Abstract
It is estimated that Helicobacter pylori infects the stomachs of over 50% of the world's population and if not treated may cause chronic gastritis, peptic ulcer disease, gastric adenocarcinoma and gastric B-cell lymphoma. The aim of this study was to enhance the mucosal and systemic immune responses against the H. pylori antigens cytotoxin-associated gene A (CagA) and neutrophil-activating protein (NAP), through combinations of mucosal and systemic immunizations in female BALB/c mice. We found that oral or intranasal (i.n.) followed by i.m. immunizations induced significantly higher serum titres against NAP and CagA compared to i.n. alone, oral alone, i.m. alone, i.m. followed by i.n. or i.m. followed by oral immunizations. However, only oral followed by i.m. immunizations induced anti-NAP antibody-secreting cells in the stomach. Moreover, mucosal immunizations alone or in combination with i.m., but not i.m. immunizations alone, induced mucosal immunoglobulin A (IgA) responses in faeces. Any single route or combination of immunization routes with NAP and CagA preferentially induced antigen-specific splenic interleukin-4-secreting cells and far fewer interferon-γ-secreting cells in the spleen. Moreover, i.n. immunizations alone or in combination with i.m. immunizations induced predominantly serum IgG1 and far less serum IgG2a. Importantly, we found that while both i.n. and i.m. recall immunizations induced similar levels of serum antibody responses, mucosal IgA responses in faeces were only achieved through i.n. recall immunization. Collectively, our data show that mucosal followed by systemic immunization significantly enhanced local and systemic immune responses and that i.n. recall immunization is required to induce both mucosal and systemic memory type responses.
Introduction
It is estimated that Helicobacter pylori, a Gram-negative, spiral, microaerophilic bacterium, infects the stomachs of over 50% of the world's population and if not treated it can cause, in certain individuals, chronic gastritis, peptic ulcer disease, gastric adenocarcinoma and gastric B-cell lymphoma.1 Current therapies based on a proton-pump inhibitor and antibiotics have several drawbacks, including patient compliance, antibiotic resistance and recurrence of infections. Therefore, vaccine development against infection with H. pylori has attracted much attention. Efforts for vaccine development against infection with H. pylori have focused on antigens that are involved in the pathogenesis of the bacterium such as urease, the vacuolating cytotoxin (VacA), the cytotoxin-associated antigen (CagA) and the neutrophil-activating protein (NAP).2,3
Several studies have demonstrated protection against challenge with H. pylori following oral,3–8 intranasal (i.n.)9,10 and rectal,11 as well as systemic, immunizations.12–14
The mechanisms of protection against H. pylori remain elusive. The presence of systemic and/or local H. pylori-specific antibody production has in some studies shown correlation with protection.8,15 Specifically, a role of local immunoglobulin A (IgA) secretion has been suggested in protected mice.8,16 Moreover, homing of cells expressing the mucosal homing receptor as inducers of local immunity has been regarded as important in protection.17,18 However, other studies have shown protection from H. pylori challenge in the absence of local antibody or IgA.19,20 There is increasing evidence that protection can be mediated by CD4+ T cells.17,20 It appears that infection with H. pylori results in enhanced interferon-γ (IFN-γ) and thus T helper type 1 (Th1) -type responses.21–23 These and other studies24–26 suggest that induction of a Th2-type response may reverse the course of the infection and/or pathogenicity of H. pylori. Thus, vaccine candidates that selectively induce Th2-type responses against H. pylori are sought.
Perhaps the most desirable attribute of vaccination is long-term protection against pathogens. However, in the majority of H. pylori vaccine studies the animals were challenged in the acute phase of the immune response. It is not known whether mucosal or systemic immunizations can best afford protection a long time after priming immunizations. Several studies have demonstrated that local or mucosal immunization best affords protection a long time after priming immunizations.27 Thus, mucosal immunizations may afford better protection in long-term studies. Moreover, mucosal immunizations can be performed without the use of needles, thus eliminating frequent transmission of various diseases through the re-use of contaminated needles in developing countries. Nonetheless, induction of mucosal responses through mucosal immunizations requires mucosal adjuvants, ideally non-toxic, or delivery systems that can be used for human vaccines. However, it is necessary to induce immune responses through mucosal immunization adjuvants or delivery systems. Two mutants of the heat-labile toxin (LT), elaborated from enteropathogenic Escherichia coli, were originated28 and have shown potency as a mucosal adjuvant.
In this study, we compared the mucosal route with parenteral routes of immunization and then sought to determine whether combinations of mucosal prime and systemic boost or systemic prime and mucosal boost would result in enhanced mucosal and systemic humoral immunity. For the systemic immunizations the oil in water emulsion, MF59, was used. For mucosal routes the i.n. route was compared with the oral route, using LTK63 and LTR72, respectively. Because a Th2-type immune response is desirable in an H. pylori vaccine, we investigated whether our immunization strategy induced Th1- or Th2-type responses. Moreover, we aimed to establish whether memory-type responses could be elicited by mucosal or systemic re-boosting in mice immunized with combinations of mucosal and systemic immunizations.
Materials and methods
Mice, immunizations and vaccine preparations
Female BALB/c mice were purchased from Jackson Laboratories (Bar Harbor, ME) and used at the age of 6–8 weeks at the beginning of immunizations. Immunizations were performed at 0, 10, 20, 30, 40 and/or 50 days. CagA and NAP were prepared as previously described.2,3 The LTK63 and LTR72 mutants of E. coli enterotoxin were originated and prepared as described28 and used for i.n. and oral immunizations, respectively. The doses for NAP or CagA were 10 μg each for intramuscular (i.m.), 30 μg each for i.n., and 100 μg each for oral immunizations. The i.n. immunizations were given with 10 μg LTK63 in 30 μl phosphate-buffered saline (PBS). The i.m. immunizations were given in 50 μl of the oil in water emulsion, MF59. Oral immunizations were given with 10 μg LTR72 in 500 μl 3% bicarbonate/PBS. The i.m. immunizations were given in the right thigh and i.n. immunizations were administered to the nares of non-anesthetized mice and allowed to be inhaled. Oral immunizations were performed on non-anesthetized mice through a ball-end, steel feeding tube. Sera and faecal pellets were collected at 6 days post-final immunization and the mice were killed at 7 days post final immunization to prepare single-cell suspensions from the stomach mucosa, mesenteric lymph nodes and spleen for the ELISPOT assays.
ELISPOT assay for detection of anti-CagA and anti-NAP antibody-secreting cells
Single-cell suspensions from pooled stomachs from 10 mice per group (prepared by collagenase/dispase digestion), mesenteric lymph nodes (prepared by meshing through a nylon mesh) and spleen (prepared by meshing through a nylon mesh) from 10 mice per group were added onto nitrocellulose or polyvinyldifluoride plates (Millipore, Billerca, MA) precoated with NAP (5 μg/ml PBS) or CagA (10 μg/ml PBS) and blocked with complete RPMI-1640 medium at pH 7·2, containing 10% fetal calf serum, 5 mm HEPES and antibiotics. Following overnight incubation of cells the plates were washed with PBS/0·02% Tween-20 (PBS/Tween) and biotinylated goat anti-mouse H + L immunoglobulin (Southern Biotechnology Associates, Birmingham, AL) was added in PBS/0·1% bovine serum albumin/0·02% Tween-20 (PBS/BSA/Tween) and incubated at room temperature for 2 hr. The plates were washed with PBS/Tween and incubated for 1 hr at 37° with avidin–peroxidase (Pharmingen, BD, San Diego, CA) at 1 : 1000 dilution in PBS/BSA/Tween. The plates were washed with PBS/Tween and the spots were visualized by adding 3,3 diaminobenzidine tetrahydrochloride dihydrate(DAB) in Tris–HCl (pH 7·5) buffer for 30 min The plates were washed with de-ionized H2O and air-dried. The spots were counted by a Zeiss KS automatic ELISPOT reader. Two to four wells per group and per tissue were counted. The data are presented as mean + SD of two independent experiments with pools of 10 individual mice per group and are expressed as the number of NAP- or CagA-specific antibody-secreting cells per 106 mononuclear cells.
ELISPOT assay for detection of anti-CagA and anti-NAP cytokine-secreting cells
Single-cell suspensions from pooled stomach, mesenteric lymph nodes and spleen from 10 mice per group were added onto nitrocellulose or polyvinyldifluoride plates (Millipore) precoated with rat anti-mouse interleukin-4 (IL-4; Endogen, Woburn, MA; catalogue number MM450C) or rat anti-mouse IFN-γ (catalogue number 18181D, BD/Pharmingen) and blocked with complete RPMI-1640 medium at pH 7·2, containing 10% fetal calf serum, 5 mm HEPES and antibiotics. Single-cell suspensions from the various tissues were then incubated overnight with a mixture of CagA and NAP proteins at a final concentration of 10 μg/ml. The plates were then washed with PBS/Tween and biotinylated rat anti-mouse IL-4 (Endogen, catalogue number, MM450DB) or biotinylated rat anti-mouse IFN-γ (Pharmingen, catalogue number 18112D) was added in PBS/BSA/Tween and incubated at room temperature for 2 hr. The plates were washed with PBS/Tween and incubated for 1 hr at 37° with avidin-peroxidase (Pharmingen) at 1 : 1000 dilution in PBS/BSA/Tween. The plates were washed with PBS/Tween and the spots were visualized by adding DAB substrate in Tris–HCl (pH 7·5) buffer for 30 min The reaction was stopped by washing the plates with de-ionized H2O. The plates were air-dried and the spots were counted by a Zeiss KS automatic ELISPOT reader. two to four wells per group and per tissue were counted. The data are presented as mean + SD of two independent experiments with pools of 10 individual mice per group and are expressed as the number of NAP- or CagA-specific antibody-secreting cells per 106 mononuclear cells.
Enzyme-linked immunosorbent assay (ELISA)
CagA- and NAP-specific serum IgG, IgG1, or IgG2a titres were determined by a standard colorimetric ELISA, while faecal pellet extracts were assayed for IgA using a bioluminescent immunosorbant assay (BIA) as described below. Briefly, ELISA plates (96-well U-bottom by Nunc Maxisorp, Denmark) were coated with CagA or NAP protein at 10 μg/well in PBS overnight at 4°. After washing with PBS/0·03% Tween-20 (Sigma), the wells were blocked with PBS/2% fetal calf serum for 30 min at 37°. Serum samples were added in an initial dilution of 1 : 5000 and then 1 : 2 serial dilutions were performed in the blocking reagent. A standard serum was included in each assay as a positive control. The samples and standard sera were incubated at 4° overnight and then washed with PBS/0·03% Tween-20. The plates were washed and biotinylated goat anti-mouse IgA or IgG at a 1 : 10 000 dilution was added in PBS/2% fetal calf serum and incubated for 2 hr at room temperature. The plates were then washed and avidin-horseradish peroxidase (Pharmingen) at a 1 : 1000 dilution was added and incubated at 37° for 30 min The plates were then washed with PBS/0·03% Tween-20 and developed with tetramethylbenzidine (Kirkegaard and Perry, Gaithersburg, MD) for 15 min and then stopped with 2 m HCl. The optical density of each well was measured using a Titertek instrument at 450 nm. The results are shown as average antigen-specific IgG or IgA end-point titres from 10 individual mice per group plus standard deviation. The end-point titres are reported as the reciprocal of the serum dilutions that gave an optical density of 450 nm at 0·5 ELISA absorbancy units.
Faecal pellet extracts were assayed for IgA using a BIA as previously described.29 Briefly, ELISA plates (MicroLite obtained from Dynatech, Chantilly, VA) were first coated with the NAP or CagA (5 μg/ml) overnight. After blocking (5% goat serum, 25 mm Tris–HCl, 10 mm EGTA, 150 mm KCl, 2 mg/ml BSA, 0·3% Tween-20, pH 7·5), plates were coated with 1 : 3 serially diluted faecal pellet samples in blocking buffer. The plates were developed using 1 : 1000 diluted goat anti-mouse IgA biotin conjugate. Plates were then incubated with 1 : 500 diluted streptavidin-jellyfish aequorin conjugate (SeaLite Sciences, Bogart, GA). Luminescence was triggered with 10 mm calcium acetate and measured using a luminometer (Dynatech ML3000). Quantification was based on relative light units (RLU) representing total luminescence integrated over 3 seconds (arbitrary units). Titres represent log dilution values linearly extrapolated from the log RLU data to a cut-off value at least two standard deviations above mean background.
Statistical analysis
microsoft excel statistical tools software was used to perform Student's t-test to determine statistically significant differences between the means of the groups.
Results
Enhanced mucosal and systemic humoral responses through oral priming and intramuscular boosting immunizations
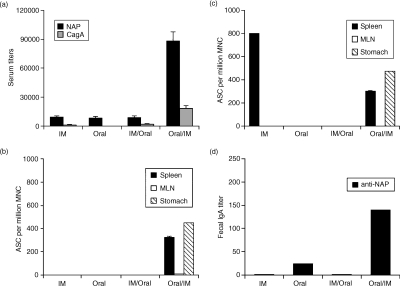
To address whether systemic, mucosal, or combinations of mucosal and systemic immunizations result in enhanced immunity, we compared i.m. alone versus oral alone versus i.m. followed by oral (i.m./oral) versus oral followed by i.m. (oral/i.m.) immunizations. We found several-fold higher anti-NAP and anti-CagA serum titres in the oral/i.m. group compared to the other groups (Fig. 1a).
Figure 1.
Enhanced systemic and mucosal humoral responses following oral priming and systemic boosting immunizations. Mice were immunized five times intramuscularly (i.m.) with NAP and CagA purified proteins in MF59 adjuvant or five times orally with a mixture of NAP and CagA purified proteins and LTR72 adjuvant, or three times orally followed by twice i.m., or twice i.m. followed by three times orally. Sera and faecal pellets were collected 6 days after the final immunization. All immunizations were performed at 2-week intervals. Single-cell suspensions were prepared from stomach, spleen and mesenteric lymph nodes (MLN) 7 days after the final immunization for the ELISPOT assay. (a) Serum IgG titres; the results are shown as mean anti-NAP and anti-CagA serum titres from 10 mice as measured by a standard colorimetric ELISA. (b) Anti-NAP and (c) anti-CagA antibody-secreting cells (ASC). The results are shown as the mean number of anti-NAP or anti-CagA ASC from pools of 10 mice + SD of a minimum of four ELISPOT wells per group per tissue. (d) Anti-NAP IgA titres in faecal extracts. The results are shown as mean anti-NAP faecal IgA titres from 10 mice as measured by a chemiluminescence ELISA. The results are representative of three independent experiments with similar results.
Next, to investigate local responses we determined the number of anti-CagA- and anti-NAP-secreting cells in local and systemic lymphoid tissues. As shown in Fig. 1(b), only oral/i.m. immunizations induced local anti-CagA humoral responses in the mucosal effector site of the stomach as well as in the local inductive site of the mesenteric lymph node. Moreover, strong systemic responses were detected in spleen. In contrast, i.m. immunizations alone did not induce local antibody-secreting cell responses in the stomach although strong systemic responses were detected in the spleen. Oral immunizations alone or i.m./oral immunizations did not induce any detectable antibody-secreting cell responses at any site. We found similar anti-NAP results, although in the i.m. group we did not detect any anti-NAP antibody-secreting cells (Fig. 1c). The numbers of total unspecific antibody-secreting cells remained similar in the stomach, MLN, or SP after each immunization (data not shown).
As an additional measure for mucosal immunity, we determined NAP-specific IgA responses in faecal extracts. We found that NAP-specific IgA titres in faecal extracts were highest in the oral/i.m. group, followed by the oral alone group (Fig. 1d). As expected, i.m. immunization alone did not induce specific IgA responses in faecal extracts. Anti-CagA IgA titres in faecal extracts were negligible in all groups tested (data not shown). These data further substantiate the enhancement of the local and mucosal responses by the oral/i.m. immunization regimen.
Collectively, these data show that when i.m. alone versus oral alone versus i.m./oral versus oral/i.m. immunizations with H. pylori CagA and NAP are compared, the oral/i.m. regimen induced the most potent antigen-specific mucosal and systemic humoral responses.
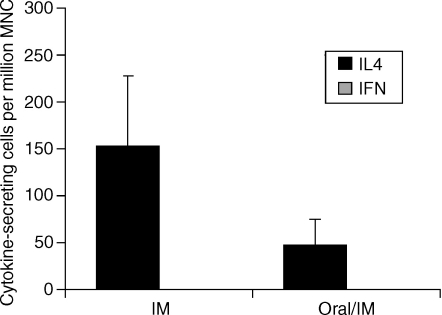
T-cell responses following oral priming and intramuscular boosting immunizations
To determine whether a Th1- or Th2-type response was induced by the combinational prime-boost versus single-route immunization, we performed the ELISPOT assay on splenocytes. We found CagA/NAP-specific IL-4-secreting cells in splenocytes isolated from mice following the oral/i.m. regimen as well as following the i.m. alone regimen (Fig. 2). In contrast, no CagA/NAP-specific IFN-γ-secreting cells were detected following either regimen. Of note, no IL-4- or IFN-γ-secreting cells were detectable in mesenteric lymph nodes or stomach. Thus, oral/i.m. or i.m. alone immunizations with CagA or NAP selectively induced a Th2-type cytokine response.
Figure 2.
Induction of Th2-type cytokine-secreting cells following oral and/or intramuscular immunizations with CagA and NAP. Mice were immunized with five intramuscular (i.m.) immunizations with NAP and CagA purified proteins in MF59 adjuvant or primed with three oral immunizations with a mixture of NAP and CagA purified proteins and LTR72 adjuvant and boosted i.m. with NAP and CagA purified proteins in MF59 adjuvant. All immunizations were performed at 2-week intervals. The mice were killed 7 days after the final immunization in each group. The number of IL4-secreting cells and IFNγ-secreting cells specific for a mixture of CagA and NAP was measured by the ELISPOT assay as described in the Materials and methods section. The results are shown as the mean number of cytokine-secreting cells from pools of 10 mice per group + SD of a minimum of four ELISPOT wells per group. The results are representative of three independent experiments with similar results.
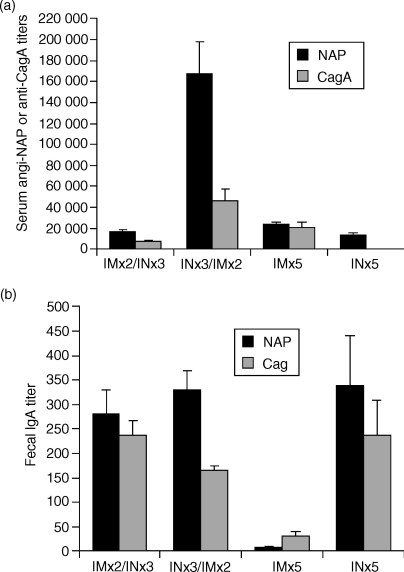
Induction of enhanced antibody responses to H. pylori antigens by i.n. followed by i.m. immunizations
Intransal immunizations generally induce better systemic immune responses and require far less antigen for vaccination compared to oral immunizations and have been shown to induce protection against H. pylori challenge. Therefore, we next investigated whether we could replace oral with i.n. immunizations. We found that three i.n. priming immunizations followed by two i.m. boosts (i.n./i.m.) significantly enhanced the serum anti-NAP and anti-CagA antibody titres several-fold compared to two i.m. primings followed by three i.n. boosts (i.m./i.n.), five i.n. alone, or five i.m. alone immunizations (P < 0·0008) (Fig. 3a). The anti-NAP serum antibody responses were generally higher, indicating that this antigen, compared to CagA, was more immunogenic for induction of serum antibody responses. These results show that the i.n. route of mucosal priming followed by the i.m. route of parenteral boosting induced enhanced anti-NAP and anti-CagA serum antibody responses compared to i.m./i.n., i.n. alone, or i.m. alone immunizations. Of note, we performed a study to determine how many i.n. and i.m. immunizations were required to induce optimal serum antibody responses. We found that two i.n. and two i.m. immunizations, as opposed to one i.n. and two i.m. or two i.n. and one i.m. immunizations, were required to induce optimal serum titres (data not shown).
Figure 3.
Enhanced humoral responses following intranasal (i.n.) priming and systemic boosting immunizations. Mice were immunized with five intramuscular (i.m.) immunizations with NAP and CagA purified proteins in MF59 adjuvant (NCMF59) or five i.n. immunizations with a mixture of NAP and CagA purified proteins and LTK63 adjuvant (NCLTK63), or primed i.m. with NCMF59 and boosted i.n. with NCLTK63, or primed i.n. with NCLTK63 and boosted i.m. with NCMF59. All immunizations were performed at 2-week intervals. Sera and faecal pellets were collected 6 days after the final immunization. (a) Mean anti-NAP (solid bars) or anti-CagA (shaded bars) serum end-point ELISA titres + SD of 10 mice per group. The results are representative of three independent experiments with 10 mice in each group with similar results. (b) Mean anti-NAP or anti-CagA faecal IgA titres from two subgroups of 10 mice each per group as measured by a chemiluminescence ELISA. The data are representative of two independent experiments with similar results.
As an indicator of the mucosal antibody responses we measured anti-NAP responses in faecal extracts. We found that i.m. immunizations alone induced very low anti-NAP IgA responses in faecal extracts (Fig. 3b). In contrast, i.n./i.m. or i.m./i.n., as well as i.n. alone, immunizations induced significantly higher anti-NAP and anti-CagA IgA titres in faecal extracts compared to the i.m. alone group. Taken together with the serum responses, the i.n./i.m. regimen induced strongly enhanced combined systemic and mucosal antibody responses compared to i.m./i.n., i.m. alone, or i.n. alone immunizations.
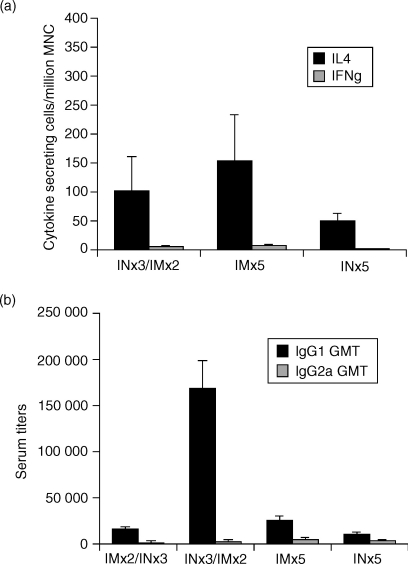
T-cell responses following i.n., i.m. and combinations of i.n. and i.m. immunizations
We next determined T-cell responses in mice following i.n. alone, i.m. alone and i.n. prime/i.m. boost immunizations. We found that in splenocytes isolated from mice following the prime/boost regimen significant numbers of CagA/NAP-specific IL-4-secreting cells were induced (Fig. 4a). In contrast, few or no CagA/NAP-specific IFN-γ-secreting cells were detected following the prime/boost regimen. Of note, no NAP/CagA-specific cytokine-secreting cells (IL-4 or IFN-γ) were detectable in the stomach mucosa, mesenteric lymph nodes, or Peyer's patches of any of the groups (data not shown). Importantly, there was no significant difference in the magnitude of Th2 or Th1 responses between the i.n.-only, i.m.-only, or i.n./i.m. groups.
Figure 4.
Induction of Th2-type cytokine-secreting cells following intranasal (i.n.) and/or intramuscular (i.m.) immunizations with CagA and NAP. Mice were immunized with five i.m. immunizations with NAP and CagA purified proteins in MF59 adjuvant (NCMF59) or five i.n. immunizations with a mixture of NAP and CagA purified proteins and LTK63 adjuvant (NCLTK63), or primed i.n. with NCLTK63 and boosted i.m. with NCMF59. All immunizations were performed at 2-week intervals. The mice were killed 7 days after the final immunization in each group. (a) The number of IL-4-secreting cells and IFN-γ-secreting cells specific for a mixture of CagA and NAP was measured by ELISPOT as described in the Materials and methods section. The results are shown as the mean number of IL-4-secreting cells from pools of 10 mice per group + SD of a minimum of four ELISPOT wells per group. The results are representative of three independent experiments with similar results. (b) Mean anti-NAP IgG1 (solid bars) or IgG2a (shaded bars) serum end-point ELISA titres + SD of 10 mice per group. The results are representative of three independent experiments with 10 mice in each group with similar results
To substantiate further the predominant Th2-type responses, we next determined anti-NAP IgG1 and IgG2a titres in the various groups. We found predominantly IgG1 and several-fold lower IgG2a serum titres in all the groups (Fig. 4b).
Collectively, these data indicate that immunizations with CagA and NAP through mucosal, parenteral, or combinations of mucosal and parenteral immunization routes selectively induce Th2-type cytokines.
Mucosal and systemic memory-type responses can be recalled through a single i.n. recall immunization
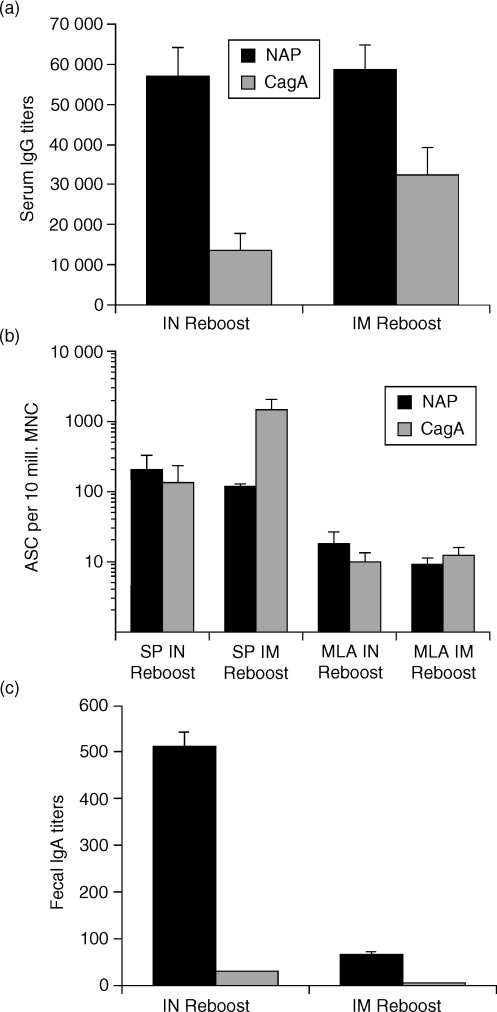
The hallmark of vaccination is to induce immunological memory, defined by the ability to respond with higher numbers of antigen-specific cells with the ability to mount a response faster upon re-encounter with specific antigens. As we had established an immunization strategy that could significantly enhance local and systemic immunity against H. pylori antigens through mucosal prime/systemic boosting, we sought to determine whether such responses could be recalled months later and whether the re-boost needs to be through the mucosal or the systemic route. To this end, we immunized mice by three i.n. and two i.m. immunizations, rested them for 4 months and then re-boosted them either i.n. or i.m. We found that anti-NAP and anti-CagA systemic IgG responses in serum were comparable, regardless of whether the mice were re-boosted i.n. or i.m. (Fig. 5a). However, anti-CagA serum responses were enhanced following i.m. re-boost compared to i.n. re-boost (Fig. 5a). As an additional measure of systemic immunity we found that splenic antibody-secreting cell responses were similar to serum IgG titres in that anti-NAP splenic antibody-secreting cells were similar following i.n. or i.m. re-boost (Fig. 5b). However, anti-CagA splenic antibody-secreting cells were enhanced several-fold following i.m. re-boost (Fig. 5b), although this difference did not reach statistical significance by a Student's t-test. Of note, local humoral responses in mesenteric lymph nodes were low and similar in both groups (Fig. 5b).
Figure 5.
Mucosal and systemic memory-type responses following nasal priming and systemic boosting can be recalled by intranasal re-boost. Mice were primed intranasally (i.n.) with a mixture of NAP and CagA purified proteins and LTK63 adjuvant (NCLTK63) and boosted intramuscularly (i.m.) with a mixture of NAP and CagA purified proteins in MF59 adjuvant (NCMF59) and rested for 4 months before given either an i.n. or i.m. re-boost with NCLTK63 or NCMF59, respectively. All immunizations were performed at 2-week intervals. Serum and faecal pellets were collected 6 days after the final immunization in each group. Cells were prepared from mice killed 7 days after the final immunization for the ELISPOT assay. (a) Serum IgG titres. The results are shown as mean anti-NAP and anti-CagA serum titres from 10 mice as measured by a standard colorimetric ELISA. (b) Anti-NAP and anti-CagA antibody-secreting cells (ASC) in spleen (SP) and mesenteric lymph nodes (MLN) as measured by the ELISPOT assay. The ASC data are means of pools of two subgroups of five mice (total of 10 mice per group) + SD of the two pools of five mice per subgroup. The results are representative of two independent experiments with 10 mice in each group with similar results. (c) Mean anti-NAP IgA end-point titres in pools of faecal extracts from 10 mice + SD of five mice per subgroup as measured by a chemiluminescence ELISA. The results are representative of two independent experiments with similar results.
To determine which route of recall immunization resulted in induction of mucosal responses we measured faecal IgA following i.n. or i.m. re-boost. We found that anti-NAP mucosal IgA responses in faecal pellets were only recalled if the mice were re-boosted i.n. (Fig. 5c). Only negligible anti-CagA faecal IgA responses were detectable in mice re-boosted i.n. or i.m. Taken together, these data show that following mucosal prime/systemic boost immunizations, the mucosal (i.n.) route of immunization is the preferred route of immunization for induction of recall humoral responses in both the mucosal and the systemic compartments.
Discussion
In this study we show that mucosal followed by parenteral immunization induces enhanced mucosal as well as systemic antibody responses. The mucosal/parenteral immunization strategy was superior to mucosal alone, parenteral alone or parenteral/mucosal routes of immunizations for the induction of systemic and mucosal antibody responses. We show this using two different mucosal routes, i.e. oral and i.n. Relatively few studies have investigated the parenteral followed by mucosal30–35 or mucosal followed by parenteral routes of immunization.32,36–41 Most of these studies used vaccinia virus or DNA for priming or boosting of animals immunized with protein, thus making comparisons with our study difficult. Interestingly, using hepatitis B surface antigen as a model protein it was found that although mucosal followed by parenteral or parenteral followed by mucosal immunizations induced higher serum antibody titres than mucosal or parenteral immunizations alone, there was no significant difference between priming mucosally or parenterally.32 However, we and others36 found that parenteral followed by mucosal was inferior to mucosal followed by parenteral routes of immunization for induction of immunity. These differences may be explained in terms of the differences between the immunogenicity of the various immunogens and adjuvants used in these studies.
Induction of mucosal responses through mucosal immunizations requires mucosal adjuvants or delivery systems. Mucosal adjuvants should ideally be non-toxic with the potential for application in human vaccines. The non-toxic mutant of the heat-labile toxin of E. coli used in this study has been successfully used as a mucosal adjuvant in several studies.42–47 There is evidence that for oral immunizations the adjuvant used should possess some ADP-ribosyltransferase activity, while for i.n. immunizations this may not be the case.42 Therefore, we chose LTR72, with reduced ADP-ribosyltransferase activity, for oral immunizations and LTK63, with no detectable ADP-ribosyltransferase activity, for i.n. immunizations.
Another important method of potentiation of immune responses through mucosal immunizations is through encapsulation of antigens in microparticles. In an effort to enhance the immune responses even further we encapsulated the NAP and CagA antigens in polylactide-co-glycotide(PLG) and found an enhancement of systemic and mucosal immune responses compared to soluble or alum-precipitated antigens, although the difference was not statistically significant (data not shown). The mechanism of enhancement of immune responses following encapsulation of antigens is not known but may be the result of protection against degradation in mucosal lumen and slow release of antigens over time.48
Presence of systemic and/or local H. pylori-specific antibody production has in some studies shown correlation with protection.8,15 We demonstrated that serum anti-NAP and anti-CagA responses are significantly enhanced following mucosal/parenteral immunizations compared to mucosal alone, parenteral alone, or parenteral/mucosal immunizations. Of note, splenic antibody-secreting cell responses were similar in mucosal/parenteral and parenteral/mucosal groups and both of these groups demonstrated significantly enhanced specific splenic antibody-secreting cells compared to mucosal alone or parenteral alone immunizations. These data underline the difference between these two systemic compartments as the serum antibodies, except from spleen, may be derived from several different compartments, including bone marrow.
A role of local IgA secretion has been suggested in protection against H. pylori in mice8 although other studies have shown protection from H. pylori challenge in the absence of local antibody or IgA.19,20 Our data show that the mucosal route of immunization, either alone or in combination with a parenteral route of immunization, selectively increased faecal IgA responses, whereas parenteral immunizations failed to induce faecal IgA responses. These data underlie the importance of mucosal immunization for the induction of mucosal responses and argue against a role of serum-derived antibodies in the faecal IgA responses.
There is increasing evidence that protection against H. pylori is mediated by CD4+ T cells.17,20 It appears that infection with H. pylori results in enhanced IFN-γ, and thus Th1-type, responses.21–23 These and other studies24–26 suggest that induction of a Th2-type response may reverse the course of infection and/or pathogenicity of H. pylori In this study, we found that our antigens, NAP and CagA, given through any routes or combination of routes induced a Th2-type response and thus may be able to counteract the Th1-type response induced during subsequent infection.
The goal of vaccination is to induce long-term protection against infection with pathogens. Although in most H. pylori vaccine studies the animals have been challenged in the acute phase of the immune response, a few studies have found long-term protection in mice after oral immunizations.49,50 In virus-related protection studies it has been demonstrated that local or mucosal immunization best affords protection a short51 or a long time after priming immunizations.52 Our data show that following establishment of immunity through mucosal/parenteral immunizations, only the mucosal route of re-boosting can induce both systemic and mucosal immune responses. In this regard, mucosal immunizations are also desired because they can be performed without the use of needles, thus eliminating the potential for transmission of various diseases through the re-use of contaminated needles.
Abbreviations
- CagA
cytotoxin-associated gene A
- i.m.
intramuscular
- i.n.
intranasal
- NAP
neutrophil-activating protein
References
- 1.Del Giudice G, Covacci A, Telford JL, Montecucco C, Rappuoli R. The design of vaccines against Helicobacter pylori and their development. Annu Rev Immunol. 2001;19:523–63. doi: 10.1146/annurev.immunol.19.1.523. [DOI] [PubMed] [Google Scholar]
- 2.Stein M, Rappuoli R, Covacci A. Tyrosine phosphorylation of the Helicobacter pylori CagA antigen after cag-driven host cell translocation. Proc Natl Acad Sci USA. 2000;97:1263–8. doi: 10.1073/pnas.97.3.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Satin B, Del Giudice G, Della Bianca V, et al. The neutrophil-activating protein (HP-NAP) of Helicobacter pylori is a protective antigen and a major virulence factor. J Exp Med. 2000;191:1467–76. doi: 10.1084/jem.191.9.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marchetti M, Arico B, Burroni D, Figura N, Rappuoli R, Ghiara P. Development of a mouse model of Helicobacter pylori infection that mimics human disease. Science. 1995;267:1655–8. doi: 10.1126/science.7886456. [DOI] [PubMed] [Google Scholar]
- 5.Marchetti M, Rossi M, Giannelli V, et al. Protection against Helicobacter pylori infection in mice by intragastric vaccination with H. pylori antigens is achieved using a non-toxic mutant of E. coli heat-labile enterotoxin (LT) as adjuvant. Vaccine. 1998;16:33–7. doi: 10.1016/s0264-410x(97)00153-9. [DOI] [PubMed] [Google Scholar]
- 6.Ghiara P, Rossi M, Marchetti M, et al. Therapeutic intragastric vaccination against Helicobacter pylori in mice eradicates an otherwise chronic infection and confers protection against reinfection. Infect Immun. 1997;65:4996–5002. doi: 10.1128/iai.65.12.4996-5002.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrero RL, Thiberge JM, Kansau I, Wuscher N, Huerre M, Labigne A. The GroES homolog of Helicobacter pylori confers protective immunity against mucosal infection in mice. Proc Natl Acad Sci USA. 1995;92:6499–503. doi: 10.1073/pnas.92.14.6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee CK, Weltzin R, Thomas WD, Jr, et al. Oral immunization with recombinant Helicobacter pylori urease induces secretory IgA antibodies and protects mice from challenge with Helicobacter felis. J Infect Dis. 1995;172:161–72. doi: 10.1093/infdis/172.1.161. [DOI] [PubMed] [Google Scholar]
- 9.Corthesy-Theulaz IE, Hopkins S, Bachmann D, et al. Mice are protected from Helicobacter pylori infection by nasal immunization with attenuated Salmonella typhimurium phoPc expressing urease A and B subunits. Infect Immun. 1998;66:581–6. doi: 10.1128/iai.66.2.581-586.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weltzin R, Kleanthous H, Guirakhoo F, Monath TP, Lee CK. Novel intranasal immunization techniques for antibody induction and protection of mice against gastric Helicobacter felis infection. Vaccine. 1997;15:370–6. doi: 10.1016/s0264-410x(97)00203-x. [DOI] [PubMed] [Google Scholar]
- 11.Kleanthous H, Myers GA, Georgakopoulos KM, et al. Rectal and intranasal immunizations with recombinant urease induce distinct local and serum immune responses in mice and protect against Helicobacter pylori infection. Infect Immun. 1998;66:2879–86. doi: 10.1128/iai.66.6.2879-2886.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gottwein JM, Blanchard TG, Targoni OS, et al. Protective anti-Helicobacter immunity is induced with aluminum hydroxide or complete Freund's adjuvant by systemic immunization. J Infect Dis. 2001;184:308–14. doi: 10.1086/322032. [DOI] [PubMed] [Google Scholar]
- 13.Guy B, Hessler C, Fourage S, Rokbi B, Millet MJ. Comparison between targeted and untargeted systemic immunizations with adjuvanted urease to cure Helicobacter pylori infection in mice. Vaccine. 1999;17:1130–5. doi: 10.1016/s0264-410x(98)00332-6. [DOI] [PubMed] [Google Scholar]
- 14.Guy B, Hessler C, Fourage S, Haensler J, Vialon-Lafay E, Rokbi B, Millet MJ. Systemic immunization with urease protects mice against Helicobacter pylori infection. Vaccine. 1998;16:850–6. doi: 10.1016/s0264-410x(97)00258-2. [DOI] [PubMed] [Google Scholar]
- 15.Ferrero RL, Thiberge JM, Labigne A. Local immunoglobulin G antibodies in the stomach may contribute to immunity against Helicobacter infection in mice. Gastroenterology. 1997;113:185–94. doi: 10.1016/s0016-5085(97)70094-5. [DOI] [PubMed] [Google Scholar]
- 16.Czinn SJ, Cai A, Nedrud JG. Protection of germ-free mice from infection by Helicobacter felis after active oral or passive IgA immunization. Vaccine. 1993;11:637–42. doi: 10.1016/0264-410x(93)90309-l. [DOI] [PubMed] [Google Scholar]
- 17.Michetti M, Kelly CP, Kraehenbuhl JP, Bouzourene H, Michetti P. Gastric mucosal alpha (4) beta (7)-integrin-positive CD4 T lymphocytes and immune protection against helicobacter infection in mice. Gastroenterology. 2000;119:109–18. doi: 10.1053/gast.2000.8548. [DOI] [PubMed] [Google Scholar]
- 18.Quiding-Jarbrink M, Ahlstedt I, Lindholm C, Johansson EL, Lonroth H. Homing commitment of lymphocytes activated in the human gastric and intestinal mucosa. Gut. 2001;49:519–25. doi: 10.1136/gut.49.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blanchard TG, Czinn SJ, Redline RW, Sigmund N, Harriman G, Nedrud JG. Antibody-independent protective mucosal immunity to gastric helicobacter infection in mice. Cell Immunol. 1999;191:74–80. doi: 10.1006/cimm.1998.1421. [DOI] [PubMed] [Google Scholar]
- 20.Ermak TH, Giannasca PJ, Nichols R, et al. Immunization of mice with urease vaccine affords protection against Helicobacter pylori infection in the absence of antibodies and is mediated by MHC class II-restricted responses. J Exp Med. 1998;188:2277–88. doi: 10.1084/jem.188.12.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohammadi M, Czinn S, Redline R, Nedrud J. Helicobacter-specific cell-mediated immune responses display a predominant Th1 phenotype and promote a delayed-type hypersensitivity response in the stomachs of mice. J Immunol. 1996;156:4729–38. [PubMed] [Google Scholar]
- 22.Mohammadi M, Nedrud J, Redline R, Lycke N, Czinn SJ. Murine CD4 T-cell response to Helicobacter infection. TH1 cells enhance gastritis and TH2 cells reduce bacterial load. Gastroenterology. 1997;113:1848–57. doi: 10.1016/s0016-5085(97)70004-0. [DOI] [PubMed] [Google Scholar]
- 23.Lindholm C, Quiding-Jarbrink M, Lonroth H, Hamlet A, Svennerholm AM. Local cytokine response in Helicobacter pylori-infected subjects. Infect Immun. 1998;66:5964–71. doi: 10.1128/iai.66.12.5964-5971.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saldinger PF, Porta N, Launois P, et al. Immunization of BALB/c mice with Helicobacter urease B induces a T helper 2 response absent in Helicobacter infection. Gastroenterology. 1998;115:891–7. doi: 10.1016/s0016-5085(98)70261-6. [DOI] [PubMed] [Google Scholar]
- 25.Berg EL, Mullowney AT, Andrew DP, Goldberg JE, Butcher EC. Complexity and differential expression of carbohydrate epitopes associated with l-selectin recognition of high endothelial venules. Am J Pathol. 1998;152:469–77. [PMC free article] [PubMed] [Google Scholar]
- 26.Smythies LE, Waites KB, Lindsey JR, Harris PR, Ghiara P, Smith PD. Helicobacter pylori-induced mucosal inflammation is Th1 mediated and exacerbated in IL-4, but not IFN-gamma, gene-deficient mice. J Immunol. 2000;165:1022–9. doi: 10.4049/jimmunol.165.2.1022. [DOI] [PubMed] [Google Scholar]
- 27.Gallichan WS, Rosenthal KL. Long-term immunity and protection against herpes simplex virus type 2 in the murine female genital tract after mucosal but not systemic immunization. J Infect Dis. 1998;177:1155–61. doi: 10.1086/515286. [DOI] [PubMed] [Google Scholar]
- 28.Giuliani MM, Del Giudice G, Giannelli V, Dougan G, Douce G, Rappuoli R, Pizza M. Mucosal adjuvanticity and immunogenicity of LTR72, a novel mutant of Escherichia coli heat-labile enterotoxin with partial knockout of ADP-ribosyltransferase activity. J Exp Med. 1998;187:1123–32. doi: 10.1084/jem.187.7.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ugozzoli M, O'Hagan DT, Ott GS. Intranasal immunization of mice with herpes simplex virus type 2 recombinant gD2: the effect of adjuvants on mucosal and serum antibody responses. Immunology. 1998;93:563–71. doi: 10.1046/j.1365-2567.1998.00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mantis NJ, Kozlowski PA, Mielcarz DW, Weissenhorn W, Neutra MR. Immunization of mice with recombinant gp41 in a systemic prime/mucosal boost protocol induces HIV-1-specific serum IgG and secretory IgA antibodies. Vaccine. 2001;19:3990–4001. doi: 10.1016/s0264-410x(01)00115-3. [DOI] [PubMed] [Google Scholar]
- 31.Kowalczyk DW, Wlazlo AP, Shane S, Ertl HC. Vaccine regimen for prevention of sexually transmitted infections with human papillomavirus type 16. Vaccine. 2001;19:3583–90. doi: 10.1016/s0264-410x(01)00070-6. [DOI] [PubMed] [Google Scholar]
- 32.McCluskie MJ, Weeratna RD, Payette PJ, Davis HL. Parenteral and mucosal prime-boost immunization strategies in mice with hepatitis B surface antigen and CpG DNA. FEMS Immunol Med Microbiol. 2002;32:179–85. doi: 10.1111/j.1574-695X.2002.tb00551.x. [DOI] [PubMed] [Google Scholar]
- 33.Marx PA, Compans RW, Gettie A, et al. Protection against vaginal SIV transmission with microencapsulated vaccine. Science. 1993;260:1323–7. doi: 10.1126/science.8493576. [DOI] [PubMed] [Google Scholar]
- 34.Israel ZR, Gettie A, Ishizaka ST, et al. Combined systemic and mucosal immunization with microsphere-encapsulated inactivated simian immunodeficiency virus elicits serum, vaginal, and tracheal antibody responses in female rhesus macaques. AIDS Res Hum Retroviruses. 1999;15:1121–36. doi: 10.1089/088922299310412. [DOI] [PubMed] [Google Scholar]
- 35.Moldoveanu Z, Vzorov AN, Huang WQ, Mestecky J, Compans RW. Induction of immune responses to SIV antigens by mucosally administered vaccines. AIDS Res Hum Retroviruses. 1999;15:1469–76. doi: 10.1089/088922299309982. [DOI] [PubMed] [Google Scholar]
- 36.Eo SK, Gierynska M, Kamar AA, Rouse BT. Prime-boost immunization with DNA vaccine. mucosal route of administration changes the rules. J Immunol. 2001;166:5473–9. doi: 10.4049/jimmunol.166.9.5473. [DOI] [PubMed] [Google Scholar]
- 37.Kanellos TS, Byarugaba DK, Russell PH, Howard CR, Partidos CD. Naked DNA when co-administered intranasally with heat-labile enterotoxin of Escherichia coli primes effectively for systemic B- and T-cell responses to the encoded antigen. Immunol Lett. 2000;74:215–20. doi: 10.1016/s0165-2478(00)00257-1. [DOI] [PubMed] [Google Scholar]
- 38.Bruhl P, Kerschbaum A, Eibl MM, Mannhalter JW. An experimental prime-boost regimen leading to HIV type 1-specific mucosal and systemic immunity in BALB/c mice. AIDS Res Hum Retroviruses. 1998;14:401–7. doi: 10.1089/aid.1998.14.401. [DOI] [PubMed] [Google Scholar]
- 39.Lee CK, Soike K, Giannasca P, Hill J, Weltzin R, Kleanthous H, Blanchard J, Monath TP. Immunization of rhesus monkeys with a mucosal prime, parenteral boost strategy protects against infection with Helicobacter pylori. Vaccine. 1999;17:3072–82. doi: 10.1016/s0264-410x(99)00144-9. [DOI] [PubMed] [Google Scholar]
- 40.Bergmeier LA, Mitchell EA, Hall G, Cranage MP, Cook N, Dennis M, Lehner T. Antibody-secreting cells specific for simian immunodeficiency virus antigens in lymphoid and mucosal tissues of immunized macaques. Aids. 1998;12:1139–47. doi: 10.1097/00002030-199810000-00005. [DOI] [PubMed] [Google Scholar]
- 41.Lehner T, Tao L, Panagiotidi C, et al. Mucosal model of genital immunization in male rhesus macaques with a recombinant simian immunodeficiency virus p27 antigen. J Virol. 1994;68:1624–32. doi: 10.1128/jvi.68.3.1624-1632.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neidleman JA, Vajdy M, Ugozzoli M, Ott G, O'Hagan D. Genetically detoxified mutants of heat-labile enterotoxin from Escherichia coli are effective adjuvants for induction of cytotoxic T cell responses against HIV-1 gag-p55. Immunology. 2000;101:154–60. doi: 10.1046/j.1365-2567.2000.00090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Partidos CD, Salani BF, Pizza M, Rappuoli R. Heat-labile enterotoxin of Escherichia coli and its site-directed mutant LTK63 enhance the proliferative and cytotoxic T-cell responses to intranasally co-immunized synthetic peptides. Immunol Lett. 1999;67:209–16. doi: 10.1016/s0165-2478(99)00013-9. [DOI] [PubMed] [Google Scholar]
- 44.Ryan EJ, McNeela E, Murphy GA, Stewart H, O'Hagan D, Pizza M, Rappuoli R, Mills KH. Mutants of Escherichia coli heat-labile toxin act as effective mucosal adjuvants for nasal delivery of an acellular pertussis vaccine: differential effects of the nontoxic AB complex and enzyme activity on Th1 and Th2 cells. Infect Immun. 1999;67:6270–80. doi: 10.1128/iai.67.12.6270-6280.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pizza M, Giuliani MM, Fontana MR, et al. Mucosal vaccines. Non toxic derivatives of LT and CT as mucosal adjuvants. Vaccine. 2001;19:2534–41. doi: 10.1016/s0264-410x(00)00553-3. [DOI] [PubMed] [Google Scholar]
- 46.Pizza M, Giuliani MM, Fontana M, et al. LTK63 and LTR72, two mucosal adjuvants ready for clinical trials. Int J Med Microbiol. 2000;290:455–61. doi: 10.1016/S1438-4221(00)80064-8. [DOI] [PubMed] [Google Scholar]
- 47.Beignon AS, Briand JP, Rappuoli R, Muller S, Partidos CD. The LTR72 mutant of heat-labile enterotoxin of Escherichia coli enhances the ability of peptide antigens to elicit CD4 (+) T cells and secrete gamma interferon after coapplication onto bare skin. Infect Immun. 2002;70:3012–9. doi: 10.1128/IAI.70.6.3012-3019.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vajdy M, O'Hagan DT. Microparticles for intranasal immunization. Adv Drug Deliv Rev. 2001;51:127–41. doi: 10.1016/s0169-409x(01)00167-3. [DOI] [PubMed] [Google Scholar]
- 49.Myers GA, Ermak TH, Georgakopoulos K, et al. Oral immunization with recombinant Helicobacter pylori urease confers long-lasting immunity against Helicobacter felis infection. Vaccine. 1999;17:1394–403. doi: 10.1016/s0264-410x(98)00387-9. [DOI] [PubMed] [Google Scholar]
- 50.Sutton P, Danon SJ, Walker M, Thompson LJ, Wilson J, Kosaka T, Lee A. Post-immunisation gastritis and Helicobacter infection in the mouse: a long term study. Gut. 2001;49:467–73. doi: 10.1136/gut.49.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vajdy M, Gardner J, Neidleman J, Cuadra L, Greer C, Perri S, O'Hagan D, Polo JM. Human immunodeficiency virus type 1 Gag-specific vaginal immunity and protection after local immunizations with sindbis virus-based replicon particles. J Infect Dis. 2001;184:1613–6. doi: 10.1086/324581. [DOI] [PubMed] [Google Scholar]
- 52.Gallichan W, Rosenthal K. Long-lived cytotoxic T lymphocyte memory in mucosal tissues after mucosal but not systemic immunization. J Exp Med. 1996;184:1879–90. doi: 10.1084/jem.184.5.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]