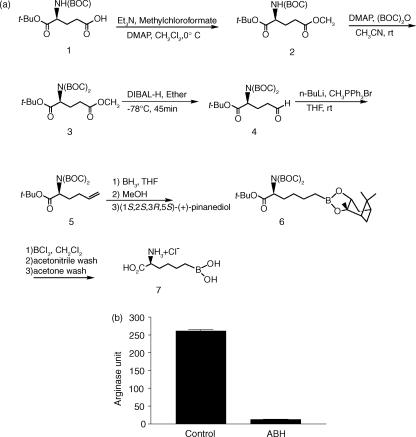
Arginase can be effectively inhibited by l-arginine analogues. We have recently demonstrated that the boronic acid analogue of arginine, ABH, is one of the most potent, stable and specific arginase inhibitors available.11–13 ABH was synthesized using a modified method as described in Fig. 5(a). When tested in vitro, ABH can completely block the arginase activity in the liver homogenate (Fig. 5b). Unlike other arginine analogues, ABH does not inhibit NOS, and is effective in blocking arginase activity in vivo.13
Figure 5.
ABH is a potent inhibitor of arginase. (a) Synthesis of ABH. As reported previously11–13 (2)-(S)-amino-6-boronohexanoic acid (ABH) was synthesized using l-glutamic acid derivative (S)-5-(tert-butoxy)-4-[(tert-butoxycarbonyl)amino]-5-oxopentanoic acid (S)-1, as the starting material. However, we modified the previous method to improve the overall yield. Briefly, esterification of (S)-1 using methyl chloroformate by treatment with triethylamine and a catalytic amount of DMAP in dry methylene chloride provided (S)-1-tert-butyl-5-methyl-[(2-tert-butoxycarbonyl)amino]pentanedioate 2 with 92% yield. Synthesis of di-BOC-(S)-3 was carried out by treatment with di-tert-butyl dicarbonate in the presence of DMAP in acetonitrile with 81% yield. The subsequent reduction of ester (S)-3 was performed with diisobutylaluminium hydride (DIBAL-H) in ether to provide the aldehyde (S)-4 with 71% yield. The Wittig reaction of aldehyde (S)-4 with the ylide generated from methyl triphenylphosphonium bromide and n-BuLi in tetrahydrofuran (THF) at 0° gave olefin (S)-5 with 33% yield. Accordingly, olefin (S)-5 was prepared for hydroboration followed by treatment with methanol to quench unreacted borane and protected with (1S,2S,3R,5S)-(+)-pinanediol. Complete deprotection with BCl3 yielded 2-(S)-amino-6-boronohexanoic acid 7 with an overall yield of 27% for the two steps (from 5 to 7). The overall yield of this synthesis is 4·7%, which is significantly increased compared with the 1·3% yield reported by Baggio et al.11 (b) Inhibition of arginase by ABH. Liver homogenates of naïve mouse were prepared in 5 ml PBS. Ten µl homogenates were treated with or without (control) 100 µm of ABH in PBS. Arginase activity was measured as described in Methods. Data presented are mean ± SD of arginase unit from three independent samples.
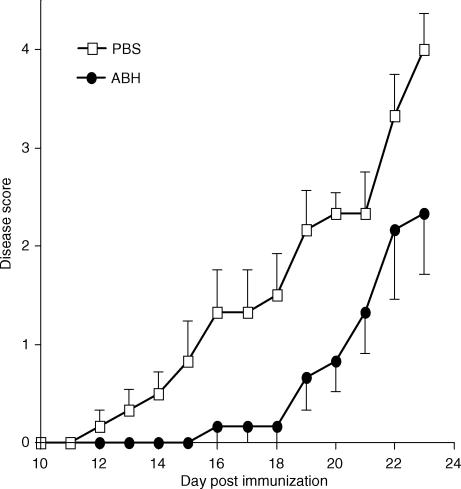
To directly examine the roles of arginase in EAE, we immunized C57BL/6 mice with MOG38-50 peptide and treated them with a daily intraperitoneal injection of 200 µm ABH. Using the specific arginase assay (Fig. 3), we have determined that the half-life of ABH in C57BL/6 mice is approximately 8 hr. The treatment started at the day of immunization and continued for 23 consecutive days. Mice were monitored for signs of EAE by physical examination. Figure 6 illustrates the disease courses of control and ABH-treated mice. EAE developed in all control mice that received PBS, starting approximately 12 days after the immunization and reached a maximal clinical score of 3·5 12 days later. By day 23, less than 18% of the mice in this group recovered from EAE, while 25% of the mice died from the disease (Table 1). By contrast, although mice treated with ABH also developed EAE, the onset of the disease was significantly delayed and the maximal disease score was significantly reduced (Table 1). Most mice in ABH-treated group started to recover 9 days after the disease onset, and few died from EAE (Table 1). In one experiment, mice were treated with ABH beyond the peak of the disease when 50% of mice in PBS-treated group (with a maximum disease score of 3·17 ± 1·2) and 80% of mice in the ABH-treated group (with a maximum disease score of 2·6 ± 0·5) showed signs of disease recovery. Twenty-three days after the immunization, the mean clinical score of PBS-treated mice dropped to 3 ± 1·5, whereas the mean clinical score of ABH-treated group dropped to 1·2 ± 0·4 (P < 0·05). Thus, ABH treatment not only delayed the onset but also reduced the severity of the disease.
Figure 6.
ABH ameliorates EAE. Two groups of C57BL/6 mice, 6 mice per group, were immunized with MOG38-50 peptide to induce EAE. Starting from the day of immunization, mice received daily intraperitoneal injection of either PBS or 200 µm ABH in PBS for a total of 23 days. Data presented are representative of two experiments. The differences between the two groups are statistically significant (P < 0·05) after day 14 as determined by the Mann–Whitney test.
Table 1.
ABH ameliorates EAE
| Treatments | ||
|---|---|---|
| Parameters | PBS | ABH |
| Day of onset | 13·3 ± 2·6 | 19·3 ± 1·9 |
| Incidence | 12/12 | 6/6 |
| Mean maximum score | 3·5 ± 1·1 | 2·3 ± 1·5 |
| Mortality | 3/12 | 1/6 |
Mice were treated and examined as described in Fig. 6. The differences in day of onset and mean maximum score between PBS and ABH groups are statistically significant (P < 0·01) as determined by anova. The experiment was repeated twice with similar results.
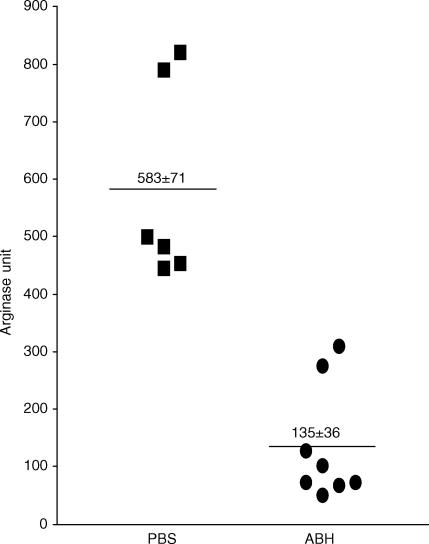
To confirm that ABH indeed reduces the arginase activities in vivo, we also tested the arginase levels in the spinal cord of mice treated with ABH or PBS. As shown in Fig. 7, ABH treatment significantly reduced the arginase activities in the spinal cord. These results suggest that ABH is effective in inhibiting arginase activities in the central nervous system.
Figure 7.
ABH inhibits arginase activities in the spinal cord. Two groups of mice were treated as described in Fig. 6 and killed 1 hr after the last injection of PBS or ABH in PBS. Whole spinal cord homogenates were prepared from each mouse in 2 ml PBS. Fifty-µl homogenates were tested for arginase activity as described in Materials and Methods. Results shown are arginase activities of individual spinal cord. The horizontal bars represent means of each group. The differences between the groups are statistically significant (P < 0·01) as determined by anova.
Arginase inhibition alters immune responses in vivo
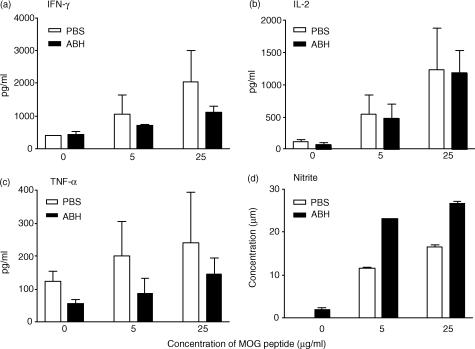
To determine the effect of arginase blockade on anti-MOG immune responses, we collected splenocytes from control and ABH-treated mice 1 hr after the last ABH injection, and tested them in vitro for their cytokine and nitric oxide production in response to MOG. As shown in Fig. 8, splenocytes from ABH-treated mice produced less IFN-γ and tumour necrosis factor-α than the control splenocytes in response to MOG peptide. However, they produced higher levels of NO, which suggests that arginase blockade may enhance the NO pathway of arginine metabolism. Thus, arginase blockade selectively altered immune responses to MOG in vivo.
Figure 8.
ABH alters immune responses to MOG. Two groups of C57BL/6 mice, six mice per group, were immunized with MOG38-50 peptide to induce EAE. Starting from the day of immunization, mice received daily i.p. injection of either PBS or 200 µm ABH in PBS for a total of 23 days. Mice were killed 1 hr after the last injection of ABH or PBS. Splenocytes were harvested and tested as described in Methods. Data shown are mean + SD of triplicate cultures. For cultures with MOG peptide, the differences between PBS and ABH groups are statistically significant (P < 0·05) for all the parameters except IL-2 as determined by anova. Results are representative of two experiments.
Discussion
This project was inspired by our unexpected discovery that Arg I was the most significantly up-regulated gene in the spinal cord during EAE. Using Northern blot and arginase enzyme assay, we further demonstrated that not only arginase mRNA, but also arginase protein was up-regulated in EAE. The mechanisms whereby Arg I is up-regulated in the CNS are not clear, although immune responses against self myelin antigens are likely involved. Two arginase isoforms are expressed in mammals: the cytosolic Arg I and the mitochondrial Arg II. Arg I is normally expressed at high levels only in the liver and plays an essential role in the urea cycle.4 Although Arg I has also been found in other organs, its exact role in extrahepatic cells and tissues is not clear.
The roles of arginase in immune responses have been studied primarily in the context of infectious diseases. It has been reported that Arg I induction in macrophages is required for Leishmania proliferation inside the host, and inhibiting arginase by N(omega)-hydroxy-l-arginine (NOHA) prevents the growth of the parasite.14,15 Similarly, in mice infected with Schistosoma mansoni, arginase appears to play important roles in regulating inflammation and pathogenesis of the disease.16 In these systems, arginase expression can be up-regulated by NOS regulators such as IL-4, IL-10, prostaglandin E2, and transforming growth factor-β1,17 while IFN-γ may suppress its expression.16 Moreover, arginase and NOS 2 are preferentially used by M-1 and M-2 macrophages, respectively, and the balance between the two enzymes may dictate the types of functions of macrophages in infection.17 Thus, the roles of arginase in immunity may be closely related to those of NOS.
Both arginase and NOS use and compete for l-arginine as their substrate. While arginase converts arginine into ornithine and urea, NOS converts arginine into NO. During the past decade, NO has merged as an important mediator of many physiological and pathophysiological processes. NO can act as a potent cytotoxic effector molecule in immune defence against infections and in autoimmune diseases against self-tissues. NO can also induce cytokine gene expression, modulate apoptosis, and regulate lymphocyte migration.18,19 Astrocytes and microglia are considered to be the main source of NO in CNS lesions during EAE.20 Although it was initially proposed that NO may induce oligodendrocyte death leading to demyelination,21 recent studies suggest that NO may also play a protective role in EAE. Thus, inhibition of NO production in EAE does not always ameliorate the disease. Instead, under certain circumstances, depletion of NOS 2 leads to increased pathology.22–26 In the Lewis rat model of EAE, T-cell cytokines such as IFN-γ can activate dendritic cells to produce NO, which in turn shuts off T-cell responses by inducing T-cell apoptosis, leading to disease recovery.27 Additionally, it has been reported that linsidomine (SIN-1), a nitric oxide donor, can ameliorate EAE in Lewis rats.28 Thus, our understanding of NO in EAE and MS has shifted from the early belief that it is purely a destructive molecule contributing to disease pathology, to a current more balanced view of NO as an immune regulator, which can both promote and prevent autoimmune inflammation depending on its dosage and context of action.
Results reported here suggest for the first time that arginase may play a crucial role in EAE. Inhibiting arginase in mice altered immune responses to MOG and ameliorated EAE. Because NO production in mice treated with arginase inhibitor ABH was moderately enhanced (Fig. 8), it is possible that the effect of arginase on EAE was partially mediated through the NOS pathway. Alternatively, because l-ornithine, a product of arginase pathway, is a necessary metabolite for the production of polyamine and proline, which control cell proliferation and collagen production, respectively, arginase may also regulate EAE through NO-independent pathways. Further studies are required to delineate these issues.
Acknowledgments
This work was supported by grants from the National Institutes of Health, USA (AI50059, NS40188, and NS40447 to YHC, and GM49758 to DWC).
References
- 1.Miller SD, Karpus WJ. The immunopathogenesis and regulation of T-cell-mediated demyelinating diseases. Immunol Today. 1994;15:356–61. doi: 10.1016/0167-5699(94)90173-2. [DOI] [PubMed] [Google Scholar]
- 2.Zamvil SS, Steinman L. The T lymphocyte in experimental allergic encephalomyelitis. Annu Rev Immunol. 1990;8:579–621. doi: 10.1146/annurev.iy.08.040190.003051. [DOI] [PubMed] [Google Scholar]
- 3.Carmody RJ, Hilliard B, Maguschak K, Chodosh LA, Chen YH. Genomic scale profiling of autoimmune inflammation in the central nervous system: the nervous response to inflammation. J Neuroimmunol. 2002;133:95–107. doi: 10.1016/s0165-5728(02)00366-1. [DOI] [PubMed] [Google Scholar]
- 4.Jenkinson CP, Grody WW, Cederbaum SD. Comparative properties of arginases. Comp Biochem Physiol. 1996;114:107–32. doi: 10.1016/0305-0491(95)02138-8. [DOI] [PubMed] [Google Scholar]
- 5.Liu TS, Hilliard B, Samoilova EB, Chen Y. Differential roles of Fas ligand in spontaneous and actively induced autoimmune encephalomyelitis. Clin Immunol. 2000;95:203–11. doi: 10.1006/clim.2000.4861. [DOI] [PubMed] [Google Scholar]
- 6.Hilliard B, Wilmen A, Seidel C, Liu TS, Goke R, Chen Y. Roles of TNF-related apoptosis-inducing ligand in experimental autoimmune encephalomyelitis. J Immunol. 2001;166:1314–9. doi: 10.4049/jimmunol.166.2.1314. [DOI] [PubMed] [Google Scholar]
- 7.Munder M, Eichmann K, Moran JM, Centeno F, Soler G, Modolell M. Th1/Th2-regulated expression of arginase isoforms in murine macrophages and dendritic cells. J Immunol. 1999;163:3771–7. [PubMed] [Google Scholar]
- 8.Xiao BG, Xu LY, Yang JS, Huang YM, Link H. An alternative pathway of nitric oxide production by rat astrocytes requires specific antigen and T cell contact. Neurosci Lett. 2000;283:53–6. doi: 10.1016/s0304-3940(00)00914-9. [DOI] [PubMed] [Google Scholar]
- 9.Hilliard B, Mason N, Xu L, Sun J, Lamhamedi-Cherradi S-E, Liou H-C, Hunter C, Chen Y. Critical roles of c-Rel in autoimmune inflammation and helper T cell differentiation. J Clin Invest. 2002;110:843–50. doi: 10.1172/JCI15254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song K, Chen Y, Goke R, Wilmen A, Seidel C, Goke A, Hilliard B, Chen Y. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is an inhibitor of autoimmune inflammation and cell cycle progression. J Exp Med. 2000;191:1095–104. doi: 10.1084/jem.191.7.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baggio R, Elbaum D, Kanyo ZF, Carroll PJ, Cavalli RC, Ash DE, Christianson DW. Inhibition of Mn2+2-arginase by borate leads to the design of a transition state analog inhibitor, 2(S)-amino-6-boronohexanoic acid. J Am Chem Soc. 1997;119:8107–8. [Google Scholar]
- 12.Cox JD, Baggio RF, Emig FA, et al. Biochemical and functional profile of a newly developed potent and isozyme-selective arginase inhibitor. Biochemistry. 2001;40:2678–88. [PubMed] [Google Scholar]
- 13.Baggio R, Emig FA, Christianson DW, Ash DE, Chakder S, Rattan S. Biochemical and functional profile of a newly developed potent and isozyme-selective arginase inhibitor. J Pharmacol Exp Ther. 1999;290:1409–16. [PubMed] [Google Scholar]
- 14.Iniesta V, Gomez-Nieto LC, Corraliza I. The inhibition of arginase by N(omega)-hydroxy-1-arginine controls the growth of Leishmania inside macrophages. J Exp Med. 2001;193:777–84. doi: 10.1084/jem.193.6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iniesta V, Gomez-Nieto LC, Molano I, Mohedano A, Carcelen J, Miron C, Alonso C, Corraliza I. Arginase I induction in macrophages, triggered by Th2-type cytokines, supports the growth of intracellular Leishmania parasites. Parasite Immunol. 2002;24:113–8. doi: 10.1046/j.1365-3024.2002.00444.x. [DOI] [PubMed] [Google Scholar]
- 16.Hesse M, Modolell M, La Flamme AC, Schito M, Fuentes JM, Cheever AW, Pearce EJ, Wynn TA. Differential regulation of nitric oxide synthase-2 and arginase-1 by type 1/type 2 cytokines in vivo: granulomatous pathology is shaped by the pattern of 1-arginine metabolism. J Immunol. 2001;167:6533–44. doi: 10.4049/jimmunol.167.11.6533. [DOI] [PubMed] [Google Scholar]
- 17.Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164:6166–73. doi: 10.4049/jimmunol.164.12.6166. [DOI] [PubMed] [Google Scholar]
- 18.Bogdan C. The multiplex function of nitric oxide in (auto) immunity. J Exp Med. 1998;187:1361–5. doi: 10.1084/jem.187.9.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kolb H, Kolb-Bachofen V. Nitric oxide in autoimmune disease: cytotoxic or regulatory mediator? Immunol Today. 1998;19:556–61. doi: 10.1016/s0167-5699(98)01366-8. [DOI] [PubMed] [Google Scholar]
- 20.Hewett SJ, Misko TP, Keeling RM, Behrens MM, Choi DW, Cross AH. Murine encephalitogenic lymphoid cells induce nitric oxide synthase in primary astrocytes. J Neuroimmunol. 1996;64:201–8. doi: 10.1016/0165-5728(95)00178-6. [DOI] [PubMed] [Google Scholar]
- 21.Smith KJ, Kapoor R, Felts PA. Demyelination: the role of reactive oxygen and nitrogen species. Brain Pathol. 1999;9:69–92. doi: 10.1111/j.1750-3639.1999.tb00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cowden WB, Cullen FA, Staykova MA, Willenborg DO. Nitric oxide is a potential down-regulating molecule in autoimmune disease: inhibition of nitric oxide production renders PVG rats highly susceptible to EAE. J Neuroimmunol. 1998;88:1–8. doi: 10.1016/s0165-5728(98)00040-x. [DOI] [PubMed] [Google Scholar]
- 23.O'Brien NC, Charlton B, Cowden WB, Willenborg DO. Nitric oxide plays a critical role in the recovery of Lewis rats from experimental autoimmune encephalomyelitis and the maintenance of resistance to reinduction. J Immunol. 1999;163:6841–7. [PubMed] [Google Scholar]
- 24.Fenyk-Melody JE, Garrison AE, Brunnert SR, Weidner JR, Shen F, Shelton BA, Mudgett JS. Experimental autoimmune encephalomyelitis is exacerbated in mice lacking the NOS2 gene. J Immunol. 1998;160:2940–6. [PubMed] [Google Scholar]
- 25.Sahrbacher UC, Lechner F, Eugster HP, Frei K, Lassmann H, Fontana A. Mice with an inactivation of the inducible nitric oxide synthase gene are susceptible to experimental autoimmune encephalomyelitis. Eur J Immunol. 1998;28:1332–8. doi: 10.1002/(SICI)1521-4141(199804)28:04<1332::AID-IMMU1332>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 26.Okuda Y, Sakoda S, Fujimura H, Yanagihara T. Nitric oxide via an inducible isoform of nitric oxide synthase is a possible factor to eliminate inflammatory cells from the central nervous system of mice with experimental allergic encephalomyelitis. J Neuroimmunol. 1997;73:107–16. doi: 10.1016/s0165-5728(96)00194-4. [DOI] [PubMed] [Google Scholar]
- 27.Xiao BG, Huang YM, Xu LY, Ishikawa M, Link H. Mechanisms of recovery from experimental allergic encephalomyelitis induced with myelin basic protein peptide 68-86 in Lewis rats. a role for dendritic cells in inducing apoptosis of CD4+ T cells. J Neuroimmunol. 1999;97:25–36. doi: 10.1016/s0165-5728(99)00041-7. [DOI] [PubMed] [Google Scholar]
- 28.Xu LY, Yang JS, Link H, Xiao BG. SIN-1, a nitric oxide donor, ameliorates experimental allergic encephalomyelitis in Lewis rats in the incipient phase: the importance of the time window. J Immunol. 2001;166:5810–6. doi: 10.4049/jimmunol.166.9.5810. [DOI] [PubMed] [Google Scholar]