Abstract
To establish the feasibility of inducing a protective immune response against a chlamydial genital infection in animals with different genetic backgrounds, groups of C3H/HeN (H-2k), BALB/c (H-2d) and C57BL/6 (H-2b) mice, were immunized intranasally with elementary bodies (EB) of the Chlamydia trachomatis mouse pneumonitis biovar. Following the intranasal immunization strong Chlamydia-specific humoral and cell-mediated immune (CMI) responses were detected in the three strains of mice. Eight weeks following immunization the animals were challenged with C. trachomatis in the genital tract. Vaginal cultures showed that the three strains of mice immunized with EB were significantly protected in comparison to the sham immunized animals. To determine the ability of this immunization protocol to protect against infertility six weeks after the genital challenge the animals were mated. Mice of the three strains immunized with EB showed significant protection as demonstrated by the number of animals that were fertile, and the number of embryos present in their uterine horns, in comparison to the sham immunized mice.
Introduction
Chronic abdominal pain, ectopic pregnancy and infertility are some of the most devastating complications that can occur in women following a Chlamydia trachomatis genital infection.1–7 The reasons for some individuals developing these long-term sequelae are not well understood. The quantity of the infecting inoculum, the hormonal balance and the stage of the menstrual cycle at the time of the infection probably play a significant role on the outcome of the acute infection. In addition, the immunogenetic background of the individual may account for the apparent differences in the susceptibility to develop long-term sequelae.4,6,7 C. trachomatis can be treated with several types of antibiotics, however, a majority of the infections in females are asymptomatic, and even in those that are symptomatic, implementation of therapeutic measures may be too late to prevent the development of sequelae.4,6,7 For these reasons significant efforts have focused on the development of a vaccine.8–14
Experience with several types of vaccines against infectious pathogens has shown that, in addition to the specific immunization protocol, induction of a protective immune response is dependent on the genetic background of the individual.15,16 As a result of the widespread presence of chlamydial infections throughout the world this consideration is particularly important in the case of a vaccine for C. trachomatis. Strains of mice with various genetic backgrounds have been shown to have different susceptibilities to a chlamydial infection.17–22 For example, when C3H/HeN, BALB/c and C57BL/6 mice where tested for their susceptibility to a C. trachomatis intravaginal infection the C3H/HeN were found to be the most susceptible while the C57BL/6 were the most resistant.19 In addition, the course and outcome of the infection differed markedly between various strains of mice.17–19,21 Furthermore, significant differences in the innate and acquired immune responses have been reported between these three strains of mice following a genital and a pulmonary infection.17,18,21,22 We have previously shown that intranasal immunization of BALB/c mice with elementary bodies (EB) of C. trachomatis mouse pneumonitis (MoPn) results in an immune response that can protect the animals against a genital challenge.23,24 Thus, here we wanted to determine if it was possible to induce a protective immune response against a C. trachomatis genital challenge in three genetically unique strains of mice that have marked differences in susceptibility and immune response to a chlamydial infection.
Materials and methods
Organisms
The C. trachomatis MoPn strain Nigg II was obtained from the American Type Culture Collection (Manassas, VA) and grown in HeLa-229 cells as previously described.23–25 The stocks of EB were titrated in HeLa-229 cells.23
Animal immunization and challenge
Seven- to 8-week-old female BALB/c (H-2d), C3H/HeN (H-2k) and C57BL/6 (H-2b) mice were purchased from Simonsen Laboratory (Gilroy, CA). All protocols were approved by, the University of California, Irvine, Animal Care and Use Committee.
BALB/c and C57BL/6 mice were immunized intranasally (i.n) with 104C. trachomatis MoPn inclusion-forming units (IFU)/mouse, while C3H/HeN were immunized with 10 IFU/mouse.23 The number of IFU used for inoculation was 10-fold lower than the LD50 for each strain of mice (unpublished data). As a negative control mice were inoculated with HeLa-229 cell extracts. Eight weeks after the initial immunization, animals were anaesthetized with methoxyflurane and a lateral abdominal incision was made to inoculate 105 IFU of C. trachomatis MoPn into the left ovarian bursa,20,23,26 The right ovarian bursa was used as a control and inoculated with mock-infected HeLa-229 cell extracts. All experiments were repeated.
Vaginal swabs and genital cultures
Vaginal swabs for C. trachomatis MoPn were collected at weekly intervals following the challenge and resuspended in 200 µl of sterile 0·2 m sucrose, 20 mm sodium phosphate (pH 7·2), and 5 mm glutamic acid (SPG).23 Two aliquots from each specimen (100 and 10 µl of SPG) were cultured in McCoy cells grown in 48-well plates by centrifugation at 1000 g for 1 hr at room temperature. Following incubation at 37° the chlamydial inclusions were stained at 30 hr with a rabbit polyclonal anti-C. trachomatis MoPn serum prepared in our laboratory.23
Immunoassays
Blood was collected by periorbital or heart puncture and the serum pooled for each group of animals. Vaginal samples were collected by washing the vagina twice with 20 µl of phosphate-buffered saline (PBS; pH 7·2) and were pooled for each group.23 All immunoassays were performed with the pooled serum or vaginal washes from each group.
The C. trachomatis MoPn specific antibody titre in sera and vaginal washes was determined by an enzyme-linked immunosorbent assay (ELISA) as previously described.23 Briefly, 96-well plates were coated with 100 µl/well of C. trachomatis MoPn EB containing 10 µg/ml of protein in PBS, and 100 µl of serum or 50 µl of vaginal wash was added per well in threefold serial dilutions. After incubation at 37° for 1 hr and washing, the ELISA plates were incubated with horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG), IgG1, IgG2a, IgG2b, IgG3, IgM or IgA (Southern Biotechnology Associates, Inc. Birmingham, AL). The binding was measured in an ELISA reader (Bio-Rad Laboratories, Richmond, CA) using 2-2′-azinobis(3-ethylbenzthiazoline-6-sulphonic acid) as the substrate.
The ability of serum to neutralize in vitro the infectivity of C. trachomatis MoPn EB was determined as described.27 Briefly, C. trachomatis MoPn (104 IFU) was added to threefold serial dilutions of the serum made with 5% (v/v) guinea-pig sera in Ca2+-, Mg2+-free PBS. After incubation at 37° for 45 min, the mixture was inoculated by centrifugation onto HeLa-229 cells. The chlamydial inclusions were stained as described above.23 The inclusions were counted at a magnification of 200×, and the 50% inhibition was calculated using as a control the sera from the animals inoculated with HeLa cell extracts.
For immunoblotting C. trachomatis MoPn EB were resolved by sodium dodecyl sulphate– polyacrylamide gel electrophoresis (SDS–PAGE).28 Approximately, 250 µg of purified EB were loaded on a 7·5-cm wide slab gel. Following transfer to nitrocellulose membranes, antibody binding was detected with horseradish peroxidase-conjugated goat antimouse antibody developed with 0·01% hydrogen peroxide and 4-chloro-1-naphthol.23
The lymphocyte proliferation assay (LPA) was performed as previously described.23 Briefly, the spleens and inguinal and iliac lymph nodes of two to four mice from each group were collected, teased, and splenocytes enriched for T cells by passage over a nylon wool column. Accessory cells for antigen presentation were prepared by irradiating (3000 rad; 137Cs) syngeneic unseparated spleen cells and incubating them with various ratios of C. trachomatis MoPn EB. Concanavalin A (Con A) was used as a positive stimulant, and HeLa cell extracts and tissue culture media as negative controls. At the end of 4 days of incubation, 1·0 µCi of [methyl-3H]thymidine (47 Ci/mmol; Amersham; Arlington, Heights, IL) in 25 µl of RPMI-1640 was added per well, and the incorporation of [3H] was measured using a scintillation counter (Beckman Instruments, Fullerton, CA).
To determine the frequency of Chlamydia-specific proliferative T and Chlamydia-specific B cells, spleens were harvested, teased and single cell suspensions plated. The frequency of Chlamydia-specific proliferativeT-cells was determined by limited dilution analysis (LDA) while the antibody secreting B cells were enumerated using an enzyme-linked immunospot (ELISPOT) assay. The LDA was performed as previously described.29 Briefly, freshly collected lymphocytes were serially diluted eightfold and plated in 12 duplicates in 96-well microtitre plates. Radiated splenocytes were added as feeder cells to all the wells. EB were then added to a set of plates at a ratio of 10 EB for one feeder cell and the control duplicate set of plates received no antigen. The plates were incubated for 4 days and for the last 20 hr of incubation 3H-thymidine (1 µCi/well) was added, the cells harvested and counted on a Beckman scintillation counter. Frequencies of Chlamydia-specific proliferative T cells at the 99·9% confidence level were calculated using a software program (courtesy of Dr J. Whittum-Hudson, Wayne State University School of Medicine, Detroit, MI).
To perform the ELISPOT assay sterile flat-bottom 96-well ELISA plates were coated with 50 µl of C. trachomatis MoPn EB (1 mg/ml) in 10 mm PBS (pH 7·4) at 4°.24 The plates were then washed with sterile PBS and the nonspecific sites were blocked with sterile PBS–bovine serum albumin for 1 hr at 37°. To each well 2 × 105 spleen cells were added in 0·1 ml of RPMI-1640 containing 10% fetal bovine serum and 50 µm 2-β-mercaptoethanol. The cells were subsequently incubated at 37° overnight, stained with isotype-specific antibodies and counted.23
The levels of interferon-γ (IFN-γ) and interleukin-4 (IL-4) were determined using commercial kits (IFN-γ from BDPharMingen, San Diego, CA; IL-4 from Endogen, Cambridge, MA) in supernatants from splenic T cells stimulated as described above.24
Fertility studies
Six weeks after the intrabursal challenge, groups of four female mice were caged with a proven breeder male mouse for 18 days.23 Pregnancy was assessed, by determining the increase in weight of the mice. Pregnant mice were euthanized at days 14–17 of gestation. Animals that did not become pregnant during the first mating were mated for a second time. Finally, all non-pregnant mice were killed 2 weeks after the end of the second mating. The number of embryos in both uterine horns was counted at the time the mice were killed.
Statistical analyses
The two-tailed unpaired Student's t-test, Mann–Whitney's U-test, and the Fisher's exact test were used for analysis with the Statview IV (Abacus, Berkeley, CA) software program.
Results
Immune response to intranasal inoculation with C. trachomatis MoPn EB
The three strains of mice were intranasally immunized with a dose of C. trachomatis that was 10-fold lower than their respective LD50. The results of the antibody response at 8 weeks following i.n. immunization are shown in Table 1. High titres of antichlamydial IgG and IgA antibodies in the serum were detected in the three strains of mice while the titres of IgM were relatively low. Determination of the different IgG subclasses of antibodies show that all the three strains had a predominant T helper 1 (Th1) response as shown by the ratio of IgG2a/IgG1 antibodies. However, while in the BALB/c and the C3H/HeN mice the ratio was 9 (65 610/7290 and 21 870/2 430, respectively), in the C57BL/6 mice the ratio was 27 (7290/270). Furthermore, while in the BALB/c and C3H/HeN mice the highest titres of antibody were of the IgG2a subtype in the C57BL/6 mice the IgG2b had the highest levels. The vaginal secretions of the BALB/c mice had the highest levels of IgA antibodies while the C57BL/6 mice had the highest levels of IgA antibodies in serum. Neutralizing antibodies were detected in the three strains of mice with the serum in the C3H/HeN having more than a twofold higher neutralization activity.
Table 1.
Antibody responses in three strains of mice the day before the genital challenge
| Mouse strain | Anti-C. trachomatis MoPn EB ELISA titre in | Serum neutralizing titre | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Serum | Vagina | |||||||||
| IgG | IgM | IgA | IgG1 | IgG2a | IgG2b | IgG3 | IgG | IgA | ||
| C3H/HeN | 7290 | 30 | 2430 | 2430 | 21 870 | 2430 | 810 | 81 | 81 | 4050 |
| C57BL/6 | 21 870 | 30 | 7290 | 270 | 7290 | 21 870 | 810 | 27 | 81 | 1350 |
| BALB/c | 21 870 | 30 | 2430 | 7290 | 65 610 | 2430 | 2430 | 81 | 243 | 1350 |
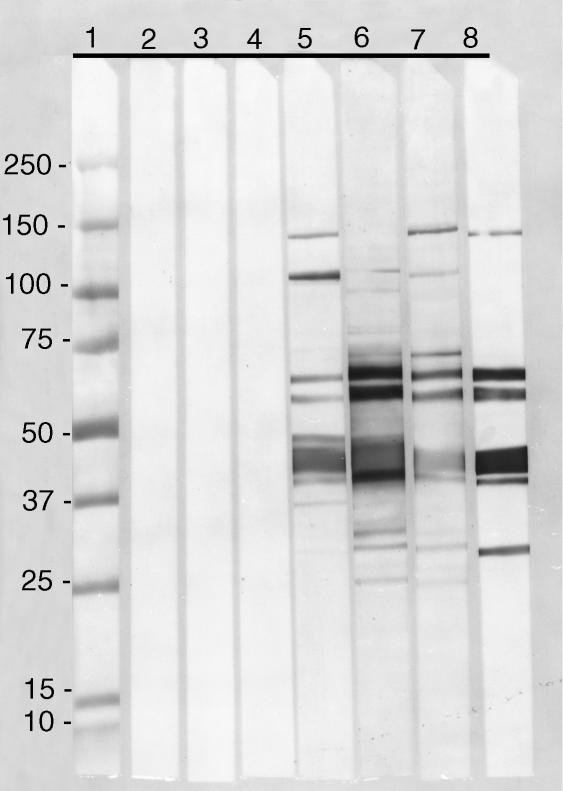
To further define the specificity of the antibody response, serum samples were tested by immunoblot against C. trachomatis EB (Fig. 1). Antibodies from the three strains reacted with a 120 000 MW band, the 60 000 MW cysteine-rich protein (crp) doublet, major outer membrane protein (MOMP), the 28 000 MW protein and lipopolysaccharide (not shown) when tested against EB. In addition, the serum from the C3H/HeN and BALB/c mice reacted with bands at 150 000 and 24 000 MW, while serum from the C57BL/6 and BALB/c mice reacted with a band at 70 000 MW.
Figure 1.
Immunoblot of serum samples using C. trachomatis MoPn EB as the antigen performed on a 5–20% gradient SDS–PAGE. Lane 1, MW markers (×1000). Lanes 2, 3, and 4, preimmunization serum samples from C3H/HeN, C57BL/6 and BALB/c mice, respectively. Lanes 5, 6 and 7 serum samples collected 8 weeks after i.n. immunization from C3H/HeN, C57BL/6 and BALB/c mice, respectively. Lane 8, monoclonal antibodies against the 150 000 MW, the 60 000 MW crp, MOMP and the 28 000 MW proteins of the C. trachomatis MoPn serovar.
Table 2 depicts the lymphoproliferative responses of splenocytes and lymphocytes from the iliac and inguinal lymph nodes the day before the intrabursal challenge. The negative controls are the mice sham immunized with HeLa cell extracts. When EB were used as the antigenic stimuli, a significant T-cell proliferative response was observed in the splenocytes and the lymph nodes from the three strains of mice immunized i.n. with EB, while the sham immunized animals had no significant response. Overall, the immunized and sham immunized mice had similar T-cell responses to HeLa cells, Con A and the tissue culture medium that were used as controls.
Table 2.
Lymphoproliferative responses in T cells from splenocytes and iliac and inguinal lymph nodes the day before the genital challenge
| Mouse strain | Group | Enriched T-cells from the spleen (Δ CPM × 1000 ± 1 SD) | Lymphocytes from iliac and inguinal lymph nodes (Δ CPM × 1000 ± 1 SD) | ||
|---|---|---|---|---|---|
| EB* | Con A† | EB | Con A | ||
| C3H/HeN | Immunized | 35 ± 12‡ | 83 ± 32 | 7 ± 0·5‡ | 43 ± 1 |
| Sham immunized | 0·3 ± 0·3 | 81 ± 18 | 0·2 ± 0·01 | 155 ± 168 | |
| C57BL/6 | Immunized | 14 ± 0·8‡ | 38 ± 3 | 23 ± 4‡ | 40 ± 21 |
| Sham immunized | 3 ± 1 | 43 ± 6 | 0·01 ± 0·01 | 77 ± 49 | |
| BALB/c | Immunized | 23 ± 4‡ | 139 ± 15 | 16 ± 2‡ | 160 ± 10 |
| Sham immunized | 0·8 ± 0·2 | 107 ± 5 | 0·8 ± 0·3 | 204 ± 116 | |
CPM, counts per minute.
UV-inactivated C. trachomatis MoPn EB were added at a ratio of 10 : 1 to the irradiated splenocytes (APC).
5 µg/ml concanavalin A (Con A).
P < 0·05 by Student's t-test compared to the corresponding sham immunized group.
The frequency of Chlamydia-specific proliferative T cells was very similar in the three strains of mice (Table 3). Also, the number of antibody secreting B cells was similar in the three strains of mice except for the IgG-secreting cells that were lower in the C3H/HeN mice.
Table 3.
Frequency of Chlamydia-specific proliferative T-cells and antibody secreting B-cells in three strains of mice the day before the genital challenge
| Mouse strain | Frequency of Chlamydia-specific proliferative T- cells (±1SD)/106 | Frequency of Chlamydia-specific antibody secreting B-cells (± 1SD)/106 splenocytes† | ||
|---|---|---|---|---|
| Group | Splenocytes* | IgG | IgA | |
| C3H/HeN | Immunized | 30 ± 0·6 | 35 ± 14 | 170 ± 28 |
| Sham immunized | 2 ± 0·08 | 0 ± 0 | 2 ± 3 | |
| C57BL/6 | Immunized | 33 ± 0·6 | 160 ± 63 | 173 ± 10 |
| Sham immunized | 4 ± 0·09 | 0 ± 0 | 2 ± 3 | |
| BALB/c | Immunized | 28 ± 0·5 | 95 ± 0 | 208 ± 3 |
| Sham immunized | 6 ± 0·14 | 2 ± 3 | 5 ± 0 | |
Precursor frequency of Chlamydia-specific proliferative T-cells was measured by limiting dilution analysis.
Frequency of Chlamydia-specific antibody secreting B-cells was enumerated by an ELISPOT assay.
Production of cytokines by splenocytes, harvested 8 weeks after the i.n. inoculation, was measured following stimulation with MoPn EB (Table 4). In the three strains of mice the levels of IFN-γ were significantly higher than the levels of IL-4.
Table 4.
In vitro production of cytokines by splenocytes in three strains of mice the day before the genital challenge
| Mouse strain | Group | IL-4 (pg/ml ± 1SD) | IFN-γ (ng/ml ± 1SD) | ||
|---|---|---|---|---|---|
| EB* | Medium | EB | Medium | ||
| C3H/HeN | Immunized | <0·1 | <0·1 | 94 ± 16† | <0·03 |
| Sham immunized | <0·1 | <0·1 | <0·03 | <0·03 | |
| C57BL/6 | Immunized | 2 ± 1 | <0·1 | 112 ± 34† | <0·03 |
| Sham immunized | <0·1 | <0·1 | <0·03 | <0·03 | |
| BALB/c | Immunized | 17 ± 0 | <0·1 | 96 ± 9† | <0·03 |
| Sham immunized | <0·1 | <0·1 | <0·03 | <0·03 | |
UV-inactivated C. trachomatis MoPn EB were added at a ratio of 10 : 1 to the irradiated splenocytes (APC).
P < 0·05 by the Student's t-test compared to the corresponding sham immunized group.
Course of the vaginal shedding of C. trachomatis following the intrabursal challenge
Following the intrabursal challenge vaginal cultures were collected from the three strains of mice. As shown in Table 5, none of the C3H/HeN and C57BL/6 mice immunized i.n. with EB had positive vaginal cultures following the intrabursal challenge. In contrast, in the control groups inoculated with HeLa cell extracts, 100% (14 of 14) of the C3H/HeN mice, and 66·7% (12 of 18) of the C57BL/6 mice had positive cultures during the 6-week period of observation (P < 0·05). Of the BALB/c mice immunized i.n. with EB only 12·5% (2 of 16) of the animals shed C. trachomatis vaginally while 100% (20 of 20) of the controls had positive vaginal cultures (P < 0·05). In addition, for the first 3 weeks after challenge the EB immunized BALB/c mice shed significantly less IFU than the mice inoculated with the HeLa cell extracts (P < 0·05). Furthermore, in the EB immunized BALB/c mice the length of time the mice had positive vaginal cultures was shorter, 2 weeks versus 4 weeks, than in the HeLa inoculated group. All mice had negative vaginal cultures during the 5th and 6th week of observation.
Table 5.
Results of vaginal cultures of three strains of mice following the genital challenge: % mice with positive vaginal culture and mean ± 1 SE IFU of C. trachomatis MoPn per mouse per week
| Mouse strain | Group | No mice/ group | Week 1 | Week 2 | Week 3 | Week 4 | Total % mice with positive culture | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| % | IFU | % | IFU | % | IFU | % | IFU | ||||
| C3H/HeN | Immunized | 17 | 0* | 0† | 0* | 0† | 0* | 0† | 0 | 0 | 0* |
| Sham immunized | 14 | 85·7 | 34 433 ± 26 711 | 57·1 | 30 750 ± 26 912 | 50·0 | 746 ± 726 | 14·3 | 18 ± 10 | 100·0 | |
| C57BL/6 | Immunized | 18 | 0* | 0† | 0* | 0† | 0* | 0† | 0 | 0 | 0* |
| Sham immunized | 18 | 27·8 | 2424 ± 775 | 66·7 | 1006 ± 134 | 44·4 | 4156 ± 1373 | 16·6 | 9498 ± 3158 | 66·7 | |
| BALB/c | Immunized | 16 | 6·3* | 3 ± 3† | 6·3* | 250 ± 250† | 0* | 0† | 0 | 0 | 12·5* |
| Sham immunized | 20 | 80·0 | 8131 ± 1818 | 65·0 | 7965 ± 1781 | 25·0 | 32 396 ± 7244 | 15·0 | 119 ± 27 | 100·0 | |
P < 0·05 by the Fisher's exact test compared to the sham immunized, challenged mice.
P < 0·05 by the Mann–Whitney U-test compared to the sham immunized, challenged mice.
Fertility studies
The C3H/HeN, C57BL/6 and BALB/c mice immunized i.n. with EB were protected as shown by the fact that when compared with the sham immunized non-challenged group no statistically significant differences in fertility rates were observed (P > 0·05) (Table 6). In addition, the immunized challenged animals from the three strains of mice had much higher fertility rates than the sham immunized challenged animals (P < 0·05). The most striking differences in this respect were in the C3H/HeN mice. In this group while 100% of the EB immunized mice had bilateral fertility 0% of the sham immunized challenged animals were fertile.
Table 6.
Fertility studies of three strains of mice
| Mouse strain | Group | No. mice with bilateral fertility/total mice (%) | Mean ± 1 SD no. embryos per mouse per uterine horn | |
|---|---|---|---|---|
| Right* | Left† | |||
| C3H/HeN | Immunized, challenged | 17/17 (100) | 3·5 ± 2·0 | 2·9 ± 1·5 |
| Sham immunized, challenged | 0/14 (0)‡ | 0·9 ± 2·4§ | 0·1 ± 0·3§ | |
| Sham immunized, non-challenged | 17/19 (89·5) | 3·2 ± 1·9 | 3·4 ± 1·1 | |
| C57BL/6 | Immunized, challenged | 15/18 (83·3) | 3·7 ± 2·4 | 3·2 ± 2·3 |
| Sham immunized, challenged | 3/18 (16·7)‡ | 2·4 ± 3·0 | 0·2 ± 0·4§ | |
| Sham immunized, non-challenged | 9/11 (81·8) | 3·8 ± 2·6 | 3·8 ± 1·9 | |
| BALB/c | Immunized, challenged | 16/16 (100) | 4·4 ± 2·0 | 3·4 ± 1·5 |
| Sham immunized, challenged | 3/20 (15)‡ | 3·1 ± 3·1 | 0·6 ± 0·6§ | |
| Sham immunized, non-challenged | 20/21 (95·2) | 4·1 ± 2·0 | 3·0 ± 1·7 | |
Inoculated with HeLa cell extracts.
Inoculated with 105 IFU of C. trachomatis MoPn.
P < 0·05 by Fisher's exact test compared to the sham immunized, non-challenged mice.
P < 0·05 by Student's t-test compared to the sham immunized, non-challenged mice.
Mice that were i.n. immunized with EB had similar number of embryos in the left uterine horn (challenged) when compared with the sham immunized non-challenged mice (P > 0·05) (Table 6). In contrast, the average number of embryos per mouse found in the left uterine horn was statistically significantly lower in the three strains of sham immunized challenged mice when compared with the animals that were sham immunized and non-challenged (P < 0·05). Furthermore, no significant differences were observed among these two groups, immunized and challenged and sham immunized non-challenged, when comparing the number of embryos found in the right (control) and left (challenged) uterine horns. Also, only in the C3H/HeN mice the number of embryos in the right horn was significantly decreased in the animals that were sham immunized and challenged, supporting the higher susceptibility of this strain of mice to a chlamydial infection.
Discussion
Here we have for the first time shown that i.n. immunization with C. trachomatis EB, of three genetically different strains of mice, can result in the induction of an immune response that is protective against a genital challenge. Mice that were immunized had minimal or no vaginal shedding of Chlamydia following the genital challenge. Furthermore, the immunized mice had fertility rates that were equal to control animals that were not challenged. These findings are very encouraging particularly because these three strains of mice differ significantly in susceptibility to a chlamydial infection and the immune responses vary markedly from strain to strain.17–19,21,22
Both the cell-mediated and the humoral immune responses play a role in controlling chlamydial infections.16,30–35 Work using knockout mice and adoptive transfer of lymphocytes indicates that CD4+ cells play a greater role than CD8+ cells in resolving a chlamydial genital infection.31,32 In the three strains of mice that we tested the cell mediated immune response elicited by the intranasal immunization was very strong. In the three groups of mice the frequency of Chlamydia-specific proliferative T and B cells, and the lymphoproliferative responses of T cells from the spleen and lymph nodes, were significant following stimulation with EB. Based on the analysis of the levels of the different IgG subtypes in serum it appears that the three strains of mice mounted predominantly a Th1 response. The predominance of a Th1 response was also supported by the measurement of cytokines produced by splenocytes in response to a stimulus with chlamydial EB.
Interestingly, Morrison and Morrison33 have shown that immunity to a chlamydial genital reinfection does not rely exclusively on CD4+ or CD8+ cells. Mice that were infected in the genital tract with C. trachomatis MoPn, and were subsequently depleted of CD4+ and CD8+ cells using monoclonal antibodies, were found to be as resistant to a chlamydial genital reinfection as immunocompetent wild type mice. Based on these findings, these authors concluded that B cells probably play a predominant role in resistance to a chlamydial genital reinfection.33 A caveat with these experiments is that the mice were treated with progesterone before the primary infection and the secondary challenge with Chlamydia and thus, most likely there was a significant bias towards a Th2 response (see below).
In addition to the neutralizing antibodies present in the mucosal surface, the non-specific anatomical defences and the components of the innate immune response, are the only barriers in the initial encounter with the pathogen that can protect against infection.8,16,30 In this respect, we detected the presence of IgA chlamydial specific antibodies in the vaginal washes from the three strains of mice. Passively transferred neutralizing monoclonal IgA antibodies able to protect in a murine model against a genital chlamydial challenge have been described.34 Current vaccines however, for the most part do not protect against infection but protect only against disease.36 In the case of chlamydial genital infections a vaccine that can limit the spread of the organisms to the lower genital tract will probably be efficacious in protecting against the long-term sequelae resulting from infection of the upper genital tract. The ability of this type of vaccine however, to protect from transmission to the sexual partners will be limited. Thus, it is encouraging that the immunized C3H/HeN and C57BL/6 mice had negative vaginal cultures during the 6 weeks of observation. This apparent ‘sterilizing immunity’ will have to be further validated before we can reach a final conclusion since we can not exclude at this point the possibility that the animals were shedding Chlamydia below the detection level.
Based on the results obtained several years ago, using viable and inactivated C. trachomatis EB to protect against trachoma, utilizing the whole organism as a vaccine is currently not an acceptable approach.11 More specifically it was shown, that immunized individuals that were not fully protected when they were subsequently exposed to C. trachomatis, developed a severe autoimmune reaction when re-exposed to the organism.11 Efforts to identify the chlamydial antigen(s) involved in this reaction suggest that the 60 000 MW heat-shock protein (hsp) may play a role in eliciting autoimmunity.37 The fact that three genes have been detected in Chlamydia with similarity to the hsp 60 000 MW family poses interesting questions, not only about their functional role in the organism, but also about their potential role in pathogenesis.38 A positive aspect of our immunization protocol is that it did not result in functional damage to the genital tract. The fertility rates in the animals that were immunized and challenged were equal to those observed in the fertility control group of mice that were sham immunized and non-challenged.
As a result of the autoimmune reaction observed in previous vaccination trials several attempts have been made at producing a subunit vaccine against genital infections. So far these efforts have provided some positive results. For example, Pal et al.39 showed that a preparation of the chlamydial outer membrane protein can induce a protective immune response against a genital challenge. On the other hand, attempts to use single antigens, in particular MOMP, have yielded contradictory results.38–43 The apparent discrepancies between the data obtained by several laboratories using various approaches to prepare the antigen are not well understood. In this respect, it has been proposed that the preservation of conformational epitopes in MOMP may be critical for inducing protection.14,39 The recent publication indicating that a folded C. trachomatis MoPn MOMP preparation purified from EB can induce protection against a genital challenge supports this hypothesis.41 Furthermore, other antigens may be needed to elicit a protective immune response.13
In this respect, in addition to the MOMP two other proteins, as detected by Western blots, elicited antibodies in the three strains of mice including the 60 000 MW crp, and a high molecular weight protein of approximately 120 000 MW that may correspond to one of the polymorphic membrane proteins (PMP).13,44 Another possible PMP with a molecular weight of 150 000 MW induced antibodies in C3H/HeN and BALB/c mice but no antibodies to this protein were detected in the C57BL/6 mice. There are nine PMP genes in C. trachomatis that are thought to code for proteins ranging in size from approximately 900–1500 amino acids in length. Since some of these proteins appear to be surface exposed it has been proposed that they may be able to induce a protective immune response.13 Relevant to this at least two of these candidate PMPs elicited an immune response in this model. However, whether or not any one of these antigens, that when administered as part of an EB can induce an immune response in animals with different genetic backgrounds, will be able to elicit a protective response when delivered as purified antigens, will have to be tested.
Of the murine models currently available to test vaccine candidates we decided to use the intrabursal model in spite of the obvious shortcoming of the route of inoculation.19,45,46 This model, originally described by Barron et al.45 offers the advantage that all strains of mice so far tested, independent of age, develop infertility following a relatively low challenge with the C. trachomatis MoPn serovar.23 Furthermore, by inoculating directly the upper genital tract we are delivering a direct and thus, a more stringent challenge to the anatomical site that we are primarily interested in protecting. The intravaginal model, although it follows the natural route of infection, requires a larger dose of C. trachomatis MoPn and certain strains of mice appear to be completely resistant to develop infertility if they are inoculated past a certain age.19,47 To overcome this problem the mice can be treated with progesterone before they are challenged with Chlamydia.46 Treatment with progesterone, however, induces a significant alteration of the immune system including, among others, severe suppression and a shift from a Th1 to a Th2 response.48–50 Hence, when testing a vaccine candidate this is a significant shortcoming because progesterone may abrogate or mask the protective response induced by the immunization. Thus, currently we do not have an ideal animal model to test vaccine candidates against chlamydial genital infections. However, we expect that if different groups of investigators test vaccine candidates in several models, conclusions will be reached that can be applied to larger segments of the human population than if only one model is characterized.
In conclusion, our results suggest that immunization with EB may induce a protective response in hosts with a variety of genetic backgrounds against a genital challenge with C. trachomatis. However, further work is required to extend these observations as immunization protocols utilizing subunit vaccines, or non-viable vaccines, may not be effective at achieving protection in certain hosts with a unique genetic make-up. This animal model however, could help determine the vaccination protocols that are required to stimulate the immune system to generate a protective response in hosts with different genetic backgrounds.
Acknowledgments
This work was supported by Public Health Service grant AI-32248 from the National Institute of Allergy and Infectious Diseases.
References
- 1.Grayston JT, Wang SP. New knowledge of Chlamydiae and the diseases they cause. J Infect Dis. 1975;132:87–105. doi: 10.1093/infdis/132.1.87. [DOI] [PubMed] [Google Scholar]
- 2.Paavonen J, Eggert-Kruse W. Chlamydia trachomatis: impact on human reproduction. Human Reprod Update. 1999;5:433–47. doi: 10.1093/humupd/5.5.433. [DOI] [PubMed] [Google Scholar]
- 3.Schachter J. Infection and disease epidemiology. In: Stephens RS, editor. Chlamydia: Intracellular Biology, Pathogenesis, and Immunity. Washington, DC: ASM Press; 1999. pp. 139–69. [Google Scholar]
- 4.Stamm WE. Chlamydia trachomatis infections of the adult. In: Holmes KK, et al., editors. Sexually Transmitted Diseases. New York: McGraw-Hill; 1999. pp. 407–22. [Google Scholar]
- 5.Cates W and the American Social Health Association Panel. Estimates of the incidence and prevalence of sexually transmitted diseases in the United States. Sex Transm Dis. 1999;26:S2–7. doi: 10.1097/00007435-199904001-00002. [DOI] [PubMed] [Google Scholar]
- 6.Westrom L, Joesoef R, Reynolds D, Hagdu A, Thompson SE. Pelvic inflammatory disease and fertility. a cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopy. Sex Transm Dis. 1992;19:185–92. [PubMed] [Google Scholar]
- 7.Westrom L, Mardh PA. Chlamydial salpingitis. Br J Bull. 1990;39:145–50. doi: 10.1093/oxfordjournals.bmb.a071806. [DOI] [PubMed] [Google Scholar]
- 8.Bavoil PM, Hsia RC, Rank RG. Prospects for a vaccine against Chlamydia genital disease I-Microbiology and pathogenesis. Bull Inst Pasteur. 1996;9:5–54. [Google Scholar]
- 9.de la Maza MA, de la Maza LM. A new computer model for estimating the impact of vaccination protocols and its application to the study of Chlamydia trachomatis genital infections. Vaccine. 1995;13:119–27. doi: 10.1016/0264-410x(95)80022-6. [DOI] [PubMed] [Google Scholar]
- 10.de la Maza LM, Peterson EM. Vaccines for Chlamydia trachomatis infections. Curr Opin Invest Drugs. 2002;3:980–6. [PubMed] [Google Scholar]
- 11.Grayston JT, Wang SP. The potential for vaccine against infection of the genital tract with Chlamydia trachomatis. Sex Transm Dis. 1978;5:73–7. doi: 10.1097/00007435-197804000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Stagg AJ. Vaccines against Chlamydia: approaches and progress. Mol Med Today. 1998;4:166–73. doi: 10.1016/s1357-4310(98)01232-5. [DOI] [PubMed] [Google Scholar]
- 13.Stephens RS. Chlamydial genomics and vaccine antigen discovery. J Infect Dis. 2001;181(Suppl. 3):S521–3. doi: 10.1086/315631. [DOI] [PubMed] [Google Scholar]
- 14.Igietseme JU, Eko FO, Black CM. Contemporary approaches to designing and evaluating vaccines against Chlamydia. Exper Rev Vaccines. 2003;2:129–46. doi: 10.1586/14760584.2.1.129. [DOI] [PubMed] [Google Scholar]
- 15.Ada G. The immunology of vaccination. In: Plotkin SA, Orenstein WA, editors. Vaccines. Philadelphia: W.B. Saunders; 1999. pp. 28–39. [Google Scholar]
- 16.Rank RG. Models of immunity. In: Stephens RS, editor. Chlamydia: Intracellular Biology, Pathogenesis, and Immunity. Washington, DC: American Society for Microbiology; 1999. pp. 239–95. [Google Scholar]
- 17.Darville T, Andrews CW, Laffoon KK, Shymasani W, Kishen LR, Rank RG. Mouse strain-dependent variation in the course and outcome of chlamydial genital tract infection is associated with differences in host response. Infect Immun. 1997;65:3065–73. doi: 10.1128/iai.65.8.3065-3073.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Darville T, Andrews CW, Sikes JD, Fraley PL, Rank RG. Early local cytokine profiles in strains of mice with different outcomes from chlamydial genital tract infection. Infect Immun. 2001;69:3556–61. doi: 10.1128/IAI.69.6.3556-3561.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de la Maza LM, Pal S, Khamesipour A, Peterson EM. Intravaginal inoculation of mice with the Chlamydia trachomatis mouse pneumonitis biovar results in infertility. Infect Immun. 1994;62:2094–7. doi: 10.1128/iai.62.5.2094-2097.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tuffrey M, Falder P, Gale J, Taylor-Robinson D. Salpingitis in mice induced by human strains of Chlamydia trachomatis. Br J Exp Pathol. 1986;67:605–16. [PMC free article] [PubMed] [Google Scholar]
- 21.Yan X, HayGlass KT, Brunham RC. Genetically determined differences in IL-10 and IFN-γ response correlate with clearance of Chlamydia trachomatis mouse pneumonitis infection. J Immunol. 1996;156:4338–44. [PubMed] [Google Scholar]
- 22.Stagg AJ, Tuffrey M, Woods C, Wunderink E, Knight SC. Protection against ascending infection of the genital tract by Chlamydia trachomatis is associated with recruitment of major histocompatibility complex class II antigen-presenting cells into uterine tissue. Infect Immun. 1998;66:3535–44. doi: 10.1128/iai.66.8.3535-3544.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pal S, Fielder TJ, Peterson EM, de la Maza LM. Protection against infertility in a BALB/c mouse salpingitis model by intranasal immunization with the mouse pneumonitis biovar of Chlamydia trachomatis. Infect Immun. 1994;62:3354–62. doi: 10.1128/iai.62.8.3354-3362.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pal S, Peterson EM, de la Maza LM. Intranasal immunization induces long-term protection in mice against a Chlamydia trachomatis genital challenge. Infect Immun. 1996;64:5341–8. doi: 10.1128/iai.64.12.5341-5348.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nigg C. An unidentified virus which produces pneumonia and systemic infection in mice. Science. 1942;99:49–50. doi: 10.1126/science.95.2454.49-a. [DOI] [PubMed] [Google Scholar]
- 26.Swenson CE, Schachter J. Infertility as a consequence of chlamydial infection of the upper genital tract in female mice. Sex Trans Dis. 1984;11:64–7. doi: 10.1097/00007435-198404000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Peterson EM, Zhong G, Carlson E, de la Maza LM. Protective role of magnesium in the neutralization by antibodies of Chlamydia trachomatis infectivity. Infect Immun. 1988;56:885–91. doi: 10.1128/iai.56.4.885-891.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schagger H, Von Jagow G. Tricine-sodium dodecyl sulphate polyacrylamide gel electrophoresis for the separation of protein range 1–100 kDa. Anal Biochem. 1987;166:368–79. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 29.Pal S, Pu Z, Huneke RB, Taylor HR, Whittum-Hudson JA. Chlamydia-specific lymphocytes in conjunctiva during ocular infection: limiting dilution analysis. Regional Immunol. 1991;3:171–6. [PubMed] [Google Scholar]
- 30.Brunham RC, Kuo CC, Cles L, Holmes KK. Correlation of host immune response with quantitative recovery of Chlamydia trachomatis from the human endocervix. Infect Immun. 1983;39:1491–4. doi: 10.1128/iai.39.3.1491-1494.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Igietseme JU, Ramsey KH, Magee DM, Williams DM, Kincy TJ, Rank RG. Resolution of murine chlamydial genital infection by the adoptive transfer of a biovar-specific Th1 lymphocyte clone. Reg Immunol. 1993;5:317–24. [PubMed] [Google Scholar]
- 32.Morrison RP, Caldwell HD. Immunity to murine chlamydial genital infection. Infect Immun. 2002;70:2741–51. doi: 10.1128/IAI.70.6.2741-2751.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morrison SG, Morrison RP. Resolution of secondary Chlamydia trachomatis genital tract infection in immune mice with depletion of both CD4+ and CD8+ cells. Infect Immun. 2001;69:2643–9. doi: 10.1128/IAI.69.4.2643-2649.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pal S, Theodor I, Peterson EM, de la Maza LM. Monoclonal immunogloblin A antibody to the major outer membrane protein of the Chlamydia trachomatis mouse pneumonitis biovar protects mice against a chlamydial genital challenge. Vaccine. 1997;15:575–82. doi: 10.1016/s0264-410x(97)00206-5. [DOI] [PubMed] [Google Scholar]
- 35.Ramsey KH, Rank RG. Resolution of chlamydial genital infection with antigen specific T-lymphocyte lines. Infect Immun. 1991;59:925–31. doi: 10.1128/iai.59.3.925-931.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roizman B. Introduction: objectives of herpes simplex virus vaccines seen from historical perspective. Rev Infect Dis. 1991;13:S892–4. doi: 10.1093/clind/13.supplement_11.s892. [DOI] [PubMed] [Google Scholar]
- 37.Morrison RP, Belland RJ, Lyng K, Caldwell HD. Chlamydial disease pathogenesis. The 57-kDa chlamydial hypersensitivity antigen is a stress response protein. J Exp Med. 1989;170:113–22. doi: 10.1084/jem.170.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stephens RS. Genomic autobiographies of Chlamydiae. In: Stephens RS, editor. Chlamydia: Intracellular Biology, Pathogenesis and Immunity. Washington DC: American Society for Microbiology; 1999. pp. 9–28. [Google Scholar]
- 39.Pal S, Theodor I, Peterson EM, de la Maza LM. Immunization with an acellular vaccine consisting of the outer membrane complex of Chlamydia trachomatis induces protection against a genital challenge. Infect Immun. 1997;65:3361–9. doi: 10.1128/iai.65.8.3361-3369.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pal S, Barnhart KM, Wei Q, Abai AM, Peterson EM, de la Maza LM. Vaccination of mice with DNA plasmids coding for the Chlamydia trachomatis major outer membrane protein elicits an immune response but fails to protect against a genital challenge. Vaccine. 1999;17:459–65. doi: 10.1016/s0264-410x(98)00219-9. [DOI] [PubMed] [Google Scholar]
- 41.Pal S, Theodor I, Peterson EM, de la Maza LM. Immunization with the Chlamydia trachomatis mouse pneumonitis major outer membrane protein can elicit a protective immune response against a genital challenge. Infect Immun. 2001;69:6240–7. doi: 10.1128/IAI.69.10.6240-6247.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tuffrey M, Alexander F, Conlan W, Woods C, Ward M. Heterotypic protection of mice against chlamydial salpingitis and colonization of the lower genital tract with a human serovar F isolate of Chlamydia trachomatis by prior immunization with recombinant serovar L1 major outer-membrane protein. J Gen Microbiol. 1992;148:1707–15. doi: 10.1099/00221287-138-8-1707. [DOI] [PubMed] [Google Scholar]
- 43.Zhang DJ, Yang X, Berry J, Shen C, McClarty G, Brunham RC. DNA vaccination with the major outer-membrane protein gene induces acquired immunity to Chlamydia trachomatis (mouse pneumonitis) infection. J Infect Dis. 1997;176:1035–40. doi: 10.1086/516545. [DOI] [PubMed] [Google Scholar]
- 44.Longbottom D, Russel M, Jones GE, Lainson FA, Herring AJ. Identification of a multigene family coding for the 90 kDa proteins of the ovine abortion subtype of Chlamydia psittaci. FEMS Microbiol Lett. 1996;142:277–81. doi: 10.1111/j.1574-6968.1996.tb08443.x. [DOI] [PubMed] [Google Scholar]
- 45.Barron AL, White HJ, Rank RG, Soloff BL, Moses EB. A new animal model for the study of Chlamydia trachomatis genital infection of mice with the agent of mouse pneumonitis. J Infect Dis. 1981;143:63–6. doi: 10.1093/infdis/143.1.63. [DOI] [PubMed] [Google Scholar]
- 46.Tuffrey M, Taylor-Robinson D. Progesterone as a key factor in the development of a mouse genital-tract infection with Chlamydia trachomatis. FEMS Microbiol Lett. 1981;12:111–5. [Google Scholar]
- 47.Pal S, Peterson EM, de la Maza LM. Susceptibility of mice to a vaginal infection with Chlamydia trachomatis mouse pneumonitis is dependent on the age of the animal. Infect Immun. 2001;69:5203–6. doi: 10.1128/IAI.69.8.5203-5206.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grossman JC. Interactions between the gonadal steroids and the immune system. Science. 1985;227:257–61. doi: 10.1126/science.3871252. [DOI] [PubMed] [Google Scholar]
- 49.Kaushic C, Zhou F, Murdin AD, Wira CR. Effects of estradiol and progesterone on susceptibility and early immune responses to Chlamydia trachomatis infection in the female reproductive tract. Infect Immun. 2000;68:4207–16. doi: 10.1128/iai.68.7.4207-4216.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Piccinni MP, Giudizi MG, Biagiotti R, et al. Progesterone favors the development of human T helper cells producing Th2-type cytokines and promotes both IL-4 production and membrane CD30 expression in established Th1 cell clones. J Immunol. 1995;155:128–33. [PubMed] [Google Scholar]