Abstract
Coeliac disease (CD), a gastrointestinal illness characterized by intestinal malabsorption, results from gluten intolerance accompanied with immunological responses towards gliadin, an ethanol-soluble protein fraction of wheat and other cereals. The role of gliadin in eliciting immune responses in CD is still partly unclear; however, the occurrence of anti-gliadin in the sera of patients suffering from CD correlates well with clinical symptoms. In this work we report the construction of isotype-specific, phage-displayed scFv libraries from peripheral blood lymphocytes of a patient with CD and from a healthy control individual. VH and VL chains were amplified by reverse transcription–polymerase chain reaction (RT–PCR) using a set of oligonucleotides recognizing all human variable gene families. The three scFv libraries (IgA, IgG and IgM) were selectively enriched for gliadin-binding phage. After four rounds of affinity selection, polyclonal enrichment of gliadin-binding phage was observed in all libraries from the CD patient but in none from the healthy donor. Phagemid particles generated from single clones were demonstrated to be gliadin-specific, as shown by strongly positive enzyme-linked immunosorbent assay (ELISA) and BiaCore signals. The VH and VL chains from samples of these monoclonal isotype-specific phage were sequenced to identify the most common variable regions used by the immune system to elicit antibody responses against gliadin.
Introduction
Coeliac disease (CD), or gluten-sensitive enteropathy, has historically been considered as an uncommon gastrointestinal condition. However, recent screening studies in several European countries suggest a prevalence of CD in adults in the range of one per 100–300 individuals.1 CD is triggered by dietary gluten of wheat, rye and barley.2 Gluten can be fractionated into ethanol-soluble prolamines (gliadin, secalin, hordein) and ethanol-insoluble glutenins.3 One of the most common clinical parameters in CD is the occurrence of serum antibodies recognizing gliadin and a structure on the surface of smooth muscle cells, called endomysium.4 The endomysial autoantigen has been recently identified as tissue transglutaminase (tTG), an enzyme located in several different types of tissue.5,6 The exact role of tTG in CD is still unclear, but it is proposed that this enzyme is involved in the activation of gliadin peptides,7 thus leading to their toxicity. Strong evidence currently points to humoral T-lymphocyte-mediated immune mechanisms, resulting in activation of cellular and humoral responses, as key elements in the pathogenesis of CD.3 Different peptide fragments of gliadin have been shown to be presented to T cells in the intestine by human leucocyte antigen (HLA) class II molecules.8 Simultaneous modification of these peptides by tTG may lead to an increased immunogenicity.6 The occurrence of antibodies against both gliadin and tTG is thought to trigger the onset of an autoimmune reaction, leading to chronic inflammation and flattening of the jejunal mucosa and, hence, malnutrition. The intestinal immune system plays a dominant role in the degradation of the morphological and functional properties of the intestinal mucosa in CD. However, the exact pathogenesis of the mucosal lesion in the disease is still partly unknown. CD is considered to occur as the result of interplay of genetic and environmental factors, explaining the wide spectrum of clinical manifestations ranging from asymptomatic phenotypes to severe symptoms of malabsorption,3 including chronic diarrhoea, abdominal distension, weakness, malaise and weight loss.4
Phage display of antibody repertoires has been shown to be a valuable tool for using to study the antibody responses in different pathological conditions.9 To achieve this, the whole antibody repertoire of a donor is amplified by reverse transcription–polymerase chain reaction (RT–PCR) using a set of primers that enable amplification of human variable antibody regions of the heavy (VH) and light (VL) chains.10 As the primers are anchored on constant regions of the antibody genes, isotype-defined amplification products can be distinguished and the exact VH and VL configuration of positive binding clones can be determined by sequencing of the insert subcloned into phagemid vectors. Phagemids for the display of antibody genes can have different formats, allowing display of antibody fragments in different configurations.11 In this study we chose the scFv format, where the VH and VL gene products are coupled by a flexible polypeptide linker and fused to protein pIII of filamentous phage. Upon infection of the host organism (Escherichia coli) with helper phage, phage particles displaying the scFv constructs on their surface, linked to the genetic information required for their production, are generated and screened for antigen specificity by repeated steps of affinity enrichment (reviewed in ref. 12). The aim of the present study was to extend molecular dissection of immunological responses to isotype-specific gliadin antibodies (anti-gliadin) in CD.
Materials and methods
Isolation of peripheral blood mononuclear cells, mRNA and library construction
Peripheral blood mononuclear cells (PBMC), from a healthy individual and from a patient with CD, were isolated from 5 ml of fresh blood either by density-gradient centrifugation on Ficoll13 or by selective lysis of erythrocytes using a commercial kit (Qiagen blood RNA kit; Qiagen, Hilden, Germany). For preparation of total RNA, samples of 107 PBMC were processed using the RNA easy kit (Qiagen) following the protocols recommended by the manufacturer. mRNA was prepared from 7-µg samples of total RNA using the OligoTex mRNA kit (Qiagen). cDNA was generated using a first-strand synthesis kit (SuperscriptII; GibcoBRL, Paisley, UK) and (dT)15 priming, according to the manufacturer's protocol. Polymerase chain reaction (PCR) amplification of the immunoglobulin VH and VL genes was carried out in a total reaction volume of 50 µl, with 1 ng of cDNA as template and a set of specific primers, as described in ref. 14. PCR products were purified by agarose-gel electrophoresis and gel extraction.14 Approximately 20 ng of each first PCR product was used as template for the introduction of flanking restriction sites in a subsequent PCR.14 Products were digested with either XhoI/HindIII (for VL) or MluI/NotI (for VH) and subcloned into appropriately restricted, phosphatase-treated phagemid vector pSEX81 (F. Breitling; GenBank accession number: Y14584), in two steps. First, VL sublibraries were generated, DNA was isolated from phagemid, restricted with MluI/NotI and used to clone the VH repertoires in a second step. Nine different isotype-specific ligation mixtures were used for transformation of E. coli XL1 Blue cells (Stratagene, LaJolla, CA) yielding libraries containing between 1 × 107 and 5 × 108 primary clones. For screening, the libraries harbouring the VH chains of the IgM, IgA and IgG isotype were pooled to obtain libraries only distinguished by the isotype of the heavy chain, but not by the light chain (these libraries were formally termed A, G and M, and isolated single clones were labelled with the letter of the library followed by a number, e.g. A2 for clone IgA no. 2).
Antigens and affinity selection of gliadin-binding phagemids
Commercially available gliadin (crude ethanol extract from wheat gluten; G3375; Sigma Chemical Co., St Louis, MO) was re-extracted with 70% ethanol/10% acetic acid for 48 hr for further purification, centrifuged at 50 000 g and the supernatant lyophilized for long-term storage. Lyophilized extract was reconstituted in ddH2O, cleared by centrifugation (5 min, 15 000 g) and the soluble protein concentration determined according to ref 15. Recombinant α-gliadin was prepared as described below.
Phage were prepared from glycerol stocks of library-transformed E. coli XL1 Blue cells, grown to an optical density at 550 nm (OD550) of ≈ 0·5 in 5 ml of 2*TY broth (Bio101, Carlsbad, CA) supplemented with 12·5 µg/ml tetracycline and 100 µg/ml ampicillin (2*TY AT), by superinfection with helper phage VCSM13 (Stratagene). After 1 hr of incubation (37°, at 200 r.p.m. on an orbital shaker), 45 ml of 2*TY AT broth containing kanamycin (60 µg/ml) was added and incubation continued overnight (37°, at 250 r.p.m. on an orbital shaker). Phage were isolated from supernatants by precipitation with polyethylenglycol 6000/NaCl and centrifugation, as described previously,14 pelleted and dissolved in 3 ml of Tris-buffered saline (TBS). Suspensions were cleared by centrifugation (3 min, 10 000 g), supernatants transferred to fresh tubes and the number of colony-forming units (CFU) determined by plating serial dilutions on agar plates.16
Biopanning was performed in ImmunoTubes (Nunc, Roskilde, Denmark) that had been coated with 5 µg of extracted or recombinant gliadin in phosphate-buffered saline (PBS) and blocked with 2% skimmed milk powder in TBS. Phage (1010 CFU), diluted in blocking buffer, were added to blocked tubes. After incubation (2 hr, 37°), the ImmunoTubes were washed with 1% Tween in TBS and ddH2O, with the stringency increasing with the number of panning rounds (see below). Bound phage were eluted with 0·5 ml of 0·1-m HCl (pH 2·2 adjusted with solid glycine), neutralized with 60 µl of 1-m Tris–HCl (pH 8·0) and used for infection of freshly cultured, exponentially growing E. coli XL1 Blue cells. The titre of the eluted phage was determined in CFU by spreading serial dilutions of the freshly infected bacteria onto agar plates containing ampicillin and tetracyline. Phage rescue and biopanning were repeated three to four times, as described previously.16
Heterologous expression of gliadin
A cDNA library of wheat was constructed in the phagemid vector pJuFo17 and affinity enriched with immobilized serum IgE from patients suffering from wheat allergy. Several different clones, of which some also encoded α-gliadin, were identified (after screening and sequencing) as IgE-binding proteins. The α-gliadin coding sequence was subcloned into the high-level expression vector, pQE30 (Qiagen), produced as hexahistidine-tagged protein in E. coli and affinity purified by immobilised metal affinity chromatography (IMAC)18 using standard procedures (Qiagen). The purified recombinant gliadin was used for screening the CD antibody libraries, as described for gliadin extracted from wheat.
Phage enzyme-linked immunosorbent assay (ELISA)
ELISA plates were coated overnight with 500 ng/well of gliadin in PBS. After blocking with 2% milk powder in TBS, ≈ 109 phagemid particles per well (10 µl) were added in 140 µl of blocking buffer containing 0·5% Tween. After incubation and washing, bound phage were detected with a horseradish peroxidase (HRP)-conjugated monoclonal anti-M13 antibody (no. 27-9421-01; Amersham Pharmacia Biotech, Bucks., UK). ELISA plates were developed with 2′,2′-azino-bis(3-ethylbenz-thiazoline-6-sulphonicacid) diammonium salt (No A1888; Sigma Chemical Co.) in 0·05-m citric acid (pH 4·0 adjusted with NaOH, at a concentration of 0·22 mg/ml of the chromophore) to visualize bound phage. Plates were read in an ELISA plate reader (Molecular Devices, Menlo Park, CA) at 410 nm. Several different ELISA set-ups were run, including serial twofold dilutions of phage or coated antigen and inhibition ELISA. For inhibition experiments, phage were preincubated with different concentrations of soluble gliadin in a separate, blocked ELISA plate for 30 min at room temperature, prior to transfer to an ELISA plate for detection of binding.
Surface plasmon resonance analysis
Using the BIAcore system (Biacore, Uppsala, Sweden) the binding and specificity of the polyclonal phagemid particles, and of selected single clones, were measured.19 For the immobilization of gliadin, the sensor chip CM-5 was used. The chip was activated with EDC/NHS solution (Biacore) and gliadin covalently immobilized on the surface by normal human serum (NHS)-mediated covalent coupling of functional groups. Gliadin was coated in two steps to a final resonance response of 1136 resonance units (RU) corresponding to a total amount of ≈ 1 ng of gliadin bound on the chip. Measurements were performed by injecting phagemid particles displaying scFv (109 CFU) diluted in HEPES-buffered saline (10 mm HEPES; Biorad, Hercules, CA) for 1 min, followed by elution for 100 seconds. Binding curves were calculated using the bia-viewer software program. The CM-5 chip was regenerated for 5 min using solutions and protocols provided by the supplier.
DNA sequencing and analysis
The V genes from different scFv-displaying clones were sequenced by the chain-termination method,20 using primers located on the vector. The isotype membership was confirmed using sequence analysis software (Omiga 2·0 and gcg 10·0; Accelrys, Cambridge, UK) and database searches.
Results
Library construction and quality control
The isotype-specific scFv libraries were constructed from cDNA obtained from whole-blood PBMC of a voluntary, untreated CD patient and a healthy donor; PBMC were collected after obtaining written consent and according to ethical guidelines. VH and VL chains were amplified by PCR using 5′ primers recognizing all human V genes. For amplification of VH chains, 3′ primers (specific for IgA, IgG and IgM) were used to generate isotype-specific libraries.10 VL amplification products were first cloned into the phagemid vector, pSEX81, to obtain a light-chain repertoire library (2 × 108 primary clones), which was further converted into isotype-specific scFv sublibraries by insertion of the appropriate VH amplification products. For each IgA, IgG and IgM isotype, serial independent ligations were performed and only transformation batches with >5 × 107 transformants/µg of DNA were used. For screening purposes, the three best transformations of each isotype were pooled to obtain primary libraries of ≈ 3 × 108 independent clones for IgA, IgG and IgM, respectively. Thirty randomly selected clones from each pooled library were shown, by PCR amplification, to contain >95% full-length scFv inserts. Restriction analysis performed with BstNI9 generated a different restriction pattern for each clone, further confirming the diversity of the libraries. The libraries were reported according to the VH isotype (e.g. A, G and M), and single clones isolated from the different libraries were referenced by the isotype followed by a number.
Biopanning
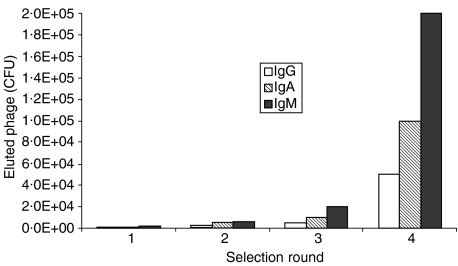
Four consecutive rounds of biopanning were carried out to isolate gliadin-specific phagemids of the different isotypes. Following an increasing stringency strategy, the washing time was increased from direct liquid removal in the first two biopanning rounds to standing times, of the washing fluid, of up to 5 min in the two following rounds. The number of washing steps was increased from 10 to 25, by an increment of five for each round of panning. All isotype-specific libraries, containing the antibody repertoire of the CD patient, showed selective enrichment of clones containing full-length scFv constructs (Fig. 1). Biopanning of the libraries constructed from the immunoglobulin repertoire of the healthy control individual failed to show enrichment above background, as expected (data not shown).
Figure 1.
Isotype-specific phage enrichment during subsequent rounds of affinity selection. The number of phage was determined as colony-forming units (CFU) after infection of freshly grown Escherichia coli XL1 Blue cells. Notably, the absolute number of eluted phage significantly depends on the isotype library used for screening.
The biopanning with recombinant gliadin was stopped after three cycles, because no evidence for phage enrichment was observed. Lack of specific enrichment was shown by PCR analysis of 40 single clones. In 15 cases, no amplification product was detectable, whereas 25 clones showed large deletions within the scFv insert. Therefore, we conclude that the phagemids selected are not specific for gliadin and probably represent background phage overgrowing the libraries.21
Antigen specificity of the selected phage
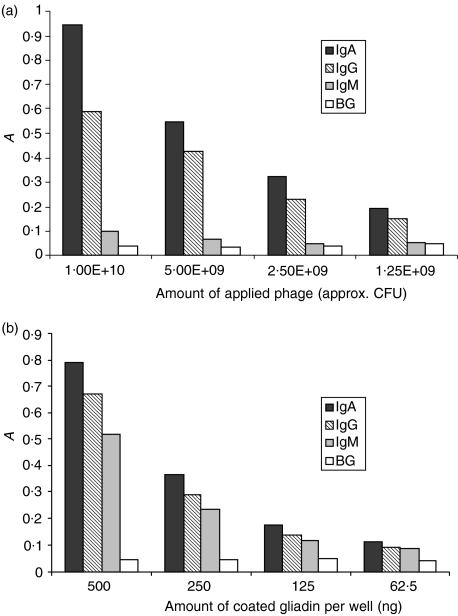
Polyclonal phage, obtained by selective enrichment of the isotype-specific libraries, were tested in an ELISA for their ability to bind to natural gliadin. As shown in Fig. 2(a), the polyclonal mixtures showed a titration-dependent binding to gliadin. Figure 2(b) shows the same experiment where the number of phage was kept constant (109 CFU) and the coated antigen diluted 1 : 1 throughout the plate. This experiment excludes non-specific binding of the phage to the plastic surface or to the blocking agents. In both experiments, the absorbance (A) followed the linear titrations of both phage and antigen, indicating gliadin specificity of the polyclonal scFv-displaying phage. Inhibition ELISAs were performed with phage rescued from single clones to confirm the antigen specificity of the selected scFvs. As shown in Fig. 3, increasing the concentration of gliadin in the fluid phase reduced the specific binding of the scFv-displaying phage to solid phase-coated gliadin, in a concentration-dependent manner, whereas inhibition with non-specific antigen (bovine serum albumin) and recombinant gliadin did not influence the binding capacity of the phage. A maximum inhibition of ≈ 60% was obtained at a concentration of free α-gliadin of 260 nm, which is the range of the maximal solubility of α-gliadin in aqueous, physiological solutions. Some selected clones were used in an ELISA-inhibition experiment, using a serum pool of CD patients with high gliadin-specific IgA titres. Serum was serially diluted in a gliadin-coated plate and, after 30 min of preincubation, 1010 phage were added to each well. This serum-inhibition experiment clearly shows that with decreasing serum concentration an increasing amount of gliadin-specific phage can bind to the antigen (Fig. 4). Whether this behaviour is a result of steric hindrance or (as expected) competition for binding to shared epitopes, remains to be elucidated.
Figure 2.
(a) Binding of isotype-specific polyclonal phage mixtures obtained after four rounds of affinity selection to immobilized gliadin (400 ng/well). A total of 1010 phagemid particles were serially diluted 1 : 1 throughout an enzyme-linked immunosorbent assay (ELISA) plate and visualized with horseradish peroxidase (HRP)-labelled anti-M13 monoclonal antibody (mAb). The first four dilutions for each isotype are shown. As background (BG), phagemid particles rescued from the original, unselected libraries were used. (b) Binding of isotype-specific polyclonal phage mixtures (as in panel a) to decreasing amounts of coated gliadin and constant phage load (1010 phagemid particles/well).
Figure 3.
Competition enzyme-linked immunosorbent assay (ELISA) of different scFv phage clones selected from the IgA (▪, ×), IgG (•, –) and IgM (○) libraries for binding to solid-phase coated gliadin, showing increasing inhibition with increasing gliadin concentrations in fluid phase. (♦) shows the inhibition of phage A21, with bovine serum albumin (BSA) used as a negative control.
Figure 4.
Serum-inhibition enzyme-linked immunosorbent assay (ELISA). Dilutions of a serum pool from coeliac disease (CD) patients with a high immunoglobulin A (IgA) anti-gliadin titre (260 IU/ml) were preincubated on a gliadin-coated plate. A constant amount of single clone phage IgA (♦, ▪), IgG (▴) and IgM (•) was applied to each well and the binding curves were determined. Low-affinity binders derived from the IgM library seem to be much more susceptible to inhibition by serum antibodies than high-affinity IgA and IgG binders. The lack of binding inhibition of a mixture of gliadin-positive IgA, IgG and IgM phage to gliadin, by serum of a healthy donor used as a negative control, is shown (×).
Surface plasmon resonance analysis
To clearly demonstrate the specificity of the selected scFv-carrying phage for gliadin, surface plasmon resonance measurements were performed using the BIAcore system.19 The relative binding capacity of different scFv-displaying phage for α-gliadin increased, on average, by 90 RUs during the association phase, and an average of 80 ± 5 RUs remained as the relative binding value after 100 seconds of elution, indicating a remarkable increase in the binding affinity. In contrast, measurements of polyclonal phagemid particles rescued from the unselected libraries or helper phage (as negative controls) showed only marginal binding between 0 and 5 RUs after elution. The binding curves (data not shown) indicated a high relative affinity of the ELISA-positive scFv-displaying clone for the α-gliadin extract.
Sequence analysis of the isotype-specific VH and VL genes
Six ELISA-positive clones of the IgA, four of the IgG and three of the IgM isotype were sequenced and shown to contain full-length VH and VL scFv fragments. The cDNA and deduced amino acid sequence of representative clones for each isotype were submitted to GenBank/EMBL (accession numbers: AJ557893, AJ557894, AJ557895 and AJ557896). Interestingly, all light chains were of the Lambda type, belonging to several of the 10 λ variable-region families, in spite of the fact that the isotype-specific libraries used for screening contained an equivalent number of VH-λ- and VH-κ-displaying phage. This is in contrast to previous work9 reporting usage of both λ and κ light chains in α-gliadin-selected repertoire libraries and may represent a restricted immunological response of the patient to gliadin. With one exception, represented by an IgG clone showing a VH region belonging to family 1, all other clones were restricted to the VH-3 family. Neither bias is the result of a lack of diversity in the library because PCR amplification with the family-specific VH and VL primers yielded comparable amplification signals (data not shown).
Discussion
Dietary exposure to wheat gliadin and homologous proteins in rye, barley and oats plays an important role in the development of immunological responses in CD. Antibodies to gliadin of the IgA and IgG isotypes were the first diagnostic antibodies used to confirm clinically suspected CD and are still widely used as diagnostic tools despite the fact that more specific tests are now available.22 In this study we constructed and screened antibody repertoire libraries from a CD patient and a healthy donor for the presence of binders to natural gliadin extract and recombinant α-gliadin. The α-gliadin coding sequence was cloned from a cDNA library of wheat displayed on the phage surface23 and affinity enriched with serum IgE of individuals suffering food allergy to wheat, unrelated to CD (M. Weichel, unpublished).
In this study we confirmed that the antibody response, to gliadin, of an individual suffering from untreated CD followed the predictions that can be drawn from the clinical manifestation of the disease. The fact that enrichment of the CD libraries with extracted (partially deamidated) gliadin was successful indicates that immune responses specific for this type of gliadin are elicited in CD individuals, but not in unaffected persons. This is not dissimilar to the commonly accepted theory of T-cell-mediated immune response and the involvement of tTG in humoral responses to dietary gliadin.3 In contrast to previously described work,9 investigating the IgA responses in CD, we focused on the enrichment and investigation of isotype-dissected phage libraries. The phage pool isolated from the IgA library yielded a high percentage of ELISA-positive phage (79%), which was comparable to the number of positive phage isolated from the IgM pool (83%) (Table 1). In contrast, selection from the IgG library yielded a high background of non-specific phage and a relatively low number of specific binders (21%) (Table 1). Phage reaching A values of at least 10 times higher than background levels were considered as ELISA positive. Among positive clones, phage reaching A values of >0·7 were considered to be high-affinity binders and reached 90, 80 and 15% of the positive clones for IgA, IgG and IgM, respectively (Table 1). This is in agreement with the immunological response to gliadin in CD, which has been shown to elicit antibody of all three isotypes.24 It is remarkable that only libraries selected with natural gliadin extract showed enrichment, whereas no specific enrichment was detected with recombinant gliadin. The fact that natural gliadin is mandatory for recognition by the cloned CD antibody fragments is further supported by the lack of inhibitory effect of recombinant gliadin in ELISA-inhibition assays.
Table 1.
Analysis of isotype-specific binding of scFv single clones to gliadin
| Isotype | Number of clones tested | Clones with an A of >0·1* | Clones with an A of >0·7‡ |
|---|---|---|---|
| IgA | 48 | 38 (79%) | 34 (90% of positives) |
| IgG | 48 | 10 (21%) | 8 (80% of positives) |
| IgM | 48 | 40 (83%) | 8 (15% of positives) |
Clones with absorbance (A) values of >0·1 (5 × background value) were considered as positive.
Clones with A values of >0·7 were considered to be high-affinity binders.
In conclusion, we have shown that phage display of combinatorial antibody scFv libraries represents a suitable approach for the isotype-specific dissection of immune responses occurring in a pathological condition. Furthermore, we demonstrated that only natural gliadin, partially deamidated under acidic conditions (as expected to occur during the ingestion of food gliadin in the stomach), is recognized by the cloned antibodies. A majority of the gliadin ELISA-positive phage isolated, mainly of the IgA and IgG isotype, showed strong A signals and high RU values in surface plasmon resonance measurements, confirming the high antigen specificity of the isolated scFv antibody fragments.25
Acknowledgments
We are grateful to Dr Isabelle Dahinden (Laboratory of Food Chemistry, University of Bern, Switzerland) for supplying PBMCs of a CD patient, and to Dr Helene Joller-Jemelka and Prof. Peter Grob, University Hospital Zürich, for the supply of sera from CD patients. We are indebted to Dr Frank Breitling (DKFZ, Heidelberg, Germany) for his generous gift of the phagemid vector, pSEX81, to Dr Päivi Holopainen and Dr Sabine Flückiger for fruitful discussions and to Dr Gerald Walter for careful comments on the manuscript.
References
- 1.Fasano A, Catassi C. Current approaches to diagnosis and treatment of celiac disease: an evolving spectrum. Gastroenterology. 2001;120:636–51. doi: 10.1053/gast.2001.22123. [DOI] [PubMed] [Google Scholar]
- 2.Shewry PR, Miles MJ, Tatham AS. The prolamin storage proteins of wheat and related cereals. Prog Biophys Mol Biol. 1994;61:37–59. [PubMed] [Google Scholar]
- 3.Schuppan D. Current concepts of celiac disease pathogenesis. Gastroenterology. 2000;119:234–42. doi: 10.1053/gast.2000.8521. [DOI] [PubMed] [Google Scholar]
- 4.Collin P, Kaukinen K, Maki M. Clinical features of celiac disease today. Dig Dis. 1999;17:100–9. doi: 10.1159/000016911. [DOI] [PubMed] [Google Scholar]
- 5.Dieterich W, Ehnis T, Bauer M, Donner P, Volta U, Riecken EO, Schuppan D. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat Med. 1997;3:797–801. doi: 10.1038/nm0797-797. [DOI] [PubMed] [Google Scholar]
- 6.Marzari R, Sblattero D, Florian F, Tongiorgi E, Not T, Tommasini A, Ventura A, Bradbury A. Molecular dissection of the tissue transglutaminase autoantibody response in celiac disease. J Immunol. 2001;166:4170–6. doi: 10.4049/jimmunol.166.6.4170. [DOI] [PubMed] [Google Scholar]
- 7.Molberg O, McAdam SN, Korner R, et al. Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gut-derived T cells in celiac disease. Nat Med. 1998;4:713–7. doi: 10.1038/nm0698-713. [DOI] [PubMed] [Google Scholar]
- 8.O'Keeffe J, Mills K, Jackson J, Feighery C. T cell proliferation, MHC class II restriction and cytokine products of gliadin-stimulated peripheral blood mononuclear cells (PBMC) Clin Exp Immunol. 1999;117:269–76. doi: 10.1046/j.1365-2249.1999.00973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sblattero D, Florian F, Not T, Ventura A, Bradbury A, Marzari R. Analyzing the peripheral blood antibody repertoire of a celiac disease patient using phage antibody libraries. Hum Antibodies. 2000;9:199–205. [PubMed] [Google Scholar]
- 10.Welschof M, Terness P, Kolbinger F, et al. Amino acid sequence based PCR primers for amplification of rearranged human heavy and light chain immunoglobulin variable region genes. J Immunol Methods. 1995;179:203–14. doi: 10.1016/0022-1759(94)00286-6. [DOI] [PubMed] [Google Scholar]
- 11.Little M, Kipriyanov SM, Le Gall F, Moldenhauer G. Of mice and men: hybridoma and recombinant antibodies. Immunol Today. 2000;21:364–70. doi: 10.1016/s0167-5699(00)01668-6. [DOI] [PubMed] [Google Scholar]
- 12.Hoogenboom HR, de Bruine AP, Hufton SE, Hoet RM, Arends JW, Roovers RC. Antibody phage display technology and its applications. Immunotechnology. 1998;4:1–20. doi: 10.1016/s1380-2933(98)00007-4. [DOI] [PubMed] [Google Scholar]
- 13.de Rock E, Taylor N. An easy method of layering blood over Ficoll–Paque gradients. J Immunol Methods. 1977;17:373–4. doi: 10.1016/0022-1759(77)90120-x. [DOI] [PubMed] [Google Scholar]
- 14.Little M, Dübel S, Breitling F. Recent developments in antibody engineering. In: Reischl eU., editor. Molecular Diagnosis of Infectious Diseases. Vol. 13. Totowa, NJ: Humana Press; 1997. [Google Scholar]
- 15.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 16.Crameri R, Achatz G, Weichel M, Rhyner C. Direct selection of cDNAs by phage display. Methods Mol Biol. 2002;185:461–9. doi: 10.1385/1-59259-241-4:461. [DOI] [PubMed] [Google Scholar]
- 17.Crameri R, Suter M. Display of biologically active proteins on the surface of filamentous phages: a cDNA cloning system for selection of functional gene products linked to the genetic information responsible for their production. Gene. 1993;137:69–75. doi: 10.1016/0378-1119(93)90253-y. [DOI] [PubMed] [Google Scholar]
- 18.Crowe J, Dobeli H, Gentz R, Hochuli E, Stuber D, Henco K. 6xHis-Ni-NTA chromatography as a superior technique in recombinant protein expression/purification. Methods Mol Biol. 1994;31:371–87. doi: 10.1385/0-89603-258-2:371. [DOI] [PubMed] [Google Scholar]
- 19.Zeder-Lutz G, Benito A, Van Regenmortel MH. Active concentration measurements of recombinant biomolecules using biosensor technology. J Mol Recognit. 1999;12:300–9. doi: 10.1002/(SICI)1099-1352(199909/10)12:5<300::AID-JMR467>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 20.Sanger F. Determination of nucleotide sequences in DNA. Science. 1981;214:1205–10. doi: 10.1126/science.7302589. [DOI] [PubMed] [Google Scholar]
- 21.Rhyner C, Kodzius R, Crameri R. Direct selection of cDNAs from filamentous phage surface display libraries: potential and limitations. Curr Pharm Biotechnol. 2002;3:13–21. doi: 10.2174/1389201023378535. [DOI] [PubMed] [Google Scholar]
- 22.Stern M. Comparative evaluation of serologic tests for celiac disease: a European initiative toward standardization. J Pediatr Gastroenterol Nutr. 2000;31:513–9. doi: 10.1097/00005176-200011000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Rozynek P, Sander I, Appenzeller U, Crameri R, Baur X, Clarke B, Bruning T, Raulf-Heimsoth M. TPIS – an IgE-binding wheat protein. Allergy. 2002;57:463. doi: 10.1034/j.1398-9995.2002.23649.x. [DOI] [PubMed] [Google Scholar]
- 24.O'Mahony S, Arranz E, Barton JR, Ferguson A. Dissociation between systemic and mucosal humoral immune responses in coeliac disease. Gut. 1991;32:29–35. doi: 10.1136/gut.32.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luger E, Lamers M, Achatz-Straussberger G, Geisberger R, Infuhr D, Breitenbach M, Crameri R, Achatz G. Somatic diversity of the immunoglobulin repertoire is controlled in an isotype-specific manner. Eur J Immunol. 2001;31:2319–30. doi: 10.1002/1521-4141(200108)31:8<2319::aid-immu2319>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]