Abstract
T-cell homing within germinal centres (GCs) is required for humoral B-cell responses. However, the mechanisms implicated in the recruitment of T cells into the GC are not completely understood. Here we show, by immunohistology, and Northern and Western blots, that in vivo human GC B lymphocytes can express CxC and CC chemokines. Moreover, B-cell subset-specific experiments reveal that interleukin (IL)-8 and regulated on activation, normal, T-cell expressed, and secreted (RANTES) are predominantly expressed by GC centroblast and centrocytes, suggesting that chemokine expression is essential at stages in which B-lymphocytes engage in active antigen-dependent interactions with T lymphocytes. In keeping with this hypothesis, we show that the T cells recruited into the GC correlatively express the receptors for IL-8 and RANTES. We propose that chemokine expression is a key B-cell function that facilitates T-lymphocyte recruitment into the GCs and supports cognate B-cell : T-cell encounters. Moreover, our data are consistent with the impaired homing of T cells to secondary lymphoid organs in mice that are either deficient in CC and CxC chemokines or their receptors.
Introduction
T-cell-dependent immune responses require the cognate interactions of antigen (Ag)-specific B and T lymphocytes.1,2 B-cell activation in the extrafollicular T-cell-rich areas of secondary lymphoid organs enables the generation of short-lived plasma cells and the recruitment of germinal centre (GC) precursors into the B-cell follicles.3,4 Upon GC reaction, centroblasts proliferate and mutate in the dark zone.5–10 Centroblasts then progress into non-proliferating centrocytes, which undergo Ag-driven selection and differentiate into either memory or plasma B cells.1,11–14 Mature lymphocytes recirculate continuously within the lymphoid organs and display differential tissue tropism.15,16 For example, in human tonsils, B lymphocytes reside and move within three major histological compartments: the follicular mantle; GCs; and the intraepithelial region.1 T lymphocytes, on the other hand, are activated in the extrafollicular areas, migrate to the secondary follicles and are subsequently recruited into the GC apical light zone.1 Chemotactic signals within GCs are critical for maintaining cell recirculation throughout the secondary follicular microenvironment and for recruiting lymphocyte subsets into the sites where immune responses take place.17–19 While T-cell homing and trafficking within lymphoid organs have been the subject of more comprehensive studies, migration and chemokine production by B lymphocytes has only recently started to be investigated.20–22
The present study reveals that, in vivo, human GC B lymphocytes can express chemokines that belong to the CxC and CC gene superfamilies,23,24 represented herein by interelukin (IL)-8 and regulated on activation, normal, T-cell expressed, and secreted (RANTES), respectively. Because these two chemokines are predominantly expressed at maturation stages in which B- and T-lymphocyte interactions are critical for Ag-dependent responses, we postulate that expression of CxC and CC chemokines by B-cell centrocytes are important for the recruitment of T lymphocytes into the secondary follicles.
Materials and methods
Fluorescence-activated cell sorting of B-cell subsets
Tonsils were obtained as discarded biological material, according to institutional guidelines. All experiments were preformed in triplicate, and a minimum of three tonsil specimens were examined in the present study. Cell suspensions were subjected to T-cell depletion, using anti-CD2, -CD3 and -CD4 monoclonal antibodies (mAbs) (Pharmigen, San Diego, CA) in combination with magnetic beads coupled to anti-mouse immunoglobulin G (IgG) (Miltenyi Biotech, Auburn, CA), as described in the manufacturer's protocol. Differential expression of immunoglobulin D (IgD) was used to separate B lymphocytes into IgD+ and IgD− cells by fluorescence-activated cell sorting (FACS). B-lymphocyte subpopulations were further purified, as previously described,9,10 into five mature B-cell subsets: naive IgD+ CD38− CD23− (Bm1) and IgD+ CD38− CD23+ (Bm 2); GC IgD− CD38+ CD77+ (Bm3) and IgD− CD38+ CD77− (Bm4); and memory CD38− IgD− (Bm5) B lymphocytes.
RNA extraction and Northern blotting
Total RNA was extracted using the Trizol Reagent system (Life Technologies, Gaithersburg, MD), following the manufacturer's instructions. RNA (10 µg) was electrophoresed and Northern blotted, as previously described.25 Equal RNA loading was routinely monitored by the presence of distinctive 28S and 18S ribosomal RNA in ethidium bromide-stained gels. cDNA probes for IL-8 and CD23 were generated by using the reverse transcription–polymerase chain reaction (RT–PCR).
Oligonucleotides
The following oligonucleotide primers and respective cDNA probes were used: CD23 forward: 5′-AGACACAGGCTCCAAACTCCACTAA-3′ and reverse 5′-CTTCCATGTCGTCACAGGCATACCG-3′; IL-8 forward: 5′-ATGACTTCCAAGCTGGCCGTGGCTCTCTTG-3′ and reverse: 5′-TGAATTCTCAGCCCTCTTCAAAAACTTCTC-3′; RANTES forward: 5′-CCGTGCCCACATCAAGGAGTATT-3′ and reverse: 5′-CCCGGGTTCACGCCATTCT-3′; and immunoglobulin heavy chain forward: 5′-TCTGAGGTGCAGCTGGTGGAGTCTG-3′ (a consensus IgHV sequence) and reverse: 5′-TGAGGAGACGGTGACCAGGGTCCC-3′ (a consensus IgHJ sequence).
RT–PCR
Forward and reverse primers for IL-8 and RANTES were designed (as described above) and used to amplify respective transcripts from the mature B-cell subsets. Briefly, total RNA obtained from 500 cells of each fluorescence-activated cell sorted B-lymphocyte subset (Bm1 to Bm5) was reverse transcribed and amplified using appropriate RT (Life Technologies) and PCR (Perkin Elmer Corporation, Foster City, CA) kits. RANTES RT–PCR products were Southern blotted and hybridized to an end-labelled 32P-oligonucleotide probe: 5′-TGCCTGTTTCTGCTTGCTCTTGTC-3′. Expression of the immunoglobulin heavy chain (IgHV) was routinely used to monitor the RNA content in each of the five Bm subsets.
Immunohistology
Serial sections of cryopreserved tonsil tissue were blocked for 20 min at room temperature with a blocking solution (Powerblock™; Oncogene Research Products, San Diego, CA) and stained (1 : 100 dilution) with either an affinity-purified rabbit anti-human IL-8 IgG (Research Diagnostics, Inc. Flanders, NJ) or an anti-CD20 mAb (BD Pharmigen, San Diego, CA). After overnight incubation with the antibodies at 4°, the histological-section slides were washed three times with phosphate-buffered saline (PBS) containing 0·1% Tween. Alexa-594 (red fluorescence)-conjugated anti-rabbit antibody (Molecular Probes, Eugene, OR) and Alexa-488 (green fluorescence)-conjugated anti-mouse antibody were subsequently applied (1 : 1000 dilution) and the slides were incubated at room temperature for a further 60 min. After the incubation with the second antibody, the slides were washed three times with phosphate-buffered saline containing 0·1% Tween, counterstained with Gill's haematoxylin (Oncogene Research Products), mounted and analysed using fluorescence microscopy. For the expression of IL-8 and RANTES receptors, tonsil sections were reacted overnight with a 1 : 500 dilution of fluorescein-labelled anti-CXCR1 (R & D Systems, Minneapolis, MN) or a 1 : 500 dilution of fluorescein isothiocyanate (FITC)-labelled CCR5 (R & D Systems), together with a 1 : 500 dilution of phycoerythrin-labelled anti-CD3 (BD PharMingen). Slides were subsequently washed, counterstained with 4′,6-diamidine-2-phenylindol dihydrochloride (DAPI) and analysed using fluorescence microscopy. Non-immune rabbit and mouse IgGs were routinely used as negative controls.
Western blots
Equal amounts of protein lysates, extracted from naïve (IgD+ CD38−) and GC (IgD− CD38+) B cells, were resolved by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE; 12% gel) and electrotransferred onto nitrocellulose membranes. Blots were blocked with 4% non-fat dry milk in Tris-buffered saline (TBS), containing 0·2% Tween-20, for at least 1 hr at 4°. Blots were then incubated overnight with an affinity-purified rabbit anti-RANTES (diluted 1 : 500) IgG (Research Diagnostics, Inc.) at 4°, followed by three 10-min washes with TBS/Tween-20 at room temperature. Peroxidase-conjugated anti-rabbit (diluted 1 : 5000) IgG (mouse- and human-absorbed; Santa Cruz Biotechnology, Inc. Santa Cruz, CA) was added to the blots and incubated for 1 hr at room temperature. Blots were washed three times and the reactivity was revealed by chemiluminescence (Pierce, Rockford, IL), according to the manufacturer's instructions.
Results
GC calling for T-cell help
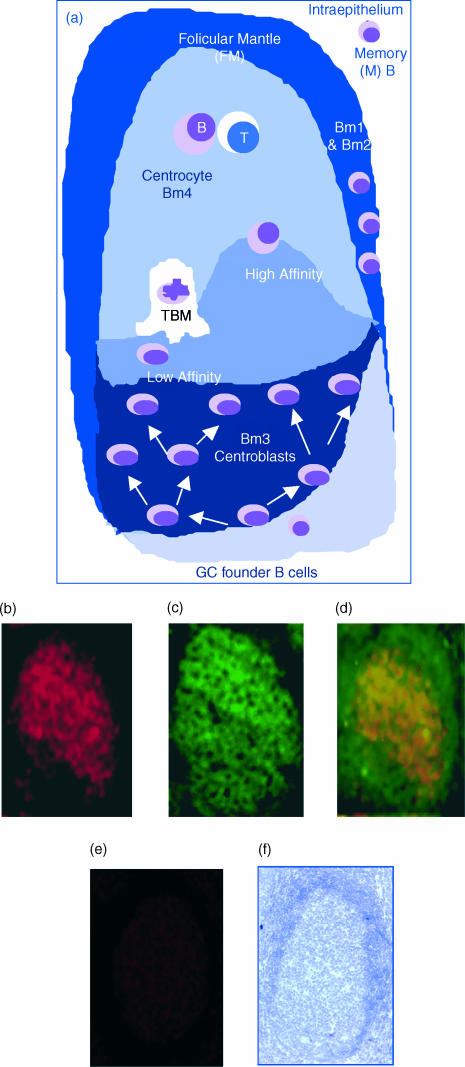
A GC reaction is initiated by the activation of naïve (Bm1 and Bm2) and memory B cells in the extrafollicular area and followed by the colonization of GC founder B cells into a follicular network (Fig. 1a). GC founder B cells undergo proliferation in the GC dark zone, which is thus populated by B-cell blasts (centroblasts, namely Bm3). Centroblasts mature into non-proliferating centrocytes (Bm4) within the light zone of GCs and ultimately differentiate into memory B cells (Bm5). As IL-8 has been previously shown to promote T-cell chemotaxis,26 and because GC follicles largely consist of B lymphocytes, we postulated that the attraction of T cells into the GC microenvironment could be B-cell dependent and IL-8 mediated. In keeping with this hypothesis, Fig. 1(b) shows a strong expression of IL-8 within the GC follicles of human tonsils, as revealed by immunofluorescence.
Figure 1.
Expression of interleukin (IL)-8 within germinal centres (GCs) by immunohistology. (a) A schematic representation of the GC reaction: an immunoglobulin M (IgM)-expressing GC founder migrates to the dark zone of a GC and undergoes clonal expansion and affinity maturation by somatic hypermutation. The mutated cells migrate to the basal light zone, where they are subjected to antigen-dependent selection. High-affinity (High) cells are selected and migrate to the apical light zone of GCs, where they encounter and present antigen (Ag) to T cells. Low affinity (Low) centroblasts undergo programmed cell death and are subsequently cleared by tingible body macrophages (TBM). (b) Reactivity of frozen tonsil (acetone-fixed) tissue sections to an affinity-pure anti-IL-8 immunoglobulin G (IgG). (c) B-cell identification by specific reactivity to an anti-CD20 monoclonal antibody (mAb). (d) Co-localization of the red and green fluorescence (anti-CD20), indicating the expression of IL-8 by CD20 B cells, as depicted by the yellow/orange merge. (e) Lack of reactivity to a non-immune IgG, used as a negative control. (f) Integrity of the tissue sections used, as determined by Gill's haematoxylin staining.
B lymphocytes are the major GC IL-8 producers
Because IL-8 is expressed by diverse cell lineages, including endothelial cells, macrophages and dendritic cells (DCs), it is uncertain whether B lymphocytes can also contribute to the expression of IL-8. However, and as demonstrated by the staining of CD20 in Fig. 1(c), GCs are populated predominantly by B lymphocytes. Moreover, recent in vitro studies have shown that B-cell activation, through the engagement of the B-cell antigen receptor (BCR), resulted in significant chemokine production.22 These findings strongly suggest that in vivo, IL-8 can also be expressed by GC B lymphocytes. Consistent with this hypothesis, Fig. 1(d) shows that indeed CD20+ GC B lymphocytes are bona fide IL-8 producers. The predominant expression of IL-8 by the GC B cells was further substantiated by the fact that while CD20 stains both follicular mantle and GC B cells (Fig. 1c, 1d), IL-8 is selectively detected in the CD20+ GC B-cell population. The bright yellow/orange fluorescence pattern, as depicted in Fig. 1(d) by the merging of IL-8 (red fluorescence) and CD20 (green fluorescence), is visible over a large area of the GC apical light zone, suggesting that IL-8 secretion is contributed by a significant number of B-cell centrocytes. The specificity of the IL-8 fluorescence staining was routinely confirmed by the absence of fluorescence when irrelevant (non-immune) antibody controls were used (Fig. 1e), despite the integrity of the human tonsil section (Fig. 1f).
While the putative expression of IL-8 by follicular dendritic cells (FDC) cannot be ruled out, the presence of CD20 at the surface of FDCs has not been satisfactorily established. FDCs possess cytoplasmic ramifications that form dense networks heavily loaded with firmly attached B lymphocytes.27 The firm embrace between FDCs and B cells has greatly impaired FDC purification and hence limited the assessment of their phenotype. Only one study has provided a report on the phenotype of highly pure FDCs, in which the lack of CD20 expression was unambiguously demonstrated.28 Our findings are in agreement with that notion and suggest that B cells are the predominant source of IL-8 within the GC follicles.
Selective IL-8 expression by CD19+ IgD− B lymphocytes
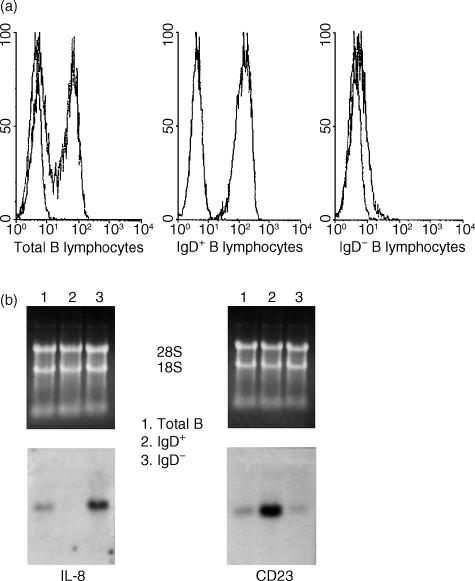
To further confirm the selective expression of IL-8 by B lymphocytes, pure CD19+ B lymphocytes were obtained from fresh human tonsils and sorted into IgD+ and IgD− subpopulations (Fig. 2a). Therefore, we first sought to compare the expression of IL-8 between CD19+ IgD+ and CD19+ IgD− cells because they comprise two major tonsil subpopulations that can be obtained in large quantities. While CD19+ IgD+ comprises naïve B cells, CD19+ IgD− comprises both GC and memory B-cell subsets. By using this approach, significant levels of RNA were purified from each subset and analysed for the expression of IL-8 without the need of transcript amplification. Figure 2(b) demonstrates the selective in vivo expression of IL-8 by the IgD− subpopulation, indicating that B-cell-derived IL-8 could be contributed by GC and memory cells. The differential expression of CD23, a phenotypic marker for naïve B lymphocytes (Fig. 2b), further confirmed the selectivity of IL-8 expression by the IgD− B cells. Taken together, these results suggest that IL-8 expression is important for B lymphocytes undergoing Ag-dependent GC responses and that its secretion may facilitate cellular interactions.
Figure 2.
Differential expression of interleukin (IL)-8 and CD23 by immunoglobulin D+ (IgD+) and IgD− B lymphocytes. (a) Flow cytometry profiles of pure IgD+ and IgD− B-cell subpopulations. IgD− cells co-localize with the mock isotype-matched control monoclonal antibody (mAb). (b) Northern blot analysis to determine the expression of IL-8 by total B cells and fluorescence-activated cell sorter (FACS) sorted IgD+ and IgD− subsets. CD23 expression was used as control for the naïve IgD+ cells. RNA loading was routinely assessed by ethidium bromide staining of the agarose gels.
IL-8 is predominantly expressed by GC centroblasts and centrocytes
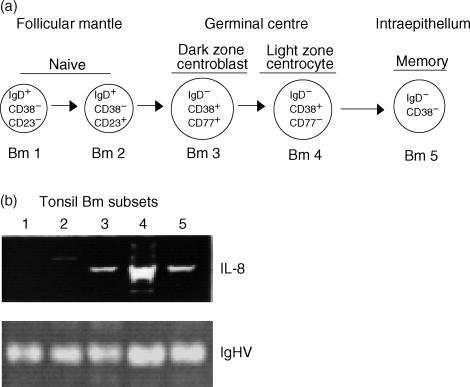
As previously reported,9,10 and as stated above, IgD+ B cells comprise the naïve cell compartment, which is populated by IgD+ CD38− CD23− (Bm1) and IgD+ CD38− CD23+ (Bm2). On the other hand, the IgD− B cells are composed of GC centroblasts (Bm3: IgD− D38+ CD77+), centrocytes (Bm4: IgD− CD38+ CD77−) and memory cells (Bm5: IgD− CD38−). To further investigate whether the expression of IL-8 by IgD− B lymphocytes occurs in a differentiation stage-specific manner, tonsil B lymphocytes were purified into five distinct mature B-lymphocyte subsets (Bm1 to Bm5). RNA from 500 cells of each Bm subset was analysed for the expression of IL-8 by RT–PCR. Figure 3 shows that within GCs, and in agreement with the immunofluorescent histology (Fig. 1), IL-8 expression is predominant at the centrocyte (Bm4) stage. These findings are in complete agreement with recent microarray studies which show selective expression of IL-8 within the GC B-cell centrocyte population.29
Figure 3.
Predominant expression of interleukin (IL)-8 by centroblast and centrocyte B-lymphocyte subsets. (a) Phenotypic scheme of mature B-cell subsets: naive Bm1 and Bm2; germinal centre (GC) Bm3 and Bm4; and memory Bm5. (b) IL-8-specific reverse transcription–polymerase chain reaction (RT–PCR), using RNA from 500 cells of each Bm subset (Bm1 to Bm5). The numbers in the figure correspond to the respective Bm subsets. RT–PCR of the immunoglobulin heavy chain (IgHV) was used herein as control for the RNA content of each subset. The results are shown as ethidium bromide staining of electrophoresed RT–PCR samples.
The lower, but relevant, expression of IL-8 by the centroblast and memory B-cell subsets may reflect a stage transition in which chemokine production, initiated at the centroblast stage, becomes maximal within centrocytes and begins to decay as the cells differentiate into memory or plasma cells. Alternatively, the expression of IL-8 by the memory B-cell subset (Bm5) may also be important for the attraction of cognate T-cell help.
RANTES contribution to GC-mediated T-cell chemoattraction
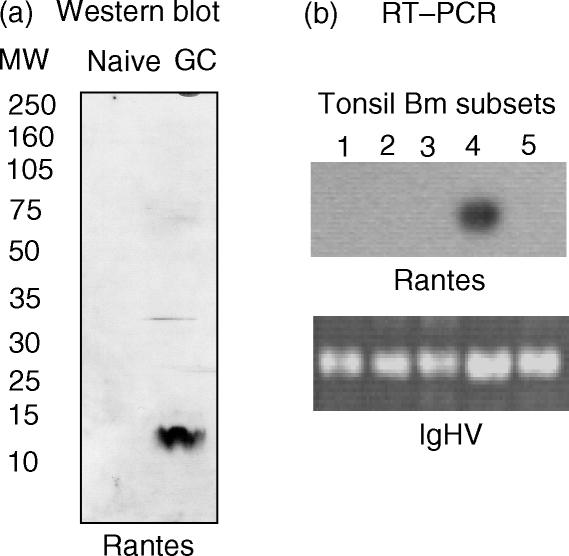
The chemokine gene superfamily consists of various subgroups,23,24 among which the CxC and CC families are probably the best characterized. The capacity of these chemokines to attract distinct leucocyte populations, including B- and T lymphocytes, has been extensively documented. While IL-8 leads the CxC subgroup, the chemokine RANTES represents the CC gene family.23,24 The selective expression of the CxC chemokine, IL-8, by the GC and memory B lymphocytes, prompted us to examine whether the expression of CC chemokines, such as RANTES, could also play a role during Ag-dependent responses. RANTES was originally identified as a T-cell chemokine by screening a cDNA library enriched for T-cell-specific genes not expressed by other cell types.30 Because our working hypothesis postulates that B-lymphocyte-mediated attraction of T lymphocytes is contributed by both CxC and CC chemokines, the expression of RANTES by the distinct B-cell subsets was also examined. An enriched and pure (98%) B-cell pool, obtained by negative selection, was sorted into naïve (IgD+ CD38−) and GC (IgD− CD38+) subsets. Protein lysates were prepared from each naïve and GC subset, and subsequently examined for the expression of the RANTES protein by Western blotting. As depicted in Fig. 4(a), GC B lymphocytes also have the capacity to produce CC chemokines such as RANTES. To determine whether, in analogy to IL-8, RANTES is also expressed in a maturation stage-specific manner that facilitates the ability of B lymphocytes to attract cognate cell interactions, we sought to examine the levels of RANTES transcripts in the five mature B-cell subsets (Bm1 to Bm5), using RT–PCR. Consistent with the predominant expression of IL-8 by the Bm4 subset, Fig. 4(b) shows that RANTES is selectively expressed by the B-cell centrocytes.
Figure 4.
Selective expression of regulated on activation, normal, T-cell expressed, and secreted (RANTES) by germinal centre (GC) B-cell subsets. (a) Western blot analysis depicting the selective expression of RANTES by IgD− CD38+ GC B cells. (b) Southern blot experiment of a RANTES-specific reverse transcription–polymerase chain reaction (RT–PCR), using total RNA from 500 cells of each of the five mature B-cell subsets. The numbers in the figure correspond to the respective Bm1 to Bm5 subsets. Again, RT–PCR amplification of the immunoglobulin heavy chain (IgHV) was used as an internal control for the RNA content of each subset. The results are autoradiographs of the RANTES hybridization reaction and ethidium bromide gel staining of the IgHV RT–PCR results, respectively.
Analogous to RANTES, IL-8 is predominantly expressed by the centrocyte subset. However, significant levels of IL-8 are also observed at the centroblast and memory stages. Because the transition from centroblast to centrocyte and from centrocyte to memory occurs rapidly (within hours), IL-8 expression by centroblasts marks the onset of Ag-driven GC B-cell activation, whereas the expression by the memory cells may be reminiscent of the ongoing centrocyte activation. The difference between IL-8 and RANTES expression at the centroblast and memory stages is probably a result of the levels of detectable expression. These results not only support the significance of the stage-specific expression of the CxC and CC chemokines by B lymphocytes, but they further suggest that IL-8 and RANTES production may be equally relevant during GC reactions.
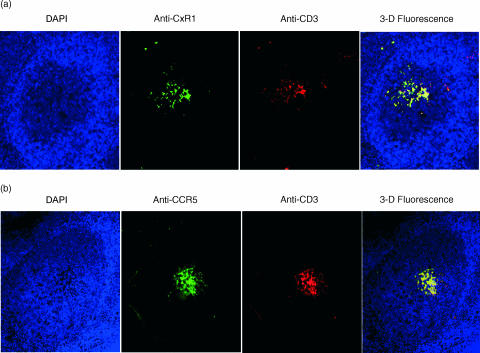
Selective GC recruitment of T cells that express CXC and CC chemokine receptors
Chemokines and their receptors have been identified as major regulators controlling the functional organization of secondary lymphoid organs.31 It is known that helper T cells (Th) comprise specialized subsets, including Th1 and Th2, which respond to chemoattraction by the expression of both CxC and CC receptors.32–34 Th cells can home to GC and engage in cognate interactions with Ag-specific B cells. While the present study consistently documents the in vivo expression of IL-8 and RANTES by B lymphocytes within GCs, their physiological relevance in the recruitment of responsive T cells that express the corresponding receptors remains to be determined. In support of the functional significance of the GC B-cell-expressed chemokines, immunohistology experiments on human tonsils (Fig. 5) demonstrate that the T cells (red fluorescence) recruited into the GC follicles indeed express both CxCR1 and CCR5 (green fluorescence). The remarkable overlap in fluorescence (shown by the yellow fluorescent merge), resulting from the concomitant CD3 and chemokine receptor detection, supports our hypothesis that T cells functionally respond to GC B-cell-mediated IL-8 and RANTES chemoattraction. These findings, together with the histological localization of CxCR1- and CCR5-expressing T cells, support the active role of GC B cells in the recruitment of cognate T-cell help.
Figure 5.
Concomitant germinal centre (GC) presence of T cells that express interleukin (IL)-8 and regulated on activation, normal, T-cell expressed, and secreted (RANTES) receptors. (a) Immunofluorescence detection of CxCR1-expressing T cells on tonsil tissue sections. (b) Histology and immunofluorescence for CCR5 expression by GC T cells. Starting from the left on both (a) and (b), the first panel depicts a histological section of a GC follicle revealed by the blue fluorescence of the 4′,6-diamidine-2-phenylindol dihydrochloride (DAPI) mount; the second panel displays the reactivity of either the anti-IL-8 receptor (CxCR1) or RANTES (CCR5) monoclonal antibodies (mAbs); the third panel reveals the detection of T cells by the reactivity to the anti-CD3 mAb; and the fourth panel is a three-dimensional merge of blue, green and red fluorescence.
Discussion
Chemokines are key molecules in the development of GC reactions because they contribute to the organization of the secondary lymphoid organ microenvironment and to lymphocyte migration. Our findings show that, in vivo, GC B lymphocytes can produce and secrete chemokines in a stage-specific manner, which strongly suggests that the recruitment of Ag-specific T-cell help is an active B-cell-dependent process. Because B lymphocytes present antigen to T cells at the GC apical light zone,1–5 the expression of IL-8 and RANTES, at the centrocyte stage, supports the hypothesis that a B-lymphocyte-mediated attraction facilitates cognate B- and T-cell interactions. Moreover, B cells exhibit a relatively lower rate of migration and tend to stay within one GC follicle, while T cells respond more avidly to chemotactic signals and can migrate to proximal and distal follicles,35 thus indicating that T cells could be susceptible to B-lymphocyte-mediated attraction. In this context, the in vivo production and secretion of chemokines by Ag-driven B lymphocytes could result in the generation of a gradient to attract Ag-specific T cells into the histological GC areas in which effective B- and T-cell interactions can take place.
The aim of the present study is to demonstrate that in vivo human GC B cells are major producers of chemokines of the CxC and CC gene families, and IL-8 and RANTES are presented as primary members of the respective families. While several chemokines have been reported to be involved in T-cell chemotaxis, the B-cell-dependent recruitment and maintenance of T cells within GCs has only started to be investigated.
While it is conceivable that IL-8 may act as an autocrine factor to promote the survival of GC B cells, previous studies, using freshly purified human T cells, have demonstrated that T cells do migrate towards IL-8 in chemotaxis chambers.26 Moreover, the fact that GC T cells express the receptor for IL-8 supports the hypothesis that IL-8 can play a role as a chemotactic factor for T cells. Consistent with this hypothesis, it has been previously shown that the T cells recruited into GCs respond in vivo and in vitro to chemokines by expressing the corresponding receptors and by active migration.36–38 In keeping with this notion, the in vivo expression of IL-8 and RANTES by B cells, and the concomitant expression of CxCR1 and CCR5 by T cells within GCs, additionally reveal a functional ligand and receptor communication between antigen-presenting cells (APC) and effector cells.
Furthermore, chemokines play a key role in inducing cytokine expression from chemoattracted cells, including IL-4 from T cells.39 This is physiologically relevant because B-cell centrocytes obtain T-cell help to undergo isotype-switch recombination through CD40 and CD40 ligand interactions and by the secretion of specific isotype-switch factors such as IL-4 and IL-10.40 These findings provide strong support to previous in vitro studies, in which RANTES has been shown to induce Th1 cell migration.41–43 More importantly, chemokine production by B cells may not only facilitate the attraction of T cells into GCs but it may serve as a mechanism to induce the specific type of help that is required. This theory is further supported by a previous study showing that B cells and professional APCs are capable of recruiting regulatory T cells through the chemokine CCL-4.44 Cognate cell interactions within GC secondary follicles not only help B lymphocytes, but also provide critical signals for the growth and survival of Ag-specific T cells.45
The data presented herein strongly suggest that the in vivo expression of chemokines by centrocytes facilitates T-lymphocyte recruitment into the GCs and supports their encounter with B lymphocytes. Chemoattraction within GCs may represent a relevant function of B lymphocytes during secondary immune responses, in which both B and T lymphocytes mutually benefit from their cognate interactions.
Human tonsils are therapeutically removed as a result of chronic inflammation. This fact alone exposes the extraordinary diversity in antigen-driven responses and the enormous heterogeneity in the phenotypes and functions of the T cells recruited into tonsil GCs, including Th1, Th2 and the new ‘follicular T helper’ subset.31 In keeping with this notion, the complexity of the GC reaction in the human tonsils is at least phenomenal. Therefore, the real-time capture of fresh and untouched histology, biochemistry and gene expression of tonsil sections and pure B-cell subsets is the closest in vivo documentation of the physiology of GC B cells in the chemokine-mediated recruitment and retention of T-cell help that has been obtained in humans.
Given the complexity and diversity of the GC reaction in human tonsils, which is likely to accumulate a broad repertoire of T cells that include Th1, Th2 and follicular Th cells, substantive differences between human and mouse models are anticipated. Nevertheless, our work strongly supports the remarkable observation by Fillatreau et al., in which a stable ratio of four T cells to 100 B cells is consistently present within the GC follicles.46 In line with their conclusions, the present study proposes that the strong correlation of the number of T cells accumulating within the B-cell follicles indicates that the process is limited by factors present in the follicles. Such factors might be constitutively delivered by B cells, follicular stroma cells (such as FDCs), or DCs present within the follicle. While our study does not dispute FDC and DC function, our data show that human GC B cells are very capable of actively producing chemoattracting factors in vivo.
Moreover, our findings are additionally supported by the notion that genetic alterations in either the CC and CXC chemokines, or their receptors, lead to profound defects in the capacity of T cells to home into secondary lymphoid organs. For instance, in the spontaneous mutant plt–/– (paucity of lymph node T cells) mice, which lack CC gene expression, T cells migrate poorly to the secondary follicles. Consistently, transfer of T cells lacking CC or CXC receptors reveal a marked suppression in their ability to home into wild-type lymph nodes and Peyer's patches.47
In conclusion, the data reported in the present study provide a novel view of B- and T-cell encounters within GCs in which the B lymphocytes promote the recruitment of the type of help that is required. Such a view lends additional support to the notion of GC B-lymphocyte self-determination, as documented previously by their capacity to autonomously engage in homotypic signalling.48
Acknowledgments
The authors are grateful to Drs John I. Hamilton and Jun Wang for their critical reading of the manuscript. This work was supported by grant 6161-03 from the Leukaemia and Lymphoma Society and by grant AI056125-01 from the National Institutes of Health. J.C.S., D.X.N. and R.R. were supported by the Smith predoctoral fellowship. L.G.-R. and R.R. are graduate students from the Department of Immunology, School of Biological Sciences, at the Autonomous University of Nuevo Leon, Mexico.
References
- 1.MacLennan ICM. Germinal centers. Annu Rev Immunol. 1994;12:117–39. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- 2.Kelsoe G. The germinal center: a crucible for lymphocyte selection. Semin Immunol. 1996;8:179–84. doi: 10.1006/smim.1996.0022. [DOI] [PubMed] [Google Scholar]
- 3.Liu Y-J, Zhang J, Lane PJL, Chan EY-T, MacLennan ICM. Sites of specific B-cell activation in primary and secondary responses to T-cell-dependent and T-cell-independent antigens. Eur J Immunol. 1991;21:2951–62. doi: 10.1002/eji.1830211209. [DOI] [PubMed] [Google Scholar]
- 4.Jacob J, Kassir R, Kelsoe G. In situ studies of the primary immune response to [4-hydroxy-3-nitrophenyl] acetyl. I. The architecture and dynamics of responding cell populations. J Exp Med. 1991;173:1165–75. doi: 10.1084/jem.173.5.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berek C, Berger A, Apel M. Maturation of immune responses in germinal centers. Cell. 1991;67:1121–9. doi: 10.1016/0092-8674(91)90289-b. [DOI] [PubMed] [Google Scholar]
- 6.Jacob J, Kelsoe G, Rajewsky K, Weiss U. Intraclonal generation of antibody mutants in germinal centers. Nature. 1991;354:389–92. doi: 10.1038/354389a0. [DOI] [PubMed] [Google Scholar]
- 7.Kuppers R, Zhao M, Hansmann M-L, Rajewsky K. Tracing B cell development in human germinal centers by molecular analysis of single cells picked from histological sections. EMBO J. 1993;12:4955–67. doi: 10.1002/j.1460-2075.1993.tb06189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McHeyzer-Williams MG, McLean MJ, Lalor PA, Nossal GJV. Antigen-driven B cell differentiation in vivo. J Exp Med. 1993;178:295–307. doi: 10.1084/jem.178.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pascual V, Liu YJ, Magalski A, de Bouteiller O, Banchereau J, Capra JD. Analysis of somatic mutation in five B cell subsets of human tonsil. J Exp Med. 1994;180:329–39. doi: 10.1084/jem.180.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu YJ, Malisan F, de Bouteiller O, et al. Within germinal centers, isotype switching of immunologlobulin genes occur after the onset of somatic mutation. Immunity. 1996;4:241–50. doi: 10.1016/s1074-7613(00)80432-x. [DOI] [PubMed] [Google Scholar]
- 11.Kroese FGM, Timens W, Nieuwenhuls P. Germinal center reaction and B lymphocytes: morphology and function. In: Grundmann E, Vollmer E, editors. Current Topics in Pathology. Vol. 84. New York: Springer-Verlag; 1990. pp. 103–48. Reaction Pattern of the Lymph Node. [DOI] [PubMed] [Google Scholar]
- 12.Liu Y-J, Johnson GD, Gordon J, MacLennan ICM. Germinal centres in T-cell-dependent antibody responses. Immunol Today. 1992;13:17–21. doi: 10.1016/0167-5699(92)90199-H. [DOI] [PubMed] [Google Scholar]
- 13.Thorbecke GJ, Amin AR, Tsiagbe VK. Biology of germinal centers in lymphoid tissue. FASEB J. 1994;8:832–40. doi: 10.1096/fasebj.8.11.8070632. [DOI] [PubMed] [Google Scholar]
- 14.Martinez-Valdez H, Guret C, de Bouteiller O, Fugier I, Banchereau J, Liu YJ. Human germinal center B cells express the apoptosis-inducing genes fas, c-myc, P53, and Bax, but not the survival gene bcl-2. J Exp Med. 1996;183:971–7. doi: 10.1084/jem.183.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–6. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 16.Springer TA. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu Rev Physiol. 1995;57:827–72. doi: 10.1146/annurev.ph.57.030195.004143. [DOI] [PubMed] [Google Scholar]
- 17.Rollins BJ. Chemokines. Blood. 1997;90:909–28. [PubMed] [Google Scholar]
- 18.Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines-CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- 19.Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 20.Corcione A, Ottonello L, Tortolina G, et al. Recombinant tumor necrosis factor enhances the locomotion of memory and naive B lymphocytes from human tonsils through the selective engagement of the type II receptor. Blood. 1997;90:4493–501. [PubMed] [Google Scholar]
- 21.Nielsen LS, Frydenberg J, Lind M, Deleuran M, Stengaard-Pedersen K, Deleuran B. CD19-selected B lymphocytes synthesize, secrete and migrate in the presence of IL-8. TNF-alpha and gammaIP-10 are also B lymphocyte migratory factors. Cytokine. 1997;9:747–53. doi: 10.1006/cyto.1997.0226. [DOI] [PubMed] [Google Scholar]
- 22.Krzysiek R, Lefevre EA, Zou W, Foussat A, Bernard J, Portier A, Galanaud P, Richard Y. Antigen receptor engagement selectively induces macrophage inflammatory protein-1α (MIP-1α) and MIP-1β chemokine production in human B cells. J Immunol. 1999;162:4455–63. [PubMed] [Google Scholar]
- 23.Matsushima K, Morishita K, Yoshimura T, et al. Molecular cloning of a human monocyte derived neutrophil chemotactic factor (MDNCF) and the induction of MDNCF mRNA by interleukin 1 and tumor necrosis factor. J Exp Med. 1988;167:1883–93. doi: 10.1084/jem.167.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hornung F, Giuseppe S, Lenardo MJ. TNF-α-induced secretion of C-C chemokines modulates C-C chemokines receptor 5 expression on peripheral blood lymphocytes. J Immunol. 2000;164:6180–7. doi: 10.4049/jimmunol.164.12.6180. [DOI] [PubMed] [Google Scholar]
- 25.Guzman-Rojas L, Sims JC, Rangel R, Guret C, Sun Y, Alcocer JM, Martinez-Valdez H. PRELI, the human homologue of the avian px19, is expressed by germinal center B-lymphocytes. Int Immunol. 2000;12:607–12. doi: 10.1093/intimm/12.5.607. [DOI] [PubMed] [Google Scholar]
- 26.Schratzberger P, Dunzendorfer S, Reinisch N, Kahler CM, Wiedermann CJ. Interleukin-8-induced human peripheral blood B-lymphocyte chemotaxis in vitro. Immunol Lett. 1997;58:167–70. doi: 10.1016/s0165-2478(97)00085-0. [DOI] [PubMed] [Google Scholar]
- 27.Petrasch S, Brittinger G, Wacker HH, Schmitz J, Kosco-Vilbois M. Follicular dendritic cells in non-Hodgkin's lymphomas. Leuk Lymphoma. 1994;15:33–43. doi: 10.3109/10428199409051675. [DOI] [PubMed] [Google Scholar]
- 28.Schriever F, Freedman AS, Freeman G, Messner E, Lee G, Daley J, Nadler LM. Isolated human follicular dendritic cells display a unique antigenic phenotype. J Exp Med. 1989;169:2043–58. doi: 10.1084/jem.169.6.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klein U, Tu Y, Stolovitzky GA, et al. Transcriptional analysis of the B cell germinal center reaction. Proc Natl Acad Sci USA. 2003;100:2639–44. doi: 10.1073/pnas.0437996100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schall TJ, Jongstra J, Dyer BJ, Clayberger C, Davis MM, Krensky AM. A human T cell-specific molecule is a member of a new gene family. J Immunol. 1988;1:1018–25. [PubMed] [Google Scholar]
- 31.Breitfeld D, Ohl L, Kremmer E, Ellwart J, Sallusto F, Lipp M, Forster R. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med. 2000;192:1545–52. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim CH, Rott LS, Clark-Lewis I, Campbell DJ, Wu L, Butcher EC. Subspecialization of CXCR5+ T cells: B helper activity is focused in a germinal center-localized subset of CXCR5+ T cells. J Exp Med. 2001;193:1373–81. doi: 10.1084/jem.193.12.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med. 2000;192:1553–62. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nanki T, Lipsky PE. Stromal cell-derived factor-1 is a costimulator for CD4+ T cell activation. J Immunol. 2000;164:5010–4. doi: 10.4049/jimmunol.164.10.5010. [DOI] [PubMed] [Google Scholar]
- 35.Bowman EP, Campbell JJ, Soler D, Dong Z, Manlongat N, Picarella D, Hardy RR, Butcher EC. Developmental switches in chemokine response profiles during B cell differentiation and maturation. J Exp Med. 2000;191:1303–18. doi: 10.1084/jem.191.8.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tani K, Su SB, Utsunomiya I, Oppenheim JJ, Wang JM. Interferon gamma maintains the binding and functional capacity of receptors for IL-8 on cultured human T cells. Eur J Immunol. 1998;28:502–7. doi: 10.1002/(SICI)1521-4141(199802)28:02<502::AID-IMMU502>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 37.Xu L, Kelvin DJ, Ye GQ, Taub DD, Ben-Baruch A, Oppenheim JJ, Wang JM. Modulation of IL-8 receptor expression on purified human T lymphocytes is associated with changed chemotactic responses to IL-8. J Leukoc Biol. 1995;57:335–42. doi: 10.1002/jlb.57.2.335. [DOI] [PubMed] [Google Scholar]
- 38.Utsunomiya I, Tani K, Gong W, Oppenheim JJ, Wang JM. Differential expression of binding sites for chemokine RANTES on human T lymphocytes. Eur J Immunol. 1997;27:1406–12. doi: 10.1002/eji.1830270617. [DOI] [PubMed] [Google Scholar]
- 39.Lukacs NW, Chensue SW, Karpus WJ, Lincoln P, Keefer C, Strieter RM, Kunkel SL. C-C chemokines differentially alter interleukin-4 production from lymphocytes. Am J Pathol. 1997;150:1861–8. [PMC free article] [PubMed] [Google Scholar]
- 40.Malisan F, Briere F, Bridon JM, Harindranath N, Mills FC, Max EE, Banchereau J, Martinez-Valdez H. Interleukin-10 induces immunoglobulin G isotype switch recombination in human CD40-activated naïve B-lymphocytes. J Exp Med. 1996;183:937–47. doi: 10.1084/jem.183.3.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Siveke JT, Hamann A. T helper 1 and T helper 2 cells respond differentially to chemokines. J Immunol. 1998;160:550–4. [PubMed] [Google Scholar]
- 42.Ding Z, Xiong K, Issekutz TB. Regulation of chemokine-induced transendothelial migration of T lymphocytes by endothelial activation: differential effects on naive and memory T cells. J Leukoc Biol. 2000;67:825–33. doi: 10.1002/jlb.67.6.825. [DOI] [PubMed] [Google Scholar]
- 43.Ding Z, Xiong K, Issekutz TB. Chemokines stimulate human T lymphocyte transendothelial migration to utilize VLA-4 in addition to LFA-1. J Leukoc Biol. 2001;693:458–66. [PubMed] [Google Scholar]
- 44.Bystry RS, Aluvihare V, Welch KA, Kallikourdis M, Betz AG. B cells and professional APCs recruit regulatory T cells via CCL4. Nat Immunol. 2001;2:1126–32. doi: 10.1038/ni735. [DOI] [PubMed] [Google Scholar]
- 45.Ansel KM, McHeyzer-Williams LJ, Ngo VN, McHeyzer-Williams MG, Cyster JG. In vivo-activated CD4 T cells upregulate CXC chemokine receptor 5 and reprogram their response to lymphoid chemokines. J Exp Med. 1999;190:1123–34. doi: 10.1084/jem.190.8.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fillatreau S, Gray D. T cell accumulation in B cell follicles is regulated by dendritic cells and is independent of B cell activation. J Exp Med. 2003;197:195–206. doi: 10.1084/jem.20021750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gunn MD, Kyuwa S, Tam C, Kakiuchi T, Matsuzawa A, Williams LT, Nakano H. Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization. J Exp Med. 1999;189:451–60. doi: 10.1084/jem.189.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Siddiqa A, Sims-Mourtada JC, Guzman-Rojas L, Rangel R, Guret C, Madrid-Marina V, Sun Y, Martinez-Valdez H. Regulation of CD40 and CD40 ligand by the AT-hook transcription factor AKNA. Nature. 2001;410:383–7. doi: 10.1038/35066602. [DOI] [PubMed] [Google Scholar]