Abstract
The immunoregulatory cytokine, interleukin-10 (IL-10), has been shown to inhibit the maturation of human myeloid dendritic cells (DC). In the present study, we demonstrate that IL-10 has paradoxical effects on the maturation of murine myeloid bone marrow-derived DC. On the one hand, IL-10 inhibits the maturation of murine myeloid DC. The addition of IL-10 to granulocyte–macrophage colony-stimulating factor (GM-CSF)-supported murine BM-derived DC cultures reduced the frequency of major histocompatibility complex (MHC) class IIbright cells. These IL-10-pretreated DC have a reduced capacity to stimulate T cells in an allogeneic mixed leucocyte reaction. On the other hand, however, and in contrast to the effects of IL-10 on human DC, we found that the addition of IL-10 from the initiation of the culture onwards induced an up-regulation of the expression of the costimulatory molecules CD40, CD80 and CD86 on murine myeloid DC, as compared to DC generated with GM-CSF only. Moreover, a subpopulation of IL-10-pretreated MHC class IIdim DC lacked the capacity to take up dextran-fluorescein isothiocyanate (FITC), a feature of DC maturation. Taken together, our data demonstrate that the generation of murine myeloid DC in the presence of IL-10 results in a population of incompletely matured MHC class IIdim CD80+ CD86+ DC. These DC lack T-cell stimulatory capacity, suggesting a role for IL-10 in conferring tolerogenic properties on murine myeloid DC.
Introduction
Dendritic cells (DC) represent a heterogeneous population of professional antigen-presenting cells (APC), which function as sentinels of the immune system. Immature DC are characterized by a high capacity for antigen capture and processing, and low-level expression of major histocompatibility complex (MHC) class II and costimulatory molecules. During maturation, DC gradually lose their capacity to capture and process antigen, and simultaneously acquire high level expression of MHC class II and costimulatory molecules, thus enabling them to initiate antigen-specific T-cell-mediated immune responses.1,2 It is well established that DC are pivotal for the initiation of primary immune responses and play a key role in the modulation of the immune response by regulation of the T-helper (Th)1/Th2 balance.1,2 In addition, DC also play a crucial role in the establishment and maintenance of T-cell tolerance in the periphery.3 The capacity of DC to initiate and modulate immune responses, or to induce and maintain peripheral tolerance, depends on their maturational state, the kinetics of activation and is regulated by the nature of the pathogen and signals from their microenvironment during DC maturation.1,3–5 The induction of tolerance by immature DC has been attributed to the absence of signals from costimulatory molecules, such as CD40, CD80 and CD86.6 Recently, it has been shown that certain subpopulations of DC, expressing a more or less mature phenotype, also have the capacity to induce antigen-specific tolerance.7–9 In addition, the expression of costimulatory molecules on DC appears to be required for the tolerization of CD8+ T cells and the homeostasis of regulatory CD4+ T cells.10 It has been suggested that mature, but quiescent, DC induce tolerance, whereas activated mature DC, which have received an additional activation stimulus (via an interaction with CD4+ T cells) induce CD8+ T-cell immunity.10,11 Thus, phenotypically mature DC need not necessarily be immunogenic, but may be tolerogenic.
Culture conditions have been established for the in vitro generation of human and murine myeloid DC from progenitors in blood and bone marrow.12–14 The phenotype and functional properties of myeloid DC, propagated in vitro using granulocyte–macrophage colony-stimulating factor (GM-CSF), can be modulated by immunomodulatory cytokines. Interleukin-10 (IL-10) is a regulatory cytokine that modifies the phenotypic and functional maturation of in vitro-propagated myeloid DC and confers tolerogenic properties on human DC.15,16 IL-10 inhibits the up-regulation of expression of MHC class II, the DC maturation marker, CD83, and several costimulatory molecules, on in vitro-generated human myeloid DC. Moreover, IL-10 inhibits lipopolysaccharide (LPS)-induced interleukin-12 (IL-12) production by human DC, thus promoting the differentiation of naïve T cells into Th2 cells.17,18In vitro-generated IL-10-treated human DC are poor stimulators in the allogeneic mixed leucocyte reaction (allo-MLR) and induce a state of alloantigen-specific or peptide-specific anergy in both primed and naïve CD4+ and CD8+ human T cells.16,18,19
In the quest of establishing in vitro culture conditions for generating DC with tolerogenic properties, we analysed, in the present study, the regulatory effects of IL-10 on the phenotypic and functional characteristics of in vitro-generated murine myeloid bone marrow (BM)-derived DC. We found that exogenous IL-10 has paradoxical effects on the maturation of in vitro-generated immature murine myeloid DC. Addition of IL-10 to GM-CSF-supported murine BM-derived DC cultures induces a population of incompletely matured major histocompatibility complex (MHC) class IIdim CD80+ CD86+ DC that lack T-cell stimulatory capacity.
Materials and methods
BM preparation and DC cultures
Female BALB/c and C57BL/6 mice were purchased from Charles River Laboratories (Someren, the Netherlands) and housed under conventional conditions in the experimental animal facility of the Erasmus MC. Both food and water were available ad libitum. Housing, care and all animal experiments were performed in accordance with Dutch legal regulations, which includes approval by a local ethical committee. Femurs and tibia were isolated from BALB/c mice (10–20 weeks old) and mechanically purified from the surrounding tissue. Both epiphysial ends were cut off with scissors and the bone shafts were flushed with RPMI-1640 (Gibco BRL, Breda, the Netherlands) with 10% fetal calf serum (FCS). Mononuclear cells were separated from the single BM cell suspension by density-gradient centrifugation over Lymphoprep 1·077 (Nycomed Pharma AS, Oslo, Norway).
DC were generated from BM cells according to the protocol of Lutz et al.,12 with minor modifications. BM-derived mononuclear cells were cultured at a density of 5 × 104 cells/cm2 in T25 flasks (Corning Life Sciences BV, Schiphol-Rijk, the Netherlands) in 5 ml of RPMI-1640, containing l-glutamine, supplemented with 100 U/ml penicillin and 100 µg/ml streptomycin (Gibco BRL), 0·1 mm 2-mercaptoethanol (Sigma, Zwijndrecht, the Netherlands) and 10% of a preselected batch of heat-inactivated FCS (Gibco BRL; subsequently referred to as RPMI/FCS). The medium was supplemented with 100 U/ml recombinant mouse GM-CSF (PeproTech/Tebu, Le Perray en Yvelines, France). An additional 5 ml of RPMI/FCS, containing 100 U/ml GM-CSF, was added to the cultures on day 3. On days 6 and 8, half of the culture medium was harvested and centrifuged. The cell pellet was resuspended in 5 ml of fresh RPMI/FCS, containing 100 U/ml GM-CSF, and added back to the original cultures. On day 9, non-adherent and loosely adherent cells were harvested by vigorous pipetting. Recombinant mouse IL-10 (PeproTech or R&D systems, Oxon, UK) was added, at a final concentration of 10 U/ml, to the cultures from day 0 onwards, unless stated otherwise. In specificity control experiments, 0·2 µg/ml neutralizing anti-IL-10 polyclonal immunoglobulin (Ig) (PeproTech) was added to the cultures simultaneously with IL-10. Maturation of immature DC was induced by the addition of 1 µg/ml LPS from Escherichia coli (Sigma) on day 8 of the culture. The cells were routinely analysed on day 9 of culture.
Flow cytometric analysis
For cell-surface staining of DC, 1–5 × 105 cells were stained with the following monoclonal antibodies (mAbs): anti-CD11c–fluorescein isothiocyanate (FITC) (clone HL3), anti-MHC class II–phycoerythrin (PE) (clone M5/114), biotinylated anti-MHC class II (clone 2G9) (BD PharMingen, Heidelberg, Germany); anti-CD40–PE, anti-CD80–PE, anti-CD86–PE and anti-CD11b–PE (Beckman Coulter, Mijdrecht, the Netherlands). All incubation steps were conducted for 30 min on ice. Cells incubated with biotinylated mAb were washed and stained with streptavidin–Cy-Chrome or streptavidine-APC (BD PharMingen). Stained cells were resuspended in 500 µl of phosphate-buffered saline (PBS) containing 1% FCS. To exclude dead cells during the analysis, 2 µl of 7-aminoactinomycin D (7-AAD; Molecular Probes, Leiden, the Netherlands) was added to each tube just prior to two-, three- or four-colour flow cytometric analysis on a flow cytometer (FACS calibur; Becton-Dickinson, Immunocytometry systems, San Jose, CA) using cellquest software (Becton-Dickinson).
For analysing the antigen-uptake capacity of DC, 2 × 105 cells were incubated with 1 mg/ml dextran–FITC (Sigma), in 100 µl of RPMI-1640 containing 10% FCS, for 1 hr at 37°. The uptake was stopped by the addition of ice-cold PBS. Cells were then washed with ice-cold PBS. Background uptake of dextran–FITC was measured by incubating the cells for 1 hr on ice. Two-colour flow cytometric analysis was performed after staining the cells with MHC class II–PE.20
For the single-cell detection of intracellular IL-12, DC were stimulated with LPS (1 µg/ml) for 18 hr. Brefeldin A (10 µg/ml, Sigma) was added for the last 4 hr of the incubation to facilitate the intracellular accumulation of cytokines. Cells were washed and stained for surface MHC class II using biotinylated monoclonal anti-MHC class II followed by streptavidin–Cy-Chrome. Cells were then fixed and permeabilized using fix and perm reagents (Caltag Laboratories, Burlingame, CA) and stained for intracellular IL-12 using anti-IL-12–PE (clone C15.6; BD PharMingen).
T-cell purification and the allo-MLR
CD4+ T cells were purified from the spleen and lymph nodes of C57BL/6 mice (10–20 weeks old). Cells were stained with YTA 312 hybridoma supernatant (rat anti-mouse CD4 Ig) and resuspended in 40 µl of PBS containing 1% FCS and 2 mm EDTA per 107 cells. Ten microlitres of goat anti-rat IgG microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) was added per 107 cells, and cells were incubated for 15 min at 4°. After washing, CD4+ T cells were selected with the autoMACS (Miltenyi Biotec) using the posseld program. The purity of the selected CD4+ T cells was always found to be >90%.
CD4+ C57BL/6 T cells were cultured for 4 days in 96-well flat-bottom microtitre plates, at 2 × 105 cells/well, with titrated BM-derived BALB/c DC. Cell cultures were pulsed with 0·1 µCi/well of [3H]thymidine ([3H]TdR) (Amersham, Bucks., UK) for the last 16 hr of culture and harvested on glassfibre filters (Packard Instrument BV, Groningen, the Netherlands). Incorporated [3H]TdR was measured using a liquid scintillation counter (Packard). All cultures were performed in quadruplicate and results are expressed as mean counts per minute (c.p.m.) ± standard error of the mean (SEM). The unpaired Student's t-test was used to compare T-cell proliferation induced by one type of DC with that induced by the other types of DC, for every DC : T-cell ratio. Significance was accepted at the P < 0·05 level.
Results
Modulating effects of IL-10 on phenotype and T-cell stimulatory capacity of in vitro-generated murine myeloid DC
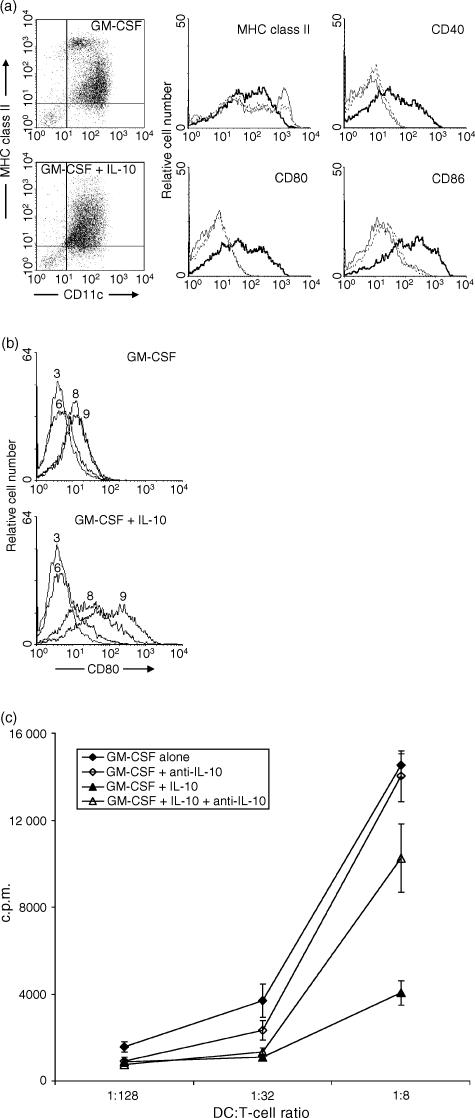
To assess the modulating effects of the immunoregulatory cytokine IL-10 on the phenotypic maturation of murine myeloid DC, we generated DC from murine BM cells with GM-CSF in the continuous presence or absence of IL-10. After 9 days of culture, non-adherent and loosely adherent cells were harvested and their phenotype was analysed. Murine BM-derived DC cultures supported with GM-CSF yielded heterogeneous populations of >80% CD11c+ cells, including a major population of immature MHC class IIdim and a minor population of mature MHC class IIbright DC, which also expressed low levels of the costimulatory molecules CD40, CD80 and CD86 (Fig. 1a), as reported previously.12,21
Figure 1.
Effects of interleukin-10 (IL-10) on the phenotype and T-cell stimulatory capacity of in vitro-generated murine myeloid bone marrow (BM)-derived dendritic cells (DC). DC were generated from BALB/c BM cells by culture in the presence of granulocyte–macrophage colony-stimulating factor (GM-CSF), with or without IL-10. Neutralizing anti-IL-10 immunoglobulin (Ig) was added simultaneously with IL-10 when indicated, as a specificity control. (a) The expression of major histocompatibility complex (MHC) class II, CD40, CD80 and CD86 was analysed by three-colour flow cytometry on day 9 of culture. Expression of costimulatory molecules by gated CD11c+ cells is shown in histograms: GM-CSF alone (solid line), GM-CSF + IL-10 (bold solid line) and GM-CSF + IL-10 + anti-IL-10 Ig (dotted line). (b) Kinetics of acquisition of CD80 by DC. Cells were analysed by flow cytometry, for the expression of CD80, at different time-points (days 3, 6, 8 and 9) after initiation of the culture. (c) IL-10 inhibits the T-cell stimulatory capacity of DC. Graded numbers of day-9 DC were cultured with purified allogeneic (C57Bl/6) CD4+ T cells. The proliferation was determined after 4 days by measuring the incorporation of [3H]thymidine. Representative examples of five experiments are shown.
As compared to DC generated with GM-CSF alone, addition of IL-10 from the initiation of the culture onwards, did not change the final yield of DC at day 9 or the percentage of CD11c+ cells. However, addition of IL-10 reduced the frequency of MHC class IIbright DC (Fig. 1a). Remarkably, the expression of the costimulatory molecules CD40, CD80 and CD86 was simultaneously upregulated by IL-10 (Fig. 1a). These studies were performed with two different sources of IL-10, and comparable results were obtained on each occasion (data not shown). To confirm that these effects were indeed caused by IL-10, we added neutralizing polyclonal anti-IL-10 Ig to the cultures. Anti-IL-10 Ig reversed the IL-10-induced phenotypic changes of the DC population (Fig. 1a).
To examine the effect of IL-10 on the timing of acquisition of costimulatory molecule expression by DC, cells were phenotyped 3, 6, 8 and 9 days after initiation of the culture. As shown in Fig. 1(b), DC generated with GM-CSF acquired low levels of expression of CD80 late during culture, i.e. on day 8 or day 9. Similar results were obtained for CD40 and CD86 (data not shown). Although IL-10 increased the level of expression of the costimulatory molecules at the end of the culture period, the timing of acquisition of costimulatory molecules was similar as compared to DC generated with GM-CSF.
To assess the effect of IL-10 on the T-cell stimulatory capacity of DC, DC generated with either GM-CSF alone or with GM-CSF + IL-10 were used as stimulator cells in an allo-MLR, using allogeneic CD4+ T cells as responder cells (Fig. 1c). DC that were generated with GM-CSF had a moderate capacity to stimulate the proliferation of allogeneic CD4+ T cells. Addition of IL-10 to the DC culture significantly inhibited the T-cell stimulatory capacity of the DC at all DC : T-cell ratios, despite the upregulated costimulatory molecule expression on these DC. Addition of neutralizing anti-IL-10 polyclonal Ig during the generation of DC restored the T-cell stimulatory capacity of IL-10-pretreated DC. As expected, anti-IL-10 Ig had no significant effect on the T-cell stimulatory capacity of DC cultured with GM-CSF alone.
IL-10 modulates DC maturation at an early stage
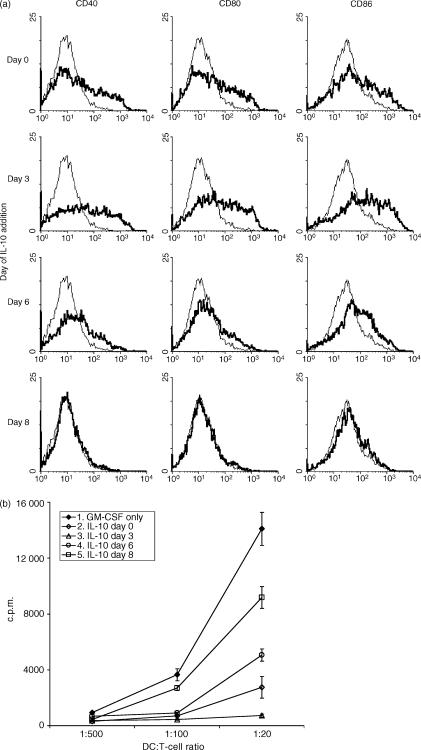
To determine at which stage of maturation IL-10 modulates the phenotype and T-cell stimulatory capacity of the DC, IL-10 was added to DC cultures at different time-points after initiation of the culture. Phenotype and T-cell stimulatory capacity of the DC populations were analysed after 9 days of culture. As already shown in Fig. 1, early addition of IL-10 (from day 0 or 3 onwards) significantly upregulated the expression of costimulatory molecules and abrogated the T-cell stimulatory capacity of the DC (Fig. 2). IL-10 was less effective when added from day 6 of culture onwards. The late addition of IL-10 (day 8) had no further effect on the expression levels of costimulatory molecules and only a minor (but still significant) effect on the T-cell stimulatory capacity of DC on day 9 (Fig. 2). The reduced effects of IL-10 on DC when added at a later time-point suggests that IL-10 exerts its effects mainly during the early maturation stages.
Figure 2.
Early addition of interleukin-10 (IL-10) modulates the phenotype and T-cell stimulatory capacity of dendritic cells (DC). DC were generated from murine bone marrow (BM) cells with granulocyte–macrophage colony-stimulating factor (GM-CSF). IL-10 was added to the cultures from day 0, 3, 6 or 8 onwards. After 9 days of culture, expression of the costimulatory molecule and T-cell stimulatory capacity of the DCs were analysed. (a) Flow cytometric analyses of CD40, CD80 and CD86 expression on gated CD11c+ DC generated either with GM-CSF alone (solid line) or with GM-CSF + IL-10 (bold line) added from different time-points onwards to the culture. (b) Purified C57Bl/6 CD4+ T cells were stimulated with graded numbers of BALB/c DC, and proliferation was determined after 4 days by measuring the incorporation of [3H]thymidine. The results are representative of three experiments.
IL-10-pretreated DC respond normally to LPS-induced maturation
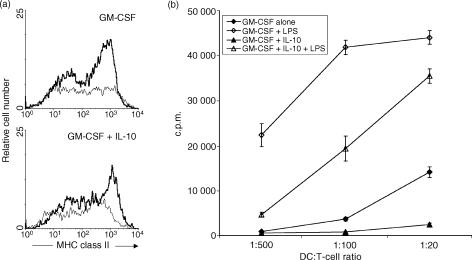
LPS is a strong inducer of maturation of immature DC. LPS is only effective when added at a late time-point, i.e. ≥day 6, to the DC cultures,22 suggesting that LPS acts mainly during the later stages of DC maturation. We therefore evaluated whether IL-10 pretreatment of DC influenced subsequent DC maturation in response to LPS. DC were generated with either GM-CSF or with GM-CSF + IL-10 for 8 days, and were then stimulated overnight with LPS. As expected, DC generated with GM-CSF alone matured in response to overnight LPS stimulation, as evidenced by an up-regulation of the expression of MHC class II and an increase of T-cell stimulatory capacity (Fig. 3). DC generated with GM-CSF + IL-10 showed an increase in MHC class II expression and T-cell stimulatory capacity in response to LPS stimulation which was similar to that of DC generated with GM-CSF alone (Fig. 3). Therefore, the IL-10-induced changes in these DC characteristics can be reversed by LPS stimulation.
Figure 3.
Interleukin-10 (IL-10)-treated dendritic cells (DC) are responsive to lipopolysaccharide (LPS)-induced maturation. DC were generated from murine bone marrow (BM) cells by incubation with either granulocyte–macrophage colony-stimulating factor (GM-CSF) or GM-CSF + IL-10, until day 8 of culture. Then, the cells were washed and stimulated overnight with LPS or with the same cytokines until day 9 of culture. (a) Flow cytometric analysis of major histocompatibility complex (MHC) class II expression of CD11c+ DC stimulated with (bold line) or without (solid line) LPS. (b) Purified C57Bl/6 CD4+ T cells were stimulated with graded numbers of BALB/c DC and proliferation was analysed after 4 days by measuring the incorporation of [3H]thymidine.
Next, we analysed whether the LPS-induced DC maturation could be inhibited by the presence of IL-10 during stimulation with LPS. Therefore, day-8 DC, generated by culture with GM-CSF + IL-10, were incubated overnight with LPS in the presence or absence of IL-10. As a control, DC were not stimulated with LPS but instead cultured from day 8–9 with GM-CSF + IL-10. When IL-10 was present during the stimulation with LPS, the LPS-induced up-regulation of MHC class II, and the LPS-induced production of IL-12, were both inhibited (Fig. 4a). Also, the LPS-induced increase in T-cell stimulatory capacity was largely inhibited when IL-10 was present during the stimulation with LPS (Fig. 4b). The LPS-induced IL-12 production, and the suppression of LPS-induced IL-12 production by IL-10, did not differ between DC generated in GM-CSF alone and DC generated in GM-CSF + IL-10 (data not shown). Thus, our data demonstrate that IL-10 inhibits the LPS-induced maturation of DC only when IL-10 is present during the LPS stimulation, confirming a recent report,23 and extend this study by demonstrating that generation of DC in the presence of IL-10 prior to LPS stimulation does not interfere with LPS-induced DC maturation.
Figure 4.
Interleukin-10 (IL-10) inhibits lipopolysaccharide (LPS)-induced maturation. Dendritic cells (DC) were generated by culture with granulocyte–macrophage colony-stimulating factor (GM-CSF) + IL-10 for 8 days. Subsequently, cells were washed and cultured overnight with GM-CSF + IL-10, GM-CSF + LPS, or GM-CSF + IL-10 + LPS. (a) On day 9, the stimulated cells were incubated for 4 hr with brefeldin A, then stained for cell-surface major histocompatibility complex (MHC) class II and intracellular IL-12 and analysed by two-colour flow cytometry. (b) Graded numbers of day 9 DC, exposed overnight to GM-CSF + IL-10, GM-CSF + LPS, or GM-CSF + IL-10 + LPS, were cocultured with purified C57Bl/6 CD4+ T cells in an allogeneic mixed leucocyte reaction (allo-MLR). T-cell proliferation was determined after 4 days by measuring the incorporation of [3H]thymidine. Representative examples of two experiments are shown.
IL-10 inhibits the antigen-uptake capacity of a subpopulation of immature MHC class IIdim DC
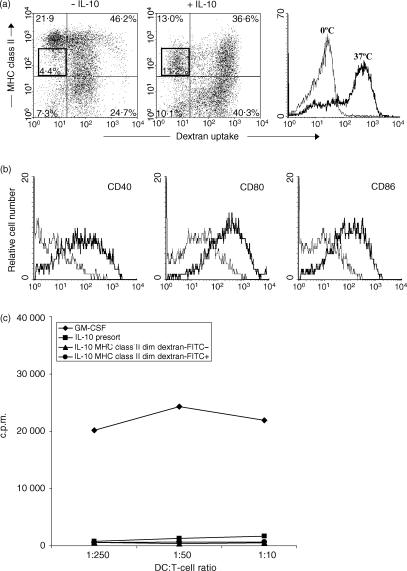
Immature DC are characterized by a high antigen-uptake capacity that is lost upon maturation.20 As we found that DC cultured with IL-10 had an upregulated expression of costimulatory molecules, corresponding to a more mature phenotype, but a decreased frequency of MHC class II bright cells and a reduced T-cell stimulatory capacity (both features of immature DC) we evaluated the antigen-uptake capacity of these IL-10-pretreated DC. In cultures generated with GM-CSF alone, the MHC class IIdim DC had a high capacity to take up dextran–FITC, whereas the majority of MHC class IIbright DC had a low capacity to take up dextran–FITC (Fig. 5), as reported previously.12 The majority of IL-10-pretreated DC were MHC class IIdim and possessed a high dextran–FITC uptake capacity, comparable with the MHC class IIdim DC within the DC population generated without IL-10. Remarkably, a subpopulation of the MHC class IIdim in the IL-10-pretreated DC population lacked the capacity to take up dextran–FITC (Fig. 5).
Figure 5.
Antigen-uptake capacity of dendritic cells (DC), generated by culture with granulocyte–macrophage colony-stimulating factor (GM-CSF), with or without interleukin-10 (IL-10). DC were cultured with GM-CSF, with or without IL-10. On day 9, DC were incubated with dextran–fluorescein isothiocyanate (FITC) for 1 hr at 37°, or at 0° as a control. Afterwards, the cells were washed twice with ice-cold medium. (a) Cells were stained for major histocompatibility complex (MHC) class II expression and analysed on a flow cytometer, using 7-aminoactinomycin D (7-AAD) to exclude dead cells. (b) Expression of CD40, CD80 and CD86 on gated MHC class IIdim dextran–FITCnegative cells (thin line) and on gated MHC class IIdim dextran–FITCpositive cells (thick line) was analysed by four-colour flow cytometry (dextran–FITC/CD40– CD80– or CD86–phycoerythrin (PE)/7-AAD/MHC class II biotin + streptavidin-allophycocyanin (APC)]. (c) MHC class IIdim dextran–FITCnegative cells and MHC class IIdim dextran–FITCpositive cells were sorted using a FACScalibur. Graded numbers of both sorted and the presort DC were cultured with purified allogeneic CD4+ T cells. Proliferation was assessed after 4 days by measuring the incorporation of [3H]thymidine.
To further characterize the MHC class IIdim, dextran–FITCnegative subpopulation and compare its features with the MHC class IIdim, dextran–FITCpositive subpopulation, we analysed the expression of the costimulatory molecules CD40, CD80 and CD86 on both subpopulations using four-colour flow cytometry (dextran–FITC/CD40, CD80 or CD86–PE, 7-AAD, MHC class II–biotin + streptavidin–allophycocyanin (APC)). As shown in Fig. 5(b), the MHC class IIdim, dextran–FITCnegative subpopulation expressed low levels of costimulatory molecules as compared to the MHC class IIdim, dextran–FITCpositive subpopulation.
The T-cell stimulatory capacity of both subpopulations was assessed by sorting the MHC class IIdim, dextran–FITCnegative and the MHC class IIdim, dextran–FITCpositive subpopulations, and using both sorted populations as stimulator cells in an allogeneic MLR (Fig. 5c). Like the total population of IL-10-pretreated DC, both sorted subpopulations lacked T-cell stimulatory capacity.
Discussion
In the present study, we analysed the effects of IL-10 on phenotypic and functional characteristics of murine myeloid BM-derived DC. Our data show that IL-10, when added early to the DC generation cultures, induced:
An up-regulation of the expression of costimulatory molecules.
A reduction in the frequency of MHC class IIbright cells.
A reduced capacity to stimulate CD4+ T cells in an allo-MLR.
Lack of dextran–FITC uptake capacity in a subpopulation of MHC class IIdim cells.
Therefore, IL-10 has paradoxical effects as regards DC maturation. As a result, IL-10 induces incompletely mature murine myeloid BM-derived DC that possess certain characteristics of mature DC and also certain features of immature DC.
Although several studies in both humans15,16 and mice24 have demonstrated the down-regulation of costimulatory molecules on DC and Langerhans cells by IL-10, we found that IL-10 specifically upregulated the expression of CD40, CD80 and CD86 on murine myeloid BM-derived DC. Our data agree with those of Morel et al.25 who reported an IL-10-induced up-regulation of several costimulatory and adhesion molecules on human monocyte-derived DC. These differences in effects of IL-10 on costimulatory molecule expression may be a result of the origin of the DC, the culture conditions and/or the maturation status of the DC. Fully mature human DC are resistant to the action of IL-10.3,16 Our kinetic studies demonstrate that up-regulation of costimulatory molecule expression by IL-10 depends on its early addition to the DC-generation cultures. This would indicate that IL-10 modifies the maturation process of DC precursors, but that it does not affect mature DC. Indeed, the late addition of IL-10 to differentiated (day 6 or day 8) DC had a minimal or no effect. The acquisition of costimulatory molecule expression by DC occurs towards the end of the culture (day 8 or day 9). Early addition of IL-10 does not accelerate the acquisition of costimulatory molecule expression. This may be a result of the fact that at earlier time-points a major fraction of the cells are still DC precursors that lack costimulatory molecule expression. Taken together, these results suggest that IL-10 exerts its modulating effects at early stages of DC differentiation, but that the DC precursor cells need to differentiate into immature/mature DC before the effects of IL-10 on costimulatory molecule expression become apparent.
Inhibition of T-cell stimulatory capacity is a well-known effect of IL-10 on DC. In vitro-generated IL-10-treated human DC are poor stimulators in the allo-MLR and induce a state of alloantigen-specific or peptide-specific anergy in both primed and naïve CD4+ and CD8+ T cells.16,18,19 IL-10 pretreatment also inhibits the capacity of murine and human Langerhans cells to stimulate the proliferation of primary T cells and Th1 clones.26,27 We now show that IL-10 also inhibits the T-cell stimulatory capacity of murine myeloid BM-derived DC. In previous studies, the reduced T-cell stimulatory capacity correlated with, and was attributed to, the low level of expression of costimulatory molecules. In our study, the IL-10-treated murine myeloid DC lack the capacity to induce T-cell proliferation, despite upregulated expression levels of CD40, CD80 and CD86. Our data suggest that IL-10 may be a factor that promotes murine myeloid DC maturation to a certain extent and then prevents the further maturation of DC, resulting in incompletely mature DC that lack T-cell stimulatory capacity. Recent studies have shown that certain populations of phenotypically mature DC, i.e. expressing high levels of CD40, CD80 and CD86 molecules and/or MHC class II molecules, may induce T-cell unresponsiveness and possess tolerogenic properties.7,8 Phenotypically mature pulmonary DC, which were CD80high CD86high CD40dim MHC class IIdim, mediate tolerance induced by respiratory exposure to antigen.8 Phenotypically mature DC, generated with tumour necrosis factor (TNF)-α, have been shown to induce regulatory T cells and induce antigen-specific protection of mice from autoimmunity.7 Moreover, the expression of costimulatory molecules on DC appears to be required for the tolerization of CD8+ T cells.10 Thus, phenotypically mature DC, expressing equal amounts of costimulatory molecules, may possess completely different T-cell stimulatory capacities. The expression of costimulatory molecules is therefore insufficient to discriminate between DC with and without T-cell stimulatory capacity. It has been suggested that a third, as yet undefined, signal is required to convert a T-cell non-stimulatory/tolerogenic DC into a T-cell stimulatory/immunogenic DC.10,11 This third signal may be delivered by CD4+ T cells. The genes in the DC that are involved in this regulatory switch in phenotypically mature DC remain to be identified. The culture conditions and thereby the quality of the DC maturation stimulus is probably a decisive factor for the development of the different T-cell stimulatory capacities of DC. Our findings are consistent with previous data showing that production of IL-10 by tumour cells or tumour-infiltrating lymphocytes might serve as a mechanism for tumour-induced anergy, possibly by modulation of DC function. The tolerogenic potential of liver-derived DC has also been attributed to the microenvironmental factors, including IL-10 and transforming growth factor (TGF)-β, in the liver.
The capacity of DC to take up exogenous antigens is gradually lost during DC maturation. In heterogeneous DC populations, the immature MHC class IIdim cells are characterized by a high capacity to ingest exogenous antigens, whereas the more mature MHC class IIbright cells are delineated by a low antigen-uptake capacity.12,20 A proportion of the MHC class IIbright cells were still able to ingest dextran–FITC. This suggests that the up-regulation of MHC class II expression and the loss of antigen-uptake capacity during DC maturation does not occur synchronically. The acquisition of MHC class IIbright expression appears to occur faster than the loss of antigen-uptake capacity. In the present study, we demonstrate that IL-10 inhibits the dextran–FITC uptake capacity of a subpopulation of MHC class IIdim cells. Simultaneously, IL-10 inhibits the development of MHC class IIbright DC. Apparently, IL-10 interferes with the maturation-associated up-regulation of MHC class II expression, but not with the maturation-associated loss of antigen-uptake capacity, resulting in a unique subpopulation of MHC class IIdim, dextran–FITC uptakenegative DC. Our data suggest that the antigen-uptake capacity and the up-regulation of MHC class II expression are regulated independently during DC maturation.
In conclusion, we demonstrate that exogenous IL-10 has paradoxical effects as regards maturation of in vitro-generated immature murine myeloid DC. Addition of IL-10 to GM-CSF-supported murine BM-derived DC cultures induces a population of incompletely matured MHC class IIdim CD80+ CD86+ DC that lack T-cell stimulatory capacity. The tolerogenic properties of these IL-10-treated DC are currently explored in vitro and in vivo in allogeneic transplantation models.
Abbreviations
- allo-MLR
allogeneic mixed leucocyte reaction
- 7-AAD
7-aminoactinomycin D
- APC
antigen-presenting cells
- BM
bone marrow
- DC
dendritic cells
- FCS
fetal calf serum
- FITC
fluorescein isothiocyanate
- GM-CSF
granulocyte–macrophage colony-stimulating factor
- IL-10
interleukin-10
- LPS
lipopolysaccharide
- mAbs
monoclonal antibodies
- MHC
major histocompatibility complex
- PBS
phosphate-buffered saline
- PE
phycoerythrin
- Th
T helper
- [3H]TdR
[3H]thymidine
References
- 1.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–61. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 3.Jonuleit H, Schmitt E, Steinbrink K, Enk AH. Dendritic cells as a tool to induce anergic and regulatory T cells. Trends Immunol. 2001;22:394–400. doi: 10.1016/s1471-4906(01)01952-4. [DOI] [PubMed] [Google Scholar]
- 4.Kalinski P, Hilkens CM, Wierenga EA, Kapsenberg ML. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol Today. 1999;20:561–7. doi: 10.1016/s0167-5699(99)01547-9. [DOI] [PubMed] [Google Scholar]
- 5.Dhodapkar MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J Exp Med. 2001;193:233–8. doi: 10.1084/jem.193.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morelli AE, Hackstein H, Thomson AW. Potential of tolerogenic dendritic cells for transplantation. Semin Immunol. 2001;13:323–35. doi: 10.1006/smim.2001.0328. [DOI] [PubMed] [Google Scholar]
- 7.Menges M, Rossner S, Voigtlander C, et al. Repetitive injections of dendritic cells matured with tumor necrosis factor alpha induce antigen-specific protection of mice from autoimmunity. J Exp Med. 2002;195:15–21. doi: 10.1084/jem.20011341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akbari O, DeKruyff RH, Umetsu DT. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat Immunol. 2001;2:725–31. doi: 10.1038/90667. [DOI] [PubMed] [Google Scholar]
- 9.Lutz M, Schuler G. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. 2002;23:445. doi: 10.1016/s1471-4906(02)02281-0. [DOI] [PubMed] [Google Scholar]
- 10.Albert ML, Jegathesan M, Darnell RB. Dendritic cell maturation is required for the cross-tolerization of CD8+ T cells. Nat Immunol. 2001;2:1010–7. doi: 10.1038/ni722. [DOI] [PubMed] [Google Scholar]
- 11.Blankenstein T, Schuler T. Cross-priming versus cross-tolerance: are two signals enough? Trends Immunol. 2002;23:171–3. doi: 10.1016/s1471-4906(02)02185-3. [DOI] [PubMed] [Google Scholar]
- 12.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 13.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romani N, Reider D, Heuer M, Ebner S, Kampgen E, Eibl B, Niederwieser D, Schuler G. Generation of mature dendritic cells from human blood. An improved method with special regard to clinical applicability. J Immunol Methods. 1996;196:137–51. doi: 10.1016/0022-1759(96)00078-6. [DOI] [PubMed] [Google Scholar]
- 15.Buelens C, Willems F, Delvaux A, Pierard G, Delville JP, Velu T, Goldman M. Interleukin-10 differentially regulates B7-1 (CD80) and B7-2 (CD86) expression on human peripheral blood dendritic cells. Eur J Immunol. 1995;25:2668–72. doi: 10.1002/eji.1830250940. [DOI] [PubMed] [Google Scholar]
- 16.Steinbrink K, Wolfl M, Jonuleit H, Knop J, Enk AH. Induction of tolerance by IL-10-treated dendritic cells. J Immunol. 1997;159:4772–80. [PubMed] [Google Scholar]
- 17.Buelens C, Verhasselt V, De Groote D, Thielemans K, Goldman M, Willems F. Human dendritic cell responses to lipopolysaccharide and CD40 ligation are differentially regulated by interleukin-10. Eur J Immunol. 1997;27:1848–52. doi: 10.1002/eji.1830270805. [DOI] [PubMed] [Google Scholar]
- 18.Steinbrink K, Graulich E, Kubsch S, Knop J, Enk AH. CD4(+) and CD8(+) anergic T cells induced by interleukin-10-treated human dendritic cells display antigen-specific suppressor activity. Blood. 2002;99:2468–76. doi: 10.1182/blood.v99.7.2468. [DOI] [PubMed] [Google Scholar]
- 19.Steinbrink K, Jonuleit H, Muller G, Schuler G, Knop J, Enk AH. Interleukin-10-treated human dendritic cells induce a melanoma-antigen-specific anergy in CD8(+) T cells resulting in a failure to lyse tumor cells. Blood. 1999;93:1634–42. [PubMed] [Google Scholar]
- 20.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lutz MB, Suri RM, Niimi M, Ogilvie AL, Kukutsch NA, Rossner S, Schuler G, Austyn JM. Immature dendritic cells generated with low doses of GM-CSF in the absence of IL-4 are maturation resistant and prolong allograft survival in vivo. Eur J Immunol. 2000;30:1813–22. doi: 10.1002/1521-4141(200007)30:7<1813::AID-IMMU1813>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 22.Lutz MB, Kukutsch NA, Menges M, Rossner S, Schuler G. Culture of bone marrow cells in GM-CSF plus high doses of lipopolysaccharide generates exclusively immature dendritic cells which induce alloantigen-specific CD4 T cell anergy in vitro. Eur J Immunol. 2000;30:1048–52. doi: 10.1002/(SICI)1521-4141(200004)30:4<1048::AID-IMMU1048>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 23.Haase C, Jorgensen TN, Michelsen BK. Both exogenous and endogenous interleukin-10 affects the maturation of bone-marrow-derived dendritic cells in vitro and strongly influences T-cell priming in vivo. Immunology. 2002;107:489–99. doi: 10.1046/j.1365-2567.2002.01529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang CH, Furue M, Tamaki K. B7-1 expression of Langerhans cells is upregulated by proinflammatory cytokines, and is downregulated by interferon-gamma or by interleukin-10. Eur J Immunol. 1995;25:394–8. doi: 10.1002/eji.1830250213. [DOI] [PubMed] [Google Scholar]
- 25.Morel AS, Quaratino S, Douek DC, Londei M. Split activity of interleukin-10 on antigen capture and antigen presentation by human dendritic cells: definition of a maturative step. Eur J Immunol. 1997;27:26–34. doi: 10.1002/eji.1830270105. [DOI] [PubMed] [Google Scholar]
- 26.De Smedt T, Van Mechelen M, De Becker G, Urbain J, Leo O, Moser M. Effect of interleukin-10 on dendritic cell maturation and function. Eur J Immunol. 1997;27:1229–35. doi: 10.1002/eji.1830270526. [DOI] [PubMed] [Google Scholar]
- 27.Peguet-Navarro J, Moulon C, Caux C, Dalbiez-Gauthier C, Banchereau J, Schmitt D. Interleukin-10 inhibits the primary allogeneic T cell response to human epidermal Langerhans' cells. Eur J Immunol. 1994;24:884–91. doi: 10.1002/eji.1830240416. [DOI] [PubMed] [Google Scholar]