Abstract
Sh-CRIT-ed1 is a potent anti-complement peptide that inhibits the classical complement-activation pathway by interfering with the formation of the C3-convertase complex, C4b2a. C2 is an essential serum glycoprotein that provides the catalytic subunit of the C3 and C5 convertases of the classical pathways of complement activation. Because only in its C4-bound state is C2a capable of cleaving its physiological protein substrates C3 and C5, the interaction of Sh-CRIT-ed1 with C2 plays a decisive role of inhibition in the classical complement-activation process. However, the role of individual Sh-CRIT-ed1 amino acid residues in C2 binding is not fully understood. We constructed nine recombinant Sh-CRIT-ed1 (rSh1) analogues, substituted at conserved residues, and evaluated their anti-complement and C2-binding activities. Results from glutathione S-transferase (GST) pull-down and haemolytic assays suggested that residues 10K, 17E, 19K and 26Y are critical for the interaction of rSh1 with C2. We then constructed an improved anti-complement peptide by duplicating Sh-CRIT-ed1 C-terminal motifs (17H–26Y). This linear homodimer (rH17d) was more potent than rSh1 with respect to binding to C2 and anti-complement activity (the 50% inhibitory concentration value was ≈1·2 µm versus ≈6·02 µm for rSh1). Furthermore, rH17d showed higher anti-complement activity in vivo, providing additional evidence that this duplication is a more effective inhibitor of complement activation than rSh1. Taken together, these results identify four key residues in rSh1 and strongly suggest that rH17d is a potent inhibitor of complement activation that may have therapeutic applications.
Introduction
Complement activation is essential for immune defence against pathogens. However, uncontrolled complement activation may cause severe inflammatory tissue destruction in situations such as autoimmune disease,1 acute respiratory distress syndrome,2 Alzheimer's disease,3,4 stroke,5 heart attack,6 burns7 and reperfusion injuries.8,9 Therefore, there is a pressing need for therapeutically effective complement inhibitors.10 A number of such inhibitors are currently being tested, including recombinant forms of naturally occurring inhibitors, antibodies against complement components, inhibitors of serine proteases, and anaphylatoxin analogues.11–13 Recently, a relevant membrane protein (Sh-CRIT) was discovered on the plasma membrane of the Schistosoma parasite, the causative agent for bilharzia, a tropical disease that infects ≈200 million people worldwide.14 Sh-CRIT-ed1, a 26-residue fragment from the N-terminal extracellular domain of Sh-CRIT, binds C2 via the C2a segment. C2 is an essential serum glycoprotein that provides the catalytic subunit of the C3 and C5 convertases of the classical pathways of complement activation. Because only in its C4-bound state is C2a capable of cleaving its physiological protein substrates C3 and C5, the interaction of Sh-CRIT-ed1 with C2 plays a decisive role of inhibition in the classical complement-activation process. Sh-CRIT-ed1 inhibits the classical pathway of complement activation by interfering with the formation of the C3 convertase complex, C4b2a.15 Sh-CRIT-ed1 exhibits 42% similarity to the β-chain of C4b,16 suggesting that this peptide may serve as a potent anti-complement drug. Indeed, previous studies demonstrate that recombinant Sh-CRIT-ed1 (rSh1) is an attractive anti-complement drug candidate,17 although little is known about the relative importance of residues within the peptide. In the present study, rSh1, nine different rSh1 analogues and rH17d [a linear homodimer constructed by duplicating the C-terminal motif (17H–26Y) of Sh-CRIT-ed1], were tested for anti-complement and C2-binding activities using haemolytic and GST pull-down assays. Four residues that are required for C2 binding and inhibition of complement activation were identified. In addition, rSh1 and rH17d were tested for inhibition of complement haemolysis in vivo, and increased inhibition by rH17d relative to rSh1 suggest that rH17d may represent a candidate investigatory drug for inhibiting complement activation.
Materials and methods
Construction and purification of rSh1, rSh1 analogues and rH17d
The glutathione S-transferase (GST) fusion protein system (pGEX-KG; Amersham Pharmacia Biotech, Uppsala, Sweden) was used to produce large quantities of rSh1 and its analogues in Escherichia coli. This system is often used to stably express small peptides and protect them against degradation.18–20 For production of the recombinant peptides, 10 pairs of different oligonucleotides (Fig. 1) were synthesized, as previously described,17 and individually phosphorylated with T4 polynucleotide kinase in kinase buffer containing 0·5 m Tris–HCl (pH 7·5), 0·1 m MgCl2 and 50 mm dithiothreitol (DTT). After incubation at 37° for 2 hr, the oligonucleotides were mixed in an annealing buffer containing 50 mm NaCl, 1 mm EDTA (pH 8·0) and 10 mm Tris–HCl. The mixture was boiled for 3 min and cooled slowly to room temperature. The annealed DNA was cloned into the pGEX-KG expression vector, which lends itself to purification and thrombin cleavage.21 Precultured transformed E. coli (BL21) were inoculated (1 : 50) into Luria–Bertani (LB) broth containing ampicillin and 0·4% glucose and incubated at 37° until the absorbance (A) at 600 nm reached 0·5–0·6. Recombinant peptide expression was then induced with 0·4 mm isopropyl-β-thiogalactopyranoside (IPTG) and the cells were incubated for a further 4 hr (A600 = 1·7). Cells were then centrifuged (5000 g at 4°) resuspended in a 1 : 20 volume of 100 mm ammonium bicarbonate (pH 8·0) and lysed by four sonications of 30-seconds each (Labsonic U.B.; Braun Biotech International GmbH, Melsungen, Germany). The lysate was centrifuged (15 000 g, 4°, 20 min) to remove the insoluble fraction. The supernatant was incubated with glutathione–Sepharose 4B resin (Amersham Pharmacia Biotech) with gentle mixing for 30 min at room temperature, followed by centrifugation at 500 g for 3 min. The peptide-bound resin was washed four times with 100 mm ammonium bicarbonate buffer to remove unbound proteins, and the peptide was eluted by cleavage with 0·04 U of thrombin in 20 mm Tris–HCl (pH 8·4), containing 150 mm NaCl and, 2·5 mm CaCl2, at 22° for 22 hr. The flow-through was filtered by using a 0·45-µm syringe filter (Corning NY 14831; Corning, GmbH & Co. KG, Neustadt, Germany) to separate the desired peptide from the thrombin. Recombinant (r)GST-Sh1 and its analogues (for the GST pull-down assays) were obtained by washing the bound resin with glutathione elution buffer (20 mm reduced glutathione in 50 mm Tris–HCl, pH 8·0) before thrombin proteolysis.
Figure 1.
Sequence of the primers used to generate the mutant forms of Sh-CRIT-ed1.
GST pull-down assay
GST pull-down assays were performed by using a modification of the method of Lee et al.22 Briefly, aliquots (500 µl) of normal human serum (NHS) (diluted 1 : 10 in 20 mm Tris–HCl, pH 8·0) were mixed with a constant amount of glutathione-Sepharose 4B resin preloaded with 1, 4 or 10 µg of rGST-Sh1, its analogues or control GST. After four washes with a solution containing 20 mm Tris–HCl (pH 8·0), 1 mm EDTA and 0·1 m NaCl, the bound proteins were eluted by cleavage with 0·04 U of thrombin. The eluted proteins were subjected to sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) on a 10% gel and detected by Western blotting using the enhanced chemiluminescence (ECL) kit (Amersham, Arlington Heights, IL) with monoclonal antibody (mAb) HYB50-5 against C2 (Antibodyshop, c/o Statens Serum Institute, Copenhagen, Denmark) and anti-mouse immunoglobulin G (IgG) secondary antibody-linked horseradish peroxidase (Amersham Pharmacia Biotech).
Haemolytic assay for anti-complement activity
Inhibition of complement pathway-based haemolysis was determined by using a modification of the method of Kweon et al.23 Different concentration ranges (0·1 to ≈20 µm) of rSh1 and its analogues were added to 50 µl of freshly prepared gelatin veronal buffer (GVB2+, pH 7·4) and preincubated with 100 µl of NHS (diluted 1 : 40 in GVB2+) for 30 min at 37°. Residual haemolytic activity was measured by the addition of 50 µl of immunoglobulin M (IgM)-sensitized sheep erythrocytes (2 × 108 cells/ml; Nippon Biotest Laboratory, Tokyo, Japan). As a control, NHS was incubated with GVB2+ in the absence of peptide. The degree of complement haemolysis (amount of released haemoglobin) was measured in the supernatant at 405 nm on a microtitre plate reader (Bio-Rad, Hercules, CA). After correcting for background, the degree of specific lysis was converted to Z units, as follows:
which is proportional to the number of haemolytically effective molecules per erythrocyte. Activity comparisons were based on Z units/µg of rSh1 or rSH1 analogue.
Forssman systemic shock
To determine the in vivo anti-complement activity of rSh1 and the linear homodimer, rH17d, Forssman systemic shock was provoked in male guinea-pigs (250–300 g; Japan SLC, Hartley, Japan) in the presence or absence of the peptides, and prolongation in time-to-survival was measured. Forssman shock was provoked by intravenous injection of haemolysin (α-haemolysin from Staphylococcus aureus;24 Sigma, St Louis, MO) by using a modification of the method of Ueda et al.25 All experimental procedures were carried out in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals. Guinea-pigs were sedated with intramuscular ketamine (80 mg/kg) and anaesthetized with 1% halothane using an anaesthesia machine to connect the cannula in the vein of the leg. Haemolysin and inhibitor were administered in the jugular vein as a bolus. Haemolysin (80 µg/kg) was injected first, followed by the inhibitor, and a stopwatch was used to measure the length of survival prolongation. Animals were judged to be dead when their heart had stopped beating. In order to introduce the appropriate shock in animals within several minutes, a suitable haemolysin dose was determined before the experiments. Test compounds (rSh1 and rH17d; the doses had been determined by preliminary experiments) were injected intravenously into animals just prior to the injection of haemolysin. The survival time was continuously monitored.
Results
rSh1 inhibits complement haemolysis and binds C2
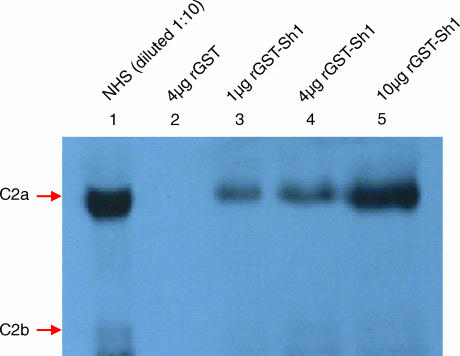
We previously reported that 10 µm rSh1 has 83·2% anti-complement activity in the haemolytic assay and that the 50% inhibitory concentration (IC50) for rSh1 is 6·02 µm. To address C2 binding, a GST pull-down assay was conducted with NHS and purified recombinant rGST-Sh1 bound to glutathione–Sepharose. rGST-Sh1 interacted with C2, as demonstrated by Western blot analysis using the mAb HYB50-5 against C2 (Fig. 2). GST alone did not bind C2.
Figure 2.
Interaction of C2 protein with recombinant glutathione S-transferase-Sh1 (rGST-Sh1) in vitro. Western blot showing C2 that was bound to GST fusion proteins retained on the glutathione–Sepharose resin. Normal human serum (NHS; 10-fold dilution) was loaded in lane 1.
Construction of rSh1 analogues, and their anti-complement activities
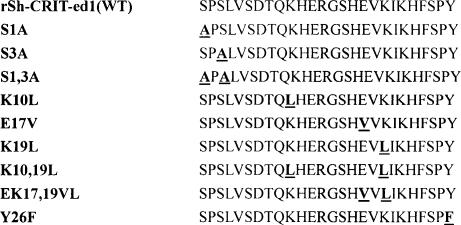
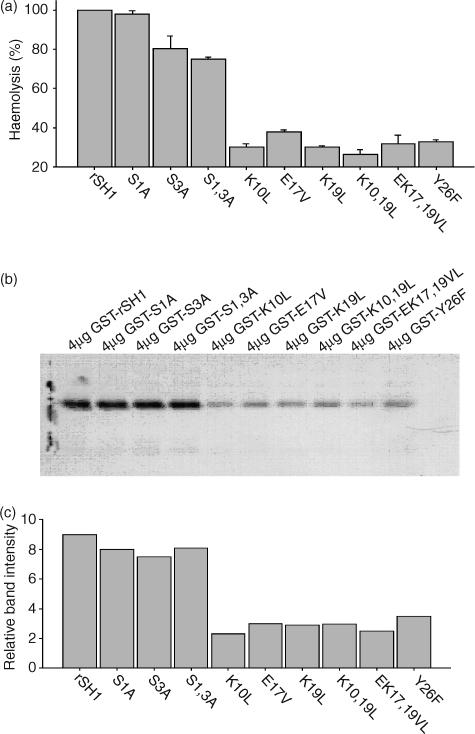
Sequence comparison between rSH1 sequences and human and mouse C4 β-chains revealed that rSH1 residues 1S, 3S, 10K, 17E, 19K and 26Y are conserved (Fig. 3). To determine the importance of each of these residues with regard to the rSh1–C2 interaction, analogous residues were substituted individually or in combination (Fig. 4). These rSH1 analogues were expressed in E. coli at levels similar to rSH1 (≈5–10 mg/l of culture). The relative anti-complement activity of these analogues was assessed by the complement haemolysis assay averaged across triplicate samples (Fig. 5a). Analogues in which 1S and 3S were replaced with alanine (S1A, S3A, S1,3A) retained significant anti-complement activity. However, substitutions at other positions (including mutants K10L, E17V, K19L, K10,19L, EK17,19VL, Y26F) resulted in substantially decreased activity. To confirm that the anti-haemolytic activity of the analogues was the result of inhibition of C3 convertase formation, a GST pull-down assay was performed. The ability of rSH1 and its analogues to bind C2 was compared quantitatively by Western blot (Fig. 5b). Relative band intensities indicated a striking similarity between C2-binding activity (Fig. 5c) and anti-complement activity (Fig. 5a).
Figure 3.
Alignment of rSh1 and rH17d with the equivalent regions in human (hu), mouse (mo) and carp C4 proteins. Important consensus residues in the sequence are shown in boxes. rSh1 residues substituted in mutagenesis experiments are shown in red. Dashes indicate gaps introduced into the sequence to facilitate alignment.
Figure 4.
Nomenclature and amino acid sequence of 1–26 segment analogues examined in this study.
Figure 5.
Haemolytic assay of complement inhibition by rSh1 analogues, and the C2-binding assay. (a) Comparison of the haemolytic activities of recombinant wild-type (rSh1) and rSh1 analogues substituted at various conserved residues. Results are expressed as a percentage of wild-type haemolytic activity. (b) Western blot showing C2 that was bound to glutathione S-transferase (GST)–analogue fusions retained on glutathione–Sepharose. (c) Quantitative PhosphorImager analysis of gels shown in (b). The relative band intensity was determined by quantifying the optical density at 595 nm in each gel lane (Gel-Pro v3·1; MediaCybernetics, Carlsbad, CA).
rH17 homodimer inhibits haemolysis and binds C2
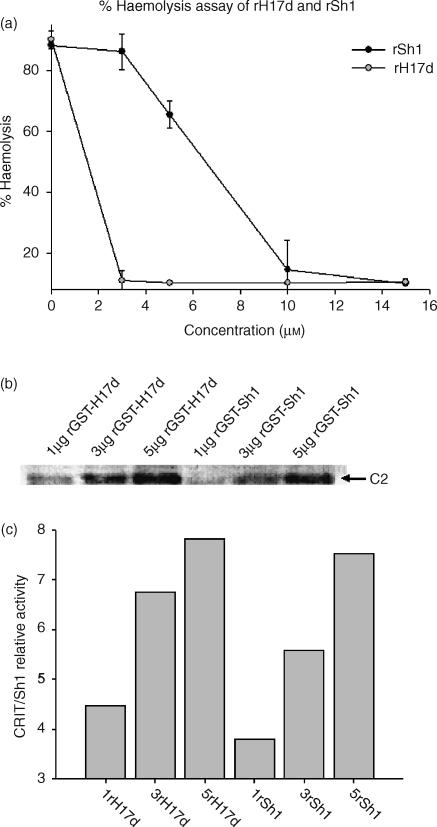
Although the CRIT-H17 motif occurs only once within Sh-CRIT-ed1, it has been found to act as a site of dimerization in many other receptors. To investigate the role of dimerization of the CRIT-H17 motif in haemolysis, the C-terminal 11 amino acids of Sh-CRIT-ed1 (with homology to the C4 α-chain CRIT-ed1 domain; see refs 26, 27) were allowed to duplicate. This homodimer was denoted rH17d. Both rSh1 and rH17d inhibited complement activation in a dose-dependent manner, although rH17d showed a fivefold higher anti-complement activity (IC50 = ≈1·2 µm versus ≈6·02 µm for rSh1; Fig. 6a). In addition, a GST pull-down assay showed that rH17d bound C2 much more strongly than did rSh1 (Fig. 6b, 6c), providing further evidence that rH17d inhibited complement activation more effectively than rSh1.
Figure 6.
Haemolytic assay of complement inhibition by linear homodimer rH17d, and C2 binding by rH17d. (a) Comparison of inhibition of complement activation by rSh1 and rH17d across a range of concentrations. The percentage of lysis is based on the degree of sheep red blood cell (SRBC) lysis, evaluated by quantification of haemoglobin release, as measured by A405. (b) C2 binding by recombinant glutathione S-transferase (rGST)-Sh1 and rGST-H17d. Western blot analysis showing C2 that was retained on the glutathione–Sepharose resin in the presence of varying amounts of rGST-Sh1 and rGST-H17d. (c). Quantitative PhosphorImager analysis of gels shown in (b). The relative band intensity was determined by quantifying the optical density at 595 nm in each gel lane (Gel-Pro v3·1, MediaCybernetics, Carlsbad, CA).
Forssman systemic shock
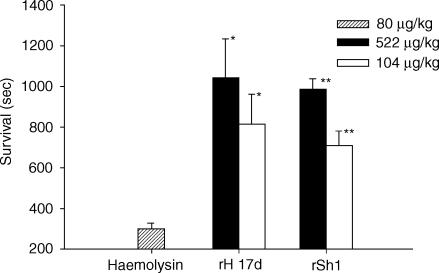
To determine the anti-complement activities of rSh1 and rH17d in vivo, Forssman systemic shock was induced in guinea pigs, and survival time was measured following treatment with rSh1 or rH17d (Fig. 7). The optimal shock-inducing dose of haemolysin was determined to be 80 µg/kg, and rSh1 and rH17d significantly inhibited Forssman systemic shock at 104 µg/kg (P < 0·005) and 522 µg/kg (P < 0·001), respectively. Animals treated with rH17d or rSh1 (522 µg/kg) lived 3·5 times and three times longer, respectively, than those treated with haemolysin alone. These results suggest that rH17d is a more effective inhibitor of death than rSh1 under these conditions. The difference between rH17d and rSh1, with respect to the delay of death, occurred at all doses tested (data not shown).
Figure 7.
Effects of rSh1 and rH17d on survival time during Forssman systemic shock. rSh1 and rH17d were intravenously injected into guinea pigs (n = 3) just prior to the induction of Forssman systemic shock. The results show the mean values ± standard deviation (SD) of three independent experiments. Significant differences from the control group were determined using the sum test (*P < 0·005, **P < 0·001).
Discussion
The rH17 homodimer, rSh1 and nine rSh1 analogues substituted at one or more conserved residues were produced as soluble peptides at high yields (≈5–10 mg/l of culture). These peptides were evaluated for their anti-complement activities, both in vitro and in vivo. A GST pull-down assay confirmed that rSh1 and some of its analogues bound C2 as well as the native protein, Sh-CRIT, suggesting that these recombinant peptides may inhibit the classical complement-activation pathway by interfering with the formation of the complement C3 convertase, C4b2a. However, six substituted analogues (K10L, E17V, K19L, K10,19L, EK17,19VL and Y26F) showed three- to fourfold less C2 binding than rSh1, clearly indicating that residues 10K, 17E, 19K and 26Y are important in binding C2. In addition, double mutations at these key residues significantly reduced C2 binding (Fig. 5). These results are in good agreement with recent work16 demonstrating that an 11-residue peptide (H17 – Y27) within the C2-binding ed1 region, along with the K10 residue, constitutes the ligand-binding site. These residues are normally found at the binding interface (e.g. lysine or phenylalanine)28 or at binding hotspots (tryptophan, tyrosine or arginine)29 of proteins. Taken together, these results indicate that residues 10K, 17E, 19K and 26Y are important for the interaction between Sh-CRIT-ed1 and C2 for activation of the classical complement pathway. Using this information, we developed an anti-complement peptide homodimer with characteristics superior to those of rSh1. The rH17d homodimer contained the C-terminal 11 residues of Sh-CRIT-ed1 (H17 – Y27). This homodimer not only maintained the required 10K, 17E, 19K and 26Y residues, but also matched the β-turn structure of human C4β, which is known to be involved in intermolecular interactions. In the complement haemolytic assay, rH17d was nearly fivefold more effective than rSh1 at inhibiting haemolysis (Fig. 6a). Interestingly, rH17d was also more effective than rSh1 with respect to C2 binding (Fig. 6b, 6c). Furthermore, during the in vivo Forssman systemic shock assay in guinea-pigs, rH17d promoted survival to a greater extent than rSh1 (Fig. 7), confirming the increased inhibitory effects of this homodimer.
Further work will be required to evaluate the exact role of rH17d as an anti-complement drug candidate and to understand its mode of action. Although the CRIT-H17 motif occurs only once within Sh-CRIT-ed1, two CRIT-H17 motifs may be presented for ligand binding within other receptors.16 In the case of peptides that compete with thrombopoietin for binding to its receptor, the peptide dimer stimulates in vitro proliferation and promotes a greater increase in platelet count in normal mice relative to the peptide monomer.30 In the case of erythropoietin, a peptide dimer induces an almost perfect twofold dimerization of the receptor.31 Hence, in these cases, peptide dimers may interact more favourably with their target proteins in a manner similar to that of rH17d. The results of three independent approaches, namely the haemolytic assay, C2 interaction assay and in vivo assay of Forssman systemic shock, all strongly suggest that rH17d is a more potent complement inhibitor than rSh1, indicating that rH17d may be an attractive candidate anti-complement drug. Furthermore, because rH17d is selective for the classical complement pathway, this homodimer may allow the complement system to function in a therapeutically relevant manner, thus maintaining the ability to defend against pathogens via an alternative pathway.10
Acknowledgments
This research was supported by the Brain Korea 21 project funded by the Korean Ministry of Education. In vivo data were provided by the Kolon Central Research Institute (KCRI).
References
- 1.Kalli KR, Hsu P, Fearon DT. Therapeutic uses of recombinant complement protein inhibitors. Springer Semin Immunopathol. 1994;15:417. doi: 10.1007/BF01837368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robbins RA, Russ WD, Rasmussen JK, Clayton MM. Activation of the complement system in the adult respiratory distress syndrome. Am Rev Respir Dis. 1987;135:651. doi: 10.1164/arrd.1987.135.3.651. [DOI] [PubMed] [Google Scholar]
- 3.Bradt BM, Kolb WP, Cooper NR. Complement-dependent proinflammatory properties of the Alzheimer's disease β-peptide. J Exp Med. 1998;188:431. doi: 10.1084/jem.188.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rogers J, Cooper NR, Webster S, et al. Complement activation by beta-amyloid in Alzheimer-disease. Proc Natl Acad Sci USA. 1992;89:10016. doi: 10.1073/pnas.89.21.10016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bishop MJ, Giclas PC, Guidotti SM, Su ML, Chi EY. Complement activation is a secondary rather than a causative factor in rabbit pulmonary artery ischemia/reperfusion injury. Am Rev Respir Dis. 1991;143:386. doi: 10.1164/ajrccm/143.2.386. [DOI] [PubMed] [Google Scholar]
- 6.Kilgore KS, Friedrichs GS, Homeister JW, Lucchesi BR. The complement system in myocardial ischaemia/reperfusion injury. Cardiovasc Res. 1994;28:437. doi: 10.1093/cvr/28.4.437. [DOI] [PubMed] [Google Scholar]
- 7.Gallinaro R, Cheadle WG, Applegate K, Polk HC., Jr The role of the complement system in trauma and infection. Surg Gynecol Obstet. 1992;174:435. [PubMed] [Google Scholar]
- 8.Beranek JT. Terminal complement-complex in myocardial reperfusion injury. Cardiovasc Res. 1997;33:495. doi: 10.1016/s0008-6363(96)00251-9. [DOI] [PubMed] [Google Scholar]
- 9.Weiser MR, Williams JP, Moore FD, Kobzik L, Ma M, Hechtmant B, Carroll MC. Reperfusion injury of ischemic skeletal muscle is mediated by natural antibody and complement. J Exp Med. 1996;183:2343. doi: 10.1084/jem.183.5.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sahul A, Lambris JD. Complement inhibitors: a resurgent concept in anti-inflammatory therapeutics. Immunopharmacology. 2000;49:133. doi: 10.1016/s0162-3109(00)80299-4. [DOI] [PubMed] [Google Scholar]
- 11.Ember JA, Hugli TE. Complement factors and their receptors. Immunopharmacology. 1997;38:3. doi: 10.1016/s0162-3109(97)00088-x. [DOI] [PubMed] [Google Scholar]
- 12.Kirschfink M. Controlling the complement system in inflammation. Immunopharmacology. 1997;38:51. doi: 10.1016/s0162-3109(97)00057-x. [DOI] [PubMed] [Google Scholar]
- 13.Makrides SC. Therapeutic inhibition of the complement system. Pharmacol Rev. 1998;50:87. [PubMed] [Google Scholar]
- 14.Inal JM. Schistosoma TOR (trispanning orphan receptor), a novel, antigenic surface receptor of the blood-dwelling, Schistosoma parasite. Biochim Biophys Acta. 1999;1445:283. doi: 10.1016/s0167-4781(99)00051-2. [DOI] [PubMed] [Google Scholar]
- 15.Inal JM, Sim RB. A Schistosoma protein, Sh-CRIT, is a novel inhibitor of complement which binds human C2. FEBS Lett. 2000;470:131. doi: 10.1016/s0014-5793(00)01304-1. [DOI] [PubMed] [Google Scholar]
- 16.Inal JM, Schifferli JA. Complement C2 receptor inhibitor trispanning and the β-chain of C4 share a binding site for complement C2. J Immunol. 2002;168:5213. doi: 10.4049/jimmunol.168.10.5213. [DOI] [PubMed] [Google Scholar]
- 17.Oh KS, Na DK, Kweon MH, Sung HC. Expression and purification of the anticomplementary peptide Sh-CRIT-ed1 (formerly Sh-TOR-ed1) as a tetramultimer in Escherichia coli. Protein Exp Purif. 2003;27:202. doi: 10.1016/s1046-5928(02)00598-3. [DOI] [PubMed] [Google Scholar]
- 18.Kuliopulos A, Walsh CT. Production, purification and cleavage of tandem repeats of recombinant peptides. J Am Chem Soc. 1994;116:4599. [Google Scholar]
- 19.Lewis RV, Hinman M, Kothakota S, Fournier MJ. Expression and purification of a spider silk protein: a new strategy for producing repetitive proteins. Protein Exp Purif. 1996;7:400. doi: 10.1006/prep.1996.0060. [DOI] [PubMed] [Google Scholar]
- 20.Oh KS, Park YS, Sung HC. Expression and purification of an ACE-inhibitory peptide multimer from synthetic DNA in Escherichia coli. J. Microbiol Biotechnol. 2002;12:59. [Google Scholar]
- 21.Guan KL, Dixon JE. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with Glutathione S-Transferase. Anal Biochem. 1991;192:262. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
- 22.Lee KY, Broker TR, Chow LT. Transcription factor YY1 represses cell-free replication from human papillomavirus origins. J Virol. 1998;72:4911. doi: 10.1128/jvi.72.6.4911-4917.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kweon MH, Lim WJ, Yang HC, Sung HC. Characterization of two glucans activating an alternative complement pathway from the fruiting bodies of mushroom Pleurotus ostreatus. J Microbiol Biotechnol. 2000;10:267. [Google Scholar]
- 24.Gemmell CG, Peterson PK, Townsend K, Quie PG, Kin Y. Biological effects of the interaction of staphylococcal alpha-toxin with human serum. Infect Immun. 1982;38:981. doi: 10.1128/iai.38.3.981-985.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ueda N, Midorikawa A, Ino Y, Oda M, Nakamura K, Suzuki S, Kurumi M. Inhibitory effects of newly synthesized active center-directed trypsin-like serine protease inhibitors on the complement system. Inflamm Res. 2000;49:42. doi: 10.1007/PL00000202. [DOI] [PubMed] [Google Scholar]
- 26.Oglesby TJ, Accavitti MA, Volanakis JE. Evidence for a C4b binding site on the C2b domain of C2. J Immunol. 1988;141:926. [PubMed] [Google Scholar]
- 27.Xu Y, Volanakis JE. Contribution of the complement control protein modules of C2 in C4b binding assessed by analysis of C2/factor B chimeras. J Immunol. 1997;158:5958. [PubMed] [Google Scholar]
- 28.Tsai CJ, Lin SL, Wolfson HJ, Nussinov R. Protein–protein interfaces: a statistical analysis of the hydrophobic effect. Protein Sci. 1997;6:53. doi: 10.1002/pro.5560060106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bogan AA, Thorn KS. Anatomy of hot spots in protein interfaces. J Mol Biol. 1998;280:1. doi: 10.1006/jmbi.1998.1843. [DOI] [PubMed] [Google Scholar]
- 30.Cwirla SE, Balasubramanian P, Duffin DJ, et al. Peptide agonist of the thrombopoietin receptor as potent as the natural cytokine. Science. 1997;276:1696. doi: 10.1126/science.276.5319.1696. [DOI] [PubMed] [Google Scholar]
- 31.Livnah O, Stura EA, Johnson DL, et al. Functional mimicry of a protein hormone by a peptide agonist: the EPO receptor complex at 2.8. Science. 1996;273:464. doi: 10.1126/science.273.5274.464. [DOI] [PubMed] [Google Scholar]