Abstract
CD40/CD154 interaction is essential for both humoral and cellular immune response. We investigated whether this interaction could be altered in patients with kidney failure who are known to present an impaired immune response. To that aim, we measured the levels of the soluble form of CD40 (sCD40), which is known to interfere with CD40/CD154 interaction, in 43 chronic renal failure patients, 162 hemodialysed patients, and 83 healthy donors. Uraemic and haemodialysed patients presented a three- and fivefold increase, respectively, of the antagonist soluble form of CD40 in their serum, when compared to healthy subjects. Serum sCD40 levels correlated with those of creatinine in uraemic non-haemodialysed patients. While sCD40 is widely excreted in urine of healthy individuals, it is not eliminated by dialysis sessions on classic membranes. The return to a normal kidney function in nine haemodialysed patients who received renal transplantation, leads to a rapid decrease of serum sCD40 levels. This natural sCD40 exhibited multimeric forms and was able to inhibit immunoglobulin production by CD154-activated B lymphocytes in vitro. Furthermore, the positive correlation we observed between the serum levels of sCD40 and the deficient response to hepatitis B vaccination in uraemic patients suggests that sCD40 also compromises the humoral response in vivo.
Introduction
Patients with chronic renal failure (CRF) display several features of immune response deficiency such as recurrent bacterial infections1 or enhanced prevalence of cancer compared with the general population (for review see 2). They present an altered immune response to hepatitis B vaccination revealed by lower seroconversion rates and anti-hepatitis B surface antigen (HBs) titres than healthy subjects.3,4 Despite reinforced vaccination protocols, 20–40% of these patients fail to produce protective antibody titres and, even in responders, the duration of protection is shorter than in healthy subjects.5,6
The in vitro response of T cells to mitogens or to allogeneic stimulation is notably decreased in uraemic patients compared to healthy subjects (for review see 7). Despite this diminished response capacity, T lymphocytes from uraemic patients display signs of activation, such as an up-regulation of CD25, closely related to uraemia even before they become haemodialysed. It is assumed that the functional capacities of uraemic patients' monocytes/macrophages are diminished8 despite their enhanced production of inflammatory cytokines.9 Notably, defective expression of CD86 hampers their costimulatory function toward T cells.10 Recently, a genotype of interleukin-10 (IL-10) promoter associated with a low production of this cytokine by monocytes was correlated with the absence of response to hepatitis B vaccination in haemodialysed patients.11 Little is known about B-cell functions in uraemic patients. Studies have shown a diminished production of antibody to specific stimuli in CRF patients12 but it is difficult to determine the origin of these altered responses.
The CD40/CD154 couple plays a critical role both in humoral and cellular immune response. CD40 is a 50 000 MW membrane glycoprotein that belongs to the tumor necrosis factor (TNF) receptor superfamily (for reviews see 13–16). It is expressed by a wide range of cells such as B cells, endothelial cells, epithelial cells (notably renal epithelial tubular cells), monocytes/macrophages, dendritic cells and fibroblasts. The ligand for CD40, CD154 (CD40L, gp39) is a 33 000 MW glycoprotein, a member of the TNF superfamily, transiently expressed on the surface of activated T cells predominantly belonging to the CD4+ T-cell subset.17 Basophils, mast cells, eosinophils, natural killer cells and platelets have also been shown to express CD154.
CD40 triggering by CD154 is essential for B-cell growth and differentiation, and immunoglobulin class switching and somatic hypermutations.18,19 It is also required for antigen-presenting cell (f) activation as it induces costimulatory molecules and cytokine synthesis.20,21 Signals resulting from CD40/CD154 interaction are bidirectional as costimulation through CD154 induces short-term activation and cytokine production in T cells.22,23 The importance of this system is highlighted by the X-linked hyper-immunoglobulin M (IgM) syndrome. This disease caused by a loss-of-function mutation in the CD154 locus is accompanied by a drastic alteration of the humoral response characterized by low immunoglobulin levels, an inability to form germinal centres, and an increased susceptibility to recurrent infections.24–27
A natural antagonist of CD40/CD154 interaction is the soluble form of CD40 (sCD40) which has been shown to inhibit the binding of CD154 to CD40 in vitro.28 The aim of the present study was thus to investigate the presence of sCD40 in the serum of uraemic individuals and to analyse its possible contribution to the altered humoral immune response in these patients as tested by vaccinal response.
Materials and methods
Patients and blood samples
Characteristics of the 162 haemodialysed patients, the 43 uraemic individuals, and the 83 healthy donors included in the transversal study comparing serum sCD40 levels are presented in Table 1. Nine kidney transplanted patients were longitudinally monitored for 3 months post-transplantation (sex ratio M/F = 2·3, mean age = 42·5 ± 8·3). Blood samples were taken on an heparinized tube at day 0, then daily after transplantation for 20 days and then monthly for 3 months. For uraemic individuals, samples used were retrieved from frozen sera collection. All patients gave their informed consent to participate in the study.
Table 1.
Characteristics of the patients included in the retrospective study
| Haemodialysed patients (n = 162) | Uraemic patients (n = 43) | Healthy donors (n = 83) | |
|---|---|---|---|
| Sex ratio (M/F) | 1·50 | 2·58 | 1·25 |
| Age (years, median (range)) | 59 (17–89) | 58 (32–84) | 33 (20–67) |
| Primary renal disease (n) | NR* | ||
| Glomerular | 43 | 6 | |
| Tubulointerstitial | 32 | 4 | |
| Vascular | 32 | 6 | |
| Hereditary† | 28 | 13 | |
| Diabetes | 9 | 2 | |
| ND‡ | 17 | 12 | |
| Residual diuresis (n) | |||
| <500 ml | 117 | ||
| >500 ml | 45 | ||
NR: not relevant.
Mostly autosomal-dominant polycystic kidney disease.
ND: not determined.
Antibodies and reagents
cDNA constructs coding the CD40Fc chimeric molecule (extracellular domain of human CD40 fused with the Fc fragment of human IgG128) or for rsCD40 (extracellular domain of CD40, amino acid 1-19328), and the anti-CD40 monoclonal antibody (mAb; mAb89)29 were kindly provided by Schering-Plough Laboratory for Immunological Research (Dardilly, France). CD40Fc and sCD40 were produced in transiently transfected COS cells and immunopurified on a mAb89 column (Hi-Trap, Pharmacia Biotech, Les Ullis, France). The anti-CD40 3B2 and 7C3 mAb were obtained in our laboratory by immunization of mice with CD40Fc. The phycoerythrin (PE)-conjugated anti-CD40 mAb (clone EA-5) was purchased from Calbiochem (Meudon, France). The soluble trimeric rCD154 was purchased from Bender MedSystems (Vienna, Austria). The PE-conjugated anti-CD154 (clone TRAP-1) was purchased from Immunotech (Marseille, France). The anti-CD154 blocking mAb (clone 24-31) was purchased from Calbiochem. Recombinant IL-10 was kindly provided by Schering-Plough (Dardilly, France), and IL-2 by Chiron Corporation (Paris, France).
Enzyme-linked immunosorbent assay (ELISA) for detection of soluble CD40
ELISA was adapted from van Kooten et al.28 except that the antibody used for revelation was the biotinylated anti-CD40 3B2 antibody detected by streptavidin peroxidase (Amersham, Saclay, France, 1/1000). Recombinant sCD40 (see above) served as the standard for the calibration curve. The plates were developed with tetramethyl benzidine (Sigma, St Quentin Fallavier, France) and the reaction was stopped with H2SO4 2 n. Plates were read at 450 nm and 570 nm. Each of the samples were measured in duplicate and the mean concentration was calculated. The detection limit of the ELISA was around 10 pg/ml. All samples were tested in a control ELISA in which biotinylated 3B2 was replaced by an unrelated biotinylated isotypic mAb at 1 µg/ml. Samples found positive in the control ELISA (about 10% for all sera tested and none for urine, as previously described by Hennig et al.30) were considered as false positive and eliminated from the study. For detection of multimeric from of sCD40, an homotypic ELISA was set up using the same anti-CD40 antibody for capture and revelation (i.e. mAb89 and biotinylated mAb89, respectively).
Immunoaffinity purification of sCD40
A 1-ml Hi-Trap column (Pharmacia Biotech) was coupled to 2 mg of purified anti-CD40 mAb89 antibody according to the manufacturer's instructions. For natural sCD40 purification, 6 l of supernatant from the Epstein–Barr virus (EBV)-transformed lymphoblastoid cell line JY cultured for 2 days in serum-free conditions were ultrafiltered using Biomax© polysulphone membrane (Millipore SA, Saint Quentin Yuelines, France; MW cutoff of 10 000) at 4° according to the manufacturer's instructions. Two hundred ml of the concentrated supernatant was then loaded on an unconjugated Hi-Trap column (to remove non-specific fixation) followed by the mAb89 column which was then washed with 5 ml of phosphate-buffered saline (PBS) without Ca2+, Mg2+, KCl 1 m, Tween-20 0·02%, pH 7·5. One millilitre fractions were then eluted with a 0·1-m glycine HCl pH 2·5 solution immediately neutralized with 1 m Tris-HCl pH 8·0 buffer and dialysed twice against 5 l of PBS. Finally, 4 µg/ml of natural sCD40 was purified as determined by ELISA. Coupled mAb89 was not released from the column as determined by Western Blot analysis using an anti-mouse immunoglobulin horseradish peroxidase-conjugated antibody.
Flow cytometry analysis
For direct staining of sCD40 fixation on CD154, 5 × 104 Jurkat-CD154 cells (Jurkat cells stably transfected with cDNA encoding human CD15431) were incubated with 1·5 µg/ml of purified sCD40, or 25 µg/ml of CD40Fc for 30 min at 4°, washed and stained with EA-5 anti-CD40. To test the inhibitory effect of sCD40 on the binding of CD154 on CD40, 5 µg/ml of soluble CD154 was incubated (v/v) with 1·5 µg/ml of purified sCD40 or PBS bovine serum albumin (BSA) 0·1% for 1 hr at 4° under shaking. Then, 5·104 EBV-transformed JY cells were incubated with this mixture for 30 min at 4°, washed and labelling was revealed with an anti-CD154 mAb. Analyses were performed on a FACScan (Becton Dickinson, San Jose, CA) apparatus.
Production of immunoglobulin by purified tonsil B cells
B cells were isolated from tonsils as described earlier.32 Purity was always >95%. Fifty thousand purified tonsil B cells were incubated in 96-well flat-bottomed plates in the presence of IL-10 (50 ng/ml) and IL-2 (10 U/ml) together with 5 × 103 mouse fibroblastic L cells stably transfected with the cDNA encoding for the human CD15433 (kind gift from Schering-Plough Laboratory) or non-transfected control cells, as previously described.34 For mixed lymphocyte reaction, 2 × 104 tonsil B cells were cultured with IL-10 (50 ng/ml) and IL-2 (10 U/ml) in the presence of 4 × 104γ-irradiated (30 Gy) allogeneic peripheral blood mononuclear cells (PBMC). Five µg/ml of blocking anti-CD154 antibody, irrelevant antibody or purified natural sCD40 (1 µg/ml) were added at the onset of the culture. As a control for sCD40 specific activity, we added to the culture a sCD40-depleted fraction (ΔsCD40) which was prepared as follows: an aliquot (300 µl) of natural purified sCD40 used in the immunoglobulin production experiments (1 µg/ml) was incubated with 40 µl of cyanogen bromide (CNBR)-activated sepharose beads (Pharmacia AB, Orsay, France) covalently coupled with the non-blocking anti-CD40 antibody (7C3) at 4° under agitation. Three rounds of depletion with fresh beads were performed and sCD40-depleted fraction was dialysed against 2 l of PBS. Immunoglobulin production was assessed after 7 days of culture by ELISA, as previously described.35
Sephacryl S-200 gel filtration
Purified sCD40 from EBV-transformed JY supernatant or rsCD40 at a final concentration of 500 ng/ml (1 ml) were fractionated through a Sephacryl S-200 column (Pharmacia AB: 16 × 60 cm, bed volume (Vo) = 120 ml, excluded volume (Ve) = 35 ml). Fifty to 60 eluate fractions of 1 ml were collected after the first Vo and calibrated for molecular size by use of the following marker proteins: carbonic anhydrase, 29 000 MW; albumin, 66 000 MW; alcohol dehydrogenase, 150 000 MW; and β-amylase, 200 000 MW (Sigma Aldrich).
Hepatitis B vaccination
Three subcutaneous injections of 40 µg hepatitis B vaccine (ENGERIX B20, Smithkline-Beecham, Nanterre, France) were administered to 17 haemodialysed patients (see Table 2) who did not present with anti-hepatitis B antibody before the vaccination. One month separated each injection and control of immunization was monitored 1 month after the third injection by measuring serum anti-HBs IgG levels by ELISA (ENZYGNOST HBs Immunoglobulin Kit, Dade Behring S.A, Paris, France). Response to hepatitis B vaccination was considered as positive if anti-HBs antibody levels were above 10 IU/l (detection threshold of the ELISA). In parallel, concentration of serum sCD40 were determined before each vaccine injection and at the time of anti-HBs antibody titre determinations in blood.
Table 2.
Characteristics of the patients included in the hepatitis B vaccination study
| Non-responsive (n = 8) | Responsive (n = 9) | |
|---|---|---|
| Sex ratio (M/F) | 3 | 8 |
| Age (years, median (range)) | 65·5 (38–90) | 58 (17–76) |
| Primary renal disease (n) | ||
| Tubulointerstitial | 3 | 3 |
| Vascular | 0 | 1 |
| Hereditary | 1 | 1 |
| Diabetes | 2 | 3 |
| ND | 2 | 1 |
| Duration of the treatment bydialysis (years, median (range)) | 2 (1–3) | 1 (1–5) |
Statistical analysis
Data are expressed as medians (range). Continuous variables were compared with the non-parametric Mann–Whitney U-test for unpaired variables or by the Wilcoxon rank sum test for paired variables. Correlation significance was assessed by the Z-test. All statistical calculations were made with the Statview software package (Abacus Concepts). Differences were considered to be significant at P < 0·05.
Results
Serum sCD40 concentration is increased in patients with CRF
The presence of sCD40 was evaluated by ELISA in serum and urine of 10 healthy subjects. Low levels of sCD40 were found in the serum (0·37 (0·07–0·76) ng/ml) contrasting to the high amounts measured in urine (1·02 (0·45–3·81) ng/ml) corresponding to an excretion of 1100 (300–5500) ng per 24 hr.
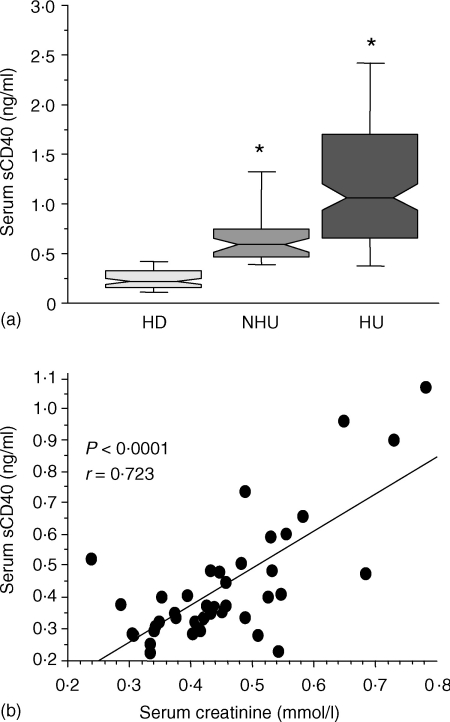
As this result suggested that sCD40 might be eliminated by the kidney in physiological conditions, we then examined the impact of an impaired renal function on the concentration of this molecule in the serum. Soluble CD40 was measured in a population of patients with chronic renal failure, comprising haemodialysed patients (n = 162) and non-haemodialysed uraemic patients (n = 43), who were compared to the group of healthy donors (n = 83). Figure 1a reveals that levels of sCD40 were 2·5-fold higher in uraemic patients 0·58 (0·34–1·98) ng/ml when compared to healthy donors (0·22 (0·04–1·00) ng/ml). In these patients, sCD40 levels correlated with serum creatinine concentration (Fig. 1b r = 0·53, P = 0·0002).
Figure 1.
Increased levels of serum sCD40 in patients with kidney failure and correlation with creatininemia. (a) Serum sCD40 levels in healthy donors (HD), non-haemodialysed uraemic patients (NHU) and haemodialysed uraemic patients (HU) were determined by ELISA. Results are shown as medians, 25th and 75th percentiles (bottom and top of histograms), and 10th and 90th percentiles (bottom and top range bars). *P < 0·0001, determined using a Mann–Whitney test between patients and healthy donors. Soluble CD40 levels in HU patients were detected just before the onset of dialysis session. (b) Serum sCD40 levels of the NHU were correlated with creatininemia. Correlation was determined with the Z-test.
The increased serum sCD40 levels were further amplified in haemodialysed patients reaching 1·07 (0·15–9·27) ng/ml (Fig. 1a). For each individual, sCD40 levels just before and after the haemodialysis session on cellulosic or synthetic membrane were similar demonstrating that serum sCD40 is not eliminated through this process (1·05 (0·15–9·27) before versus 1·13 (0·11–8·74) ng/ml after, n = 156, P = 0·1). In these patients, the absence of residual diuresis was associated with high levels of sCD40 (P = 0·0002; data not shown). Also, elevated sCD40 amounts correlated with the duration of the treatment by dialysis (P = 0·0002; data not shown). In contrast, initial nephropathy did not have any influence on sCD40 level (data not shown).
All together, these data indicated that there is an accumulation of sCD40 during the progression of the renal failure, process which culminates in anuric long-term haemodialysed patients.
Serum sCD40 returns to normal level when renal function is restored by kidney transplantation
To directly address the involvement of the kidney on sCD40 elimination, we examined the evolution of serum concentration of this molecule in patients with end-stage renal failure when they return to normal kidney function, in the course of a renal transplantation. In nine patients studied longitudinally from day 0 until several months post-transplantation, serum sCD40 levels abruptly dropped to basic levels within the very first days following the kidney graft (Fig. 2a and b), and then stabilised at the levels seen in healthy subjects. Simultaneously, the urine excretion of sCD40 was high during the first 2–3 days and then decreased to the normal values (Fig. 2c). In all the patients studied, the close kinetics of sCD40 and creatinine blood levels post-transplantation indicated that the early decrease of sCD40 also closely correlated with the return to a normal kidney function (Fig. 2c).
Figure 2.
Evolution of sCD40 levels in serum of kidney transplant recipients after the graft. Serum and urine sCD40 levels, and creatininemia were measured longitudinally in nine kidney recipients just before and for 3 months after transplantation. All patients recovered normal kidney function at day 11 (mean ± SD = 11·4 ± 7·2 days) as showed by normal creatininemia values. sCD40 levels from one representative patient (a), and before or at day 20 after transplantation for all patients (b). sCD40 levels in urine (○) and serum (•), and creatininemia (□) from one representative patient (c).
Natural sCD40 binds CD154 and inhibits CD40/CD154 interaction in vitro
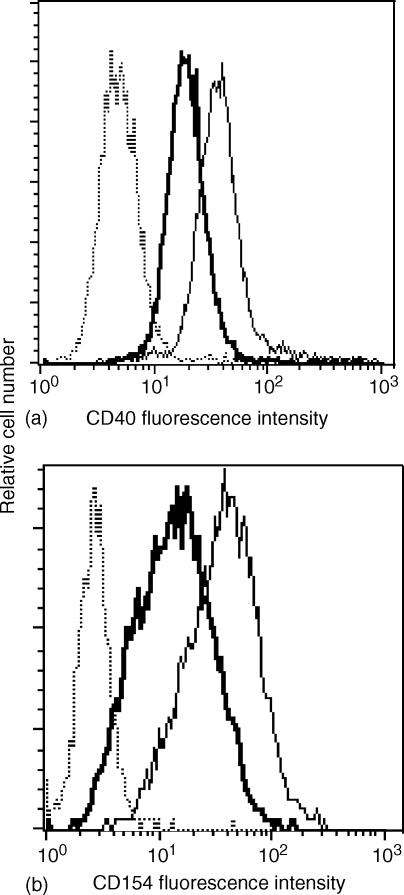
To analyse its biological activity, natural sCD40 was purified from the lymphoblastoid JY B-cell line culture supernatant. Similarly to the CD40Fc chimeric molecule, purified natural sCD40 was fully able to bind its ligand constitutively expressed on the surface of CD154-transfected Jurkat cells (Fig. 3a). By contrast, rsCD40 (up to 100 µg/ml) never bound membrane CD154 (data not shown). Furthermore, incubation of sCD40 with a soluble recombinant form of CD154 partially inhibited binding of the latter on membrane CD40 expressed by an EBV-transformed B-cell line (Fig. 3b).
Figure 3.
Soluble CD40 binds to CD154 and inhibits CD40/CD154 interaction. (a) 5 × 104 JKCD154 cells were incubated with 1·5 µg/ml of affinity purified natural sCD40 from JY supernatant (thick line), 25 µg/ml of CD40Fc (thin line) or PBS 0·1% BSA (dotted line) and labelling was revealed with PE-conjugated EA5 anti-CD40 mAb recognizing CD40 even when it is coupled to CD154. (b) Recombinant soluble CD154 (5 µg/ml) was preincubated (v:v) with 1·5 µg/ml of sCD40 (thick line) or PBS 0·1% BSA (thin line). This mixture was then incubated with 5 × 104 CD40 expressing EBV-transformed B cells. Bound soluble CD154 was revealed with an anti-CD154 PE-conjugated antibody. The dotted line represents the labelling with the isotypic control.
As in vitro stimulation of B lymphocytes with CD154, IL-2 and IL-10 has been previously shown to induce their differentiation into immunoglobulin-secreting cells34,36 we examined the effect of purified natural sCD40 in this setting. As shown in Fig. 4(a), on a 7-day culture period sCD40 was able strongly to inhibit the production of immunoglobulins by activated tonsil B lymphocytes. To control that this inhibition was not mediated by a putative contaminant purified together with sCD40, we specifically depleted sCD40 from the sample using beads covalently coupled to an anti-CD40 mAb different from that used for sCD40 purification (see Materials and methods). As measured by ELISA this procedure depleted 80% of sCD40. Accordingly, sCD40 depletion nearly fully restored immunoglobulin production in vitro, showing that the observed antagonistic function is indeed mediated by sCD40 itself (Fig. 4a). To further confirm the antagonistic effect of sCD40 in a model in which CD154 is not artificially expressed, tonsil B cells were activated with gamma-irradiated allogenic PBMC in the presence of IL-2 and IL-10 and IgG production was measured in culture supernatant at day 7. As shown in Fig. 4(b), sCD40 prevented the IgG production induced by allogeneic PBMC as efficiently as the blocking anti-CD154 mAb did, whereas partial depletion of sCD40 nearly fully restored IgG production.
Figure 4.
Soluble CD40 inhibits immunoglobulin production by tonsil B cells activated with CD154. (a) 5 × 104 purified tonsil B cells were cultured with IL-10 (50 ng/ml) and IL-2 (10 IU/ml) in the presence of untransfected L cells or with CD154 transfected L cells with or without 1 µg/ml of purified natural sCD40 or the same sample depleted for sCD40 (only 0·2 µg/ml sCD40 remaining). (b) 4 × 104 irradiated allogeneic PBMC were cultured with 2 × 104 purified tonsil B cells in the presence of IL-10 (50 ng/ml) and IL-2 (10 U/ml) with 1 µg/ml of purified natural sCD40 or the same sample depleted for sCD40 (ΔsCD40, only 0·2 µg/ml sCD40 remaining), or with 5 µg/ml of blocking anti-CD154 mAb or irrelevant control mAb. After 7 days of culture, supernatants were harvested and total immunoglobulin or IgG production was determined by ELISA as described in materials and methods. Results are expressed in mean ± SD of a triplicate culture and is representative of at least three independent experiments.
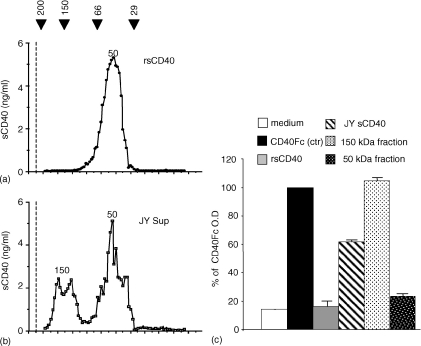
Natural sCD40 presents oligomeric forms by contrast to rsCD40
Since the efficiency of the rsCD40 to block the CD154 signal has been correlated with its degree of oligomerization37 we examined whether natural sCD40 is oligomerized. For this purpose we used gel filtration analysis (Fig. 5a and b) and also an homotypic ELISA (in which mAb89 is used for capture and revelation) allowing only the detection of at least dimeric form of sCD40, as proved by positive signal obtained with the dimeric CD40Fc (Fig. 5c). As seen in Fig. 5(a), gel filtration experiments revealed that rsCD40 was 50 000 MW and is monomeric as it is not detected in the homotypic ELISA (Fig. 5c). With respect to natural sCD40 purified from JY culture supernatant, the most abundant form similarly displayed a MW of 50 000, but an additional higher MW form of 150 000 is also evidenced. This is most probably a multimeric form of sCD40 since it is detected in the homotypic ELISA in contrast to the 50 000 MW form. It is noteworthy that similar 150 000 and 50 000 MW forms were also found in sCD40 purified from urine of healthy subjects (data not shown).
Figure 5.
Natural sCD40 exists under multimeric forms. Gel filtration profiles of rsCD40 (a) and purified natural sCD40 (b). One ml (500 ng) of sCD40 purified from JY cell line supernatant or rsCD40 were run through a Sephadex S-200 column. Eluate fractions were tested for the presence of sCD40 by ELISA as described above. Black Arrows correspond to molecular weights of proteins used for the calibration curve (see Materials and methods). Numbers indicate approximate molecular weight of each form. Dotted lines correspond to the void volume (Vo = 35 ml). (c) 4 ng/ml of CD40Fc, rsCD40, purified sCD40 from JY culture supernatant before (JY sCD40) or after gel filtration separation (150 000 or 50 000 MW fraction) were analysed in homotypic ELISA. CD40Fc was taken as the CD40 dimerized control and values are expressed as percentage of the optical density obtained with the CD40Fc control.
Taken together these results highlight a heterogeneity between natural and rsCD40.
High levels of blood sCD40 are associated with an absence of humoral immune response to hepatitis B vaccination in haemodialysed patients
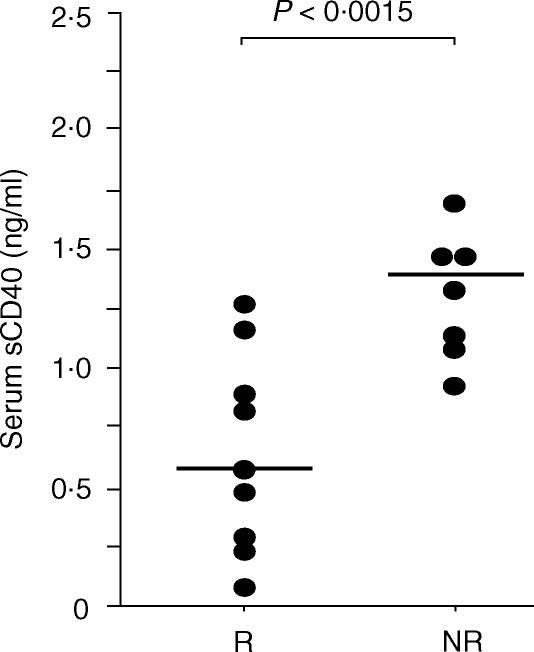
Since natural sCD40 is able to inhibit in vitro immunoglobulin production mediated by CD40/CD154 interaction, we analysed if the increase of this molecule in the serum of haemodialysed patients could be correlated to an impaired humoral immune response in vivo. During a 6-month period, all (n = 17) haemodialysed patients who were given hepatitis B vaccination were consecutively included without any selection criteria in a monthly monitoring for their blood amount of sCD40. Soluble CD40 was assayed just before each vaccine injection and one month after the last boost when the response of patients to the vaccination was evaluated by measuring the anti-HBs IgG levels. As sCD40 levels did not change over the entire period time for each individual patient (data not shown), we took the mean of the four values obtained as the amount of sCD40 for one patient. As presented in Fig. 6, patients who responded to vaccination displayed significantly lower levels of sCD40 in the serum (0·57 (0·08–1·17) ng/ml) when compared to patients who were not immunized despite vaccination (1·39 (0·93–2·27) ng/ml; P = 0·0015). These results support the hypothesis of an in vivo involvement of the accumulation of sCD40 in the impairment of humoral immune response in haemodialysed patients.
Figure 6.
Serum sCD40 levels in chronic haemodialysed patients in the course of Hepatitis B vaccination and correlation to their vaccinal response status. Two groups of haemodialysed patients were set up according to their response to hepatitis B vaccination. Responsive patients (R, n = 9) presented >10 IU/l of anti-HBs IgG one month after last vaccine injection whereas Non-responsive patients (NR, n = 8) presented <10 IU/l. sCD40 values of each patients correspond to the mean of the four sCD40 values obtained during the vaccination. Lines represent the median values of sCD40 for all the patients of each group. *P < 0·0015 determined using a Mann–Whitney test.
Discussion
Numerous studies have well documented the crucial role of CD40/CD154 interaction for a productive humoral immune response (for review see 38). The rapid up- and down-regulation of CD154 on the surface of T cells is an obvious and important way of control. In a first step, CD154 is quickly expressed upon T-cell receptor engagement39 and in a second step the CD40 itself contributes to down-regulating CD154 expression on T cells as sustained interaction between CD40/CD154 leads to endocytosis of the ligand.40 However, only a few studies have focused on the role that natural sCD40 might play on the CD40/CD154 interaction. In vitro, sCD40 has been shown to be produced by B lymphocytes cocultured with anti-CD3 activated T cells and to inhibit the recognition between CD154 and CD40Fc through competition.28 In the present study, the large amounts of sCD40 found in urine of healthy individuals suggested that it is actively produced under physiological conditions and may play an important role in regulating CD40/CD154 interactions in vivo. The aim of this study was to delineate the regulatory role of natural sCD40 and its impact on humoral immunity in uraemic patients who are well known for presenting altered immune functions.
We found that uraemia is associated with high levels of serum sCD40, confirming a previous study by Schwabe et al.41 Several features indicated that an impaired urine excretion of sCD40 as a result of renal dysfunction is the most likely direct cause for the accumulation of sCD40 in the serum of uraemic patients. First, sCD40 is massively secreted in urine of healthy subjects while it is not eliminated by dialysis sessions. Second, high serum sCD40 levels closely correlated with elevated creatininaemia in non-haemodialysed uraemic patients, and with diuresis loss and duration under extrarenal epuration in haemodialysed patients. Third, when patients received a kidney graft, sCD40 was rapidly eliminated in urine, and serum levels dropped to basic values in the very first days following transplantation, in close association with recovery of renal function monitored by creatininaemia. Little is known about the mechanism underlying sCD40 production. A recent report revealed post-transcriptional regulation of murine and human CD40 expression through alternative splicing leading to the production of several isoforms among which a soluble CD40 molecule.42 However, a shedding of the membrane protein through proteolytic cleavage cannot be ruled out, as it has been demonstrated for other members of TNF receptor family: TNF receptor II43 and CD30.44 The conditions favourable to the production of the soluble rather than the membrane form of CD40 are unknown.
Our data show that purified natural sCD40 is able to bind CD154, as previously demonstrated for sCD40 produced in T–B-cell cocultures.28 This was in accordance with detection of sCD40 in the serum of mouse mammary tumour virus infected mice, which binds and induces the internalization of CD154 on the surface of T cells.45 Furthermore, we demonstrated that sCD40 inhibited CD154 ligation onto membrane CD40 and strongly diminished the production of immunoglobulins by CD154-activated B lymphocytes. The antagonistic effect of natural sCD40 contrasted with the absence of functional effect of rsCD40 which is unable to bind CD154 (data not shown). Gel filtration experiments showed that natural sCD40 has a high MW (150 000) form, which might be oligomerized molecules never found with rsCD40. Hence, it is tempting to speculate that these high MW forms are highly biologically active ones. This hypothesis is supported by the work of Holler and colleagues37 who engineered a polymeric sCD40 molecule that displays a higher inhibition activity than dimeric CD40Fc on CD154-mediated proliferation of B cells. The close correlation we observed between high levels of sCD40 and the weak response to hepatitis B vaccination in haemodialysed patients, together with the in vitro data, are suggestive of a role for sCD40 in inhibiting the humoral response. Of interest, injections of a recombinant soluble form of CD40 (the CD40Fc chimeric molecule) in mice inhibit primary antigen-specific IgG response and memory B-cell development.46 Moreover, CD154 expression has been shown to be a limiting factor in the induction of high-affinity antibody and antibody-class switching in CD154 transgenic mice.47 In haemodialysed patients, high levels of sCD40 could compete for the ligation of membrane CD40 on CD154 thus resulting in inhibition of antibody production.
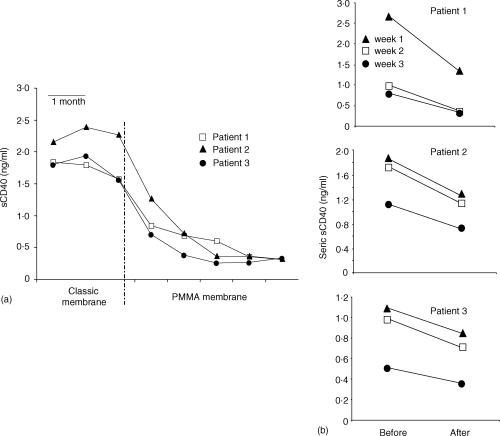
CD40 triggering participates both to the humoral response through direct activation of B lymphocytes, and to the cellular response through induction of B7 expression on antigen-presenting cells instrumental for CD28-full activation of T cells. In addition, triggering of CD154 induces direct activation of T cells. 22,23 Consequently, sCD40 may interfere with each part of the immune response, and may be an important contributor to the humoral and cellular immune dysfunction seen in uraemic patients. Finally, our results identified sCD40 as a new potential uraemic toxin which might be involved in the immune dysfunction associated with chronic renal failure. In this context, the easiest way of restoring an efficient humoral immune response would be to increase the dialytic clearance of sCD40 by the use of a membrane with higher permeability and better adsorbance capacities. In this regard, we obtained promising preliminary results with polymethylmethacrylate (PMMA) membranes, which have been shown to exhibit higher permeability capacity because of an enhanced porosity.48 Indeed, three patients displaying high seric sCD40 levels when dialysed on classical membranes (cellulosic or synthetic) showed a dramatic decrease of these sCD40 concentrations as soon as they were dialysed over a PMMA membrane (Fig. 7a). sCD40 levels dropped during the haemodialysis session (Fig. 7b) indicating that the PMMA membrane is able to eliminate sCD40 from the serum and might thus be useful to improve a patient's response to vaccination.
Figure 7.
Effect of PMMA (BK-F) membrane on the elimination of sCD40 from serum of haemodialysed patients. (a) Longitudinal study of sCD40 levels. Soluble CD40 concentrations were monthly determined by ELISA in the serum of three patients who were dialysed on classical membranes before to be dialysed on BK-F membrane. Blood samples were taken after the dialytic session. (b) Per dialytic elimination of seric sCD40 during haemodialysis on BK-F membrane. Soluble CD40 was measured in the serum taken weekly just before and after one dialysis session on BK-F.
Studying the pathological condition of chronic renal failure should be of interest in understanding the fundamental role of sCD40 in physiological humoral response.
Acknowledgments
We are indebted to P. Chauveau, C. Combe and V. de Précigout for efficient collaboration. We thank D. Coulon for technical assistance in ultrafiltration experiments. We are grateful to the patients who participated in this study.
Cécile Contin was supported by a grant from the Fondation pour la recherche Médicale and the Ligue Nationale contre le Cancer.
References
- 1.Higgins RM. Infections in a renal unit. Q J Med. 1989;70:41–51. [PubMed] [Google Scholar]
- 2.Vamvakas S, Bahner U, Heidland A. Cancer in end-stage renal disease: potential factors involved – editorial. Am J Nephrol. 1998;18:89–95. doi: 10.1159/000013314. [DOI] [PubMed] [Google Scholar]
- 3.Crosnier J, Jungers P, Courouce AM, et al. Randomised placebo-controlled trial of hepatitis B surface antigen vaccine in French haemodialysis units: II, Haemodialysis patients. Lancet. 1981;1:797–800. doi: 10.1016/s0140-6736(81)92679-9. [DOI] [PubMed] [Google Scholar]
- 4.Kohler H, Arnold W, Renschin G, Dormeyer HH, Meyer zum Buschenfelde KH. Active hepatitis B vaccination of dialysis patients and medical staff. Kidney Int. 1984;25:124–8. doi: 10.1038/ki.1984.18. [DOI] [PubMed] [Google Scholar]
- 5.Benhamou E, Courouce AM, Jungers P, Laplanche A, Degos F, Brangier J, Crosnier J. Hepatitis B vaccine: randomized trial of immunogenicity in hemodialysis patients. Clin Nephrol. 1984;21:143–7. [PubMed] [Google Scholar]
- 6.Jungers P, Chauveau P, Courouce AM, Devillier P, Excler JL, Bailleux F, Saliou P. Immunogenicity of the recombinant GenHevac B Pasteur vaccine against hepatitis B in chronic uremic patients. J Infect Dis. 1994;169:399–402. doi: 10.1093/infdis/169.2.399. [DOI] [PubMed] [Google Scholar]
- 7.Descamps-Latscha B, Chatenoud L. T cells and B cells in chronic renal failure. Semin Nephrol. 1996;16:183–91. [PubMed] [Google Scholar]
- 8.Ruiz P, Gomez F, Schreiber AD. Impaired function of macrophage Fc gamma receptors in end-stage renal disease [see comments] N Engl J Med. 1990;322:717–22. doi: 10.1056/NEJM199003153221102. [DOI] [PubMed] [Google Scholar]
- 9.Girndt M, Sester U, Kaul H, Kohler H. Production of proinflammatory and regulatory monokines in hemodialysis patients shown at a single-cell level. J Am Soc Nephrol. 1998;9:1689–96. doi: 10.1681/ASN.V991689. [DOI] [PubMed] [Google Scholar]
- 10.Girndt M, Sester M, Sester U, Kaul H, Kohler H. Defective expression of B7-2 (CD86) on monocytes of dialysis patients correlates to the uremia-associated immune defect. Kidney Int. 2001;59:1382–9. doi: 10.1046/j.1523-1755.2001.0590041382.x. [DOI] [PubMed] [Google Scholar]
- 11.Girndt M, Sester U, Sester M, Deman E, Ulrich C, Kaul H, Kohler H. The interleukin-10 promoter genotype determines clinical immune function in hemodialysis patients. Kidney Int. 2001;60:2385–91. doi: 10.1046/j.1523-1755.2001.00062.x. [DOI] [PubMed] [Google Scholar]
- 12.Degiannis D, Mowat AM, Galloway E, Tsakiris D, Briggs JD, Junor BJ, Parrott DM. In vitro analysis of B lymphocyte function in uraemia. Clin Exp Immunol. 1987;70:463–70. [PMC free article] [PubMed] [Google Scholar]
- 13.Banchereau J, Bazan F, Blanchard D, et al. The CD40 antigen and its ligand. Annu Rev Immunol. 1994;12:881–922. doi: 10.1146/annurev.iy.12.040194.004313. [DOI] [PubMed] [Google Scholar]
- 14.Noelle RJ, Ledbetter JA, Aruffo A. CD40 and its ligand, an essential ligand-receptor pair for thymus- dependent B-cell activation. Immunol Today. 1992;13:431–3. doi: 10.1016/0167-5699(92)90068-I. [DOI] [PubMed] [Google Scholar]
- 15.Durie FH, Foy TM, Masters SR, Laman JD, Noelle RJ. The role of CD40 in the regulation of humoral and cell-mediated immunity. Immunol Today. 1994;15:406–11. doi: 10.1016/0167-5699(94)90269-0. [DOI] [PubMed] [Google Scholar]
- 16.van Kooten C, Banchereau J. CD40-CD40 ligand. J Leukoc Biol. 2000;67:2–17. doi: 10.1002/jlb.67.1.2. [DOI] [PubMed] [Google Scholar]
- 17.Hollenbaugh D, Grosmaire LS, Kullas CD, et al. The human T cell antigen gp39, a member of the TNF gene family, is a ligand for the CD40 receptor: expression of a soluble form of gp39 with B cell co-stimulatory activity. EMBO J. 1992;11:4313–21. doi: 10.1002/j.1460-2075.1992.tb05530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clark EA, Ledbetter JA. How B and T cells talk to each other. Nature. 1994;367:425–8. doi: 10.1038/367425a0. [DOI] [PubMed] [Google Scholar]
- 19.Rousset F, Garcia E, Banchereau J. Cytokine-induced proliferation and immunoglobulin production of human B lymphocytes triggered through their CD40 antigen. J Exp Med. 1991;173:705–10. doi: 10.1084/jem.173.3.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stout RD, Suttles J. The many roles of CD40 in cell-mediated inflammatory responses. Immunol Today. 1996;17:487–92. doi: 10.1016/0167-5699(96)10060-i. [DOI] [PubMed] [Google Scholar]
- 21.Grewal IS, Flavell RA. A central role of CD40 ligand in the regulation of CD4+ T-cell responses. Immunol Today. 1996;17:410–4. doi: 10.1016/0167-5699(96)10030-x. [DOI] [PubMed] [Google Scholar]
- 22.Blair PJ, Riley JL, Harlan DM, et al. CD40 ligand (CD154) triggers a short-term CD4 (+) T cell activation response that results in secretion of immunomodulatory cytokines and apoptosis. J Exp Med. 2000;191:651–60. doi: 10.1084/jem.191.4.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cayabyab M, Phillips JH, Lanier LL. CD40 preferentially costimulates activation of CD4+ T lymphocytes. J Immunol. 1994;152:1523–31. [PubMed] [Google Scholar]
- 24.Aruffo A, Farrington M, Hollenbaugh D, et al. The CD40 ligand, gp39, is defective in activated T cells from patients with X-linked hyper-IgM syndrome. Cell. 1993;72:291–300. doi: 10.1016/0092-8674(93)90668-g. [DOI] [PubMed] [Google Scholar]
- 25.Allen RC, Armitage RJ, Conley ME, et al. CD40 ligand gene defects responsible for X-linked hyper-IgM syndrome [see comments] Science. 1993;259:990–3. doi: 10.1126/science.7679801. [DOI] [PubMed] [Google Scholar]
- 26.DiSanto JP, Bonnefoy JY, Gauchat JF, Fischer A, de Saint Basile G. CD40 ligand mutations in x-linked immunodeficiency with hyper-IgM [see comments] Nature. 1993;361:541–3. doi: 10.1038/361541a0. [DOI] [PubMed] [Google Scholar]
- 27.Kroczek RA, Graf D, Brugnoni D, et al. Defective expression of CD40 ligand on T cells causes ‘X-linked immunodeficiency with hyper-IgM (HIGM1)’. Immunol Rev. 1994;138:39–59. doi: 10.1111/j.1600-065x.1994.tb00846.x. [DOI] [PubMed] [Google Scholar]
- 28.van Kooten C, Gaillard C, Galizzi JP, Hermann P, Fossiez F, Banchereau J, Blanchard D. B cells regulate expression of CD40 ligand on activated T cells by lowering the mRNA level and through the release of soluble CD40. Eur J Immunol. 1994;24:787–92. doi: 10.1002/eji.1830240402. [DOI] [PubMed] [Google Scholar]
- 29.Valle A, Zuber CE, Defrance T, Djossou O, De Rie M, Banchereau J. Activation of human B lymphocytes through CD40 and interleukin 4. Eur J Immunol. 1989;19:1463–7. doi: 10.1002/eji.1830190818. [DOI] [PubMed] [Google Scholar]
- 30.Hennig C, Rink L, Fagin U, Jabs WJ, Kirchner H. The influence of naturally occurring heterophilic anti-immunoglobulin antibodies on direct measurement of serum proteins using sandwich ELISAs. J Immunol Methods. 2000;235:71–80. doi: 10.1016/s0022-1759(99)00206-9. [DOI] [PubMed] [Google Scholar]
- 31.Dechanet J, Grosset C, Taupin JL, Merville P, Banchereau J, Ripoche J, Moreau JF. CD40 ligand stimulates proinflammatory cytokine production by human endothelial cells. J Immunol. 1997;159:5640–7. [PubMed] [Google Scholar]
- 32.Defrance T, Vanbervliet B, Aubry JP, et al. B cell growth-promoting activity of recombinant human interleukin 4. J Immunol. 1987;139:1135–41. [PubMed] [Google Scholar]
- 33.Garrone P, Neidhardt EM, Garcia E, Galibert L, van Kooten C, Banchereau J. Fas ligation induces apoptosis of CD40-activated human B lymphocytes. J Exp Med. 1995;182:1265–73. doi: 10.1084/jem.182.5.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fluckiger AC, Garrone P, Durand I, Galizzi JP, Banchereau J. Interleukin 10 (IL-10) upregulates functional high affinity IL-2 receptors on normal and leukemic B lymphocytes. J Exp Med. 1993;178:1473–81. doi: 10.1084/jem.178.5.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Defrance T, Vanbervliet B, Aubry JP, Banchereau J. Interleukin 4 inhibits the proliferation but not the differentiation of activated human B cells in response to interleukin 2. J Exp Med. 1988;168:1321–37. doi: 10.1084/jem.168.4.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arpin C, Dechanet J, Van Kooten C, et al. Generation of memory B cells and plasma cells in vitro. Science. 1995;268:720–2. doi: 10.1126/science.7537388. [DOI] [PubMed] [Google Scholar]
- 37.Holler N, Kataoka T, Bodmer JL, et al. Development of improved soluble inhibitors of FasL and CD40L based on oligomerized receptors. J Immunol Methods. 2000;237:159–73. doi: 10.1016/s0022-1759(99)00239-2. [DOI] [PubMed] [Google Scholar]
- 38.Marshall LS, Aruffo A, Ledbetter JA, Noelle RJ. The molecular basis for T cell help in humoral immunity. CD40 and its ligand, gp39. J Clin Immunol. 1993;13:165–74. doi: 10.1007/BF00919969. [DOI] [PubMed] [Google Scholar]
- 39.Roy M, Waldschmidt T, Aruffo A, Ledbetter JA, Noelle RJ. The regulation of the expression of gp39, the CD40 ligand, on normal and cloned CD4+ T cells. J Immunol. 1993;151:2497–510. [PubMed] [Google Scholar]
- 40.Yellin MJ, Sippel K, Inghirami G, et al. CD40 molecules induce down-modulation and endocytosis of T cell surface T cell–B cell activating molecule/CD40-L. Potential role in regulating helper effector function. J Immunol. 1994;152:598–608. [PubMed] [Google Scholar]
- 41.Schwabe RF, Engelmann H, Hess S, Fricke H. Soluble CD40 in the serum of healthy donors, patients with chronic renal failure, haemodialysis and chronic ambulatory peritoneal dialysis (CAPD) patients. Clin Exp Immunol. 1999;117:153–8. doi: 10.1046/j.1365-2249.1999.00935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tone M, Tone Y, Fairchild PJ, Wykes M, Waldmann H. Regulation of CD40 function by its isoforms generated through alternative splicing. Proc Natl Acad Sci U S A. 2001;98:1751–6. doi: 10.1073/pnas.98.4.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dri P, Gasparini C, Menegazzi R, et al. TNF-Induced shedding of TNF receptors in human polymorphonuclear leukocytes: role of the 55-kDa TNF receptor and involvement of a membrane-bound and non-matrix metalloproteinase. J Immunol. 2000;165:2165–72. doi: 10.4049/jimmunol.165.4.2165. [DOI] [PubMed] [Google Scholar]
- 44.Hansen HP, Dietrich S, Kisseleva T, et al. CD30 shedding from Karpas 299 lymphoma cells is mediated by TNF-alpha- converting enzyme. J Immunol. 2000;165:6703–9. doi: 10.4049/jimmunol.165.12.6703. [DOI] [PubMed] [Google Scholar]
- 45.Sharma MD, Leite de Moraes M, Zavala F, Pontoux C, Papiernik M. Induction and inhibition of CD40–CD40 ligand interactions: a new strategy underlying host-virus relationships. J Immunol. 1998;161:5357–65. [PubMed] [Google Scholar]
- 46.Gray D, Dullforce P, Jainandunsing S. Memory B cell development but not germinal center formation is impaired by in vivo blockade of CD40–CD40 ligand interaction. J Exp Med. 1994;180:141–55. doi: 10.1084/jem.180.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perez-Melgosa M, Hollenbaugh D, Wilson CB. Cutting edge: CD40 ligand is a limiting factor in the humoral response to T cell-dependent antigens. J Immunol. 1999;163:1123–7. [PubMed] [Google Scholar]
- 48.Arakawa M. Long-term multicentre study on beta 2-microglobulin removal by PMMA. Nephrol Dial Transplant. 1991;6(Suppl. 2):69–74. [PubMed] [Google Scholar]