Abstract
Amongst the families of intracellular molecules that chaperone and assist with the trafficking of other proteins, notably during conditions of cellular stress, heat shock protein (hsp) 70 is one of the most studied. Although its name suggests that expression is exclusively induced during cellular hyperthermia, members of the hsp70 family of proteins can be constitutively expressed and/or induced by a range of other cellular insults. The ubiquitous presence of hsp70 in eukaryotic and prokaryotic cells, combined with its high degree of sequence homology and intrinsic immunogenicity, have prompted the suggestion that inappropriate immune reactivity to hsp70 might lead to pro-inflammatory responses and the development of autoimmune disease. Indeed, hsp70 has been shown to be a potent activator of innate immunity and aberrant expression of hsp70 in certain organs promotes immunopathology. However, studies also suggest that hsp70 might have immunotherapeutic potential, as hsp70 purified from malignant and virally infected cells can transfer and deliver antigenic peptides to antigen-presenting cells to elicit peptide-specific immunity and, in contrast to its reported pro-inflammatory effects, the administration of recombinant hsp70 can attenuate experimental autoimmune disease. This review focuses on the immunoregulatory capacity of hsp70 and its potential therapeutic value.
Introduction
The heat shock proteins are evolutionarily ancient families of molecules that are present in, and considered essential to the survival of, all prokaryotic and eukaryotic cell types. Their name derives from the original observations that their expression was induced following exposure of cells to elevated temperatures.1,2 However, it has subsequently been demonstrated that many of these molecules are constitutively expressed, and those that are inducible can be induced by a variety of physical and biochemical stressors such as ultraviolet and gamma radiation, bacterial and viral infection, certain chemicals and drugs, hypoxia and glucose deprivation.3
During steady-state conditions, heat shock proteins fulfil a variety of functions, including the intracellular assembly, folding and translocation of oligomeric proteins.4 Under conditions of cellular stress they act as cytoprotective agents by binding to misfolded proteins and thus protecting them from denaturation.5 Heat shock proteins are also involved in a number of events associated with embryonic development.6 There is considerable conservation in the genetic sequence of molecules in the same families across species and the perception is that functions of heat shock proteins are conserved across the phyla, however, this might not necessarily be the case.
Heat shock proteins are immunodominant molecules and a significant element of the immune response to pathogenic micro-organisms is directed towards heat shock-protein-derived peptides.7,8 The conserved nature of heat shock proteins, combined with their inherent immunogenicity, has prompted the suggestions that they might act as autoantigens that are capable of initiating and driving inflammatory processes,7 and that immune recognition of cross-reactive heat shock protein epitopes provides a link between infection and autoimmunity.9 However, in contrast to its reported pro-inflammatory effects, the administration of recombinant heat shock protein 70 (hsp70) can attenuate experimental autoimmune disease.10–12 In addition to a possible role as immunoregulatory molecules, heat shock proteins might also be useful as immunotherapeutic agents, as hsp70 purified from malignant cells or re-constituted with viral peptides can elicit tumour and viral peptide-specific immunity.13–15
A number of heat shock proteins have been implicated in the induction of pro-inflammatory immune responses and the delivery of antigenic peptides to antigen-presenting cells; however, it is on the immunoregulatory capacity and immunotherapeutic potential of hsp70 that this article focuses.
The hsp70 family
Heat shock proteins are categorized into different families that are named on the basis of their approximate molecular weight (e.g. the 70 000 MW hsp70 family) and they are localized in various intracellular compartments (Table 1). In mammalian cells, hsp70 is located within the cytoplasm, as is its constitutively expressed and only slightly inducible form, hsc70. The hsp70 is believed to act as part of a protein degradation system16 and it might aid antigen presentation via major histocompatibility complex (MHC) class I. Depending on their nature, heat shock protein-associated peptides are presented in a transporter associated with antigen presentation (TAP) complex-dependent or -independent manner, as demonstrated by lactacystein inhibition.17 It is now known that N-terminal extended epitopes can be processed by N-terminal amino peptidases in the cytosol, whereas peptides with C-terminal extensions often require proteasomal activity.18
Table 1.
Mammalian heat shock protein families and their intracellular location
| Major family, and members | Intracellular localization |
|---|---|
| Small heat shock proteins | |
| αB-crystallin | cytoplasm |
| hsp27 | cytoplasm/nucleus |
| Heme oxygenase, hsp32 | cytoplasm |
| hsp40 | |
| hsp40 | cytoplasm/nucleus |
| hsp47 | endoplasmic reticulum |
| hsp60 (or chaperonins) | |
| hsp60 | mitochondria |
| TCP-1 | cytoplasm |
| hsp70 | |
| Inducible: hsp70, hsp70hom | cytoplasm/nucleus |
| Cognate/constitutive: hsc70 | cytoplasm/peroxisome |
| Grp78/BiP | endoplasmic reticulum |
| mt-hsp70/Grp75 | mitochondria |
| hsp 90 | |
| hsp90 (α and β) | cytoplasm |
| Grp94/gp96/hsp100 | endoplasmic reticulum |
| hsp110 | |
| hsp110 (human) | nucleolus/cytoplasm |
| Apg-1 (mouse) | cytoplasm |
| hsp105 | cytoplasm |
TCP-1, tailless complex polypeptide; Grp, glucose regulated protein, hsp, heat shock protein; hsp70hom, testis-specific hsp70; BiP, immunoglobulin heavy-chain-binding protein; mt-hsp70, mitochondrial hsp70; Apg-1, protein kinase essential for autophagy.
Induction and regulation of heat shock protein expression
Heat shock protein gene transcription in response to stress is regulated by the interaction of the heat shock factor (HSF) transcription factors, with heat shock elements in the heat shock protein gene promoter regions.19,20 Four heat shock factors have been identified in vertebrates, of which HSF1 and HSF2 are ubiquitously expressed and conserved.21,22 In vertebrates, the principle heat shock factor involved in the response to physiological and environmental stress is HSF1,23,24 whereas HSF2 activity is more selective, and is primarily induced during differentiation and early development.25 HSF1 is normally present in the cytoplasm as a latent monomeric molecule which is unable to bind to DNA. Under stressful conditions, HSF1 is hyperphosphorylated in a ras-dependent manner by members of the mitogen-activated protein kinase subfamilies and converted to inducibly phosphorylated trimers that have the capacity to bind DNA.20,26,27 The phosphorylated trimers translocate from the cytoplasm to the nucleus,28 the consequences of which have been reviewed in detail elsewhere.29,30
The stress response is only transient, as a prolonged and inappropriate presence of protein-binding molecules would adversely influence protein homeostasis and a variety of intracellular functions. One mechanism by which HSF1 activity is negatively regulated is by hsp70 binding to its transactivation domain and the resultant repression of heat shock gene transcription.31 A second mechanism involves an interaction between heat shock-protein-binding factor 1 (HSBP1) with the active trimeric form of HSF1 and hsp70, thereby inhibiting the capacity of HSF1 to bind to DNA.32
Hsp70 as a regulator of adaptive immunity
Antigen processing and presentation
Expression of hsp70 influences the presentation and processing of antigen to CD4+ T cells by antigen presenting cells,33,34 and increasing the expression of hsp70 in tumour cells promotes the presentation of peptides on the tumour cell surface via an enhancement of MHC class I expression.35 hsp70 expression in tumour cells also enhances their recognition by T cells via pathways other than MHC up-regulation, such as by influencing antigen processing.36
Induction of tumour-specific immunity
As intracellular chaperones, heat shock proteins bind a large number of peptides derived from the cells from which they are isolated37,38– the so-called ‘antigenic fingerprint’ or repertoire of that cell.39 That heat shock proteins such as hsp70, hsp90, gp96, calreticulin, hsp110 and grp170 can act as carriers of protein antigens from the cell from which they are derived has been elucidated from studies demonstrating that immunization of animals with these heat shock proteins purified from tumour cells elicits effective anti-tumour immunity and protects the animals from subsequent challenge with the same, but not different, tumour cells.13,40–43 Such an approach can also be used to generate effective immunity against established tumours.44 It is now known that immunization leads to the generation of tumour-specific cytotoxic CD8+ T cells (CTLs), the specificity of which is defined by the peptides associated with the heat shock protein rather than by the heat shock protein itself.45,46
The implication of the original studies was that heat shock protein immunization was able to generate anti-tumour CTLs by transferring the antigenic peptides with which they were associated into the MHC class I antigen presentation pathway within antigen-presenting cells, a pathway usually restricted to antigens derived from the cytoplasm of the antigen-presenting cell. Such antigenic cross-presentation is now considered to be a key process in the action of heat shock proteins.47,48 One interesting observation is that although, tumour surface-expressed hsp70 is associated with tumour-specific immunity, its constitutively expressed counterpart, hsc70, is not.49 However, hsc70–peptide fusion proteins have been successfully used to immunize against CD8+ restricted T-cell epitopes,50 indicating that the functions of heat shock protein fusion proteins are distinct from those of non-covalently associated heat shock protein–peptide complexes. A role for natural killer cells in the recognition of such surface-expressed hsp70 has also been proposed.51–54
To date, tumour-derived hsp70 has shown efficacy as a vaccine in at least four different tumour models.14,15,44,55 The observation that tumour protection can be elicited in amphibians (Xenopus)56 demonstrates the evolutionarily conserved nature of these responses and strongly supports the successful translation of these strategies into the clinical environment. Recently, a range of human CTLs have been shown to be activated by hsp70 derived from melanoma cells of differing MHC origin,57 suggesting that the use of more generic heat shock protein vaccines might be feasible in situations where tumour antigens are common. Recent studies in mice also support this principle (Casey et al. in this issue).58 Impressive preclinical results have prompted clinical trials using hsp70 derived from patients' tumours (http://www.nci.nih.gov/clinicaltrials).
Induction of immunity against infectious disease
Heat shock proteins isolated from simian-virus-40-transformed and influenza-virus-infected cells, or a mixture of gp96 or hsp70 reconstituted with specific CTL epitopes from simian virus 40 and influenza virus have been shown to elicit peptide-specific cytolytic T cells and protective antiviral immunity in immunized mice.45,59–61 Similar results have been obtained using hsp70 with lymphocytic choriomeningitis virus.62 Immunity can also be induced using engineered hsp70–antigen fusion proteins, and immunization of mice with the human immunodeficiency virus 1 (HIV-1) p24 protein covalently linked to mycobacterial hsp70 elicits antibody, cytokine and lymphocyte proliferative responses.63
hsp70 receptors in adaptive immunity
The observation that microgram quantities of hsp70 are sufficient to generate substantial immunity suggests a receptor-mediated endocytosis mechanism and a high efficiency of antigen transfer. This concept was initially supported by electron microscopy, and saturation and competition binding assays.64,65 More recently, a specific receptor involved in the capacity of hsp70 to induce peptide-specific immunity has been identified as the α2-macroglobulin receptor CD91.66 CD91 is expressed by many antigen-presenting cells, including dendritic cells, and it is also a receptor for the other heat shock proteins that have the capacity to induce tumour-specific immunity by similar approaches (calreticulin, hsp90, gp96).66
Another molecule involved in the delivery of antigenic peptides to antigen-presenting cells is CD40,67,68 although its precise involvement in the mechanisms that result in heat shock-protein-induced tumour immunity has yet to be evaluated. The CD40 molecule preferentially recognizes and mediates the internalization of hsp70 in its peptide-bound state, and binding does not appear to take place in the absence of its nucleotide (ADP) and peptide substrate.67 Of particular interest is the observation that the formation of the human hsp70–CD40 complex is not inhibited by its bacterial homologue DnaK, although both molecules bind to the CD40 molecule, albeit at independent sites.67 Another distinct difference between the effects that hsp70 and DnaK have on antigen-presenting cells appears to be the capacity to deliver peptides bound to their substrate-binding site, as it is to this site on the DnaK molecule that CD40 binds and peptide delivery cannot therefore take place.67
Scavenger receptors have also been implicated in heat shock protein uptake and cross-presentation, as the receptor for oxidized low density lipoprotein (LDL) and Poly(I), namely LOX-1, has been identified as a novel heat shock protein receptor.69 LOX-1 facilitates the binding and uptake of mammalian hsp70 on human monocytes and it might be that different heat shock protein receptors co-operate to facilitate adaptive and innate immune responses: antigen-presenting cell stimulation by signalling receptors and the presentation of antigen via receptors that can take up heat shock protein–peptide complexes.69
Hsp70, autoimmunity and immunoregulation
Although the immunogenicity and sequence homology between prokaryotic and eukaryotic heat shock proteins prompted the suggestions that these proteins might act as autoantigens and be involved in the development of autoimmunity,7,9 data also suggest that self-heat shock-protein reactivity might be a physiological mechanism for regulating pro-inflammatory disease processes.70 The induction of T-cell reactivity to self-hsp60 down-regulates disease in a number of experimental arthritis models, by a mechanism that involves the induction of self-heat shock-protein-specific T helper type 2 (Th2) type CD4+ T cells producing the regulatory cytokines interleukin-4 (IL-4) and IL-10.71–75 Similar observations have been made for hsp70, in that rather than causing or exacerbating autoimmune diseases such as arthritis, immunization of rats with mycobacterial hsp70 can prevent disease.10–12
The mechanisms by which self-heat shock-protein reactivity might regulate inflammatory disease have yet to be fully elucidated, however, a number of possibilities exist and these have been reviewed in detail elsewhere.70 As has been shown for many other self-peptides, the normal T-cell repertoire includes low-affinity T cells reactive against autologous heat shock proteins.7,76–79 Self-heat shock-protein-reactive T cells recognizing self-heat shock-protein epitopes in stressed tissues via low-affinity interactions might lead to the generation of Th2 (IL-4-producing), Th3 (transforming growth factor-β-producing) or Tr1 (IL-10-producing) regulatory T-cell responses. It might also be that T-cell responses to microbial heat shock proteins in the tolerizing environment of the gut leads to a regulatory response, or that T cells recognize self-heat shock-protein epitopes as altered peptide ligands which do not fully activate T cells, and have the capacity to induce selected effector functions including the generation of regulatory cytokines.70 Indeed, a lack of prior microbial exposure has been shown to predispose animals to autoimmunity.80 Taken together these observations indicate that the immunogenicity of prokaryotic hsp70 and its considerable homology with its mammalian counterpart require that immune responses to hsp70 and other heat shock protein molecules are qualitatively distinct and tightly regulated.
Hsp70 as a ‘danger’ signal and activator of innate immunity
The ability of heat shock proteins to stimulate the innate arm of the immune system independently of chaperoned peptides has been demonstrated in studies using both microbial-derived and endogenously derived (self) heat shock proteins (mic-hsp and en-hsp, respectively). In the mammalian system, heat shock proteins have typically been regarded as being exclusively intracellular proteins, and their presence in the extracellular environment to reflect tissue damage.47,81 Thus, the release of heat shock proteins would provide the ‘danger’ signal which would activate innate and pro-inflammatory immune responses. However, the observations that hsp70 can be released from a variety of viable (non-necrotic) mammalian cell types82–84 and that hsp70 is present in the peripheral circulation of normal individuals85–87 also suggest that immune responses to mic-hsp70 and en-hsp70 are tightly and differentially controlled, and quantitatively and qualitatively different.
hsp70 receptors in innate immunity
Despite having similar potency for stimulating tumour necrosis factor-α (TNF-α) production in mouse macrophages, phylogenetically separate hsp60 species interact with murine macrophages via different recognition systems.88 The same might be true for members of other heat shock protein families, and it has been shown that human hsp70 binds to murine macrophages via the CD40 molecule, at a binding site that is distinct from that used by the bacterial hsp70 homologue DnaK.67 It is therefore important that the phylogenetic derivation of the heat shock protein under investigation is clearly indicated in reported studies.
The CD14 molecule and Toll-like receptors 2 and 4 are involved in en-hsp70-mediated monocyte activation, the consequences of which are intracellular calcium fluxes and the induction of pro-inflammatory cytokines (IL-1β, IL-6, TNF-α).84,89 A CD14-independent, but calcium-dependent response that leads to TNF-α production has also been identified.84 It has been shown that activation of antigen-presenting cells with en-hsp70 and mic-hsp70 occurs via nuclear factor-κB activation,84,90 although the phenotype of the response is not always the same (Fig. 1), and that activating capacity of the mic-hsp70 molecule resides in the C-terminal half (Fig. 2).91,92
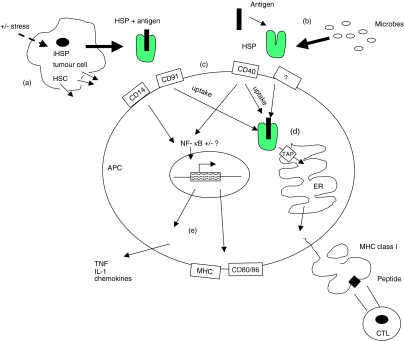
Figure 1.
Mechanisms of action of heat shock protein 70 (hsp70). (a) hsp70 can be induced within tumour cells (iHSP) and it, or its constitutive counterpart (hsc) can be released from stressed and/or dying tumour cells. Alternatively, hsp/hsc70 can be experimentally extracted from tumour cells. (b) hsp70 can be released by microbes and can experimentally be engineered to bind to antigens. (c) Interaction at the antigen-presenting cell (APC) surface leads to uptake of the hsp–antigen complex, chiefly via CD91, whilst the cell can be activated by hsp70 through CD14 and CD40 and via nuclear factor-κB. (d) Antigenic peptides are shuttled to the endoplasmic reticulum (ER) by hsp70 via TAP where they bind to MHC class I molecules for display at the cell surface. (e) Antigen-presenting cell activation is accompanied by the up-regulation of surface MHC and CD80/86 molecules and by cytokine and chemokine release.
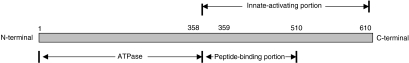
Figure 2.
Diagrammatic representation of the hsp70 molecule. A linear representation of hsp70 shows that the ATPase activity resides in the N-terminal half of the molecule, whilst the peptide-binding region is centrally located. The innate-activating portion comprises the C-terminal half of the molecule. These properties were deduced for mycobacterial hsp70.
Some concern has been expressed that the in vitro responses induced by recombinant hsp70 produced in Escherichia coli might result from the effects of lipopolysaccharide or other proteins, present as either a contaminant of the preparation or chaperoned by the heat shock protein under investigation.93,94 Endotoxin contamination has been reported to be responsible for the human hsp70 preparation-induced induction of TNF-α release from murine macrophages95 and its ability to induce the activation of human dendritic cells.96 Interestingly, similar findings have now been reported for hsp60.97 Clearly, further work must be performed to clarify this issue.
Cell stress, cell death and tumour immunogenicity
The immunogenicity of antigens expressed by dying cells is critically dependent on whether death occurs via necrosis or apoptosis. Necrosis leads to the release of intracellular components and typically induces inflammatory responses, whereas the phagocytosis of apoptotic cells results in anti-inflammatory patterns of cytokine secretion by macrophages.98–101 Apoptotic cell death is commonly an immunosuppressive phenomenon and systemic102 or local103 delivery of apoptotic cells tolerizes animals to cell-expressed antigens. Effective vaccination with apoptotic cells requires an overwhelming dose of cells, or a diminished phagocytic capacity.104 Heat shock proteins might influence the immunogenicity of dying tumour cells in a number of ways.
Tumour cells express higher levels of cytoplasmic hsp70 compared to their non-malignant counterparts and lysates of these cells efficiently induce the maturation of dendritic cells.105 There is also a correlation between the expression or release of hsp70 and non-apoptotic (necrotic) forms of cell death90,106 and cell death which occurs concomitant with heat shock protein gene expression is highly immunogenic.90,106–110 Tumour immunogenicity is also enhanced when hsp70 is over-expressed in engineered tumour cells,106 induced by heat shock,109 or are present at higher levels as a consequence of random clonal variation.49 It has also been shown that tumours over-expressing hsp70 induce more marked Th1-type immune responses.107 However, hsp70 might impart tumour cell resistance to stress and cell death by apoptosis,111,112 and indeed one study has shown that hsp70 depletion can increase the immunogenicity of rat tumour cells.113 Anti-tumour strategies based on hsp70 transfection must therefore be approached with some caution.
The precise influence that heat shock proteins have on the immunogenicity of tumour cells has yet to be fully elucidated, and might be exerted at a number of different levels. Heat shock, or the expression of hsp70, diminishes the anti-inflammatory effects of apoptotic cell phagocytosis,108 and the expression or co-administration of hsp70 enhances the immunogenicity of apoptotic cells (Gough et al., manuscript submitted).110,114 Heat shock protein expression can interfere with the induction of apoptosis113 and protect cells against what would typically be toxic stimuli, although this is not always the case.108,110,115
It might also be that the influence of heat shock proteins on tumour immunogenicity relates to the varied functions of the CD91 molecule, which has been identified as a candidate receptor for hsp70 and other heat shock proteins.66,116 CD91 appears to be a critical molecule involved in the phagocytosis of apoptotic cells117–119 via C1q of complement.117,119,120 Since CD91 is likely to be the signalling component of the calreticulin–CD91 complex,118 it is possible that heat shock protein binding to the CD91 receptor prevents assembly of the functional phagocytic complex for apoptotic cells. Such a system could explain some of the observations linking expression of heat shock proteins with decreased phagocytosis of apoptotic cells (Gough et al., manuscript submitted).108 In addition, decreased phagocytosis of apoptotic cells, whether through genetic deletion of key molecules,120 blocking macrophage uptake,104 or otherwise overwhelming phagocyte capacity104,121 enhances the immune response to antigens expressed in those cells. Cells not taken up by macrophages may proceed to secondary necrosis,100 which is a more potent immunogenic mode of cell death.106,108,122,123 Thus, in view of the controversy regarding the direct adjuvanticity of purified heat shock proteins on antigen-presenting cells,95,96 such a mechanism might yet explain the clear influence of heat shock protein gene expression in many models of immunogenicity.49,90,106–110
Conclusions
Heat shock protein 70 possesses properties that enable it to influence a variety of immunological processes. Primarily, these are manifested via its effects on immunogenicity, its capacity to chaperone antigenic peptides and deliver these into antigen-presentation pathways within antigen-presenting cells, and its ability to activate and regulate innate and adaptive immunity. The varied attributes of hsp70 suggest its rational use in immunotherapeutic strategies for the treatment of cancer and infectious disease, and for the control of inflammatory conditions such as autoimmunity. Further work aimed at exploring such facets of these ubiquitously expressed molecules is clearly warranted.
Acknowledgments
Stephen Todryk is supported by Science Foundation Ireland. Michael Gough is supported by NIH Grant R01 CA094180 and by the Mayo Foundation. Graham Pockley is supported by the British Heart Foundation, the National Heart, Lung and Blood Institute (USA) and the Association for International Cancer Research.
References
- 1.Ritossa FA. A new puffing pattern induced by temperature shock and DNP in Drosophila. Experientia. 1962;18:571–3. [Google Scholar]
- 2.Tissières A, Mitchell HK, Tracy U. Protein synthesis in salivary glands of Drosophila melanogaster: relation to chromosome puffs. J Mol Biol. 1974;84:389–98. doi: 10.1016/0022-2836(74)90447-1. [DOI] [PubMed] [Google Scholar]
- 3.Santoro MG. Heat shock factors and the control of the stress response. Biochem Pharmacol. 2000;59:55–63. doi: 10.1016/s0006-2952(99)00299-3. [DOI] [PubMed] [Google Scholar]
- 4.Hightower LE. Heat shock, stress proteins, chaperones and proteotoxicity. Cell. 1991;66:191–7. doi: 10.1016/0092-8674(91)90611-2. [DOI] [PubMed] [Google Scholar]
- 5.Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–9. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- 6.Anderson RL. Stress proteins and apoptosis in prenatal development, cancer and medicine. Cell Stress Chaperones. 1998;3:209–12. doi: 10.1379/1466-1268(1998)003<0209:spaaip>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaufmann SHE. Heat shock proteins and the immune response. Immunol Today. 1990;11:129–36. doi: 10.1016/0167-5699(90)90050-j. [DOI] [PubMed] [Google Scholar]
- 8.Young RA. Stress proteins and immunology. Annu Rev Immunol. 1990;8:401–20. doi: 10.1146/annurev.iy.08.040190.002153. [DOI] [PubMed] [Google Scholar]
- 9.Lamb JR, Bal V, Mendez-Samperio A, et al. Stress proteins may provide a link between the immune response to infection and autoimmunity. Int Immunol. 1989;1:191–6. doi: 10.1093/intimm/1.2.191. [DOI] [PubMed] [Google Scholar]
- 10.Kingston AE, Hicks CA, Colston MJ, Billingham MEJ. A 71-kD heat shock protein (hsp) from Mycobacterium tuberculosis has modulatory effects on experimental rat arthritis. Clin Exp Immunol. 1996;103:77–82. doi: 10.1046/j.1365-2249.1996.929628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanaka S, Kimura Y, Mitani A, et al. Activation of T cells recognizing an epitope of heat shock protein 70 can protect against rat adjuvant arthritis. J Immunol. 1999;163:5560–5. [PubMed] [Google Scholar]
- 12.Wendling U, Paul L, van der Zee R, Prakken B, Singh M, van Eden W. A conserved mycobacterial heat shock protein (hsp) 70 sequence prevents adjuvant arthritis upon nasal administration and induces IL-10-producing T cells that cross-react with the mammalian self-hsp70 homologue. J Immunol. 2000;164:2711–17. doi: 10.4049/jimmunol.164.5.2711. [DOI] [PubMed] [Google Scholar]
- 13.Udono H, Srivastava PK. Comparison of tumor-specific immunogenicities of stress-induced proteins gp96, hsp90 and hsp70. J Immunol. 1994;152:5398–403. [PubMed] [Google Scholar]
- 14.Udono H, Srivastava PK. Heat shock protein 70-associated peptides elicit specific cancer immunity. J Exp Med. 1993;178:1391–6. doi: 10.1084/jem.178.4.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ciupitu AM, Petersson M, Kono K, Charo J, Kiessling R. Immunization with heat shock protein 70 from methylcholanthrene-induced sarcomas induces tumor protection correlating with in vitro T cell responses. Cancer Immunol Immunother. 2002;51:163–70. doi: 10.1007/s00262-002-0263-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verma R, Chen S, Feldman R, Schieltz D, Yates J, Dohmen J, Deshaies RJ. Proteasomal proteomics. identification of nucleotide-sensitive proteasome-interacting proteins by mass spectrometric analysis of affinity-purified proteasomes. Mol Biol Cell. 2000;34:25–39. doi: 10.1091/mbc.11.10.3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castellino F, Boucher PE, Eichelberg K, Mayhew M, Rothman JE, Houghton AN, Germain RN. Receptor-mediated uptake of antigen/heat shock protein complexes results in major histocompatibility complex class I antigen presentation via two distinct pathways. J Exp Med. 2000;191:1957–64. doi: 10.1084/jem.191.11.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldberg AL, Cascio P, Saric T, Rock KL. The importance of the proteasome and subsequent proteolytic steps in the generation of antigenic peptides. Mol Immunol. 2002;39:147–64. doi: 10.1016/s0161-5890(02)00098-6. [DOI] [PubMed] [Google Scholar]
- 19.Voellmy R. Transduction of the stress signal and mechanisms of transcriptional regulation of heat shock/stress protein gene expression in higher eukaryotes. Crit Rev Euk Gene Exp. 1994;4:357–401. [PubMed] [Google Scholar]
- 20.Morimoto RI, Jurivich DA, Kroger PE, et al. Regulation of heat shock gene transcription by a family of heat shock factors. In: Morimoto RI, Tissières A, Georgopoulos C, editors. The Biology of Heat Shock Proteins and Molecular Chaperones. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1994. pp. 417–55. [Google Scholar]
- 21.Nakai A, Morimoto RI. Characterization of a novel chicken heat shock transcription factor, heat shock factor 3, suggests a new regulatory pathway. Mol Cell Biol. 1993;13:1983–97. doi: 10.1128/mcb.13.4.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarge KD, Zimarino V, Holm K, Wu C, Morimoto RI. Cloning and characterization of two mouse heat shock factors with distinct inducible and constitutive DNA-binding ability. Genes Dev. 1991;5:1902–11. doi: 10.1101/gad.5.10.1902. [DOI] [PubMed] [Google Scholar]
- 23.Sarge KD, Murphy SP, Morimoto RI. Activation of heat shock gene transcription by heat shock factor 1 involves oligomerization, acquisition of DNA-binding activity, and nuclear localization and can occur in the absence of stress. Mol Cell Biol. 1993;13:1392–407. doi: 10.1128/mcb.13.3.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zuo J, Baler R, Dahl G, Voellmy R. Activation of the DNA-binding ability of human heat shock transcription factor 1 may involve the transition from an intramolecular triple-stranded coiled-coil structure. Mol Cell Biol. 1994;14:7447–68. doi: 10.1128/mcb.14.11.7557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pirkkala L, Nykanen P, Sistonen L. Roles of the heat shock transcription factors in regulation of the heat shock response and beyond. FASEB J. 2001;15:1118–31. doi: 10.1096/fj00-0294rev. [DOI] [PubMed] [Google Scholar]
- 26.Knauf U, Newton EM, Kyriakis J, Kingston RE. Repression of human heat shock factor 1 activity at control temperature by phosphorylation. Genes Dev. 1996;10:2782–93. doi: 10.1101/gad.10.21.2782. [DOI] [PubMed] [Google Scholar]
- 27.Kim J, Nueda A, Meng YH, Dynan WS, Mivechi NF. Analysis of the phosphorylation of human heat shock transcription factor-1 by MAP kinase family members. J Cell Biochem. 1997;67:43–54. doi: 10.1002/(sici)1097-4644(19971001)67:1<43::aid-jcb5>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 28.Morimoto RI. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 1998;12:3788–96. doi: 10.1101/gad.12.24.3788. [DOI] [PubMed] [Google Scholar]
- 29.Wu C. Heat shock transcription factors. Structure and regulation. Annu Rev Cell Dev Biol. 1995;11:441–69. doi: 10.1146/annurev.cb.11.110195.002301. [DOI] [PubMed] [Google Scholar]
- 30.Nollen EA, Morimoto RI. Chaperoning signaling pathways: molecular chaperones as stress-sensing ‘heat shock’ proteins. J Cell Sci. 2002;115:2809–16. doi: 10.1242/jcs.115.14.2809. [DOI] [PubMed] [Google Scholar]
- 31.Shi Y, Mosser DD, Morimoto RI. Molecular chaperones as HSF1-specific transcriptional repressors. Genes Dev. 1998;12:654–66. doi: 10.1101/gad.12.5.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Satyal SH, Chen D, Fox SG, Kramer JM, Morimoto RI. Negative regulation of the heat shock transcriptional response by HSBP1. Genes Dev. 1998;12:1962–74. doi: 10.1101/gad.12.13.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pierce SK. Molecular chaperones in the processing and presentation of antigen to helper T cells. Experientia. 1994;50:1026–30. doi: 10.1007/BF01923457. [DOI] [PubMed] [Google Scholar]
- 34.Panjwani N, Akbari O, Barcia S, Brazil M, Stockinger B. The HSC73 molecular chaperone: involvement in MHC class II antigen presentation. J Immunol. 1999;163:1936–42. [PubMed] [Google Scholar]
- 35.Wells AD, Rai SK, Salvato MS, Band H, Malkovsky M. Hsp72-mediated augmentation of MHC class I surface expression and endogenous antigen presentation. Int Immunol. 1998;10:609–17. doi: 10.1093/intimm/10.5.609. [DOI] [PubMed] [Google Scholar]
- 36.Dressel R, Lubbers M, Walter L, Herr W, Gunther E. Enhanced susceptibility to cytotoxic T lymphocytes without increase of MHC class I antigen expression after conditional overexpression of heat shock protein 70 in target cells. Eur J Immunol. 1999;29:3925–35. doi: 10.1002/(SICI)1521-4141(199912)29:12<3925::AID-IMMU3925>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 37.Gething MJ, Sambrook J. Protein folding in the cell. Nature. 1992;355:33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- 38.Young D, Romain E, Moreno C, O'Brien R, Born W. Molecular chaperones and the immune system response. Phil Trans Royal Soc Lond. 1993;339:363–7. doi: 10.1098/rstb.1993.0035. [DOI] [PubMed] [Google Scholar]
- 39.Srivastava PK, Udono H. Heat shock protein-peptide complexes in cancer immunotherapy. Cur Opin Immunol. 1994;6:728–32. doi: 10.1016/0952-7915(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 40.Udono H, Levey DL, Srivastava PK. Cellular requirements for tumor-specific immunity elicited by heat shock proteins: tumor rejection antigen gp96 primes CD8+ T cells in vivo. Proc Natl Acad Sci USA. 1994;91:3077–81. doi: 10.1073/pnas.91.8.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Basu S, Srivastava P. Calreticulin, a peptide-binding chaperone of the endoplasmic reticulum, elicits tumor- and peptide-specific immunity. J Exp Med. 1999;189:797–802. doi: 10.1084/jem.189.5.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chandawarkar RY, Wagh MS, Srivastava PK. The dual nature of specific immunological activity of tumour-derived gp96 preparations. J Exp Med. 1999;189:1437–42. doi: 10.1084/jem.189.9.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang X-Y, Kazim L, Repasky EA, Subjeck JR. Characterization of heat shock protein 110 and glucose-regulated protein 170 as cancer vaccines and the effect of fever-range hyperthermia on vaccine activity. J Immunol. 2001;165:490–7. doi: 10.4049/jimmunol.166.1.490. [DOI] [PubMed] [Google Scholar]
- 44.Tamura Y, Peng P, Liu K, Daou M, Srivastava PK. Immunotherapy of tumors with autologous tumor-derived heat shock protein preparations. Science. 1997;278:117–20. doi: 10.1126/science.278.5335.117. [DOI] [PubMed] [Google Scholar]
- 45.Suto R, Srivastava PK. A mechanism for the specific immunogenicity of heat shock protein-chaperoned peptides. Science. 1995;269:1585–8. doi: 10.1126/science.7545313. [DOI] [PubMed] [Google Scholar]
- 46.Schild H, Arnold-Schild D, Lammert E, Rammensee H-G. Stress proteins and immunity mediated by cytotoxic T lymphocytes. Curr Opin Immunol. 1999;11:109–13. doi: 10.1016/s0952-7915(99)80019-3. [DOI] [PubMed] [Google Scholar]
- 47.Srivastava PK, Ménoret A, Basu S, Binder RJ, McQuade KL. Heat shock proteins come of age: primitive functions acquire new roles in an adaptive world. Immunity. 1998;8:657–65. doi: 10.1016/s1074-7613(00)80570-1. [DOI] [PubMed] [Google Scholar]
- 48.Colaco CALS. Towards a unified theory of immunity: dendritic cells, stress proteins and antigen capture. Cell Mol Biol. 1998;44:883–90. [PubMed] [Google Scholar]
- 49.Ménoret A, Patry Y, Burg C, Le Pendu J. Co-segregation of tumor immunogenicity with expression of inducible but not constitutive hsp70 in rat colon carcinomas. J Immunol. 1995;155:740–7. [PubMed] [Google Scholar]
- 50.Udono H, Yamano T, Kawabata Y, Ueda M, Yui K. Generation of cytotoxic T lymphocytes by MHC class I ligands fused to heat shock cognate protein 70. Int Immunol. 2001;13:1233–42. doi: 10.1093/intimm/13.10.1233. [DOI] [PubMed] [Google Scholar]
- 51.Multhoff G. Activation of natural killer cells by heat shock protein 70. Int J Hyperthermia. 2002;18:576–85. doi: 10.1080/0265673021000017109. [DOI] [PubMed] [Google Scholar]
- 52.Multhoff G, Mizzen L, Winchester CC, et al. Heat shock protein 70 (Hsp70) stimulates proliferation and cytolytic activity of natural killer cells. Exp Hematol. 1999;27:1627–36. doi: 10.1016/s0301-472x(99)00104-6. [DOI] [PubMed] [Google Scholar]
- 53.Gross C, Hansch D, Gastpar R, Multhoff G. Interaction of heat shock protein 70 peptide with NK cells involves the NK receptor CD94. Biol Chem. 2003;384:267–79. doi: 10.1515/BC.2003.030. [DOI] [PubMed] [Google Scholar]
- 54.Moser C, Schmidbauer C, Gurtler U, Gross C, Gehrmann M, Thonigs G, Pfister K, Multhoff G. Inhibition of tumor growth in mice with severe combined immunodeficiency is mediated by heat shock protein 70 (HSP70) -peptide-activated, CD94 positive natural killer cells. Cell Stress Chaperones. 2002;7:365–73. doi: 10.1379/1466-1268(2002)007<0365:iotgim>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Graner M, Raymond A, Romney D, He L, Whitesell L, Katsanis E. Immunoprotective activities of multiple chaperone proteins isolated from murine B-cell leukemia/lymphoma. Clin Cancer Res. 2000;6:909–15. [PubMed] [Google Scholar]
- 56.Robert J, Ménoret A, Basu S, Cohen N, Srivastava PK. Phylogenetic conservation of the molecular and immunological properties of the chaperone gp96 and hsp70. Eur J Immunol. 2001;31:186–95. doi: 10.1002/1521-4141(200101)31:1<186::AID-IMMU186>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 57.Castelli C, Ciupitu AM, Rini F, Rivoltini L, Mazzocchi A, Kiessling R, Parmiani G. Human heat shock protein 70 peptide complexes specifically activate anti-melanoma T cells. Cancer Res. 2001;61:222–7. [PubMed] [Google Scholar]
- 58.Casey DG, Lysaght J, James T, Bateman A, Melcher AA, Todryk SM. Heat shock protein derived from a non-autologous tumour can be used as an anti-tumour vaccine. Immunology. 2003;110:105–11. doi: 10.1046/j.1365-2567.2003.01726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blachere NE, Udono H, Janetzki S, Li Z, Heike M, Srivastava PK. Heat shock protein vaccines against cancer. J Immunother. 1993;14:352–6. doi: 10.1097/00002371-199311000-00016. [DOI] [PubMed] [Google Scholar]
- 60.Heikema A, Agsteribbe E, Wiscjut J, Huckriede A. Generation of heat shock protein-based vaccines by intracellular loading of gp96 with antigenic peptides. Immunol Lett. 1997;57:69–74. doi: 10.1016/s0165-2478(97)00048-5. [DOI] [PubMed] [Google Scholar]
- 61.Blachere NE, Li ZL, Chandawarkar RY, Suto R, Jaikaria NS, Basu S, Udono H, Srivastava PK. Heat shock protein–peptide complexes, reconstituted in vitro, elicit peptide-specific cytotoxic T lymphocyte response and tumor immunity. J Exp Med. 1997;186:1315–22. doi: 10.1084/jem.186.8.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ciupitu AT, Petersson M, O'Donnell CL, Williams K, Jindal S, Kiessling R, Welsh RM. Immunization with a lymphocytic choriomeningitis virus peptide mixed with heat shock protein 70 results in protective antiviral immunity and specific cytotoxic T lymphocytes. J Exp Med. 1998;187:685–91. doi: 10.1084/jem.187.5.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Suzue K, Young RA. Adjuvant-free hsp70 fusion protein system elicits humoral and cellular immune responses to HIV-1 p24. J Immunol. 1996;156:873–9. [PubMed] [Google Scholar]
- 64.Arnold-Schild D, Hanau D, Spehner D, Schmid C, Rammensee H-G, de la Salle H, Schild H. Receptor-mediated endocytosis of heat shock proteins by professional antigen-presenting cells. J Immunol. 1999;162:3757–60. [PubMed] [Google Scholar]
- 65.Binder RJ, Harris ML, Ménoret A, Srivastava PK. Saturation, competition, and specificity in interaction of heat shock proteins (hsp) gp96, hsp90, and hsp70 with CD11b+ cells. J Immunol. 2000;165:2582–7. doi: 10.4049/jimmunol.165.5.2582. [DOI] [PubMed] [Google Scholar]
- 66.Basu S, Binder RJ, Ramalingam T, Srivastava PK. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70 and calreticulin. Immunity. 2001;14:303–13. doi: 10.1016/s1074-7613(01)00111-x. [DOI] [PubMed] [Google Scholar]
- 67.Becker T, Hartl FU, Wieland F. CD40, an extracellular receptor for binding and uptake of Hsp70-peptide complexes. J Cell Biol. 2002;158:1277–85. doi: 10.1083/jcb.200208083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang Y, Kelly CG, Karttunen T, et al. CD40 is a cellular receptor mediating mycobacterial heat shock protein 70 stimulation of CC-chemokines. Immunity. 2001;15:971–83. doi: 10.1016/s1074-7613(01)00242-4. [DOI] [PubMed] [Google Scholar]
- 69.Delneste Y, Magistrelli G, Gauchat J, et al. Involvement of LOX-1 in dendritic cell-mediated antigen cross-presentation. Immunity. 2002;17:353–62. doi: 10.1016/s1074-7613(02)00388-6. [DOI] [PubMed] [Google Scholar]
- 70.van Eden W, van der Zee R, Paul AGA, Prakken BJ, Wendling U, Anderton SM, Wauben MHM. Do heat shock proteins control the balance of T-cell regulation in inflammatory diseases? Immunol Today. 1998;19:303–7. doi: 10.1016/s0167-5699(98)01283-3. [DOI] [PubMed] [Google Scholar]
- 71.van den Broek MF, Hogervorst EJM, van Bruggen MCJ, van Eden W, van der Zee R, van den Berg W. Protection against streptococcal cell wall induced arthritis by pretreatment with the 65kD heat shock protein. J Exp Med. 1989;170:449–66. doi: 10.1084/jem.170.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thompson SJ, Rook GAW, Brealey RJ, van der Zee R, Elson CJ. Autoimmune reactions to heat shock proteins in pristane induced arthritis. Eur J Immunol. 1990;20:2479–84. doi: 10.1002/eji.1830201118. [DOI] [PubMed] [Google Scholar]
- 73.Anderton SM, van der Zee R, Prakken B, Noordzij A, van Eden W. Activation of T cells recognizing self 60-kD heat shock protein can protect against experimental arthritis. J Exp Med. 1995;181:943–52. doi: 10.1084/jem.181.3.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Anderton SM, van Eden W. T lymphocyte recognition of hsp60 in experimental arthritis. In: van Eden W, Young D, editors. Stress Proteins in Medicine. New York: Marcel Dekker; 1996. pp. 73–91. [Google Scholar]
- 75.Paul AGA, van Kooten PJS, van Eden W, van der Zee R. Highly autoproliferative T cells specific for 60-kDa heat shock protein produce IL-4/IL-10 and IFN-γ and are protective in adjuvant arthritis. J Immunol. 2000;165:7270–7. doi: 10.4049/jimmunol.165.12.7270. [DOI] [PubMed] [Google Scholar]
- 76.Munk ME, Schoel B, Modrow S, Karr RW, Young RA, Kaufmann SHE. T lymphocytes from healthy individuals with specificity to self-epitopes shared by the mycobacterial and human 65-kilodalton heat shock protein. J Immunol. 1989;143:2844–9. [PubMed] [Google Scholar]
- 77.Cohen IR. Heat shock protein 60 and the regulation of autoimmunity. In: van Eden W, Young D, editors. Stress Proteins in Medicine. New York: Marcel Dekker; 1996. pp. 93–102. [Google Scholar]
- 78.Ramage JM, Young JL, Goodall JC, Hill Gaston JS. T cell responses to heat shock protein 60. differential responses by CD4+ T cell subsets according to their expression of CD45 isotypes. J Immunol. 1999;162:704–10. [PubMed] [Google Scholar]
- 79.Macht LM, Elson CJ, Kirwan JR, Gaston JSH, Lamont AG, Thompson JM, Thompson SJ. Relationship between disease severity and responses by blood mononuclear cells from patients with rheumatoid arthritis to human heat shock protein 60. Immunology. 2000;99:208–14. doi: 10.1046/j.1365-2567.2000.00966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rook GA, Stanford JL. Give us this day our daily germs. Immunol Today. 1998;19:113–16. doi: 10.1016/s0167-5699(97)01204-8. [DOI] [PubMed] [Google Scholar]
- 81.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 82.Hightower LE, Guidon PT. Selective release from cultured mammalian cells of heat shock (stress) proteins that resemble glia-axon transfer proteins. J Cell Physiol. 1989;138:257–66. doi: 10.1002/jcp.1041380206. [DOI] [PubMed] [Google Scholar]
- 83.Liao D-F, Jin Z-G, Baas AS, Daum G, Gygi SP, Aebersold R, Berk BC. Purification and identification of secreted oxidative stress-induced factors from vascular smooth muscle cells. J Biol Chem. 2000;275:189–96. doi: 10.1074/jbc.275.1.189. [DOI] [PubMed] [Google Scholar]
- 84.Asea A, Kraeft S-K, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK. Hsp70 stimulates cytokine production through a CD14-dependent pathway, demonstrating its dual role as a chaperone and cytokine. Nature Med. 2000;6:435–42. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- 85.Pockley AG, Shepherd J, Corton J. Detection of heat shock protein 70 (Hsp70) and anti-Hsp70 antibodies in the serum of normal individuals. Immunol Invest. 1998;27:367–77. doi: 10.3109/08820139809022710. [DOI] [PubMed] [Google Scholar]
- 86.Pockley AG, Wu R, Lemne C, Kiessling R, de Faire U, Frostegård J. Circulating heat shock protein 60 is associated with early cardiovascular disease. Hypertension. 2000;36:303–7. doi: 10.1161/01.hyp.36.2.303. [DOI] [PubMed] [Google Scholar]
- 87.Pockley AG, de Faire U, Kiessling R, Lemne C, Thulin T, Frostegård J. Circulating heat shock protein and heat shock protein antibody levels in established hypertension. J Hypertension. 2002;20:1815–20. doi: 10.1097/00004872-200209000-00027. [DOI] [PubMed] [Google Scholar]
- 88.Habich C, Kempe K, van der Zee R, Burkart V, Kolb H. Different heat shock protein 60 species share pro-inflammatory activity but not binding sites on macrophages. FEBS Lett. 2003;533:105–9. doi: 10.1016/s0014-5793(02)03772-9. [DOI] [PubMed] [Google Scholar]
- 89.Asea A, Rehli M, Kabingu E, Boch JA, Baré O, Auron PE, Stevenson MA, Calderwood SK. Novel signal transduction pathway utilized by extracellular HSP70. Role of Toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 2002;277:15028–34. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- 90.Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activates the NF-kB pathway. Int Immunol. 2000;12:1539–46. doi: 10.1093/intimm/12.11.1539. [DOI] [PubMed] [Google Scholar]
- 91.Huang Q, Richmond JF, Suzue K, Eisen HN, Young RA. In vivo cytotoxic T lymphocyte elicitation by mycobacterial heat shock protein 70 fusion proteins maps to a discrete domain and is CD4 (+) T cell independent. J Exp Med. 2000;191:403–8. doi: 10.1084/jem.191.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang Y, Kelly CG, Singh M, McGowan EG, Carrara AS, Bergmeier LA, Lehner T. Stimulation of Th1-polarizing cytokines, C-C chemokines, maturation of dendritic cells, and adjuvant function by the peptide binding fragment of heat shock protein 70. J Immunol. 2002;169:2422–9. doi: 10.4049/jimmunol.169.5.2422. [DOI] [PubMed] [Google Scholar]
- 93.Wallin RPA, Lundqvist A, Moré SH, von Bonin A, Kiessling R, Ljunggren H-G. Heat shock proteins as activators of the innate immune system. Trends Immunol. 2002;23:130–5. doi: 10.1016/s1471-4906(01)02168-8. [DOI] [PubMed] [Google Scholar]
- 94.Gaston JSH. Heat shock proteins and innate immunity. Clin Exp Immunol. 2002;127:1–3. doi: 10.1046/j.1365-2249.2002.01759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gao B, Tsan MF. Endotoxin contamination in recombinant human Hsp70 preparation is responsible for the induction of TNFalpha release by murine macrophages. J Biol Chem. 2003;278:174–9. doi: 10.1074/jbc.M208742200. [DOI] [PubMed] [Google Scholar]
- 96.Bausinger H, Lipsker D, Ziylan U, et al. Endotoxin-free heat shock protein 70 fails to induce APC activation. Eur J Immunol. 2002;32:3708–13. doi: 10.1002/1521-4141(200212)32:12<3708::AID-IMMU3708>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 97.Gao B, Tsan MF. Recombinant human heat shock protein 60 does not induce the release of tumor necrosis factor alpha from murine macrophages. J Biol Chem. 2003;278:22523–9. doi: 10.1074/jbc.M303161200. [DOI] [PubMed] [Google Scholar]
- 98.Savill J, Fadok V. Corpse clearance defines the meaning of cell death. Nature. 2000;407:784–8. doi: 10.1038/35037722. [DOI] [PubMed] [Google Scholar]
- 99.Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I. Immunosuppressive effects of apoptotic cells. Nature. 1997;390:350–1. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- 100.Fadok VA, Henson PM. Apoptosis. getting rid of the bodies. Curr Biol. 1998;8:R693–5. doi: 10.1016/s0960-9822(98)70438-5. [DOI] [PubMed] [Google Scholar]
- 101.Reiter I, Krammer B, Schwamberger G. Cutting edge. differential effect of apoptotic versus necrotic tumor cells on macrophage antitumor activities. J Immunol. 1999;163:1730–2. [PubMed] [Google Scholar]
- 102.Liu K, Iyoda T, Saternus M, Kimura M, Inaba K, Steinman RM. Immune tolerance after delivery of dying cells to dendritic cells in situ. J Exp Med. 2002;196:1091–7. doi: 10.1084/jem.20021215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Griffith TSYuX, Herndon JM, Green DR, Ferguson TA. CD95-induced apoptosis of lymphocytes in an immune privileged site induces immunological tolerance. Immunity. 1996;5:7–16. doi: 10.1016/s1074-7613(00)80305-2. [DOI] [PubMed] [Google Scholar]
- 104.Ronchetti A, Rovere P, Iezzi G, et al. Immunogenicity of apoptotic cells in vivo: role of antigen load, antigen-presenting cells, and cytokines. J Immunol. 1999;163:130–6. [PubMed] [Google Scholar]
- 105.Somersan S, Larsson M, Fonteneau JF, Basu S, Srivastava P, Bhardwaj N. Primary tumor tissue lysates are enriched in heat shock proteins and induce the maturation of human dendritic cells. J Immunol. 2001;167:4844–52. doi: 10.4049/jimmunol.167.9.4844. [DOI] [PubMed] [Google Scholar]
- 106.Melcher A, Todryk S, Hardwick N, Ford M, Jacobson M, Vile RG. Tumor immunogenicity is determined by the mechanism of cell death via induction of heat shock protein expression. Nature Med. 1998;4:581–7. doi: 10.1038/nm0598-581. [DOI] [PubMed] [Google Scholar]
- 107.Todryk S, Melcher AA, Hardwick N, Linardakis E, Bateman A, Colombo MP, Stoppacciaro A, Vile RG. Heat shock protein 70 induced during tumor cell killing induces Th1 cytokines and targets immature dendritic cell precursors to enhance antigen uptake. J Immunol. 1999;163:1398–408. [PubMed] [Google Scholar]
- 108.Gough MJ, Melcher AA, Ahmed A, et al. Macrophages orchestrate the immune response to tumor cell death. Cancer Res. 2001;61:7240–7. [PubMed] [Google Scholar]
- 109.Clark PR, Menoret A. The inducible Hsp70 as a marker of tumor immunogenicity. Cell Stress Chaperones. 2001;6:121–5. doi: 10.1379/1466-1268(2001)006<0121:tihaam>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Feng H, Zeng Y, Graner MW, Katsanis E. Stressed apoptotic tumor cells stimulate dendritic cells and induce specific cytotoxic T cells. Blood. 2002;100:4108–15. doi: 10.1182/blood-2002-05-1389. [DOI] [PubMed] [Google Scholar]
- 111.Nylandsted J, Wick W, Hirt UA, Brand K, Rohde M, Leist M, Weller M, Jaattela M. Eradication of glioblastoma, and breast and colon carcinoma xenografts by Hsp70 depletion. Cancer Res. 2002;62:7139–42. [PubMed] [Google Scholar]
- 112.van Molle W, Wielockx B, Mahieu T, Takada M, Taniguchi T, Sekikawa K, Libert C. HSP70 protects against TNF-induced lethal inflammatory shock. Immunity. 2002;16:685–95. doi: 10.1016/s1074-7613(02)00310-2. [DOI] [PubMed] [Google Scholar]
- 113.Gurbuxani S, Bruey JM, Fromentin A, et al. Selective depletion of inducible HSP70 enhances immunogenicity of rat colon cancer cells. Oncogene. 2001;20:7478–85. doi: 10.1038/sj.onc.1204948. [DOI] [PubMed] [Google Scholar]
- 114.Feng H, Zeng Y, Graner MW, Likhacheva A, Katsanis E. Exogenous stress proteins enhance the immunogenicity of apoptotic tumor cells and stimulate antitumor immunity. Blood. 2003;101:245–52. doi: 10.1182/blood-2002-05-1580. [DOI] [PubMed] [Google Scholar]
- 115.Brar BK, Stephanou A, Wagstaff MJ, Coffin RS, Marber MS, Engelmann G, Latchman DS. Heat shock proteins delivered with a virus vector can protect cardiac cells against apoptosis as well as against thermal or hypoxic stress. J Mol Cell Cardiol. 1999;31:135–46. doi: 10.1006/jmcc.1998.0857. [DOI] [PubMed] [Google Scholar]
- 116.Binder RJ, Han DK, Srivastava PK. CD91: a receptor for heat shock protein gp96. Nature Immunol. 2000;1:151–5. doi: 10.1038/77835. [DOI] [PubMed] [Google Scholar]
- 117.Ogden CA, de Cathelineau A, Hoffmann PR, Bratton D, Ghebrehiwet B, Fadok VA, Henson PM. C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J Exp Med. 2001;194:781–95. doi: 10.1084/jem.194.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Su HP, Nakada-Tsukui K, Tosello-Trampont AC, Li Y, Bu G, Henson PM, Ravichandran KS. Interaction of CED-6/GULP, an adapter protein involved in engulfment of apoptotic cells with CED-1 and CD91/low density lipoprotein receptor-related protein (LRP) J Biol Chem. 2002;277:11772–9. doi: 10.1074/jbc.M109336200. [DOI] [PubMed] [Google Scholar]
- 119.Vandivier RW, Ogden CA, Fadok VA, et al. Role of surfactant proteins A, D, and C1q in the clearance of apoptotic cells in vivo and in vitro: calreticulin and CD91 as a common collectin receptor complex. J Immunol. 2002;169:3978–86. doi: 10.4049/jimmunol.169.7.3978. [DOI] [PubMed] [Google Scholar]
- 120.Taylor PR, Carugati A, Fadok VA, et al. A hierarchical role for classical pathway complement proteins in the clearance of apoptotic cells in vivo. J Exp Med. 2000;192:59–366. doi: 10.1084/jem.192.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rovere P, Sabbadini MG, Vallinoto C, Fascio U, Zimmermann VS, Bondanza A, Ricciardi-Castagnoli P, Manfredi AA. Delayed clearance of apoptotic lymphoma cells allows cross-presentation of intracellular antigens by mature dendritic cells. J Leuk Biol. 1999;66:345–9. doi: 10.1002/jlb.66.2.345. [DOI] [PubMed] [Google Scholar]
- 122.Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumour cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191:423–33. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants. endogenous activators of dendritic cells. Nature Med. 1999;11:1249–55. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]