Abstract
Dendritic cells (DC) can be derived from monocytes in vitro by culture with granulocyte–macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4). It is unknown whether this regimen reflects DC differentiation from blood precursors under physiological conditions. Induction of DC development from monocytes by interferon-α (IFN-α) may occur in vivo during infection or inflammation and thus may represent a more physiological approach to DC differentiation in vitro. Here, we show that incubation of GM-CSF-cultured monocytes with IFN-α does not induce DC differentiation: cells maintain their original phenotype and cytokine secretion pattern. Even after stimulation with pro-inflammatory or T-cell-derived activation signals, IFN-α-treated monocytes do not develop DC characteristics. Addition of IL-4 during stimulation of IFN-α-treated monocytes results in the rapid development of DC-like cells expressing co-stimulatory molecules, CD83 and chemokine receptor CCR7, indicating that some degree of developmental plasticity is preserved. However, DC pre-activated with IFN-α are less effective in inducing allogeneic or antigen-specific autologous T-cell proliferation, produce less IL-12 and express lower levels of CCR7 compared to DC generated by culture with GM-CSF and IL-4. Incubating GM-CSF-cultured monocytes simultaneously with IFN-α and IL-4 does not affect phenotypic maturation of DC, but reduces IL-12 production upon pro-inflammatory activation. We conclude that: (1) IFN-α fails to induce DC differentiation and thus cannot replace IL-4 in generating DC from monocytes in vitro; and (2) the presence of IFN-α prior to or during differentiation of DC from monocyte precursors alters their response to maturation stimuli and may affect their capacity to stimulate T helper type 1 immune responses in vivo.
Introduction
Dendritic cells (DC) are highly specialized antigen-presenting cells (APC) playing a key role in the induction and regulation of immune responses.1 Immature DC reside in peripheral tissues where they take up and process antigen for presentation on major histocompatibility complex (MHC) molecules. Pathogen-derived components or pro-inflammatory cytokines produced at sites of infection or inflammation induce DC maturation. During the maturation process, DC alter the pattern of chemokine receptor expression thus enabling them to migrate to secondary lymphoid organs where they home to T-cell zones to initiate antigen-specific immune responses.2,3 Mature DC express high levels of co-stimulatory molecules and secrete interleukin-12 (IL-12), a key cytokine in the activation of both the innate and the adaptive immune system.4
Though it has been reported that DC maturation from monocytes can be induced by transendothelial trafficking,5 the mechanisms involved in DC differentiation from monocytes under physiological conditions are not fully understood. In vitro, DC can be derived from monocytes or CD34+ progenitor cells.6,7 Culture of monocytes for 5–7 days with granulocyte–macrophage colony-stimulating factor (GM-CSF) and IL-4 induces the development of immature DC that have to be stimulated with microbial, pro-inflammatory or T-cell-derived stimuli to develop full T-cell-stimulatory capacity.8 Whether this regimen reflects an in vivo process is unclear and it has even been suggested that IL-4-treatment may result in the generation of ‘disabled’ DC as a result of interference of IL-4 with the prostaglandin metabolism.9
Type I interferons (IFNs; interferon-α and interferon-β) are not only key mediators of the innate immune system,10 but also influence proliferation, functions and survival of different T-cell subsets11,12 and regulate IL-12 synthesis by professional APC.13–17 More than 18 genes encoding for IFN-α are known while there is only a single IFN-β gene.18 Recently, a subset of peripheral blood DC capable of producing large amounts of type I IFN after microbial stimulation, so-called ‘natural IFN-producing cells’, has been identified.19 The capability of these cells, also called ‘plasmacytoid DC’, to promote T helper cell type 1 (Th1) immune responses hints at a role of type I IFNs as linking elements of the innate and the adaptive immune system.20
Conflicting experimental data on the effects of type I IFNs on APC differentiation from monocyte-precursors have been reported. While some of the data indicate that type I IFN-induced monocyte-derived DC are potent stimulators of Th1 type immune responses in vitro and in vivo,21–24 others have reported inhibitory effects of type I IFNs on DC differentiation and IL-12 production.25,26 These divergent findings are paralelled by therapeutic effects of type I IFNs in such diverse disease entities as viral infections, malignant diseases, or multiple sclerosis.27–29 Although the exact mechanisms involved in clinical responses to IFN-treatment are not completely defined, there is increasing evidence that type I IFNs influence APC function in vivo. Blanco et al. have recently shown that DC differentiation from monocytes is induced in patients with systemic lupus erythematosus dependent on the level of IFN-α serum concentration.30
We were interested to discover whether incubation of GM-CSF-cultured monocytes with IFN-α could induce DC development and how pretreatment with IFN-α influenced the response of monocytes to different DC maturation stimuli. To this end, IFN-α-activated monocytes were compared to immature DC obtained by culture of monocytes with GM-CSF and IL-4 for expression of maturation markers and lymph-node-directing chemokine receptor CCR7, and capacity to activate allogeneic and autologous T cells, as well as cytokine secretion upon stimulation with pro-inflammatory and T-cell-derived activation signals.
Materials and methods
Reagents
The recombinant cytokines were obtained as follows: GM-CSF was purchased from Novartis (Basel, Switzerland), IL-4 from Promega (Madison, WI), tumour necrosis factor-α (TNF-α) from R & D Systems (Wiesbaden, Germany) and IL-6 from Amersham (Buckinghamshire, UK); IFN-α, IFN-γ, and IL-1β were obtained from Strathmann Biotech (Hannover, Germany). Prostaglandin E2 (PGE2) and fluorescein isothiocyanate (FITC) –dextran were purchased from Sigma-Aldrich (Steinheim, Germany), [3H]thymidine was supplied by Amersham Buchler (Freiburg, Germany).
Cell culture
All cultures of human peripheral blood mononuclear cells (PBMC) were maintained in RPMI-1640 medium (Biochrom, Berlin, Germany) supplemented with 2% human AB serum (BioWhittaker, Walkersville, MD), 2 mm l-glutamine (Life Technologies, Paisley, UK), and 50 U/ml penicillin (Sigma, Munich, Germany), hereafter referred to as complete medium.
Isolation and culture of cells
DC were generated as described.31,32 In brief, PBMC were isolated from the peripheral blood of healthy donors by Ficoll–Hypaque gradient centrifugation and subsequently allowed to adhere in culture flasks for 60 min. Initially adherent cells were harvested after overnight incubation and transferred into six-well plates (0·5 × 106−1·5 × 106 cells/ml) in fresh complete medium supplemented with 1000 U/ml GM-CSF alone, GM-CSF plus either 500 U/ml IL-4 or 500 U/ml IFN-α, or GM-CSF plus IFN-α and IL-4. For some experiments, monocytes were purified (purity > 90%) using the MACS CD14 isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany). After 6 days of culture, DC were washed extensively, resuspended in complete medium containing GM-CSF (1000 U/ml) and subsequently incubated either with a combination of pro-inflammatory cytokines (1000 U/ml TNF-α, 10 ng/ml IL-1β, 10 ng/ml IL-6 plus 1 mm PGE2) or with CD40 ligand (CD40L) -transfected and irradiated (30 Gy) 3TC cells (ratio APC to 3TC was 10 : 1) plus IFN-γ (1000 U/ml) in the presence or absence of IL-4 (500 U/ml) for 48 hr. CD40L-transfected 3TC cells were kindly provided by H. Engelmann, Insititute of Immunology, University of Munich, Germany. Autologous T cells were purified from monocyte-depleted PBMC by negative selection using the Pan T Cell Isolation Kit (Miltenyi Biotech).
Scanning electron microscopy
Scanning electron microscopy was performed in co-operation with Prof. Dr U. Welsch (Institute for Anatomy, University of Munich). Cells were spun onto standard microscopy glass slides and fixed with 2·5% glutaraldehyde in phosphate-buffered saline (pH 7·4). Following dehydration through graded ethanol, cells were critical-point-dried in CO2 and gold coated by sputtering. Samples were examined using a JSM-35-CF scanning electron microscope (JEOL, Tokyo, Japan). After electron microscopy, prints were scanned using Adobe Photoshop software (Adobe Systems, version 3·0).
Endocytic activity
Endocytic activity was assessed by adding FITC–dextran (0·5 mg/ml) to the culture medium for 30 min at 37° (unspecific binding of dextran to cell surfaces was determined by incubation on ice). Thereafter, cells were extensively washed and dextran uptake was quantified as mean fluorescence intensity (MFI) using flow cytometry.
Flow cytometry and monoclonal antibodies
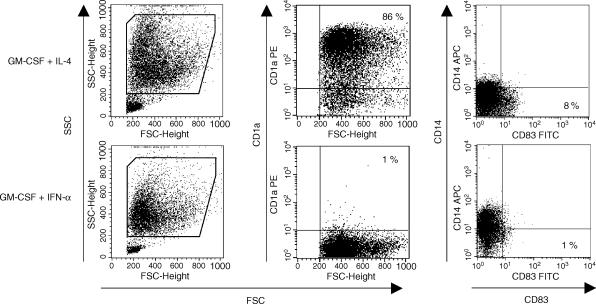
The following monoclonal antibodies were used for fluorescence-activated cell sorter (FACS) analysis: TÜ39 (anti-HLA DR, DP, DQ, FITC-conjugated), L307·4 (anti-CD80, phycoerythrin-conjugated), 2331/FUN-1 (anti-CD86, APC-conjugated), HB15e (anti-CD83, FITC-conjugated), M5E2 (anti-CD14, APC-conjugated) and HI149 (anti-CD1a, phycoerythrin-conjugated) were all from Pharmingen, San Diego, CA. CCR7 expression was determined by incubation with anti-CCR7 mAb (clone 3D12; kindly provided by R. Förster, Munich). Apoptosis was detected by staining with annexin V-FITC in combination with propidium iodide according to the manufacturer's instructions (Bender Med Systems, Vienna, Austria). Cells were analysed on a FACS-Calibur flow cytometer (Becton Dickinson, Heidelberg, Germany). Data were analysed using CellQuest software (Becton Dickinson, version 3.2·1). All FACS data are shown for viable DC after gating on forward and side scatter as indicated in Fig. 1.
Figure 1.
IFN-α treatment does not induce DC differentiation from GM-CSF-cultured monocytes. Monocytes were cultured with GM-CSF (1000 U/ml) and either IL-4 or IFN-α (each 500 U/ml). At day 6, the two different APC preparations were harvested and immunophenotype was determined by FACS analysis. For determination of surface molecule expression, viable DC were analysed after gating as indicated in the FSC/SSC panels. One representative experiment out of five is shown.
Cytokine measurements
On day 6, APC were incubated for 48 hr with the different stimuli. Subsequently, supernatants were collected for IL-12, IL-10 and TNF-α measurement using commercial enzyme-linked immunosorbent assay (ELISA) kits. IL-12 was determined using an assay that detects both IL-12 p40 and the bioactive p70 heterodimer (Bender Med Systems, Vienna, Austria). IL-10 and TNF-α were measured using OptEIA human IL-10 and human TNF-α sets (all from Pharmingen, San Diego, CA).
Proliferative T-cell response
T-cell proliferation assays were peformed in co-operation with Professor R. Wank (Institute of Immunology, University of Munich). Proliferative T-cell responses were measured as previously described with minor modifications.31,33 To determine allostimulatory capacity, APC were harvested and co-cultured in complete medium with a constant number of allogeneic non-adherent PBMC (2 × 105/200 μl) in 96-well round-bottom microtitre plates at ratios ranging from 1 : 20 to 1 : 80 in triplicates. To determine antigen-specific proliferation of autologous T cells, the different APC preparations were incubated with tetanus toxoid at day 5 at a concentration of 5 μg/ml. After 24 hr of stimulation with pro-inflammatory mediators at day 6, APC were harvested and co-cultured in complete medium with a constant number of autologous T cells (2 × 105/200 μl) in 96-well round-bottom microtitre plates at ratios ranging from 1 : 20 to 1 : 320 in triplicates. On day 5 of co-culture, the cells were pulsed with [3H]thymidine (1 μCi/well) and harvested after 18 hr onto a filtermate. The amount of incorporated [3H]thymidine was analysed in a liquid scintillation counter (Wallac, Turku, Finland).
Statistical analysis
Data are expressed as means ± SEM. Statistical significance was determined using the unpaired two-tailed Student's t-test. Differences were considered statistically significant for P < 0·01. Significance is presented for individual experiments (asterisks in figures). Statistical analysis was performed using Stat-View 4·51 software (Abacus Concepts, Calabasas, CA).
Results
IFN-α does not induce DC differentiation from monocytes cultured with GM-CSF
The adherent fraction of PBMC was cultured for 6 days either with GM-CSF (1000 U/ml) alone or GM-CSF plus IFN-α (500 U/ml), GM-CSF plus IL-4 (500 U/ml), or GM-CSF plus IFN-α and IL-4. As expected, cells cultured with GM-CSF alone maintained monocytic morphology as well as CD14 expression and did not up-regulate co-stimulatory molecules or CD83 (data not shown). GM-CSF/IL-4-cultured cells differentiated into immature DC with typical large cell bodies and blunt cytoplasmic projections showing adherence to the plastic surface and cluster formation. GM-CSF/IFN-α-incubated cells showed fewer morphological changes: cells remained smaller, were less adherent and did not show much cluster formation compared to GM-CSF/IL-4-cultured cells; cytoplasmic projections were barely visible. FACS analysis revealed a distinct pattern of forward scatter (FSC) and side scatter (SSC) of GM-CSF/IFN-α-treated cells forming a homogeneous population of smaller and less granular cells (Fig. 1, left and bottom). GM-CSF/IL-4-cultured cells displayed the typical immunophenotype of immature DC with a high percentage of CD1a+ CD83− CD14− cells (Fig. 1, top; middle and right). In contrast, GM-CSF/IFN-α-treated cells showed a different pattern of surface marker expression, indicating maintenance of a population of monocyte-/macrophage-like cells: cells did not express CD1a and more than 40% cells remained CD14+ after 6 days of culture (Fig. 1, bottom; middle and right). GM-CSF/IFN-α-incubated cells also showed significantly less expression of MHC II and the co-stimulatory molecules CD80 and CD86 compared to GM-CSF/IL-4-cultured cells (data not shown). Cells cultured with GM-CSF plus IFN-α and IL-4 showed no differences in morphology or surface marker expression compared to GM-CSF/IL-4-cultured cells, indicating that IFN-α did not interfere with phenotypic DC differentiation induced by IL-4 (data not shown).
IFN-α-treated monocytes efficiently take up soluble FITC-labelled dextran
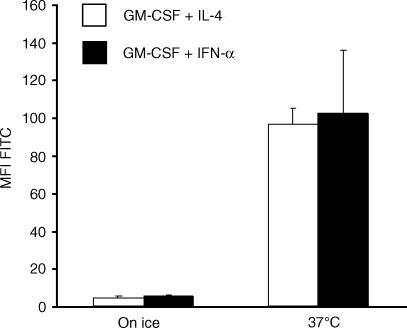
Potent APC are characterized by their capacity to take up and process antigen for presentation in the context of MHC molecules.6 To evaluate their capacity to take up soluble antigen by micropinocytosis, cells cultured with the different cytokine combinations (GM-CSF plus IFN-α or GM-CSF plus IL-4) were incubated with FITC-conjugated dextran at 37° for 30 min (controls on ice) at day 6 followed by FACS analysis. Despite the observed differences in morphology, IFN-α-treated cells showed equal endocytic capacity compared to cells cultured with IL-4 (more than 10-fold increase in FITC-MFI, Fig. 2).
Figure 2.
IFN-α-treated monocytes take up soluble FITC-labelled dextran. On day 6 of culture, the two different APC preparations (differentiated with GM-CSF plus IL-4 or IFN-α) were incubated with FITC-conjugated dextran (0·5 mg/ml) at 37° for 30 min (controls on ice) followed by FACS analysis. Data represent the mean MFI ± SEM of three independent experiments.
Presence of IL-4 during pro-inflammatory stimulation facilitates rapid development of dendritic-like cells from monocytes cultured with GM-CSF and IFN-α
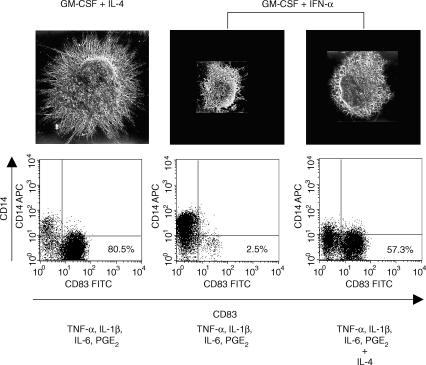
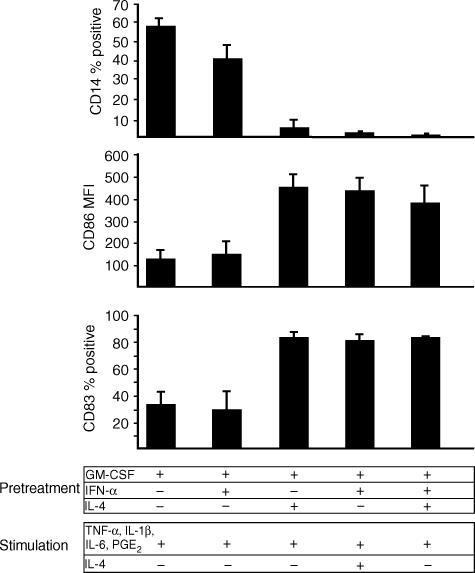
After 6 days of culture with IL-4 or IFN-α (both with GM-CSF), cells were incubated for 48 hr with a combination of pro-inflammatory mediators (TNF-α, IL-1β, IL-6 plus PGE2) known to induce DC maturation.34 Cells cultured with GM-CSF alone or cells cultured with GM-CSF plus IFN-α and IL-4 were used as controls. After stimulation, GM-CSF/IL-4-cultured cells displayed typical mature DC morphology showing fine cytoplasmic projections and homogeneously expressed the mature DC marker CD83 as well as co-stimulatory and MHC II molecules (Fig. 3, left; Fig. 4). In contrast, GM-CSF/IFN-α-treated cells remained small, did not form cytoplasmic projections and remained CD83− after stimulation (Fig. 3, middle). Cells cultured with GM-CSF alone and GM-CSF/IFN-α-treated cells showed a similar pattern of surface marker expression after stimulation indicating the preservation of a monocytic phenotype: cells remained CD14+ and expressed only low levels of CD83 as well as the co-stimulatory and MHC II molecules (Fig. 4, data not shown). Cells cultured with GM-CSF plus IFN-α and IL-4 for 6 days matured similarly to cells incubated with GM-CSF and IL-4 only, indicating that the presence of IFN-α during differentiation of DC did not prevent phenotypic maturation upon pro-inflammatory activation (Fig. 4).
Figure 3.
IL-4 facilitates rapid development of DC-like cells from IFN-α-treated monocytes. At day 6, the two different APC preparations (differentiated with GM-CSF plus IL-4 or IFN-α) were stimulated with TNF-α, IL-1β, IL-6 and PGE2 in the presence or absence of IL-4 as indicated. Cells were analysed by scanning electron microscopy and FACS analysis after 48 hr. Viable DC were analysed after gating as indicated in Fig. 1. Results of a representative FACS analysis out of five independent experiments are shown.
Figure 4.
DC-like cells derived from IFN-α-treated monocytes in the presence of IL-4 are phenotypically mature. On day 6, the different APC preparations (differentiated with GM-CSF alone, GM-CSF plus IFN-α or IL-4, GM-CSF plus IFN-α and IL-4) were stimulated for 48 hr with TNF-α, IL-1β, IL-6 and PGE2 in the presence or absence of IL-4. Expression of CD14, CD86 and CD83 was determined by flow cytometry on day 8. Viable DC were analysed after gating as indicated in Fig. 1. Results are expressed as means ± SEM of three experiments with different donors.
Next, we were interested if the presence of IL-4 – which is known to suppress macrophage differentiation from monocytes expanded with GM-CSF – could alter the response of the two different APC preparations (cells cultured with GM-CSF and either IFN-α or IL-4) to the pro-inflammatory mediators. While the continued presence of IL-4 (500 U/ml) had no effect on the phenotype of activated GM-CSF/IL-4-cultured DC (data not shown), addition of IL-4 during pro-inflammatory stimulation of GM-CSF/IFN-α-treated cells facilitated rapid DC maturation: increase in cell size and granularity, formation of cytoplasmic projections and intensive cell clustering within only 24 hr could be observed; a large proportion of cells (= 60%) expressed CD83, CD14 was down-regulated and expression of co-stimulatory and MHC II molecules increased approximately fourfold (Fig. 3, right; Fig. 4).
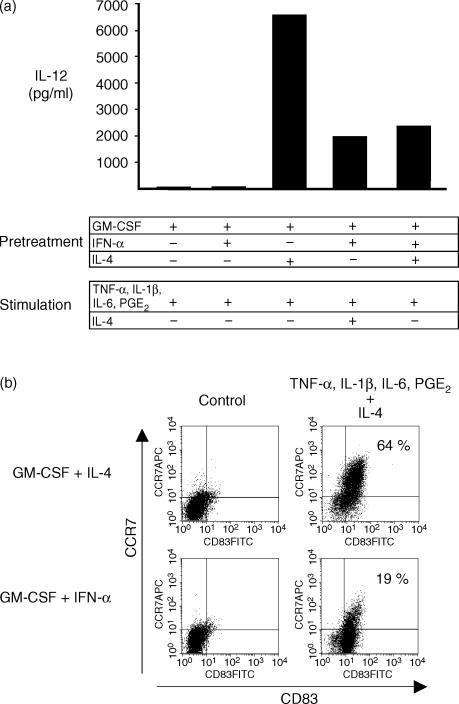
IFN-α-treated monocytes secrete low levels of IL-12 and express low levels of CCR7 upon pro-inflammatory stimulation in the presence of IL-4
IL-12 production by the different groups of APC activated with pro-inflammatory mediators was determined to assess their ability to initiate Th1 type immune responses. As expected, DC differentiated with GM-CSF and IL-4 secreted large amounts of IL-12 after stimulation (Fig. 5a). In contrast, cells cultured with GM-CSF only and cells cultured with GM-CSF plus IFN-α did not secrete IL-12 (Fig. 5a) correlating with the lack of DC surface marker expression. If IL-4 was added during stimulation of GM-CSF/IFN-α-treated cells, low levels of IL-12 could be induced. Interestingly, cells cultured with GM-CSF plus IFN-α and IL-4 showed markedly reduced levels of IL-12 production compared to GM-CSF/IL-4-derived DC indicating that the presence of IFN-α during differentiation could alter the cytokine response of DC upon encounter with maturation stimuli (Fig. 5a).
Figure 5.
DC-like cells derived from IFN-α-treated monocytes secrete low levels of IL-12 and express low levels of the chemokine receptor CCR7. On day 6, the different APC preparations (differentiated with GM-CSF alone, GM-CSF plus IFN-α or IL-4, GM-CSF plus IFN-α and IL-4) were left untreated (b, control) or stimulated for 48 hr with TNF-α, IL-1β, IL-6 and PGE2 in the presence or absence of IL-4 as indicated. On day 8, IL-12 secretion was measured by ELISA (a) and CD83/CCR7 co-expression was determined by flow cytometry (b). For FACS analysis, viable DC were analysed after gating as indicated in Fig. 1. One representative experiment out of three is shown.
Maturing DC undergo a co-ordinated switch in chemokine receptor expression, facilitating migration to secondary lymphoid organs via lymphatic vessels expressing CCR7 ligands.2 CCR7 expression by cells pre-activated with IFN-α also required the presence of IL-4 during pro-inflammatory stimulation (data not shown). However, only a minor proportion of IFN-α-treated cells (20%) up-regulated CCR7 even when IL-4 was added during activation, indicating incomplete maturation of IFN-α-pretreated DC (Fig. 5b). Interestingly, if IL-4 was added temporarily for 24 hr at day 5 of culture and removed prior to stimulation, IFN-α-treated cells also expressed DC activation markers upon pro-inflammatory activation (data not shown) indicating that IL-4 restored developmental plasticity rather than acting as a maturation factor.
Dendritic-like cells derived from IFN-α-activated monocytes show defective T-cell stimulatory capacity
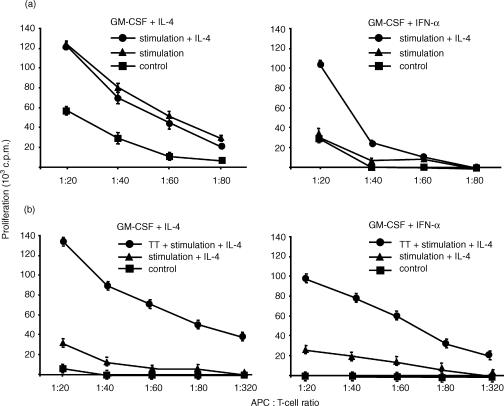
Mature DC are the most potent stimulators of antigen-specific T-cell responses. To assess the allostimulatory capacity of the different day 6 APC, standard allogeneic mixed lymphocyte reaction was performed after stimulation with pro-inflammatory cytokines plus PGE2 for 48 hr. Correlating with the expression of DC activation markers, IFN-α-treated cells were poor stimulators of allogeneic T cells unless DC maturation was induced by stimulation in the presence of IL-4 (Fig. 6a, right). However, even at high DC : T-cell ratios, IFN-α-pretreated DC-like cells induced significantly lower levels of alloreactive T-cell proliferation compared to IL-4-derived DC (Fig. 6a, left).
Figure 6.
IFN-α-pretreated DC are defective in inducing allogeneic or antigen-specific autologous T-cell proliferation. (a) To determine allostimulatory capacity, the two different APC (differentiated with GM-CSF plus IL-4 or IFN-α) were stimulated at day 6 with TNF-α, IL-1β, IL-6 and PGE2 in the presence or absence of IL-4 and co-cultured after 24 hr with the non-adherent fraction of allogeneic PBMC for 5 days. (b) To determine capacity to stimulate autologous T cells, the two different APC (differentiated with GM-CSF plus IL-4 or IFN-α) were loaded with tetanus toxoid (TT; 5 μg/ml) at day 5 and subsequently stimulated with TNF-α, IL-1β, IL-6 and PGE2 in the presence of IL-4 to induce DC maturation. After 24 hr, APC were co-cultured with purified autologous T cells. GM-CSF-cultured monocytes incubated for 6 days with IL-4 or IFN-α or unloaded, stimulated APC (differentiated with IL-4 or IFN-α) were used as controls. Cells were pulsed with [3H]thymidine at day 5 of co-culture and incorporation was measured 18 hr later. Results are representative of two experiments with different donors.
The capacity of the different APC to stimulate antigen-specific T-cell responses was determined using the recall antigen tetanus toxoid. IFN-α-treated monocytes or IL-4-cultured DC – each loaded with tetanus toxoid at day 5 or left unloaded – were stimulated with pro-inflammatory mediators at day 6 in the presence of IL-4 to induce DC maturation. After 24 hr, the different DC preparations were co-cultured with autologous T cells. Tetanus toxoid-loaded DC preactivated with IFN-α were able to induce antigen-specific autologous T-cell proliferation (Fig. 6b, right). However, levels of T-cell proliferation were markedly reduced compared to those induced by IL-4-cultured DC (Fig. 6b, left)
Monocytic cytokine production is preserved in DC pretreated with IFN-α
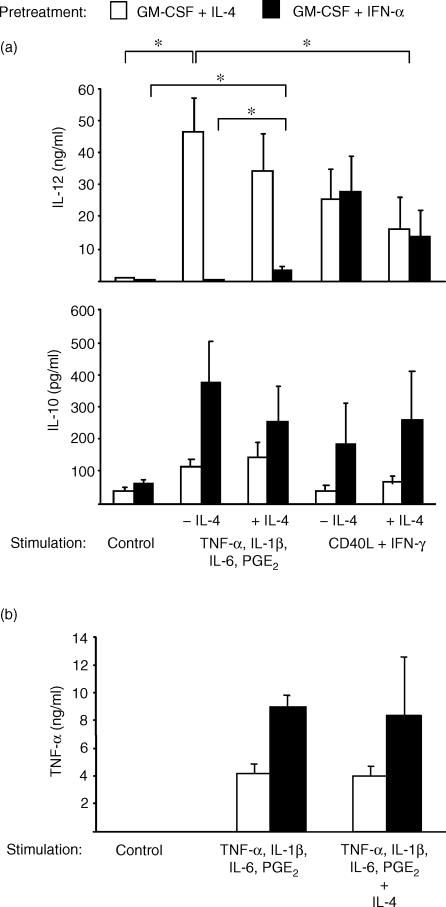
Next, we were interested in how the cytokine environment during differentiation (IL-4 versus IFN-α) influenced the cytokine production in response to different modes of activation. Day 6 APC were stimulated with pro-inflammatory mediators or CD40L plus IFN-γ for 48 hr and supernatants were obtained for cytokine measurements. As expected, DC cultured with IL-4 secreted large amounts of IL-12 (> 20 ng/ml) in response to both stimulus combinations, with maximal levels of IL-12 induced by stimulation with pro-inflammatory mediators (Fig. 7a, top; n = 4, P < 0·01). There was a trend towards reduced IL-12 production if IL-4 was added during stimulation that could be observed after activation with both stimulus combinations. However, this trend did not reach statistical significance. Higher levels of IL-12 production of IL-4-derived DC induced by pro-inflammatory mediators correlated with higher levels of expression of DC activation markers compared to stimulation with CD40L plus IFN-γ (> 80% versus 60% CD83+ cells, data not shown).
Figure 7.
IFN-α-treated monocytes maintain a monocytic cytokine secretion pattern independent of DC maturation. The different day 6 APC (differentiated with GM-CSF plus IL-4 or IFN-α) were left unstimulated (control) or activated with either TNF-α, IL-1β, IL-6 and PGE2 or CD40L plus IFN-γ in the presence or absence of IL-4 as indicated. After 48 hr, IL-12 (p40/p70) (a, top), IL-10 (a, bottom) and TNF-α (b) were measured in the supernatants by ELISA. Results are expressed as means ± SEM of four experiments with different donors (a) or as means ± SEM of two experiments with different donors (b).
IFN-α-treated cells appeared to be primed towards IL-10 production independent of the state of DC maturation: cells produced more than 200 pg/ml of IL-10 in response to all stimulus combinations tested (Fig. 7a, bottom). Even when DC maturation from IFN-α-treated cells was induced by pro-inflammatory stimulation in the presence of IL-4 (> 60% CD83+ cells, data not shown), only small amounts of IL-12 were secreted (< 5 ng/ml, Fig. 7a, top) and IL-10 production was maintained (Fig. 7a, bottom). Yet, the observed increase in IL-12 secretion was statistically significant (n = 4, P < 0·01). In contrast, activation with CD40L plus IFN-γ induced equal levels of IL-12 production in IFN-α-treated cells compared to IL-4-derived DC (Fig. 7a, top). However, this IL-12 production was not associated with the expression of DC maturation markers (< 10% CD83+ cells, data not shown). When IL-4 was added during activation with CD40L plus IFN-γ, cells expressed DC activation markers at low levels (30% CD83+ cells, data not shown), but this increase in expression of DC activation markers was associated with reduced IL-12 secretion and enhanced IL-10 production (Fig. 7a).
TNF-α was measured to confirm the preservation of a monocytic cytokine secretion pattern in IFN-α-treated cells. While unstimulated cells produced no TNF-α, pro-inflammatory cytokines plus PGE2 induced TNF-α in both APC types. However, cells pre-activated with IFN-α produced twice as much TNF-α as IL-4-derived DC (Fig. 7b, left and middle). Phenotypic DC maturation of IFN-α-treated monocytes induced by the presence of IL-4 during stimulation left high levels of TNF-α production unaffected (Fig. 7b).
Discussion
In the present study, we report that activation with IFN-α does not induce DC development from GM-CSF-cultured monocytes. Cells incubated with IFN-α for 6 days maintain a monocyte/macrophage-like immunophenotype and remain poor stimulators of allogeneic T-cell proliferation even after stimulation with pro-inflammatory mediators. Presence of IL-4 during stimulation facilitates development of DC-like cells expressing low levels of maturation markers. However, IFN-α-pretreated DC are poor inducers of allogeneic or antigen-specific autologous T-cell proliferation, fail to secrete IL-12 and express only low levels of the lymph-node-directing chemokine receptor CCR7. These findings indicate that – in the absence of other differentiation factors – IFN-α not only fails to induce DC development from monocytes expanded with GM-CSF, but pretreatment with IFN-α also prevents complete DC maturation even if developmental plasticity has been restored.
Type I IFNs influence APC function in vivo14 and may play a role during maturation of DC from monocytes during both innate and adaptive immune responses.23,30 It has been shown that type I IFNs can accelerate DC differentiation of CD34+ progenitor cells and maturation of monocyte-derived DC generated with GM-CSF and IL-4.35,36 Santini et al. have reported that incubation of monocytes with GM-CSF and type I IFN results in the development of mature DC as early as 3 days after initiation of cell culture without requiring further stimulation.23 These IFN-α-derived DC express the chemokine receptor CCR7 and migrate in response to its ligand, MIP3β, features commonly associated with DC maturation.24,37 However, only a minor proportion of monocytes expressed DC maturation markers after 3 days of culture with GM-CSF and type I IFN. Moreover, the T-cell stimulatory capacity of these IFN-derived DC was compared with that of monocytes cultured for only 3 days with GM-CSF and IL-4 and left unstimulated. Thus, it has not been conclusively shown that DC-like cells generated by incubation of monocytes with GM-CSF and type I IFN are equally or even more effective compared to fully mature IL-4-derived DC in inducing Th1 type immune responses.
Here, we show that IFN-α-treatment does not induce differentiation of functional DC, but maintains a homogeneous population of cells with monocyte/macrophage-like phenotype and low allostimulatory capacity. Even though phenotypic maturation can be induced by addition of IL-4 during differentiation or during maturation induced by pro-inflammatory stimuli, comparative analysis revealed that DC derived from IFN-α-treated monocytes are defective in their T-cell stimulatory capacity compared to stimulated, fully mature IL-4-derived DC. Moreover, low levels of CCR7 expression by IFN-α-pretreated DC indicated that maturation was incomplete. Apart from maintaining a monocyte/macrophage-like phenotype, IFN-α-treatment also preserved a monocytic cytokine secretion pattern in APC: IFN-α-treated cells primarily secreted IL-10 and TNF-α in response to various stimuli independent of DC maturation status. Although the addition of IL-4 to GM-CSF and IFN-α from the beginning of cell culture facilitated phenotypic differentiation of DC, IL-12 secretion after stimulation with pro-inflammatory mediators was reduced. It has been shown previously that human monocytes pretreated with IFN-α secrete IL-10 in response to bacterial superantigen38 and that type I IFNs inhibit IL-12 production in human PBMC in an IL-10-dependent manner.17 Taken together, these data indicate that IFN-α fails to induce DC differentiation from monocytes and reduces the capacity of monocyte-derived DC generated in the presence of IL-4 to secrete IL-12 in response to microbial and pro-inflammatory stimuli as well as their capacity to stimulate T cells. These results confirm previous reports that type I IFNs do not promote effective maturation of DC from monocyte precursors.25,26
Another striking finding made in the present study is that IFN-α-activated monocytes can differentiate very rapidly into DC if developmental plasticity has been restored by IL-4: while 48 hr of pro-inflammatory stimulation34,39 alone could not alter morphology and immunophenotype, 24 hr of activation in the presence of IL-4 sufficed to induce DC development and maturation from a large proportion of IFN-α-treated monocytes. The same effect could be observed if IFN-α-treated monocytes were cultured overnight in the presence of IL-4 prior to stimulation. To our knowledge, only one study reported maturation of DC from monocytes in a similar time period: Randolph and co-workers used a model of transendothelial trafficking to simulate migration of monocytes from tissue to lymph.5 If monocytes encountered yeast cell walls or other phagocytic factors in the collagen matrix used to simulate tissue, they acquired mature DC phenotype and allostimulatory capacity within 48 hr. Thus, the current models for in vitro generation of DC may not reflect the time span required for DC differentiation from monocyte precursors under physiological conditions.
IL-4 not only facilitated phenotypic DC maturation of IFN-α-activated monocytes, but also differentially regulated cytokine production in response to different modes of activation: upon stimulation with pro-inflammatory mediators in the presence of IL-4, IFN-α-treated monocytes not only expressed DC maturation markers, but also reduced IL-10 production and secreted low amounts of IL-12. Thus, IL-4 can restore the capacity to secrete DC cytokines in a subpopulation of IFN-α-treated monocytes indicating that some degree of functional plasticity is maintained. This is confirmed by the observation that IFN-α-treated monocytes secrete large amounts of IL-12 after stimulation with CD40L and IFN-γ, a signal imitating help by Th1 cells. However, Th1 cell signalling did not induce DC maturation and cells rather acquired an activated macrophage-like phenotype. Thus, independent of pre-activation with IFN-α, monocytes can distinguish between different – pro-inflammatory or T-cell-derived – activation signals and respond with secretion of distinct cytokine profiles. In vivo, Th1 cell help may provide a positive feedback signal for tissue-residing APC pre-activated with IFN-α to maintain a Th1 type immune response in their local environment.40
In summary, incubation with IFN-α does not induce differentiation of GM-CSF-cultured monocytes into functional DC. IL-4 can partially restore developmental plasticity of IFN-α-treated monocytes and facilitates rapid development of DC-like cells upon pro-inflammatory activation. However, CCR7 expression, IL-12 secretion and T-cell stimulatory capacity are suboptimal in DC derived from IFN-α-treated monocytes. It has been proposed that IL-4-treatment may inhibit DC functions and that incubation with type I IFNs may serve as an alternative regimen for the in vitro generation of monocyte-derived DC.9 However, our results indicate that DC developed from IFN-α-treated monocytes are defective in migration and induction of Th1 type immune responses, thus limiting their use in anti-tumour immunotherapy.
Acknowledgments
Marc Dauer is supported by a grant from the University of Munich FöFoLe no. 271 and by a grant from the Friedrich-Baur-Stiftung, Munich. We thank Simon Rothenfusser for helpful discussions and Rosemarie Kiefl for technical assistance.
References
- 1.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 2.Sozzani S, Allavena P, Vecchi A, Mantovani A. Chemokines and dendritic cell traffic. J Clin Immunol. 2000;20:151–60. doi: 10.1023/a:1006659211340. [DOI] [PubMed] [Google Scholar]
- 3.Dieu MC, Vanbervliet B, Vicari A, et al. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med. 1998;188:373–86. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trinchieri G. Interleukin-12: a cytokine at the interface of inflammation and immunity. Adv Immunol. 1998;70:83–243. doi: 10.1016/s0065-2776(08)60387-9. [DOI] [PubMed] [Google Scholar]
- 5.Randolph GJ, Beaulieu S, Lebecque S, Steinman RM, Muller WA. Differentiation of monocytes into dendritic cells in a model of transendothelial trafficking. Science. 1998;282:480–3. doi: 10.1126/science.282.5388.480. [DOI] [PubMed] [Google Scholar]
- 6.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–18. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caux C, Dezutter-Dambuyant C, Schmitt D, Banchereau J. GM-CSF and TNF-alpha cooperate in the generation of dendritic Langerhans cells. Nature. 1992;360:258–61. doi: 10.1038/360258a0. [DOI] [PubMed] [Google Scholar]
- 8.Romani N, Gruner S, Brang D, et al. Proliferating dendritic cell progenitors in human blood. J Exp Med. 1994;180:83–93. doi: 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thurnher M, Zelle-Rieser C, Ramoner R, Bartsch G, Holtl L. The disabled dendritic cell. Faseb J. 2001;15:1054–61. doi: 10.1096/fj.00-0508hyp. [DOI] [PubMed] [Google Scholar]
- 10.Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol. 1999;17:189–20. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- 11.Belardelli F, Gresser I. The neglected role of type I interferon in the T-cell response: implications for its clinical use. Immunol Today. 1996;17:369–72. doi: 10.1016/0167-5699(96)10027-X. [DOI] [PubMed] [Google Scholar]
- 12.Marrack P, Kappler J, Mitchell T. Type I interferons keep activated T cells alive. J Exp Med. 1999;189:521–30. doi: 10.1084/jem.189.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cousens LP, Orange JS, Su HC, Biron CA. Interferon-alpha/beta inhibition of interleukin 12 and interferon-gamma production in vitro and endogenously during viral infection. Proc Natl Acad Sci USA. 1997;94:634–9. doi: 10.1073/pnas.94.2.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang H, Dhib-Jalbut S. Differential induction of IL-12 by IFN-beta and IFN-gamma in human macrophages. J Interferon Cytokine Res. 1998;18:697–03. doi: 10.1089/jir.1998.18.697. [DOI] [PubMed] [Google Scholar]
- 15.Bartholome EJ, Willems F, Crusiaux A, Thielemans K, Schandene L, Goldman M. IFN-beta interferes with the differentiation of dendritic cells from peripheral blood mononuclear cells: selective inhibition of CD40-dependent interleukin-12 secretion. J Interferon Cytokine Res. 1999;19:471–8. doi: 10.1089/107999099313910. [DOI] [PubMed] [Google Scholar]
- 16.McRae BL, Beilfuss BA, van Seventer GA. IFN-beta differentially regulates CD40-induced cytokine secretion by human dendritic cells. J Immunol. 2000;164:23–8. doi: 10.4049/jimmunol.164.1.23. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Chen M, Wandinger KP, Williams G, Dhib-Jalbut S. IFN-beta-1b inhibits IL-12 production in peripheral blood mononuclear cells in an IL-10-dependent mechanism: relevance to IFN-beta-1b therapeutic effects in multiple sclerosis. J Immunol. 2000;165:548–57. doi: 10.4049/jimmunol.165.1.548. [DOI] [PubMed] [Google Scholar]
- 18.Byrnes AA, Ma X, Cuomo P, et al. Type I interferons and IL-12: convergence and cross-regulation among mediators of cellular immunity. Eur J Immunol. 2001;31:2026–34. doi: 10.1002/1521-4141(200107)31:7<2026::aid-immu2026>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 19.Siegal FP, Kadowaki N, Shodell M, et al. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–7. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 20.Cella M, Facchetti F, Lanzavecchia A, Colonna M. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive a potent TH1 polarization. Nat Immunol. 2000;1:305–10. doi: 10.1038/79747. [DOI] [PubMed] [Google Scholar]
- 21.Paquette RL, Hsu NC, Kiertscher SM, Park AN, Tran L, Roth MD, Glaspy JA. Interferon-alpha and granulocyte-macrophage colony-stimulating factor differentiate peripheral blood monocytes into potent antigen-presenting cells. J Leukoc Biol. 1998;64:358–67. doi: 10.1002/jlb.64.3.358. [DOI] [PubMed] [Google Scholar]
- 22.Radvanyi LG, Banerjee A, Weir M, Messner H. Low levels of interferon-alpha induce CD86 (B7:2) expression and accelerates dendritic cell maturation from human peripheral blood mononuclear cells. Scand J Immunol. 1999;50:499–09. doi: 10.1046/j.1365-3083.1999.00625.x. [DOI] [PubMed] [Google Scholar]
- 23.Santini SM, Lapenta C, Logozzi M, Parlato S, Spada M, Di Pucchio T, Belardelli F. Type I interferon as a powerful adjuvant for monocyte-derived dendritic cell development and activity in vitro and in Hu-PBL-SCID mice. J Exp Med. 2000;191:1777–88. doi: 10.1084/jem.191.10.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parlato S, Santini SM, Lapenta C, et al. Expression of CCR-7, MIP-3beta, and Th-1 chemokines in type I IFN-induced monocyte-derived dendritic cells: importance for the rapid acquisition of potent migratory and functional activities. Blood. 2001;98:3022–9. doi: 10.1182/blood.v98.10.3022. [DOI] [PubMed] [Google Scholar]
- 25.McRae BL, Nagai T, Semnani RT, van Seventer JM, van Seventer GA. Interferon-alpha and -beta inhibit the in vitro differentiation of immunocompetent human dendritic cells from CD14 (+) precursors. Blood. 2000;96:210–17. [PubMed] [Google Scholar]
- 26.Lehner M, Felzmann T, Clodi K, Holter W. Type I interferons in combination with bacterial stimuli induce apoptosis of monocyte-derived dendritic cells. Blood. 2001;98:736–42. doi: 10.1182/blood.v98.3.736. [DOI] [PubMed] [Google Scholar]
- 27.Neumann AU, Lam NP, Dahari H, Gretch DR, Wiley TE, Layden TJ, Perelson AS. Hepatitis C viral dynamics in vivo and the antiviral efficacy of interferon-alpha therapy. Science. 1998;282:103–7. doi: 10.1126/science.282.5386.103. [DOI] [PubMed] [Google Scholar]
- 28.Rudick RA, Cohen JA, Weinstock-Guttman B, Kinkel RP, Ransohoff RM. Management of multiple sclerosis. N Engl J Med. 1997;337:1604–11. doi: 10.1056/NEJM199711273372207. [DOI] [PubMed] [Google Scholar]
- 29.Hernberg M, Pyrhonen S, Muhonen T. Regimens with or without interferon-alpha as treatment for metastatic melanoma and renal cell carcinoma: an overview of randomized trials. J Immunother. 1999;22:145–54. doi: 10.1097/00002371-199903000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science. 2001;294:1540–3. doi: 10.1126/science.1064890. [DOI] [PubMed] [Google Scholar]
- 31.Schnurr M, Galambos P, Scholz C, Then F, Dauer M, Endres S, Eigler A. Tumor cell lysate-pulsed human dendritic cells induce a T-cell response against pancreatic carcinoma cells: an in vitro model for the assessment of tumor vaccines. Cancer Res. 2001;61:6445–50. [PubMed] [Google Scholar]
- 32.Schnurr M, Scholz C, Rothenfusser S, Galambos P, Dauer M, Robe J, Endres S, Eigler A. Apoptotic pancreatic tumor cells are superior to cell lysates in promoting cross-priming of cytotoxic T cells and activate NK and gammadelta T cells. Cancer Res. 2002;62:2347–52. [PubMed] [Google Scholar]
- 33.Schnurr M, Then F, Galambos P, Scholz C, Siegmund B, Endres S, Eigler A. Extracellular ATP and TNF-alpha synergize in the activation and maturation of human dendritic cells. J Immunol. 2000;165:4704–9. doi: 10.4049/jimmunol.165.8.4704. [DOI] [PubMed] [Google Scholar]
- 34.Feuerstein B, Berger TG, Maczek C, et al. A method for the production of cryopreserved aliquots of antigen-preloaded, mature dendritic cells ready for clinical use. J Immunol Methods. 2000;245:15–29. doi: 10.1016/s0022-1759(00)00269-6. [DOI] [PubMed] [Google Scholar]
- 35.Luft T, Pang KC, Thomas E, Hertzog P, Hart DN, Trapani J, Cebon J. Type I IFNs enhance the terminal differentiation of dendritic cells. J Immunol. 1998;161:1947–53. [PubMed] [Google Scholar]
- 36.Huang YM, Hussien Y, Yarilin D, Xiao BG, Liu YJ, Link H. Interferon-beta induces the development of type 2 dendritic cells. Cytokine. 2001;13:264–71. doi: 10.1006/cyto.2000.0835. [DOI] [PubMed] [Google Scholar]
- 37.Sallusto F, Lanzavecchia A, Mackay CR. Chemokines and chemokine receptors in T-cell priming and Th1/Th2-mediated responses. Immunol Today. 1998;19:568–74. doi: 10.1016/s0167-5699(98)01346-2. [DOI] [PubMed] [Google Scholar]
- 38.Hermann P, Rubio M, Nakajima T, Delespesse G, Sarfati M. IFN-alpha priming of human monocytes differentially regulates gram-positive and gram-negative bacteria-induced IL-10 release and selectively enhances IL-12p70, CD80, and MHC class I expression. J Immunol. 1998;161:2011–18. [PubMed] [Google Scholar]
- 39.Luft T, Jefford M, Luetjens P, et al. Functionally distinct dendritic cell (DC) populations induced by physiologic stimuli: prostaglandin E(2) regulates the migratory capacity of specific DC subsets. Blood. 2002;100:1362–72. doi: 10.1182/blood-2001-12-0360. [DOI] [PubMed] [Google Scholar]
- 40.Kato T, Hakamada R, Yamane H, Nariuchi H. Induction of IL-12 p40 messenger RNA expression and IL-12 production of macrophages via CD40–CD40 ligand interaction. J Immunol. 1996;156:3932–8. [PubMed] [Google Scholar]