Abstract
Although the expression of class II major histocompatibility complex (MHC) in retina is extremely low, it is an established fact that activated CD4 T cells, specific for retinal antigens (Ags), mediate experimental autoimmune uveoretinitis (EAU). Conversely, CD8 T cells have not been shown to recognize Ag in the retina. This study investigated whether retinal-specific Ags are detected by class I MHC-restricted CD8 T cells. Using a CD8 T-cell clone (β3) specific for an immunodominant epitope of β-galactosidase (β-gal), local Ag recognition was shown by transfer of activated β3 cells into β-gal transgenic (Tg) mice expressing β-gal in the retina (hi-arr-β-gal mice), or in the brain and eye (GFAP-β-gal mice). β-gal-positive photoreceptor cells were damaged in the retina of hi-arr-β-gal mice, and anterior segment disease was found in the eyes of GFAP-β-gal mice. Ag recognition by resting CD8 T cells was also evaluated. Recovery of 5(6)-carboxyfluorescein diacetate N-succinimidyl ester (CFSE)-labelled β3 cells from hi-arr-β-gal mice was slightly decreased compared to recovery from B10.A mice, while recovery from GFAP-β-gal mice was transiently increased. Conversely, recovery of CFSE− cells increased in hi-arr-β-gal mice, consistent with an Ag-dependent response. The CFSE content of the CFSE+ population was unchanged relative to β3 cells recovered from controls. Intracellular cytokine responses of β3 cells recovered from hi-arr-β-gal and GFAP-β-gal mice correlated with the number of cells recovered, regardless of CFSE content. Even though their production of interferon-γ and tumour necrosis factor-α was affected little by transfer into hi-arr-β-gal recipients, the ability of β3 cells to mediate delayed-type hypersesitivity was inhibited in hi-arr-β-gal mice. These results show that resting CD8 T cells are affected by the presence of Ag that originates in retina and, when activated prior to transfer, mediate pathogenic autoimmunity against retinal and other ocular targets.
Introduction
The critical role of the retina in vision, combined with its delicate and non-regenerative nature, has led to the development of endogenous mechanisms that protect the retina from the non-specific tissue damage associated with immune responses. Several mechanisms contribute to retinal immune privilege, including immunoregulatory responses that are both similar to1,2 and different from3 those observed following the inoculation of antigen (Ag) into the anterior chamber of the eye [anterior chamber-associated immune deviation (ACAID)].4 There is also Fas ligand expression in retina,5 limited class II major histocompatibility complex (MHC) expression,6 and variable thymic expression of retinal Ags.7 A regulatory role is also postulated for resident retinal microglia (MG).8
Our previous studies investigated interactions between retinal Ags and the immune system in non-inflamed eyes, avoiding mechanical disruptions. Through the transgenic (Tg) expression of Escherichia coliβ-galactosidase (β-gal) in mouse retina, we showed that resting, CD44hi, CD4 T cells, specific for an immunodominant epitope of β-gal, gave no evidence of Ag recognition when it was solely expressed in retina.9 The T cells survived for many weeks with no apparent differences when compared with T cells inoculated into non-Tg mice. Conversely, β-gal-specific activated CD4 T cells induced experimental autoimmune uveoretinitis (EAU) in mice expressing β-gal in retina.10
Issues concerning retinal immune privilege are not limited to CD4 T cells, as interactions between retinal Ags and CD8 T cells remain largely unexplored. While class I MHC expression is limited in retina, it is abundant relative to class II MHC.11–14 Immunologically quiescent retina lacks conventional dendritic cells (DCs),15 and there is no known circulation of cells with Ag-presentation potential from the parenchyma of quiet retina to lymph nodes (LNs). Although retina possesses MG and perivascular cells, similar to those found in brain,8,15,16 there is no evidence that these cells carry retinal Ags out of the retina. In this study we investigated whether Ag-specific CD8 T cells are inhibited in their recognition of retinal Ags, and if their properties are altered by residence in hosts with retinal Ag expression. Using a CD8 T-cell clone specific for an immunodominant epitope of β-gal, we found that CD8 T cells are capable of a limited response to Ag expressed in retina, which is much less vigorous than the response to Ag expressed in other sites.
Materials and methods
Mice
hi-arr-β-gal, ROSA26 and GFAP-β-gal Tg mice have been described in detail previously.3,9,10 Briefly, under control of the arrestin promoter, homozygous hi-arr-β-gal mice exhibit high levels of retinal β-gal expression (150–200 ng/retina), some pineal gland expression (<0·5 ng/pineal) and slight X-gal-detectable expression in a few cells in a small area of brain.3 No other systemic expression of β-gal has been found in hi-arr-β-gal mice. Expression of β-gal in ROSA26 mice is regulated by an embryonic promoter, leading to low, but widespread, β-gal expression that varies greatly between tissues in adult mice. GFAP-β-gal mice express β-gal primarily in the brain, eye and optic nerve, based on the activity of the GFAP promoter, but not in thymus or elsewhere.17 Little of the ocular expression in the eyes of GFAP-β-gal mice is present in the retina; most is in the optic nerve, cornea and lens.3 All β-gal Tg mice were backcrossed for at least 10 generations onto B10.A mice (Charles River, Wilmington, MA), providing an MHC known for immune reactivity to β-gal and the EAU-permissive B10 background. B10.A mice and Tg-negative littermates were used as controls. Mice were handled in accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research, and University of Minnesota animal use and care guidelines. The mice were housed under specific pathogen-free (SPF) conditions on lactose-free chow.
Generation of the β3 CD8 T-cell clone
CD8 T cells specific for an immunodominant, Ld-restricted epitope of β-gal (TPHPARIGL)18,19 were prepared from splenocytes harvested from VSC 56-vaccinated female B10.A mice. VSC 56 is a recombinant vaccinia virus expressing β-gal under control of the synthetic early/late promoter, obtained from the laboratory of Dr J. Yewdell.20 A total of 5 × 106 plaque-forming units (PFU) were inoculated intraperitoneally (i.p.) per mouse. Spleen and LN were harvested 21 days postinoculation for T cells and Ag-presenting cells (APC). Splenic APC were incubated at 37° for 1 hr with 0·01 µmβ-gal peptide, irradiated (2000 rads), washed, mixed with an equal number of non-irradiated spleen and LN cells, and cultured in RPMI-1640 supplemented with 1 mg/ml glucose, 100 µg/ml sodium pyruvate, 50 µm 2-mercaptoethanol (2-ME), 100 U/ml penicillin, 0·1 mg/ml streptomycin, 35 µg/ml gentamicin, 1× minimal essential medium (MEM), non-essential amino acids, and 5% fetal calf serum (FCS). After this initial stimulation, T-cell clones were isolated by limiting dilution. Ag-responsive clones were subjected to restimulation on a 10–14-day cycle, with 10 U/ml interleukin (IL)-2 added 24 hr after each restimulation with β-gal peptide-pulsed, irradiated, splenic APC. A cytotoxic CD8 T-cell clone was chosen and designated β3.
In vitro T-cell analysis
Proliferation assays were carried out in 96-well plates using 5 × 105 irradiated (3000 rads) splenic APCs and 5 × 104β3 CD8 T cells in 0·2 ml of RPMI-1640. Irradiated spleen cells were pulsed with β-gal peptide for 1 hr, washed and mixed with β3 cells. IL-2 (10 U/ml) was added at the 24-hr time-point, as indicated. Cells were pulsed at 48 hr with [3H]thymidine, and incorporation of [3H]thymidine was counted at 72 hr.
Cytotoxic function of β3 cells was assayed using P815 cells in a non-radioactive TdT-mediated biotin–dUTP nick-end labelling (TUNEL) assay.21–23 The P815 targets (DBA/2 mouse mastocytoma positive for Ld MHC class I) were pulsed for 1 hr with either 1 µmβ-gal peptide or medium only. The P815 cells (2 × 105/well) were washed and then cultured with Ag-activated β3 cells (0–8 × 105/well) for 2 hr. Apoptosis was measured by the TUNEL assay using flow cytometry to detect incorporation of fluorescein-dUTP by TdT into fragmented DNA using the In Situ Cell Death Detection Kit, Fluorescein (Roche Diagnostics, Indianapolis, IN) and according to the manufacturer's instructions, which were closely based on those of Sgonc et al.23 Prior to the permeabilization and fixation steps, the cell mixture was incubated with phycoerythrin (PE)-labelled anti-H-2Kk, peridinin chlorophyll protein (PerCP)-labelled anti-CD3 and allophycocyanin-labelled K-2Kd. Specific analysis of label incorporation into the P815 cells was carried out by excluding the H-2Kk- and CD3-positive B10.A T cells, and including the H-2Kd-positive P815 cells. Two positive controls were included. First, DNAse I treatment after the fixation step was used to induce breaks as a control for the TUNEL portion of the assay. Second, incubation of the P815 cells in 40 µm camptothecin for 2 hr, followed by the TUNEL assay, was used to provide a positive control for apoptosis.
CFSE labelling and adoptive transfer
Resting β3 cells were harvested from cultures, washed and resuspended to 5 × 107 cells/ml in phosphate-buffered saline (PBS). 5(6)-Carboxyfluorescein diacetate N-succinimidyl ester (CFSE; Molecular Probes, Eugene, OR) was added to a final concentration of 4 µm and the cells were incubated for 10 min at 37° with mixing. The reaction was stopped by the addition of 10–15 ml of RPMI-1640 containing 10% FCS. The cells were washed and resuspended to 5 × 107 cells/ml in saline. Recipient mice received 5 × 106 cells, in a 0·1-ml volume, via intracardiac (i.c.) injection under deep anaesthesia. Recipient mice were not irradiated.
Flow cytometry and intracellular cytokine analysis
Splenocytes were prepared from minced spleen, subjected to red blood cell lysis by the addition of 0·17 m NH4Cl, washed with PBS and resuspended to 100 µl in fluorescein-activated cell sorter (FACS) buffer (PBS containing 2% FCS and 0·02% sodium azide); 0·5–4 µl of the appropriate fluorescence-labelled antibody (Pharmingen, San Diego, CA) was then added. Splenocytes were incubated for 30 min on ice, washed, resuspended in FACS buffer and analysed in a FACS Calibur flow cytometer using cellquest software (Becton-Dickinson, San Jose, CA). Where indicated, staining of splenocytes with the APC-labelled H-2Ld/TPHPARIGL tetramer (NIAID; National Tetramer Core Facility, Atlanta, GA) was performed for 30 min, on ice, prior to staining with other antibodies. Intracellular cytokine analysis for interferon-γ (IFN-γ) and tumour necrosis factor-α (TNF-α) was carried out with kits purchased from Pharmingen and used according to the manufacturer's protocols.
Histopathology
Eyes were enucleated and fixed in neutral-buffered 10% formalin for a minimum of 48 hr, dehydrated through a graded alcohol series and embedded in paraffin. Sections were cut through the papillary-optic nerve axis and stained with haematoxylin and eosin (H & E). The slides were examined, in a blinded manner, by an ophthalmic pathologist (C.C.C.). Incidence and severity of disease were graded on a scale of 0–4, in 0·5-point increments, based on the type, size and location of lesions in the eye. The scores were as follow: Trace, limbitis and mild keratitis; Grade 1, keratitis, iridocyclitis and vitritis; Grade 2, Grade 1 + loss of peripheral photoreceptors ≤ 0·03 mm in length; Grade 3, Grade 1 + loss of peripheral photoreceptors > 0·03 mm; Grade 4, Grade 3 + retinal folds.
Ear swelling measurement of delayed-type hypersensitivity (DTH)
Using a 30-gauge needle, mice were given an intrapinna injection of 100 µg of β-gal peptide, prepared in 10 µl of saline, in the left ear, and injected with saline only in the right ear. After 24 and 48 hr, ear thickness was measured using a micrometer (Mitutoyo, no. 227-111; Mitutoyo, Kawasaki, Japan). The difference in ear thickness caused by a DTH response to Ag was determined by measuring the thickness of the left ear and subtracting the thickness of the right ear.
Results
In vitro analysis of β3 T-cell responses
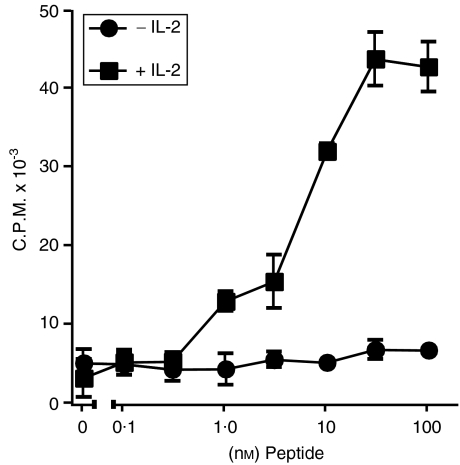
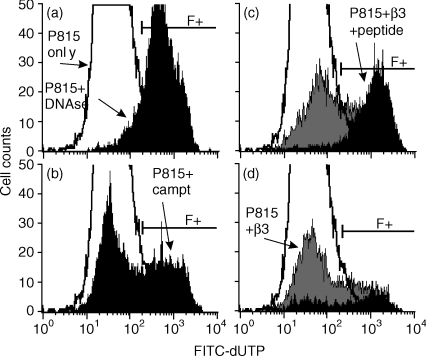
To generate MHC class I-restricted CD8 T cells, spleen and LN cells were cultured from B10.A mice 21 days after inoculation of the mice with recombinant, β-gal-expressing vaccinia virus. A clone (β3) was developed that gave a strong, IL-2-dependent response to the β-gal peptide (Fig. 1). Cytotoxic T lymphocyte (CTL) function of β3 cells was assessed by the TUNEL assay using fluorescein isothiocyanate (FITC)-conjugated thymidine and flow cytometry. Representative results are shown in Fig. 2. Increasing numbers of β3 cells resulted in higher levels of apoptosis in cultures with peptide-bearing P815 cells compared with control P815 cells alone or control P815 cells +β3 cells (Table 1). Together, these results show that β3 cells recognized β-gal peptide and were cytotoxic for cells bearing the β-gal peptide.
Figure 1.
β3 cell proliferative response. The concentrations of β-galactosidase (β-gal) peptide used to pulse the splenic antigen-presenting cells (APCs) are indicated. Interleukin-2 (IL-2) was added, as indicated, to a final concentration of 10 U/ml. c.p.m., counts per minute.
Figure 2.
Induction of apoptosis in peptide-pulsed P815 cells by β3 cells was measured by the TdT-mediated biotin–dUTP nick-end labelling (TUNEL) assay using fluorescein isothiocyanate (FITC)-conjugated dUTP and flow cytometry. (a) TUNEL control; background apoptosis in P815 cells only (P815, unshaded profile), and DNAse I control (P815 + DNAse, filled profile). (b) Positive control for apoptosis; P815 cells only (unshaded) versus P815 cells incubated with camptothecin (P815 + campto; filled profile). (c) Induction of apoptosis by 8 × 105β3 cells; P815 cells only (unshaded), P815 +β3 cells (grey shaded), and peptide-pulsed P815 cells +β3 cells (black filled). (d) Induction of apoptosis by 1 × 105β3 cells. TUNEL-labelled apoptotic cells (FITC+) are designated by the bracket (F+). The results of a single determination are shown. The results of all experiments are summarized in Table 1.
Table 1.
Analysis of the cytotoxic T-lymphocyte (CTL) function of β3 cells
| Number of β3 cells added | ||||
|---|---|---|---|---|
| 1 × 105 | 2 × 105 | 4 × 105 | 8 × 105 | |
| P815/β3 | 22·6 | 24·7 | 26·3 | 34·3 |
| P815/peptide/β3 | 60·0 | 67·4 | 86·0 | 95·1 |
Results are expressed as percentage of apoptotic P815 cells. The background apoptosis of P815 cells was 2·3%.
Local recognition of β-gal in vivo
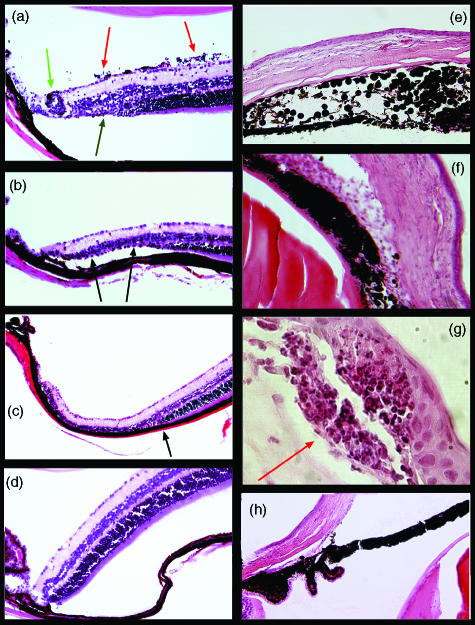
Based on EAU models, in which adoptive transfer of activated CD4 T cells specific for retinal Ags can induce retinal inflammation, β3 cells were activated in vitro with peptide-incubated APC and inoculated into β-gal Tg mice and control mice. Activated T cells damaged the retina of hi-arr-β-gal mice (Fig. 3a–3c), showing that they gained access to the target tissue and that local Ag presentation occurred. In addition to a mild ocular inflammation, including limbitis, iridocyclitis and vitritis, the most consistent finding was loss of photoreceptor cells with sharp demarcation in the peripheral retina of hi-arr-β-gal mice. A mean score of 2·0 was found. The disease incidence is summarized in Table 2. Disease induction was dependent on the transfer of activated β3 cells. Even though Ag expression in hi-arr-β-gal mice is abundantly distributed throughout the photoreceptor cells of the retina, there was selective loss of photoreceptor cells in the peripheral retina, which was accompanied with relatively little inflammatory infiltration. This contrasts with the immunopathology found on the CD4 T cells in these mice.10 The selective loss of peripheral photoreceptor cells in the hi-arr-β-gal mice may result from migration of β3 effector cells from the adjacent ciliary body, bypassing the blood–retinal barrier formed by the retinal pigment epithelium (RPE) and retinal vascular endothelium.
Figure 3.
Histopathology in hi-arr-β-gal and GFAP-β-gal mice following transfer of activated β3 T cells. (a) –(d) hi-arr-β-gal mice; (e)–(g) GFAP-β-gal mice; (h) B10.A control. (a) A hi-arr-β-gal recipient of 5 × 106 activated β3 cells harvested 23 days post-transfer. Mild vitritis (red arrow), focal retinal vasculitis (green arrow) and a small area of peripheral photoreceptor cell loss (black arrow) are shown. (b) A hi-arr-β-gal recipient of 5 × 106 activated β3 cells harvested on day 36 post-transfer. A larger area of peripheral photoreceptor cell loss (arrows), with minimal inflammation, is shown. (c) A hi-arr-β-gal recipient of 5 × 106 activated β3 cells harvested on day 34 post-transfer. The loss of photoreceptor cells in the periphery is complete and abrupt (arrow). (d) Normal retina of a hi-arr-β-gal recipient of 5 × 106 activated β3 cells harvested on day 36 post-transfer. (e) A GFAP-β-gal recipient of 5 × 106 activated β3 cells harvested 25 days post-transfer. Significant iritis resulting in destruction of the normal architecture and anterior synechiae. (f) Severe inflammation in the anterior segment, showing keratitis, iridocyclitis and limbitis, on day 25 post-transfer. (g) A focal lesion at the subepithelial space of the cornea (arrow) on day 25 post-transfer. (h) Anterior segment of a normal B10.A control.
Table 2.
Incidence of ocular pathology
| Transferred β3 T cells | ||
|---|---|---|
| Recipients | Activated | Resting |
| hi-arr-β-gal | 12/25* | 0/29† |
| GFAP-β-gal | 19/33‡ | 0/31§ |
| B10.A | 0/11¶ | 0/19** |
Values shown represent the incidence of inflammation: positive mice/total mice.
The following P values were calculated using the two-tailed χ2-test: *versus † = <0·001; ‡versus § = < ·001; *versus ‡ = 0·69 (not significant); **versus ¶ = 0·029; ‡versus ¶ = 0·016.
Disease was also found in the eyes of GFAP-β-gal mice receiving activated β3 T cells. The anterior segment diseases of keratitis and cataracts were identified, reflecting expression of β-gal in the cornea and lens epithelia. A wide range of disease severity was found, from a mild limbitis to severe, destructive inflammation of the cornea and iris (Fig. 3e–3g). The mean score of disease was 1·0. Although there is a low level of β-gal expression in retinal astrocytes, there was evidence of retinal involvement in only a few eyes. Despite extensive expression of β-gal in the brains of GFAP-β-gal mice, no neurological signs were observed, and no significant infiltrates of the central nervous system (CNS) were found (data not shown). Tissue damage resulting from Ag recognition by β3 cells is almost entirely limited to the anterior segment of these eyes. This may be a result of the abundance of DCs in the anterior segment24,25 and their relative absence from the other sites. The disparity between sites of immunopathology found upon transfer of activated, β-gal-specific CD8 T cells into the β-gal Tg mice, and the sites and levels of β-gal expression, demonstrates that there are potent differences in the local regulation and presentation between the individual tissues of the eye and CNS.
Survival and response of resting β3 cells in mice
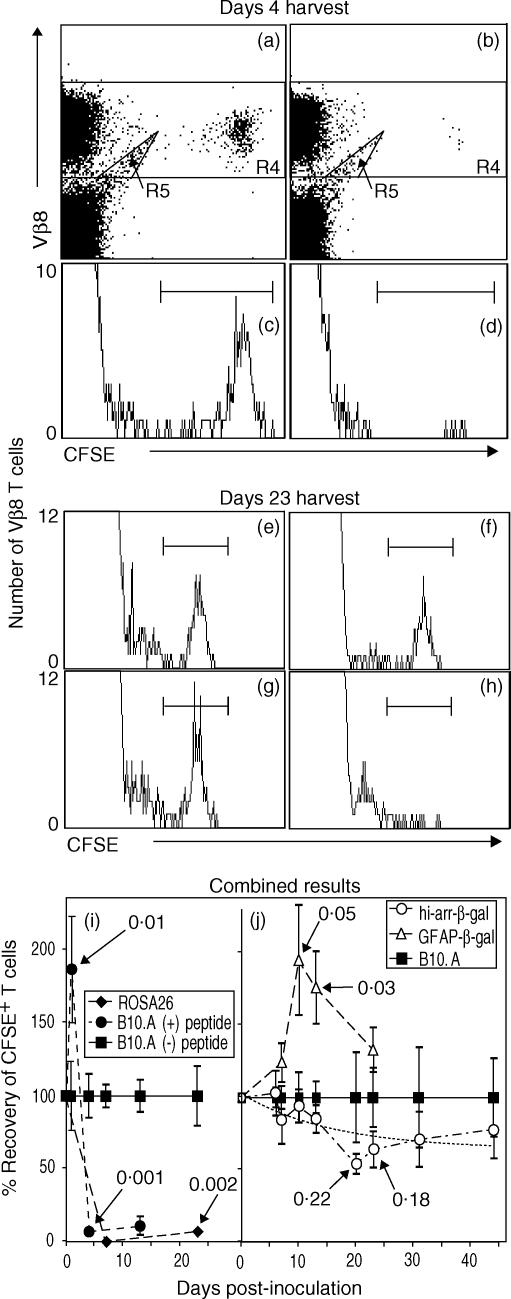
To determine if resting β3 cells recognize Ag in vivo, resting β3 cells were transferred to control and Tg mice. Spleens were harvested at intervals from 1 to 45 days post-transfer. As a control, recovery of β3 cells from B10.A mice inoculated with β-gal peptide was also analysed. Preliminary studies showed that very few β3 cells could be recovered from the LN or blood of any mouse, consistent with their lack of CD62L expression (data not shown). Representative analyses for day 4 (Fig. 4a–4d) and day 23 (Fig. 4e–4h) harvests are shown, as well as the combined results (Fig. 4i,4j). Compared with recovery from normal B10.A mice (Fig. 4a,4c), there was a 1·8-fold increase in the number of cells recovered 1 day after peptide administration, followed by the almost complete loss of CFSE+ T cells by day 4 from spleens of B10.A mice inoculated i.p. with 300 µg of β-gal peptide (Fig. 4b,4d). ROSA26 mice were also used as controls to show that endogenous β-gal in tissues without immune privilege was recognized by resting β3 cells (Fig. 4h). A summary of the results at all time-points is shown in Fig. 4(i). The rapid and almost complete loss of CFSE+ cells in the ROSA26 mice and peptide-treated mice probably results from a combination of cellular proliferation and migration out of the spleen.
Figure 4.
Survival of β3 cells in control and transgenic (Tg) mice, demonstrating recognition of β-galactosidase (β-gal) in vivo by resting β3 cells. Splenocytes were harvested at the indicated time-points and analysed, by flow cytometry, for β3 cells [CD3+ CD8+ Vβ8+ 5(6)-carboxyfluorescein diacetate N-succinimidyl ester (CFSE+) cells]. Representative analyses for harvests on day 4 (a–d) and day 23 (e–h) are shown. (a–d) Resting β3 cells were transferred to B10.A recipients on day 0. Peptide (300 µg) or saline was given on day 1, and spleens were harvested on day 4. Scatter plots were gated on lymphocyte-sized events that were further analysed for CD3+ CD8+ T cells. Positive events were then analysed for Vβ8+ CFSE-labelled events, as shown in (a) (no peptide) and (b) (with peptide). CFSE+ cells are clearly visible and were decreased in number following administration of peptide. Events in R4, excluding the artefact ‘tail’ in R5, were analysed on histograms (c, no peptide; d, with peptide) to provide the data for the combined experiments (i). No events were found in the CFSE+ region in mice not transferred with β3 cells, but the R5 ‘tail’ was still present, showing that it is not derived from the transferred cells (data not shown). (e–h) Recovery of β3 cells from control and Tg mice 23 days after transfer. The histograms were generated as described for panels (a)–(d), and show the relative changes in recovery. Recipients included: (e) B10.A; (f) hi-arr-β-gal; (g) GFAP-β-gal; and (h) ROSA26. Combined data are shown for mice expressing β-gal in non-immune-privileged sites (i) compared with expression in an immune-privileged site (hi-arr-β-gal mice) (j) For each time-point, the number of β3 cells recovered was normalized to the number recovered from normal B10.A mice at that same time-point (defined as 100%). A non-linear regression analysis for the hi-arr-β-gal mice is presented (dashed line, j). P-values for selected comparisons with β3-transferred B10.A controls are indicated. Each time-point in (i) and (j) represents a minimum of four mice, each analysed individually.
Recovery of CFSE+β3 cells from the spleen was also used to detect recognition of Ag located in immune-privileged sites. Representative results (on day 23 post-transfer) are shown in Fig. 4(e)–4(h), and suggest a low level of encounter between the CD8 T cells and β-gal of retinal origin. Compared with B10.A control mice, there was a trend towards a reduction in the number of CFSE+β3 cells recovered from hi-arr-β-gal mice by day 20 (Fig. 4j). The decline, relative to that in B10.A controls, stabilized at this level for the duration of the experiment (45 days). There was a transient increase in the number of CFSE+β3 cells recovered from GFAP-β-gal mice compared with control mice, and this difference was significant when compared to the number recovered from both normal B10.A and hi-arr-β-gal mice.
When examined for CFSE staining intensity, there were no significant differences in the geometric mean fluorescence index (GMFI) between CFSE+ cells recovered from control, hi-arr-β-gal, or GFAP-β-gal mice, even though differences were found in the number of CFSE+ cells recovered (Fig. 4e–4g). We predicted that the presence of endogenous Ag would increase the overall rate of CFSE decay, but few cells were found at intermediate CFSE staining levels. We interpret these results as evidence that individual β3 cells underwent Ag-dependent responses, which, once initiated, were sufficient to dilute their CFSE to background levels and/or lead to migration from the spleen, reducing their recovery. This is analogous to reports demonstrating that naive CD8 T cells, under conditions of limited, brief Ag exposure, are committed to a period of proliferation and acquire memory/effector functions.26–28 While the intensity of CFSE staining of the overall β3 cell population residing in hi-arr-β-gal mice declined, like that of control mice, an accumulating number of cells appear to have responded, becoming CFSE−. A proportion of these were found as an increase in the tetramer-positive cells in the spleen (see below). We propose that this type of decay in CFSE staining results from a limiting number of Ag-bearing APCs, each presenting sufficient Ag to initiate responses in only a very small fraction of the total β3 cells. If such APCs exist, it seems probable that they originate in the retina (or in the CNS, in the case of the GFAP-β-gal mice), and interact with T cells outside the eye. Regardless of the mechanism that results in loss of the β3 cells, the important result is that there are small, but measurable, consequences of T-cell/Ag interaction occurring in the hi-arr-β-gal mice.
Retinal β-gal recognition by resting β3 cells produces a small number of CFSE− tetramer+ cells
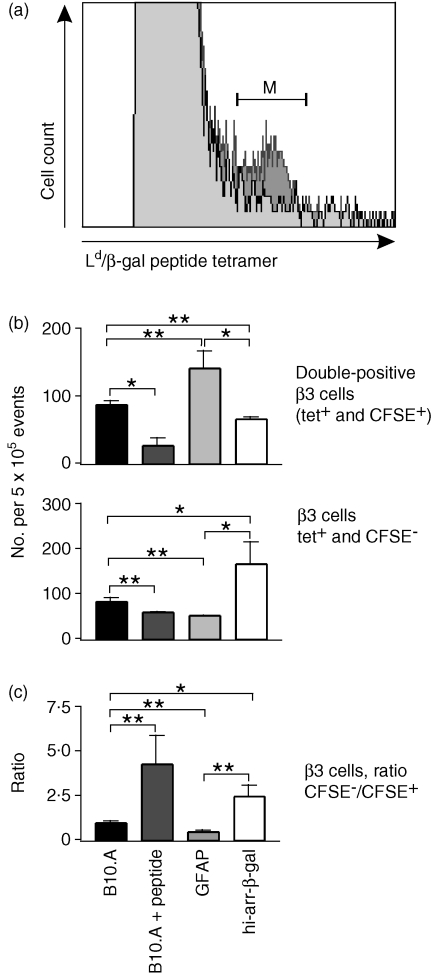
To further evaluate the recognition of retinal β-gal by CD8+ T cells, CFSE-labelled β3 cells were transferred into Tg and control mice. After 13 days, spleens were harvested. β3 cells were identified by their ability to bind an APC-labelled Ld/β-gal peptide tetramer (Fig. 5a) and then analysed for CFSE content. As a positive control for β-gal recognition, some mice received β-gal peptide 3 days after transfer. Ld tetramers are known to be difficult to use, probably based on their unusual binding properties.29 However, a small shoulder of tetramer-stained cells was found in recovered splenocytes (Fig. 5a). Although this contributed to a high background, use of controls confirmed that the β3 cells were being detected. The number of tetramer positive (tet+) CFSE+ cells, tet+ CFSE− cells and the ratio of these two populations, were compared.
Figure 5.
Analysis of transferred, 5(6)-carboxyfluorescein diacetate N-succinimidyl ester (CFSE)-labelled β3 cells. Where indicated, the mice received 300 µg of β-galactosidase (β-gal) peptide, in saline, intraperitoneally (i.p.), 3 days post-transfer. Splenocytes were harvested on day 13 and stained with anti-CD3+, anti-CD8+, and antigen-presenting cell (APC)-labelled Ld/β-gal peptide tetramer. CD3+ CD8+ T cells were identified by fluorescence-activated cell sorter (FACS) and then analysed for Ld/β-gal peptide tetramer staining and CFSE content. (a) Plot of tetramer staining on splenocytes from mice that did (dark grey) or did not (light grey) receive β3 cells. The (M) bracket represents tetramer-positive cells, which are β3 cells that are either CFSE+ or CFSE−. (b) Number of tetramer-stained β3 cells recovered from the transgenic (Tg) and control mice. The mean result is shown of four animals and six determinations. tet+, tetramer positive. (c) Ratio of CFSE− : CFSE+β3 cells from analyses, as shown in (b). The average of all experiments is shown. Significance was determined by the t-test; comparisons are indicated by the brackets. *P < 0·05; **P < 0·01.
Peptide-inoculated and normal control recipients
Relative to B10.A controls, peptide-inoculated B10.A mice showed a significant reduction in the number of CFSE+ cells, confirming the Ag responsiveness of the cells (Fig. 5b). A relatively small number of tet+ CFSE− cells were recovered from these mice, indicating that most of the progeny induced by peptide inoculation 10 days previously were not recovered from spleen, possibly owing to a combination of activation-induced cell death and migration out of the spleen. Comparison of the ratio of tet+ CFSE− with tet+ CFSE+ recovered cells (Fig. 5c) showed a 4·5-fold increase in the ratio of CFSE− to CFSE+ cells between the B10.A/peptide and B10.A control mice, consistent with greater proliferation in peptide-treated mice.
hi-arr-β-gal recipients
The recovery of CFSE+ cells from hi-arr-β-gal mice was slightly reduced relative to the recovery of CFSE+ cells from B10.A recipients (Fig. 5b). Conversely, there was a significant increase in the number of CFSE− T cells recovered from the spleens of hi-arr-β-gal mice. A 2·5-fold increase in the ratio of tet+ CFSE− to tet+ CFSE+ cells was found in hi-arr-β-gal mice compared with control mice, showing greater proliferation in hi-arr-β-gal recipients than in normal controls.
GFAP-β-gal recipients
The recovery of CFSE+ cells was increased in GFAP-β-gal mice (Fig. 5b), while CFSE− cells showed a slight decrease compared with controls (Fig. 5b). Unlike peptide-treated and hi-arr-β-gal recipients, the ratio of CFSE− : CFSE+ cells was reduced by ≈ 50% in GFAP-β-gal mice compared with B10.A controls, indicating less proliferation of β3 cells in these mice.
Based on these results, three important points can be made. First, the outcomes of β3 cell transfer to hi-arr-β-gal and GFAP-β-gal recipients were different from those of B10.A recipients. Resting CD8 T cells respond, although minimally, to Ag expressed in retina (hi-arr-β-gal mice) and to Ag expressed primarily in brain (GFAP-β-gal mice). Second, the responses of β3 cells in hi-arr-β-gal and GFAP-β-gal recipients were different from each other. Differences in the β3 T-cell response were found, even though the total amount of β-gal expressed per mouse was very similar between the two types of mice, emphasizing the importance of the different tissue origins of the Ag. Third, important perspective is provided by the β3 response in ROSA26 recipients. The response to β-gal in non-immune-privileged sites in these recipients was vigorous, as shown by the loss of > 90% of the CFSE+ cells. Recovery of β3 cells was too low to permit tetramer analysis.
Alteration of β3 effector function in Tg mice
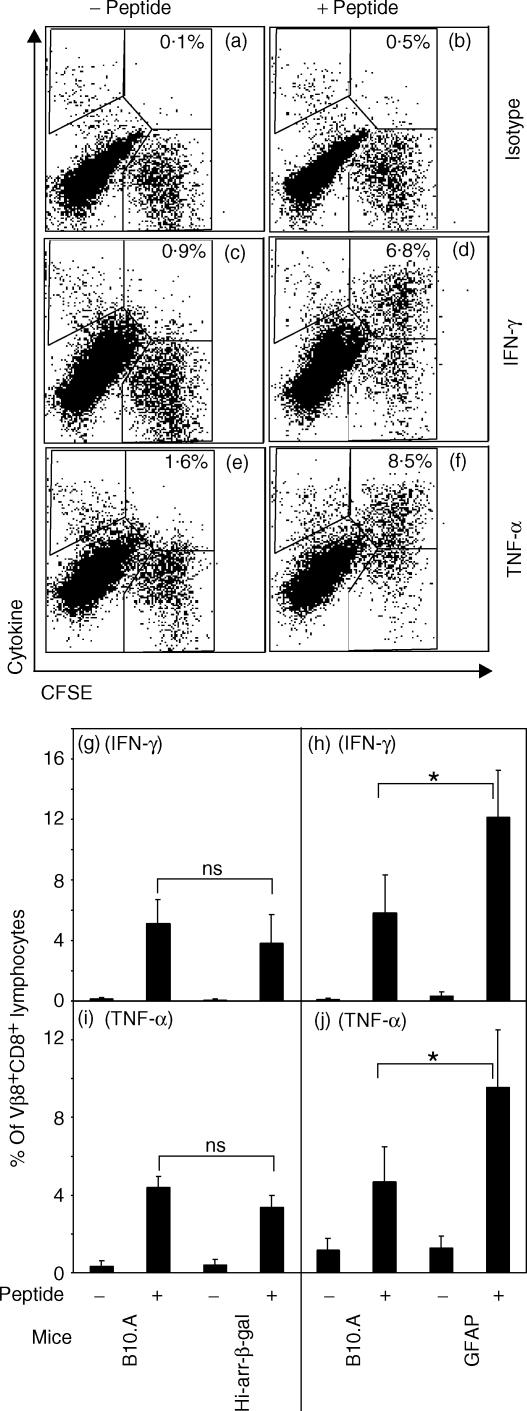
Intracellular cytokine assays for the inflammatory cytokines IFN-γ and TNF-α were used to analyse the response of β3 cells after residence in Tg or control mice. Resting CFSE-labelled β3 cells were transferred into hi-arr-β-gal, GFAP-β-gal, or control mice. After 10 days, mice were inoculated i.p. with peptide in saline. Approximately 24 hr later, splenocytes were analysed for IFN-γ and TNF-α. The frequency of cytokine-positive cells was not increased and, by 72 hr post-Ag inoculation, few CFSE+ cells could be recovered from the spleen (data not shown). The absence of a cytokine response in the β3 cells in vivo may be related to the systemic administration of a minimal peptide in the absence of ‘danger signals’. Conversely, in vitro stimulation of the recovered splenocytes with peptide-pulsed APC revealed a strong Ag response. β3 cells were identified as CD8+ Vβ8+ CFSE+ and analysed for production of IFN-γ and TNF-α (Fig. 6). The level of cytokine production, as measured by the GMFI of cytokine staining by CFSE+ cells, and the percentage of cells responding relative to the total population of Vβ8+ CD8+ lymphocytes, was compared. β3 cells responded to Ag stimulation by producing significant levels of these cytokines. Plots showing representative raw data from one GFAP-β-gal recipient are shown (Fig. 6a–6f). When transferred β3 cells were recovered and cultured without peptide, little IFN-γ or TNF-α was produced.
Figure 6.
Analysis of intracellular cytokine staining as a measure of β3 cell effector function upon recovery from hi-arr-β-gal and GFAP-β-gal mice. Splenocytes were harvested 10 days post-transfer of CFSE-labelled β3 cells and incubated with (0·1 µm) or without β-galactosidase (β-gal) peptide for 4 hr. The cells were then stained for Vβ8, CD8 and the indicated cytokine. Fluorescence-activated cell sorter (FACS) analysis for cytokines was performed by gating on lymphocyte-sized events and then gating on Vβ8+ CD8+ CFSE+ cells, using 300 000–500 000 total events. (a–f) Example of FACS analysis on splenocytes from a GFAP-β-gal recipient, showing the regions used for analysis. Percentages of β3 cells are indicated. (g–j) Summary of FACS analysis for hi-arr-β-gal and GFAP-β-gal mice versus control mice. The number of cytokine+ CFSE+ cells from the upper right region, indicated in panels (a)–(f), is given as the percentage of Vβ8+ CD8+ CFSE+ cells. The data for panels (g)–(j) is the average of three to five mice per group. *P < 0·05; NS, not significant.
A majority of CFSE+ cells substantially up-regulated cytokine production in response to peptide (from ≈ 15% in unstimulated samples to 75% in peptide-stimulated cultures). The fraction of cells that responded did not differ significantly between Tg and control recipients, but the frequency of cytokine-producing CFSE+β3 cells from hi-arr-β-gal mice was reduced (Fig. 6g,6i), consistent with the reduced recovery of CFSE+ cells shown above. Conversely, recovery of cytokine-producing CFSE+β3 cells from GFAP-β-gal mice showed a significant increase (Fig. 6h,6j) compared with controls. Comparison of the GMFI for both IFN-γ and TNF-α did not reveal consistent differences between experiments (data not shown).
Because an increased number of β3 cells were found in the tet+ CFSE− population from the spleens of the hi-arr-β-gal mice, as described above, it was possible that the cytokine production of CFSE− cells could be affected differently from that of CFSE+ cells. The weak Ld tetramer staining did not survive the manipulations required for intracellular cytokine staining, preventing direct analysis of this population. However, the cells present in the upper left region (cytokine+ CFSE− Vβ8+ CD8+Fig. 6a–6f) were counted and compared. Even though these cells cannot be positively identified as β3 cells, the number of cytokine-positive cells in this region changed in an Ag-dependent manner, consistent with response to the peptide. The isotype controls and unstimulated controls allow the background to be subtracted. The peptide-dependent, percentage increase in cytokine-positive cells (both IFN-γ and TNF-α) was significant in hi-arr-β-gal recipients relative to B10.A recipients, but there was no significant change in GFAP-β-gal recipients compared with control mice (Table 3). This is consistent with the recovery of CFSE−β3 cells from these mice (see Fig. 5), and suggests that the response of β3 cells to endogenous β-gal in hi-arr-β-gal recipients, which led to loss of CFSE staining, did not inhibit their subsequent cytokine response to Ag.
Table 3.
Cytokine response of 5(6)-carboxyfluorescein diacetate N-succinimidyl ester-negative (CFSE−) β3 cells
| Intracellular cytokine staining | |||||
|---|---|---|---|---|---|
| IFN-γ | TNF-α | ||||
| Exp. | Recipient mice | No peptide | With peptide | No peptide | With peptide |
| 1 | B10.A | 0·82 ± 0·42* | 0·51 ± 0·10 | 3·20 ± 0·61 | 2·73 ± 0·87 |
| hi-arr-β-gal | 1·85 ± 1·19 | 2·66 ± 0·74 | 2·41 ± 1·28 | 5·29 ± 1·26 | |
| NS† | 0·033 | NS | 0·020 | ||
| 2 | B10.A | 1·25 ± 1·06 | 2·16 ± 1·77 | 4·38 ± 0·68 | 3·65 ± 0·62s |
| GFAP-β-gal | 2·10 ± 0·89 | 3·09 ± 2·19 | 3·01 ± 1·50 | 2·69 ± 1·56 | |
| NS | NS | NS | NS | ||
The percentage of total CD8+ Vβ8+ from the cytokine+ CFSE− region (see Fig. 6a–6f, upper left region).
Calculated using the t-test; cells were recovered from B10.A versus hi-arr-β-gal mice and from B10.A versus GFAP-β-gal mice.
IFN-γ, interferon-γ; NS, not significant; TNF-α, tumour necrosis factor-α.
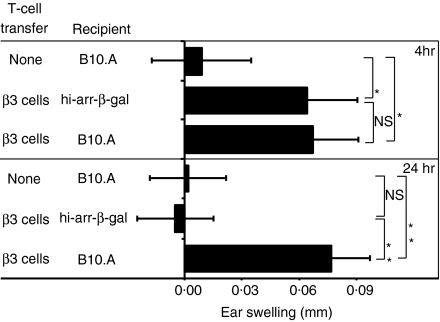
Even though the production of IFN-γ and TNF-α by β3 cells could not be used to distinguish between Tg and control recipients, the ability of β3 cells to mount a DTH response was inhibited in hi-arr-β-gal recipients. β3 cells in hi-arr-β-gal mice had a reduced ear swelling response to challenge with β-gal peptide compared with B10.A recipients (Fig. 7). The inhibition did not occur if the mice were ear challenged 4 hr after β3 transfer, but was clearly observed if ear challenge was delayed for 24 hr, indicating that the difference was induced by residence in the Tg mice. As a series of events are required to manifest a DTH response, it is possible that the difference depends on an activity distinct from the cytokines we have examined to date.
Figure 7.
Development of delayed-type hypersensitivity (DTH) following the transfer of β3 cells is inhibited in hi-arr-β-gal recipient mice. Transgenic (Tg) and control mice received 5 × 106 resting β3 cells. One group of control mice received no cells. Either 4 or 24 hr later, ears were inoculated with 100 µg of peptide or saline only. After 24 and 48 hr, swelling was measured on the ears. P-values are from t-tests comparing the indicated groups. Results are from readings taken at the 24 hr time-point. *P < 0·05; **P < 0·01; NS, not significant.
Discussion
We have used adoptive transfer of Ag-specific T cells to probe for evidence that an Ag originating in immune-privileged retinal tissue is expressed in a form recognized by T cells, i.e. that it appears in a complex with MHC molecules and at levels able to stimulate the T-cell receptor (TCR) complex with measurable consequences. In this, and in a previous study of CD4 T cells,9 CD44hi cells were chosen because of the increased ability of memory-effector cells to recirculate widely, migrate into tissues and be highly responsive to Ag.30–32 Previous results showed that resting, CD44hi, CD4 T cells specific for a class II restricted, immunodominant epitope (YVVDEANIETHGMV) of β-gal gave no evidence of recognition of their target Ag when it was expressed in retina, but recognition of β-gal in other tissues was detected by subsequent alterations in recovery of CFSE-labelled T cells. As class I MHC is expressed at much higher levels and more widely than class II MHC, especially in the retina, the present study investigated whether CD8 T cells were altered in their recognition of and response to Ag of retinal origin. Our results show that resting CD8 T cells are more capable of recognition of Ag derived from immune-privileged tissue than resting CD4 T cells. The nature and magnitude of the response was dependent on the origin of the Ag. Class I MHC provided greater opportunity for Ag presentation and recognition. Even so, we found that such interactions were greatly limited.
The site of interaction between the resting β3 cells and Ag is uncertain. Resting T cells have little access to the parenchyma of the retina,33 and there is relatively little class I MHC expression in the parenchyma of the quiet retina. Consequently, resting T-cell encounter with Ag may require that the Ag either leaks out and is picked up by APC in surrounding tissues, or exits via retinal MG or perivascular cells which have taken up Ag, left the retina and migrated to lymphoid tissue or spleen. There is presently no evidence for any cells performing this function. Characterization of T-lymphocyte recognition of Ag expressed in the retina remains critical for understanding the mechanisms of retinal immune privilege. Evidence for Ag-dependent, endogenous immunoregulatory activity caused by the retinal expression of β-gal has been reported previously.3 These results indirectly demonstrate immunorecognition of Ag expressed in normal retina. This raises difficult questions in the case of tolerance to tissue-specific Ags expressed in retina, a tissue that lies behind barriers, has little evidence of lymphoid drainage, no evidence for DC surveillance, and for which the repertoire of thymic epithelial self-expression is variable and incomplete.7,34 The daily turnover of the photoreceptor cell outer segments does provide opportunities for photoreceptor cell Ag to enter other cells. These could be either the RPE cells, which phagocytose and degrade the outer segment tips, or a population of MG recently found in the subretinal space.35 There is no evidence, to date, that RPE presents outer-segment Ags in either MHC class I or II molecules on its basal surface, which lies outside the blood–retinal barrier.
Owing to the tight junctions of the vascular endothelial cells and RPE cells that form the blood–retinal barrier, it does not seem probable that Ag simply leaks out of the retina. Leakage also seems improbable, based on the results of other studies. We have used retroviral transduction and engraftment of bone marrow cells to generate systemic S-Ag (arrestin) expression. Such expression, even at levels below detection by reverse transcription–polymerase chain reaction (RT–PCR), leads to a reduction in the susceptibility to S-Ag-induced EAU. This resistance is based on the transduced arrestin, despite the fact that arrestin is concurrently expressed in the retinas of these same animals at a level several orders of magnitude higher, but fails to provide the same degree of protection.36 Similar strategy and results have been observed for EAU induced by interphotoreceptor retinoid-binding protein (IRBP).37 These studies argue that even vanishingly small amounts of retinal Ag associated with bone marrow-derived cells outside the eye can lead to immunoregulatory responses that inhibit EAU susceptibility.
The local recognition of retinal/CNS Ag by activated T cells is less puzzling. The ability of activated, but not resting, CD4 and CD8 T cells, specific for any of several retinal Ags38 (including β-gal10), to induce retinal immunopathology by adoptive transfer, shows that local Ag recognition is possible. A study of Ag presentation in the brain provides evidence that activated T cells leave the vasculature and encounter Ag presented by CD45+ perivascular cells,39 and a similar study suggests that the same APC is active in retina.40 As there is no evidence for MHC class I expression on photoreceptor cells in which the β-gal is synthesized, we do not believe that the photoreceptor cells are the direct target of the CTLs. The photoreceptor cells are the source of Ag in the hi-arr-β-gal mice. It is most likely that microglia, or the DEC-205+ CD45+ or CD11c+ CD45+ retinal cells,15 provide the initial Ag presentation using Ag scavenged from the local environment. The ensuing response probably recruits cells from the circulation that further support the development of inflammation and ‘bystander’ damage to photoreceptor cells, which do not regenerate.
It is possible that the pattern of histopathology we have observed is caused by integration effects of the β-gal gene, as the sites of integration can alter the intended activity of the promoter. In developing the GFAP Tg mice, for the purpose of examining the effects of intragenic sequences on the promoter activity, Johnson et al.17 observed that multiple founders (B6 × SJL) produced using the C-445 construct gave expression at similar sites, including the ocular sites; the only difference was in the levels of expression at these sites. Copy number did not affect expression, and sites of expression were found to closely reproduce the expression pattern of endogenous GFAP. We backcrossed the GFAP–lacZ transgene onto B10.A, and found no change in expression.
Expression directed by the arrestin promoter has also been studied, and the elements found to produce expression in retinal photoreceptor cells have been characterized.41–44 The specificity of the promoter is robust in a variety of studies, leading to expression in tissues known to express photoreceptor cell proteins. These include rod photoreceptor cells, pinealocytes and a small number of cells in the brain.41,45 Characterization of the expression after backcrossing arrestin–lacZ mice to B10.A (hi-arr-β-gal mice) reveals the same pattern of expression.3
We have considered the possibility that the GFAP and arrestin promoters drive β-gal expression in other tissues at very low levels that we have not detected. However, the disease we have seen, to date, is consistent with the observed patterns of expression. The other consideration in the pattern of disease is the Ag-specific T cell. T-cell lines derived from two independent isolations following sensitization with the β-gal-expressing vaccinia virus show similar patterns of reactivity, although the bulk, polyclonal β4 line is more immunopathogenic than the β3 line from which the β3 clone was derived.
Two of the results suggest the possibility of peripheral tolerance induction in naive hi-arr-β-gal mice. First, the inhibition of the DTH response of transferred β3 cells suggests active tolerance in the recipients and would be consistent with our previous observation that elicitation of DTH is inhibited in primed hi-arr-β-gal mice.3 The mechanism of inhibition appears to differ from that of ACAID, in several respects. Second, the relatively mild disease found by transferring the β3 T cells would be consistent with active, peripheral tolerance limiting the immunopathogenesis. The presence of peripheral tolerance itself suggests recognition of retinal Ag in the naive mouse. We are currently testing the possibility that a DC-like cell population does sample the retinal environment and systematically delivers retinal antigens to extraocular lymphoid tissues at a low rate, providing Ag recognition of quiet retina by resting T cells, and the opportunity for Ag-specific peripheral tolerance.
Acknowledgments
The authors thank Kristin Mitchell for histology, Wes Obritsch for assistance with the FACS-based CTL assay, and Jing Xiao for X-gal staining and maintenance of the mice. This work was supported by NIH grant EY11542 (D.S.G.), Research to Prevent Blindness, the Anna Heilmaier Foundation (D.S.G.), and the MN Lions.
Abbreviations
- Ag
antigen
- ACAID
anterior chamber-associated immune deviation
- APC
antigen-presenting cell
- β-gal
β-galactosidase
- CFSE
5(6)-carboxyfluorescein diacetate N-succinimidyl ester
- CNS
central nervous system
- CTL
cytotoxic T lymphocyte
- DTH
delayed-type hypersensitivity
- EAU
experimental autoimmune uveoretinitis
- FACS
fluorescence-activated cell sorter
- FCS
fetal calf serum
- FITC
fluorescein isothiocyanate
- GFAP-β-gal
transgenic mice expressing β-gal from the glial fibrillary acidic protein promoter
- GMFI
geometric mean fluorescence index
- hi-arr-β-gal
transgenic mice expressing β-gal at a high level from the arrestin promoter
- IFN-γ
interferon-γ
- IL
interleukin
- i.p.
intraperitoneal
- LN
lymph nodes
- MG
microglia
- MHC
major histocompatibility complex
- PBS
phosphate-buffered saline
- PE
phycoerythrin
- PFU
plaque-forming units
- RPE
retinal pigment epithelium
- Tg
transgenic
- TNF-α
tumour necrosis factor-α
- TUNEL
TdT-mediated biotin–dUTP nick-end labelling
References
- 1.Jiang LQ, Jorquera M, Streilein JW. Subretinal space and vitreous cavity as immunologically privileged sites for retinal allografts. Invest Ophthalmol Vis Sci. 1993;34:3347–54. [PubMed] [Google Scholar]
- 2.Wenkel H, Streilein JW. Analysis of immune deviation elicited by antigens injected into the subretinal space. Invest Ophthalmol Vis Sci. 1998;39:1823–34. [PubMed] [Google Scholar]
- 3.Gregerson DS, Dou C. Spontaneous induction of immunoregulation by an endogenous retinal protein. Invest Ophthalmol Vis Sci. 2002;43:2984–91. [PubMed] [Google Scholar]
- 4.Streilein JW. Immunologic privilege of the eye. Springer Semin Immunopathol. 1999;21:95–111. doi: 10.1007/BF00810243. [Review] [DOI] [PubMed] [Google Scholar]
- 5.Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA. Fas ligand-induced apoptosis as a mechanism of immune privilege [see comments] Science. 1995;270:1189–92. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- 6.Forrester JV, McMenamin PG, Holthouse I, Lumsden L, Liversidge J. Localization and characterization of major histocompatibility complex class II-positive cells in the posterior segment of the eye – implications for induction of autoimmune uveoretinitis. Invest Ophthalmol Vis Sci. 1994;35:64–77. [PubMed] [Google Scholar]
- 7.Egwuagu CE, Charukamnoetkanok P, Gery I. Thymic expression of autoantigens correlates with resistance to autoimmune disease. J Immunol. 1997;159:3109–12. [PubMed] [Google Scholar]
- 8.Dick AD, Broderick C, Forrester JV, Wright GJ. Distribution of OX2 antigen and OX2 receptor within retina. Invest Ophthalmol Vis Sci. 2001;42:170–6. [PubMed] [Google Scholar]
- 9.Gregerson DS, Xiao J. Failure of memory (CD44 high) CD4 T cells to recognize their target antigen in retina. J Neuroimmunol. 2001;120:34–41. doi: 10.1016/s0165-5728(01)00406-4. [DOI] [PubMed] [Google Scholar]
- 10.Gregerson DS, Torseth JW, McPherson SW, Roberts JP, Shinohara T, Zack DJ. Retinal expression of a neo-self antigen, beta-galactosidase, is not tolerogenic, and creates a target for autoimmune uveoretinitis. J Immunol. 1999;163:1073–80. [PubMed] [Google Scholar]
- 11.Zhang J, Wu GS, Ishimoto S, Pararajasegaram G, Rao NA. Expression of major histocompatibility complex molecules in rodent retina – immunohistochemical study. Invest Ophthalmol Vis Sci. 1997;38:1848–57. [PubMed] [Google Scholar]
- 12.Akaishi K, Ishiguro S, Durlu YK, Tamai M. Quantitative analysis of major histocompatibility complex class II-positive cells in posterior segment of Royal College of Surgeons rat eyes. Jpn J Ophthalmol. 1998;42:357–62. doi: 10.1016/s0021-5155(98)00035-5. [DOI] [PubMed] [Google Scholar]
- 13.Ishimoto S, Zhang J, Gullapalli VK, Pararajasegaram G, Rao NA. Antigen-presenting cells in experimental autoimmune uveoretinitis. Exp Eye Res. 1998;67:539–48. doi: 10.1006/exer.1998.0545. [DOI] [PubMed] [Google Scholar]
- 14.Jiang HR, Lumsden L, Forrester JV. Macrophages and dendritic cells in IRBP-induced experimental autoimmune uveoretinitis in B10RIII mice. Invest Ophthalmol Vis Sci. 1999;40:3177–85. [PubMed] [Google Scholar]
- 15.Gregerson DS, Yang J. CD45-positive cells of the retina and their responsiveness to in vivo and in vitro treatment with IFN-γ or anti-CD40. Invest Ophthalmol Vis Sci. 2003;44:3083–93. doi: 10.1167/iovs.02-1014. [DOI] [PubMed] [Google Scholar]
- 16.Dick AD, Ford AL, Forrester JV, Sedgwick JD. Flow cytometric identification of a minority population of MHC class II positive cells in the normal rat retina distinct from CD45lowCD11b/c+CD4low parenchymal microglia. Br J Ophthalmol. 1995;79:834–40. doi: 10.1136/bjo.79.9.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson WB, Ruppe MD, Rockenstein EM, Price J, Sarthy VP, Verderber LC, Mucke L. Indicator expression directed by regulatory sequences of the glial fibrillary acidic protein (GFAP) gene: in vivo comparison of distinct GFAP-lacZ transgenes. Glia. 1995;13:174–84. doi: 10.1002/glia.440130304. [DOI] [PubMed] [Google Scholar]
- 18.Gavin MA, Gilbert MJ, Riddell SR, Greenberg PD, Bevan MJ. Alkali hydrolysis of recombinant proteins allows for the rapid identification of class I MHC-restricted CTL epitopes. J Immunol. 1993;151:3971–80. [PubMed] [Google Scholar]
- 19.Dick LR, Aldrich C, Jameson SC, et al. Proteolytic processing of ovalbumin and beta-galactosidase by the proteasome to yield antigenic peptides. J Immunol. 1994;152:3884–94. [PMC free article] [PubMed] [Google Scholar]
- 20.Bennink JR, Yewdell JW. Recombinant vaccinia viruses as vectors for studying T lymphocyte specificity and function. Curr Top Microbiol Immunol. 1990;163:153–84. doi: 10.1007/978-3-642-75605-4_6. [DOI] [PubMed] [Google Scholar]
- 21.Li X, Traganos F, Melamed MR, Darzynkiewicz Z. Single-step procedure for labeling DNA strand breaks with fluorescein- or BODIPY-conjugated deoxynucleotides: detection of apoptosis and bromodeoxyuridine incorporation. Cytometry. 1995;20:172–80. doi: 10.1002/cyto.990200210. [DOI] [PubMed] [Google Scholar]
- 22.Sgonc R, Wick G. Methods for the detection of apoptosis. Int Arch Allergy Immunol. 1994;105:327–32. doi: 10.1159/000236777. [DOI] [PubMed] [Google Scholar]
- 23.Sgonc R, Boeck G, Dietrich H, Gruber J, Recheis H, Wick G. Simultaneous determination of cell surface antigens and apoptosis. Trends Genet. 1994;10:41–2. doi: 10.1016/0168-9525(94)90140-6. [DOI] [PubMed] [Google Scholar]
- 24.McMenamin PG. Dendritic cells and macrophages in the uveal tract of the normal mouse eye. Br J Ophthalmol. 1999;83:598–604. doi: 10.1136/bjo.83.5.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steptoe RJ, Holt PG, McMenamin PG. Major histocompatibility complex (MHC) class II-positive dendritic cells in the rat iris. In situ development from MHC class II-negative precursors. Invest Ophthalmol Vis Sci. 1997;38:2639–48. [PubMed] [Google Scholar]
- 26.Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat Immunol. 2001;2:415–22. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tuma RA, Pamer EG. Homeostasis of naive, effector and memory CD8 T cells. Curr Opin Immunol. 2002;14:348–53. doi: 10.1016/s0952-7915(02)00338-2. [DOI] [PubMed] [Google Scholar]
- 28.van Stipdonk MJ, Lemmens EE, Schoenberger SP. Naive CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat Immunol. 2001;2:423–9. doi: 10.1038/87730. [DOI] [PubMed] [Google Scholar]
- 29.Balendiran GK, Solheim JC, Young AC, Hansen TH, Nathenson SG, Sacchettini JC. The three-dimensional structure of an H-2Ld–peptide complex explains the unique interaction of Ld with beta-2 microglobulin and peptide. Proc Natl Acad Sci USA. 1997;94:6880–5. doi: 10.1073/pnas.94.13.6880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmidt CS, Mescher MF. Peptide antigen priming of naive, but not memory, CD8 T cells requires a third signal that can be provided by IL-12. J Immunol. 2002;168:5521–9. doi: 10.4049/jimmunol.168.11.5521. [DOI] [PubMed] [Google Scholar]
- 31.Rogers PR, Dubey C, Swain SL. Qualitative changes accompany memory T cell generation: faster, more effective responses at lower doses of antigen. J Immunol. 2000;164:2338–46. doi: 10.4049/jimmunol.164.5.2338. [DOI] [PubMed] [Google Scholar]
- 32.Romanic AM, Graesser D, Baron JL, Visintin I, Janeway CA, Jr, Madri JA. T cell adhesion to endothelial cells and extracellular matrix is modulated upon transendothelial cell migration. Lab Invest. 1997;76:11–23. [PubMed] [Google Scholar]
- 33.Prendergast RA, Iliff CE, Coskuncan NM, Caspi RR, Sartani G, Tarrant TK, Lutty GA, McLeod DS. T cell traffic and the inflammatory response in experimental autoimmune uveoretinitis. Invest Ophthalmol Vis Sci. 1998;39:754–62. [PubMed] [Google Scholar]
- 34.Farr AG, Rudensky A. Medullary thymic epithelium: a mosaic of epithelial ‘self’? J Exp Med. 1998;188:1–4. doi: 10.1084/jem.188.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ng TF, Streilein JW. Light-induced migration of retinal microglia into the subretinal space. Invest Ophthalmol Vis Sci. 2001;42:3301–10. [PubMed] [Google Scholar]
- 36.McPherson SW, Roberts JP, Gregerson DS. Systemic expression of rat soluble retinal antigen induces resistance to experimental autoimmune uveoretinitis. J Immunol. 1999;163:4269–76. [PubMed] [Google Scholar]
- 37.Xu H, Wawrousek EF, Redmond TM, Nickerson JM, Wiggert B, Chan CC, Caspi RR. Transgenic expression of an immunologically privileged retinal antigen extraocularly enhances self tolerance and abrogates susceptibility to autoimmune uveitis. Eur J Immunol. 2000;30:272–8. doi: 10.1002/1521-4141(200001)30:1<272::AID-IMMU272>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 38.Gregerson DS, Obritsch WF, Fling SP, Cameron JD. S-antigen-specific rat T cell lines recognize peptide fragments of S-antigen and mediate experimental autoimmune uveoretinitis and pinealitis. J Immunol. 1986;136:2875–82. [PubMed] [Google Scholar]
- 39.Hickey W, Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 1988;239:290–2. doi: 10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- 40.Gullapalli VK, Zhang J, Pararajasegaram G, Rao NA. Hematopoietically derived retinal perivascular microglia initiate uveoretinitis in experimental autoimmune uveitis. Graefes Arch Clin Exp Ophthalmol. 2000;238:319–25. doi: 10.1007/s004170050359. [DOI] [PubMed] [Google Scholar]
- 41.Kimura A, Singh D, Wawrousek EF, Kikuchi M, Nakamura M, Shinohara T. Both PCE-1/RX and OTX/CRX interactions are necessary for photoreceptor-specific gene expression. J Biol Chem. 2000;275:1152–60. doi: 10.1074/jbc.275.2.1152. [DOI] [PubMed] [Google Scholar]
- 42.Kikuchi T, Wawrousek E, Lee T, Di Camillo S, Kusuzaki K, Shinohara T. Position or number of photoreceptor conserved element (PCE1) in arrestin promoter may be altered in molecular evolution. Invest Ophthalmol Vis Sci. 1995;36:5772. [Abstr.] [Google Scholar]
- 43.Kikuchi T, Raju K, Breitman ML, Shinohara T. The proximal promoter of the mouse arrestin gene directs gene expression in photoreceptor cells and contains an evolutionarily conserved retinal factor-binding site. Mol Cell Biol. 1993;13:4400–8. doi: 10.1128/mcb.13.7.4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mani SS, Besharse JC, Knox BE. Immediate upstream sequence of arrestin directs rod-specific expression in Xenopus. J Biol Chem. 1999;274:15590–7. doi: 10.1074/jbc.274.22.15590. [DOI] [PubMed] [Google Scholar]
- 45.Sunayashiki-Kusuzaki K, Kikuchi T, Wawrousek EF, Shinohara T. Arrestin and phosducin are expressed in a small number of brain cells. Mol Brain Res. 1997;52:112–20. doi: 10.1016/s0169-328x(97)00247-7. [DOI] [PubMed] [Google Scholar]