Abstract
Dietary oils (such as borage oil), which are rich in γ-linolenic acid (GLA), have been shown to be beneficial under inflammatory conditions. Dihomo-GLA (DGLA) is synthesized directly from GLA and forms a substrate for cyclooxygenase (COX) enzymes, resulting in the synthesis of lipid mediators (eicosanoids). In the present study, the immunomodulatory effects of DGLA were investigated and compared with those of other relevant fatty acids. Freshly isolated human peripheral blood mononuclear cells (PBMC) were cultured in fatty acid (100 µm)-enriched medium for 48 hr. Subsequently, cells were stimulated with lipopolysaccharide (LPS) for 20 hr and the cytokine levels were measured, in supernatants, by enzyme-linked immunosorbent assay (ELISA). Phospholipids were analysed by gas chromatography. Fatty acids were readily taken up, metabolized and incorporated into cellular phospholipids. Compared with the other fatty acids tested, DGLA exerted pronounced modulatory effects on cytokine production. Tumour necrosis factor-α (TNF-α) and interleukin (IL)-10 levels were reduced to 60% of control levels, whereas IL-6 levels were not affected by DGLA. Kinetic studies showed that peak levels of TNF-α, occurring early after LPS addition, were inhibited strongly, whereas IL-10 levels were not affected until 15 hr after stimulation. Both the reduction of cytokine levels and the decrease in arachidonic acid levels in these cells, induced by DGLA, were dose dependent, suggesting a shift in eicosanoid-subtype synthesis. However, although some DGLA-derived eicosanoids similarly reduced TNF-α levels, the effects of DGLA were probably not mediated by COX products, as the addition of indomethacin did not alter the effects of DGLA. In conclusion, these results suggest that DGLA affects cytokine production by human PBMC independently of COX activation.
Introduction
Dietary intake of polyunsaturated fatty acids (PUFAs) has been recognized as a useful tool for suppressing the magnitude of immune responses, which can be beneficial in inflammatory conditions.1–3 Such fatty acids include both n-6 and n-3 PUFAs, for instance γ-linolenic acid (GLA, 18:2n-6)4–6 and eicosapentaenoic acid (EPA, 20:5n-3),7–10 respectively. The mechanism by which these fatty acids exert their beneficial effects in inflammatory diseases is not completely understood. However, ex vivo stimulation of peripheral blood mononuclear cells (PBMC) from human subjects supplemented with EPA- or GLA-rich oils led to decreased production of the pro-inflammatory cytokines tumour necrosis factor-α (TNF-α) and interleukin (IL)-1β.11–13
Beside direct effects of PUFAs on cytokine production,13,14 it has been believed that a change in lipid mediator (eicosanoid) production is also involved in the effects of PUFAs.15 Eicosanoids are derived from 20-carbon (C20) PUFAs released from phospholipids16 and are synthesized via a number of enzymatic reactions. The rate-limiting enzymes in this process are cyclooxygenases (COX), which constitute synthesis of prostaglandins and thromboxanes, and lipoxygenases, which are involved in the synthesis of leukotrienes.15 In western countries, arachidonic acid (AA, 20:4n-6) is usually the main C20 fatty acid present in the phospholipids of immune cells. Therefore, AA-derived eicosanoids are produced abundantly during inflammation.17 However, EPA can effectively decrease the amount of AA present,11 and intake of GLA results in increased amounts of dihomo-γ-linolenic acid (DGLA) by elongase conversion.18 As EPA and DGLA are both C20 fatty acids, they can compete with AA for production of eicosanoids. The change in eicosanoid subclasses might relate to the effects of fish and borage oil on ex vivo cytokine production, as some eicosanoids can affect cytokine production directly.19,20 However, a recent study indicates that the shift in PGE subtypes is unlikely to be responsible for the effects of PUFAs on cytokine production.21
As a result of the difficulty in providing dietary supplementation of highly purified fatty acids, a straightforward comparison of the abilities of n-3 and n-6 PUFAs to modulate cytokine production has not yet been made. Therefore, in the present study the main n-6 and n-3 PUFAs were compared for their direct effects on cytokine production of human PBMC. In this model, fatty acid composition of cells was effectively changed and cells were stimulated to produce cytokines, as well as eicosanoids, by endotoxin. It was also investigated whether the effects of PUFAs on cytokine production are explained by the formation of different subclasses of eicosanoids.
Materials and methods
Chemicals
Phosphate-buffered saline (PBS), RPMI-1640 supplemented with l-glutamine, and 25 mm HEPES, penicillin/streptomycin and sodium pyruvate, were obtained from Gibco BRL (Life Technologies, Breda, the Netherlands). Fatty acids (free acids), lipopolysaccharide (LPS, B05:55) (±)-α-tocopherol, ascorbic acid, dimethylsulphoxide (DMSO), butylated hydroxytoluene, EDTA, prostaglandin E1 (PGE1), PGE2, propidium iodide, and boron trifluoride (BF3) were purchased from Sigma-Aldrich Chemie BV (Zwijndrecht, the Netherlands). PGE3, 15(S)-HETrE, leukotriene B4 (LTB4), thromboxane B2 (TXB2), TXB3 and carbocyclic thromboxane A2 (ccTXA2) were obtained from Cayman Chemical Company (Ann Arbor, MI). WST-1 (4-[3-(4-lodophenyl)-2-(nitrophenyl)-2H-5-tetrazolio]-1,3-benzene disulfonate) reagent was obtained from Roche Diagnostics Nederland B.V. (Almere, the Netherlands). Chloroform, methanol, hexane and iso-octane were obtained from Merck (Darmstadt, Germany). Human serum was derived from Bio-Whittaker (Verviers, Belgium). Ficoll (Ficoll–Paque®) was obtained from Amersham Pharmacia Biotech AB (Uppsala, Sweden). Palm oil was purchased from Karlshamns B.V. (Zaandijk, the Netherlands) and sunflower oil from Cargill (Minneapolis, MN). The lipid standard dinonadecanoyl-SN-glycero-phosphatidylcholine (C19:0) was from Avanti Polar Lipids (Birmingham, UK). Phospholipid extraction columns, the vacuum system and the capillary column were obtained from Varian Chrompack (Bergen op Zoom, the Netherlands).
Generation and composition of the control fat blend
The fatty acid composition of the control fat blend used in in vitro incubations is given in Table 1. For in vitro cultures, free fatty acids from the fat blend were generated by saponification. In short, triglycerides were dissolved in 1 N ethanol–KOH and heated at 90°, under reflux, for 1 hr. After acidification with concentrated HCl to pH 2–3, free fatty acids were extracted using hexane/diethylether (5 : 1) and washed in H2O. The organic phase was dried with sodium sulfate and removed by rotary evaporation. Free fatty acids were stored under nitrogen at −80° until required.
Table 1.
Total fatty acid composition of the control (fat) blend and human serum (A), and phospholipid fatty acid from peripheral blood mononuclear cells (PBMC) cultured with or without 100 µm control blend (B).
| Fatty acid (% of total) | A: total fatty acids | B: phospholipids | ||
|---|---|---|---|---|
| Control blend | Human serum | PBMC (untreated) | PBMC (control blend) | |
| 16:0 | 36·2 | 24·2 | 23·9 | 20·6 |
| 18:0 | 4·6 | 10·1 | 15·5 | 12·3 |
| 18:1n-9 | 35·0 | 17·2 | 6·7 | 8·4 |
| 18:2n-6 (LA) | 20·0 | 26·4 | 6·2 | 7·5 |
| 18:3n-6 (GLA) | 0·0 | 0·4 | 0·0 | 0·0 |
| 20:3n-6 (DGLA) | 0·0 | 1·9 | 1·1 | 1·3 |
| 20:4n-6 (AA) | 0·0 | 6·9 | 20·7 | 21·2 |
| 22:4n-6 | 0·0 | 0·3 | 2·5 | 2·6 |
| 22:5n-6 | 0·0 | 0·3 | 0·3 | 0·5 |
| 18:3n-3 (LNA) | 0·1 | 0·4 | 0·1 | 0·0 |
| 18:4n-3 (SA) | 0·0 | 0·5 | 0·0 | 0·1 |
| 20:5n-3 (EPA) | 0·0 | 0·3 | 0·2 | 0·4 |
| 22:5n-3 (DPA) | 0·0 | 0·4 | 1·7 | 2·0 |
| 22:6n-3 (DHA) | 0·0 | 1·1 | 2·1 | 2·6 |
| Others | 4·6 | 9·6 | 19·0 | 20·4 |
Data show one representative sample out of three. Intersample variation for the different fatty acids analysed was < 5%.
AA, arachidonic acid; DHA, docosahexaenoic acid; DPA, docosapentaenoic acid; DGLA, dihomo-γ-linolenic acid; EPA, eicosapentaenoic acid; GLA, γ-linolenic acid; LA, linoleic acid; LNA, α-linolenic acid; SA, stearidonic acid.
Culture of PBMCs in the presence of fatty acids
Human PBMCs from healthy donors were obtained from buffy coats and prepared by Ficoll gradient centrifugation. In short, 10 ml of Ficoll–Paque® was layered under 20 ml of peripheral blood and centrifuged at 400 g for 20 min at room temperature. The recovered PBMCs were washed with PBS/2% fetal calf serum (FCS) three times. Cells were cultured in RPMI-1640 containing 25 mm HEPES, 2 mm l-glutamine, 100 U/ml penicillin/streptomycin, 1·0 mm sodium pyruvate, 12 mm d-glucose and 20% human serum.
Free fatty acids and α-tocopherol were dissolved in 100% ethanol and stored at −80°, under nitrogen, until used. Before dissolving fatty acids, human serum was enriched with ascorbic acid and α-tocopherol to reach final concentrations of 75 and 25 µm, respectively, in culture wells. Fatty acids were dissolved by direct addition of a needed amount of fatty acid stock into prewarmed human serum. The final ethanol concentration was not permitted to exceed 0·2%. For fatty acid analysis, culture of PBMCs was performed over 3 days in six-well flat-bottom plates (1 × 107 cells per well) using a total volume of 5 ml of culture medium, in a humidified 5% CO2 atmosphere, at 37°. LPS stimulation (100 ng/ml) was performed during the final 20 hr of incubation. After incubation, the cells were washed twice with cold PBS and frozen at −80° until analysis. In order to investigate the effects of fatty acids on cytokine production, cells were cultured in 96-well plates containing 200 µl of culture medium (as described above) for 3 days. Cells were stimulated with LPS, as indicated in text. LPS stimulation (100 ng/ml) was performed during the final 20 hr of incubation.
Cytokine, PGE1 and thromboxane analysis
TNF-α, IL-6 and IL-10 levels were measured in the cell culture supernatants using enzyme-linked immunosorbent assay (ELISA) kits from Biosource (Etten-Leur, the Netherlands). PGE1 production was measured in supernatants using a competitive ELISA (R&D Systems GmbH, Wiesbaden-Nordenstadt, Germany). Thromboxanes were analysed using a competitive ELISA from Biotrak (Amersham Pharmacia Biotech, Bucks., UK). All assays were performed according to manufacturer recommendations.
Fatty acid analysis
Fatty acid extraction of 107 cells was performed according to Bligh & Dyer,22 using C19:0 as an internal standard. Phospholipids were separated by using Bond-Elut® solid-phase extraction columns and the Vac-Elut SPS 24™ system. Fatty acids were then converted to their methyl esters by incubation with 20% BF3 in ethanol at 100° for 60 min. After hexane extraction, derivatived fatty acids were dissolved in iso-octane and quantified by using gas chromatography with a capillary column (50 m × 0·25 mm, CP-SIL88-fame). Peaks were identified by reference to commercial standards.
Cell counts and viability assays
Cell counts were performed using a Coulter-Z2 cell counter (Beckman Coulter, Mijdrecht, the Netherlands). In order to include all cells present in the culture wells, adherent cells were trypsinized gently.
Cell viability was assayed using the reagent WST-1, and is based on the cleavage of the tetrazolium salt, WST-1, by the mitochondrial dehydrogenases present in viable cells. The WST-1 assay was performed according to the manufacturer's instructions (Roche Diagnostics Nederland B.V.). Each experiment was performed in parallel, in order to measure cytokine levels as well as cell viability in the same experiment. WST-1 reagent was added to the culture wells during the final 5 hr of LPS stimulation, and extinctions were measured at 450 nm.
Apoptosis/necrosis were assessed by using a propidium iodide staining kit (Sigma-Aldrich Chemie B.V.), following the recommendations of the manufacturer and together with a Coulter Epics XL flow cytometer (Beckman Coulter).
Statistical analysis
Statistical analyses were performed using a paired two-tailed Student's t-test. Significance, as indicated on the graphs, represents a P-value of < 0·05.
Results
In vitro fatty acid modulation of human PBMC
A control fat blend was composed based on the average fatty acid intake of a western diet. It contained 80% palm oil and 20% sunflower oil, providing mainly 16:0, 18:1n-9 and 18:2n-6 (Table 1). Lipid analysis of human serum was performed to determine the background of fatty acids during incubation and the values obtained were in agreement with serum fatty acid concentrations expected in a western society. Human PBMC incubated with culture medium enriched with 100 µm of control fat blend for 3 days showed only marginal changes in the fatty acid profile of cellular phospholipids compared with untreated cells (Table 1). Cell viability, studied by WST labelling, propidium iodide staining, cell counting and visual inspection, was not affected by the 3-day culture period and/or treatment with fatty acids.
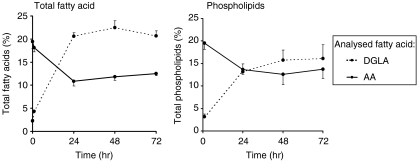
A kinetic study was performed in order to determine the incubation time necessary to induce reproducible modulation of cellular fatty acid composition of freshly isolated PBMC. Cells were cultured for several days in medium enriched with 100 µm DGLA, and LPS stimulation was performed during the last 20 hr of incubation. Fatty acid analysis of both total fatty acid extracts and phospholipid extracts showed that during a culture period of 3 days, saturation was induced in the fatty acid incorporation (Fig. 1). This resulted in the reproducible modulation of fatty acid composition of the cells. Stimulation with LPS did not affect the fatty acid composition of the cells (data not shown).
Figure 1.
Kinetics of dihomo-γ-linolenic acid (20:3n-6) (DGLA) uptake into total lipid and phospholipids of human peripheral blood mononuclear cells (PBMC) in vitro. Cells were cultured for 72 hr in the presence of 100 µm DGLA, and fatty acid analysis was performed at 1, 24, 48 and 72 hr of culture. Data represent the mean ± standard deviation (SD) (n = 3) of the percentage of total fatty acids.
n-6 and n-3 fatty acid modulation of human PBMC
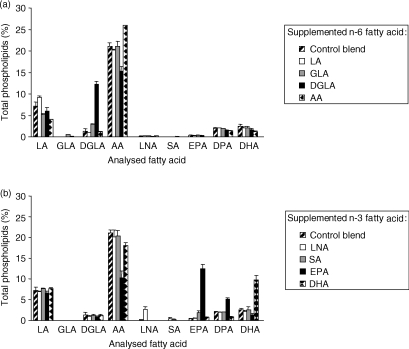
Differences in the uptake of fatty acids were observed (Fig. 2). Incubation with 100 µm DGLA, AA, EPA and docosahexaenoic acid (DHA, 22:6n-3) resulted in large increases (more than 5%) of these fatty acids in cellular phospholipids, whereas incubation with linoleic acid (LA, 18:2n-6), GLA, α-linolenic acid (LNA, 18:3n-3) or stearidonic acid (SA, 18:4n-3) induced increases of less than 3%. The metabolism of fatty acids was restricted to elongation of GLA, AA and EPA into their elongated products, whereas fatty acids that are substrates for desaturase enzymes (LA, DGLA and LNA) were not further metabolized. However, the culture of cells with SA resulted in a small increase of EPA, a transition that involves activity of both elongase and Δ5-desaturase enzymes.
Figure 2.
The effect of the n-6 fatty acids linoleic acid (LA), γ-linolenic acid (GLA), dihomo-γ-linolenic acid (DGLA) and arachidonic acid (AA) (a), and of the n-3 fatty acids α-linolenic acid (LNA), stearidonic acid (SA), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) (b), on the fatty acid composition of human peripheral blood mononuclear cells (PBMC) in vitro. Cells were cultured for 3 days in medium containing 20% human serum and 100 µm of the respective fatty acid. Lipopolysaccharide (LPS) stimulation was performed during the last 20 hr of incubation. Results represent the mean value from at least two donors (± standard deviation) per fatty acid investigated.
Differences were observed in the AA concentrations induced by incubation of the PUFAs. Control cells contained approximately 21% AA and culture with medium enriched with 100 µm AA resulted in AA concentrations of approximately 26% in cells. In contrast, EPA and DGLA reduced the AA concentrations from 21% to approximately 10% and 15%, respectively. Furthermore, DHA induced a smaller decrease in AA concentrations (from 21% to 18%), whereas culture with LA-, GLA-, LNA- or SA-enriched medium did not influence AA concentrations.
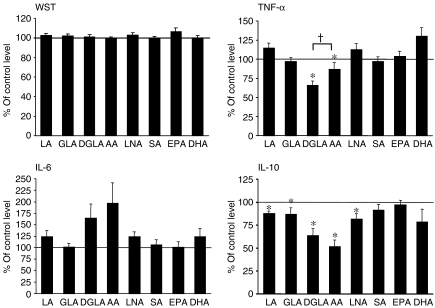
Moreover, no effects on cell viability were observed as a result of incubation with any of the fatty acids (as determined by the WST-1 assay; see Fig. 3).
Figure 3.
The effect of n-3 and n-6 fatty acids on the viability and cytokine levels of human peripheral blood mononuclear cells (PBMC) in vitro. Cells were incubated in the presence of 100 µm of the respective fatty acid, and cytokine levels were measured 20 hr after the induction of lipopolysaccharide (LPS) stimulation, as described in the Materials and methods. Cell viability (determined using the WST-1 assay) was measured during the final 5 hr of LPS stimulation, as described in the Materials and methods. The mean values (of triplicate measurements) ± standard error of the mean (SEM), from 12 blood donors, are shown. Control incubations were cells incubated with 100 µm control fat blend (Table 1), and the cytokine levels of controls were as follows: 1349 ± 251 pg/ml tumour necrosis factor-α (TNF-α); 6576 ± 2082 pg/ml interleukin (IL)-6; and 437 ± 55 pg/ml IL-10. *P < 0·05 versus control incubations. †P < 0·05 for dihomo-γ-linolenic acid (20:3n-6) (DGLA) versus arachidonic acid (AA) (one-tailed Student's t-test).
Effects of n-6 and n-3 PUFAs on cytokine production
Control cells, which were supplemented with 100 µm of the control fat blend (Table 1), produced similar amounts of cytokines compared with unsupplemented cells (data not shown). In contrast, DGLA and AA decreased TNF-α levels to approximately 66% and 87% of control levels, respectively (Fig. 3). The other n-6 fatty acids (LA and GLA), as well as the n-3 fatty acids (LNA, SA, EPA and DHA) did not alter TNF-α levels. Furthermore, none of the fatty acids tested significantly altered the levels of IL-6 (Fig. 3). Incubation with DGLA or AA induced high levels of IL-6 with five of the 12 tested donors, resulting in an overall trend towards increased IL-6 production induced by DGLA and AA. In contrast, DGLA and AA reduced IL-10 levels to approximately 60 and 50% of control levels, respectively (Fig. 3). In addition, smaller reductions of IL-10 levels were induced by LA, GLA and LNA.
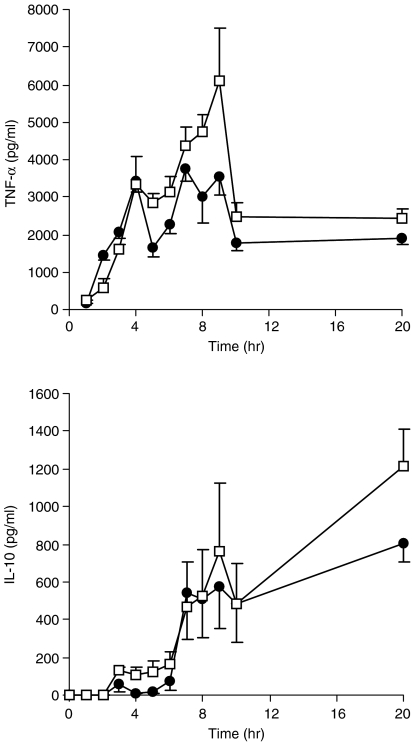
Kinetics of cytokine production altered by DGLA
In order to monitor the kinetics of the modulatory effects of DGLA, cytokine production from DGLA-incubated cells was analysed at several time-points during the time-course of LPS stimulation. In control incubations, TNF-α levels increased rapidly during the first 7 hr of incubation, and DGLA strongly inhibited this increase (Fig. 4). In contrast, significant reduction of IL-10 levels did not occur until 15 hr after LPS stimulation (Fig. 4).
Figure 4.
Effects of dihomo-γ-linolenic acid (20:3n-6) (DGLA) on the kinetics of tumour necrosis factor-α (TNF-α) and interleukin (IL)-10 levels. After culture of the cells with 100 µm Control blend or DGLA for 48 hr (Table 1), lipopolysaccharide (LPS) was added and the cytokine levels were measured in supernatants at the time-points indicated. Data represent the mean value ± standard error of the mean (SEM) from three donors using triplicate incubations.
Dose–response effects of DGLA
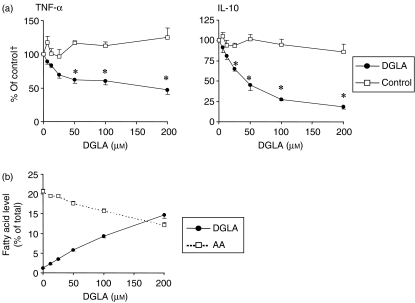
Next, we investigated whether the effects of DGLA are dose responsive. Cells were cultured with DGLA concentrations of 2·5–200 µm, and cytokine production was analysed after 20 hr of LPS stimulation. TNF-α levels were significantly reduced at DGLA concentrations of 50–200 µm, whereas IL-10 levels were reduced at DGLA concentrations of 25–200 µm (Fig. 5).
Figure 5.
Dose–response effects of dihomo-γ-linolenic acid (20:3n-6) (DGLA) on cytokine levels (a) and fatty acid composition of the phospholipid fraction (b) of human peripheral blood mononuclear cells (PBMC) in vitro. Cells were incubated with DGLA or control blend for 3 days, and cytokine or fatty acid levels were measured after 20 hr of stimulation with lipopolysaccharide (LPS). Data represent the mean value ± standard error of the mean (SEM) of cells from four (a) or five (b) blood donors. †The value calculated from cells with 0 µm control blend added to the culture medium. AA, arachidonic acid.
Dose–response analyses of the incorporation of DGLA into phospholipids showed that DGLA was incorporated into ≈ 15% of total fatty acids when cells were incubated with 200 µm DGLA. Concomitantly to the increased amounts of DGLA in phospholipids, decreased AA levels were observed.
Direct effects of eicosanoids on cytokine levels
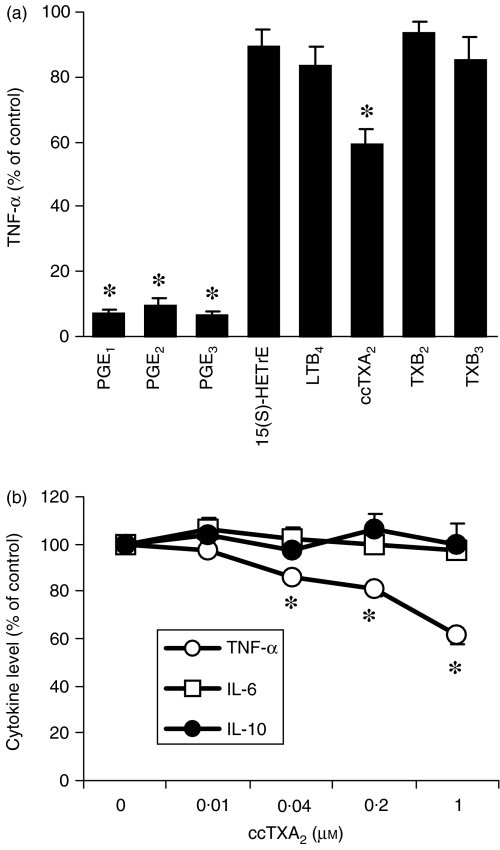
As DGLA appears to compete with AA for incorporation into cellular phospholipids, different classes of eicosanoids might be produced when COX is activated by LPS. We therefore investigated the direct effects of some eicosanoids that are expected to be produced after phospholipid enrichment with DGLA and compared these to eicosanoids derived from AA or EPA. In accordance with previous experiments,21 1 µm PGE1, PGE2 and PGE3, derived from DGLA, AA and EPA, respectively, decreased TNF-α production to almost undetectable levels (Fig. 6a). In contrast, 1 µm 15(S)-HETrE and LTB4, which are lipoxygenase products from DGLA and AA, respectively, did not affect cytokine levels. Furthermore, 1 µm TXB2 and TXB3 (COX products from AA and EPA, respectively) did not modulate cytokine levels. In contrast, the stable TXA2 analogue, ccTXA2, dose dependently inhibited TNF-α, but had no effect on IL-6 and IL-10 levels (Fig. 6b).
Figure 6.
Effects of several eicosanoids (a) and dose–response effects of carbocyclic-thromboxane-A2 (ccTXA2) (b) on cytokine levels. After a 2-day culture period, cells were exposed to the eicosanoids (1 µm) outlined and stimulated (at the same time) with lipopolysaccharide (LPS). Cytokine levels were measured in the supernatants 20 hr after the induction of LPS stimulation. Data represent mean ± standard error of the mean (SEM). (a), n = 8; (b), n = 3. Control incubations were conducted in the absence of eicosanoids.
Eicosanoid levels and effects of COX inhibition
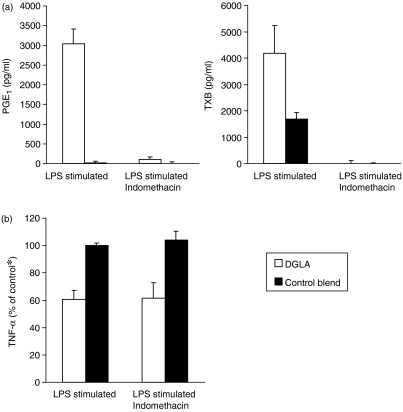
As PGE1 and the stable TXA2 analogue, ccTXA2, inhibited TNF-α production similarly to DGLA, we investigated whether the inhibition of COX activation would affect the modulatory effects of DGLA on cytokine levels. Therefore, indomethacin (10 µm) was added during LPS activation and the TNF-α levels were measured in supernatants. At this concentration, indomethacin effectively inhibited LPS-induced PGE production, without affecting cell viability (measured by WST labelling, data not shown). Indomethacin inhibited PGE1 and thromboxane production in both DGLA and control blend-supplemented cells (Fig. 7a). In the absence of indomethacin, the concentrations of these eicosanoids were higher in supernatants from DGLA-incubated cells than in control blend-treated cells. However, the addition of indomethacin did not affect the reduction of TNF-α levels induced by DGLA supplementation (Fig. 7b).
Figure 7.
Effects of dihomo-γ-linolenic acid (20:3n-6) (DGLA) on tumour necrosis factor-α (TNF-α) levels are independent of cyclooxygenase (COX) inhibition by indomethacin. Cells were cultured with 100 µm DGLA or Control blend (Table 1), stimulated with lipopolysaccharide (LPS), and the levels of prostaglandin E1 (PGE1) and thromboxane B (TXB) (Fig. 6a) or tumour necrosis factor-α (TNF-α) (Fig. 6b) were measured in supernatants 20 hr after the addition of LPS. Results represent the mean value ± standard error of the mean (SEM). (a), n = 3; (b), n = 5. *Controls were LPS-stimulated cells cultured with 100 µm Control blend in the absence of indomethacin.
Discussion
Immunomodulatory effects of PUFAs have been described in in vitro, ex vivo and in vivo studies, and the majority of results described immunosuppressive effects on human cells.1,23,24 The in vitro model used in the current study is somewhat different from other in vitro studies investigating the immunomodulatory effects of PUFA. We added free fatty acids to human serum in order to expose PBMC to fatty acids for several days. This is in contrast to other studies that used fetal bovine serum25,26 or fatty acid–bovine serum albumin complexes as a carrier,27–29 and/or used shorter prestimulus incubation times ranging from 10 min to 24 hr.13,14,26,30 In our experiments, high levels of albumin were found to affect functionality (cytokine production) of cells. An experimental set up, in which the control fatty acid blend was dissolved in human serum directly, ensured that the functionality of cells remained unchanged. Also, n-3 and n-6 fatty acids were readily taken up and incorporated into phospholipids by quiescent, unstimulated PBMC, a process that was previously described to occur only minimally in a similar study, using rat lymphocytes, which used fatty acids precoupled to albumin.31 In addition, most studies on fatty acids with incubation times of several days focus on (T-cell) mitogenic stimulation,19,29,32–37 whereas in the current study endotoxin stimulation is used. Therefore, the in vitro study described here is novel in the way that it uses freshly isolated human PBMC pretreated with PUFAs for 48 hr followed by stimulation with LPS.
In this study, we found that DGLA, but not other structurally related fatty acids (such as GLA or EPA), specifically altered cytokine levels. This rather striking finding indicates that dietary supplementation with a DGLA-rich oil may have more impact on cytokine production induced by endotoxin stimulation than the use of oils rich in any other PUFA. DGLA has previously been described to modulate immune function, although data are limited to in vitro studies. Previously reported immunomodulatory effects of DGLA on T cells were inhibited proliferation of, and IL-2 production from, human lymphocytes35,38–40 and murine T-cells,41 whereas others described DGLA not to affect the T-cell proliferation of human lymphocytes.19,28 In line with the current study, DeLuca et al.13 reported inhibition of TNF-α and IL-1β production of DGLA by LPS-stimulated human PBMC. However, the preincubation time with DGLA was only 30 min, and GLA and EPA were also shown to inhibit the production of pro-inflammatory cytokines, in contrast to the present study. Distinctions between the study of DeLuca et al. and the present study concern the differences in fatty acid incorporation and use of antioxidants to prevent lipid peroxidation during culture.
Furthermore, it was described that DGLA, used at concentrations similar to those of the present study, inhibits LTB4 production by human PBMC induced by a calcium ionophore without altering PGE2 production.42 The reduction in LTB4 synthesis was correlated with the formation of the 15 lipoxygenase product from DGLA, 15-(S)-HETrE.42,43 In the current study, both LTB4 and LTB5 appeared to be ineffective in modulating cytokine production induced by LPS activation, in contrast to other studies, involving T-cell stimulation, which reported immuno-stimulatory44–47 and also anti-inflammatory48,49 effects of leukotrienes. Moreover, 15-(S)-HETrE, did not modulate cytokine levels. These findings indicate that leukotrienes have little influence on cytokine production by monocytes or macrophages, in contrast to T cells. The TXA2 agonist, ccTXA2, specifically inhibited the LPS-induced TNF-α level, without influencing IL-6 and IL-10 levels, while cytokine levels of unstimulated cells remained low. This is in contrast to another study in which the TXA2 agonist was found to increase TNF-α production of unstimulated human monocytes, implying that TXA2 exerts pro-inflammatory activity.20 Furthermore, the inhibition of COX enzymes (by the addition of indomethacin) did not change the effects of DGLA, indicating that these effects are not a result of the altered amounts and subtypes of COX products. Overall, these findings imply that DGLA affects cytokine levels directly.
The mechanism of action whereby DGLA exerts changes in cytokine levels is, as yet, unknown. Interestingly, analysis of the total fatty acid levels showed that a large percentage of DGLA is present in cells as free fatty acids, that is, not acetylated in cellular phospholipids, which is in contrast to other PUFAs (data not shown). The large pool of unbound DGLA might directly affect a number of cellular processes, such as gene transcription and translation, and other intracellular events, such as receptor-mediated processes.50 Kinetic experiments showed that the inhibition of TNF-α by DGLA occurs during the peak levels of TNF-α, from 4 to 9 hr after LPS stimulation, whereas the onset of cytokine production was not inhibited. Furthermore, the decreased IL-10 levels found at later time-points might be directly related to the decreased peak concentrations of TNF-α, because addition of TNF-α was found to increase IL-10 levels (our own data and ref. 51).
In contrast to the general view that n-3 PUFAs are immunosuppressive, we did not observe any effects of n-3 PUFAs on cytokine production. Clearly, the lack of such effects found in this study cannot be explained by insufficient fatty acid incorporation, as EPA and DHA were both incorporated to a notable extent, which was similar to that of DGLA after DGLA supplementation. Previously published reports, describing the effects on cytokine production of human cells by EPA and DHA, merely involve decreased TNF-α and IL-1β production of endotoxin-stimulated PBMC after dietary supplementation of subjects.10–12,52,53 Nonetheless, direct effects of n-3 PUFAs on cytokine production of human cells are scarce. Together with the present data, these limited reports, which describe the direct effects of EPA and DHA in vitro, suggest that effects of EPA and DHA are only observed after in vivo metabolism of these PUFAs. However, the changes in cytokine production induced by dietary intake of n-3 or n-6 PUFAs are probably not explained by changes in PGE subtypes, as PGE1, PGE2 and PGE3 have been shown to have a similar effect on LPS-induced cytokine levels,21 and replacement of AA by EPA did not affect cytokine production.
Overall, the data obtained here clearly suggest that the production of cytokines by LPS-stimulated human PBMC can be modulated by DGLA. This can be of importance for the treatment of inflammatory diseases in which the activation of mononuclear phagocytes plays a role. Furthermore, the fact that DGLA probably exerts its effects independently from eicosanoid formation raises possibilities for the combined use of DGLA and therapy with non-steroidal anti-inflammatory drugs (NSAIDs) during harmful inflammation.
Acknowledgments
We thank Erik Hogenkamp from Numico Research B.V. (Wageningen, the Netherlands) for his technical assistance and the fatty acid analysis of human serum.
Abbreviations
- AA
arachidonic acid (20:4n-6)
- BF3
boron trifluoride
- ccTXA2
carbocyclic-thromboxane-A2
- COX
cyclooxygenase
- DGLA
dihomo-γ-linolenic acid (20:3n-6)
- DHA
docosahexaenoic acid (22:6n-3)
- DMSO
dimethylsulphoxide
- ELISA
enzyme-linked immunosorbent assay
- EPA
eicosapentaenoic acid (20:5n-3)
- FCS
fetal calf serum
- GLA
γ-linolenic acid (18:3n-6)
- IL
interleukin
- LA
linoleic acid (18:2n-6)
- LNA
α-linolenic acid (18:3n-3)
- LPS
lipopolysaccharide
- LTB4
leukotriene B4
- PBMC
peripheral blood mononuclear cells
- PBS
phosphate-buffered saline
- PGE
prostaglandin E
- PUFA
polyunsaturated fatty acid
- SA
stearidonic acid (18:4n-3)
- TNF-α
tumour necrosis factor-α
- TXB
thromboxane B
References
- 1.Calder PC, Yaqoob P, Thies F, Wallace FA, Miles EA. Fatty acids and lymphocyte functions. Br J Nutr. 2002;87:S31–48. doi: 10.1079/bjn2001455. [DOI] [PubMed] [Google Scholar]
- 2.Calder PC. Dietary fatty acids and the immune system. Lipids. 1999;34(Suppl.):S137–40. doi: 10.1007/BF02562264. [DOI] [PubMed] [Google Scholar]
- 3.Endres S. n-3 polyunsaturated fatty acids and human cytokine synthesis. Lipids. 1996;31(Suppl.):S239–42. doi: 10.1007/BF02637083. [DOI] [PubMed] [Google Scholar]
- 4.Leventhal LJ, Boyce EG, Zurier RB. Treatment of rheumatoid arthritis with gammalinolenic acid. Ann Intern Med. 1993;119:867–73. doi: 10.7326/0003-4819-119-9-199311010-00001. [DOI] [PubMed] [Google Scholar]
- 5.Leventhal LJ, Boyce EG, Zurier RB. Treatment of rheumatoid arthritis with blackcurrant seed oil. Br J Rheumatol. 1994;33:847–52. doi: 10.1093/rheumatology/33.9.847. [DOI] [PubMed] [Google Scholar]
- 6.Zurier RB, Rossetti RG, Jacobson EW, DeMarco DM, Liu NY, Temming JE, White BM, Laposata M. gamma-Linolenic acid treatment of rheumatoid arthritis: a randomized, placebo-controlled trial. Arthritis Rheum. 1996;39:1808–17. doi: 10.1002/art.1780391106. [DOI] [PubMed] [Google Scholar]
- 7.Cleland LG, French JK, Betts WH, Murphy GA, Elliott MJ. Clinical and biochemical effects of dietary fish oil supplements in rheumatoid arthritis. J Rheumatol. 1988;15:1471–5. [PubMed] [Google Scholar]
- 8.Kjeldsen-Kragh J, Lund JA, Riise T, Finnanger B, Haaland K, Finstad R, Mikkelsen K, Forre O. Dietary omega-3 fatty acid supplementation and naproxen treatment in patients with rheumatoid arthritis. J Rheumatol. 1992;19:1531–6. [PubMed] [Google Scholar]
- 9.van der Tempel H, Tulleken JE, Limburg PC, Muskiet FA, van Rijswijk MH. Effects of fish oil supplementation in rheumatoid arthritis. Ann Rheum Dis. 1990;49:76–80. doi: 10.1136/ard.49.2.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kremer JM, Lawrence DA, Jubiz W, DiGiacomo R, Rynes R, Bartholomew LE, Sherman M. Dietary fish oil and olive oil supplementation in patients with rheumatoid arthritis. Clinical and immunologic effects. Arthritis Rheum. 1990;33:810–20. doi: 10.1002/art.1780330607. [DOI] [PubMed] [Google Scholar]
- 11.Endres S, Ghorbani R, Kelley VE, et al. The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. N Engl J Med. 1989;320:265–71. doi: 10.1056/NEJM198902023200501. [DOI] [PubMed] [Google Scholar]
- 12.Endres S, Meydani SN, Dinarello CA. Effects of omega 3 fatty acid supplements on ex vivo synthesis of cytokines in human volunteers: comparison with oral aspirin and ibuprofen. World Rev Nutr Diet. 1991;66:401–6. doi: 10.1159/000419308. [DOI] [PubMed] [Google Scholar]
- 13.DeLuca P, Rossetti RG, Alavian C, Karim P, Zurier RB. Effects of gammalinolenic acid on interleukin-1 beta and tumor necrosis factor-alpha secretion by stimulated human peripheral blood monocytes: studies in vitro and in vivo. J Invest Med. 1999;47:246–50. [PubMed] [Google Scholar]
- 14.Furse RK, Rossetti RG, Zurier RB. Gammalinolenic acid, an unsaturated fatty acid with anti-inflammatory properties, blocks amplification of IL-1 beta production by human monocytes. J Immunol. 2001;167:490–6. doi: 10.4049/jimmunol.167.1.490. [DOI] [PubMed] [Google Scholar]
- 15.Robinson DR, Tateno S, Patel B, Hirai A. Lipid mediators of inflammatory and immune reactions. JPEN J Parenter Enteral Nutr. 1988;12:37S–42S. doi: 10.1177/014860718801200602. [DOI] [PubMed] [Google Scholar]
- 16.Zhou L, Nilsson A. Sources of eicosanoid precursor fatty acid pools in tissues. J Lipid Res. 2001;42:1521–42. [PubMed] [Google Scholar]
- 17.Seeds MC, Bass DA. Regulation and metabolism of arachidonic acid. Clin Rev Allergy Immunol. 1999;17:5–26. doi: 10.1007/BF02737594. [DOI] [PubMed] [Google Scholar]
- 18.Pullman-Mooar S, Laposata M, Lem D, Holman RT, Leventhal LJ, DeMarco D, Zurier RB. Alteration of the cellular fatty acid profile and the production of eicosanoids in human monocytes by gamma-linolenic acid. Arthritis Rheum. 1990;33:1526–33. doi: 10.1002/art.1780331010. [DOI] [PubMed] [Google Scholar]
- 19.Kumar GS, Das UN. Effect of prostaglandins and their precursors on the proliferation of human lymphocytes and their secretion of tumor necrosis factor and various interleukins. Prostaglandins Leukot Essent Fatty Acids. 1994;50:331–4. doi: 10.1016/0952-3278(94)90242-9. [DOI] [PubMed] [Google Scholar]
- 20.Caughey GE, Pouliot M, Cleland LG, James MJ. Regulation of tumor necrosis factor-alpha and IL-1 beta synthesis by thromboxane A2 in nonadherent human monocytes. J Immunol. 1997;158:351–8. [PubMed] [Google Scholar]
- 21.Dooper MM, Wassink L, M'Rabet L, Graus YM. The modulatory effects of prostaglandin-E on cytokine production by human peripheral blood mononuclear cells are independent of the prostaglandin subtype. Immunology. 2002;107:152–9. doi: 10.1046/j.1365-2567.2002.01474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bligh ED, Dyer WJ. A rapid method for total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–7. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 23.Calder PC. n-3 polyunsaturated fatty acids and cytokine production in health and disease. Ann Nutr Metab. 1997;41:203–34. doi: 10.1159/000177997. [DOI] [PubMed] [Google Scholar]
- 24.Kinsella JE, Lokesh B, Broughton S, Whelan J. Dietary polyunsaturated fatty acids and eicosanoids: potential effects on the modulation of inflammatory and immune cells: an overview. Nutrition. 1990;6:24–44. [discussion 59–62] [PubMed] [Google Scholar]
- 25.Galella G, Marangoni F, Rise P, Colombo C, Galli G, Galli C. n-6 and n-3 fatty acid accumulation in thp-1 cell phospholipids. Biochim Biophys Acta. 1993;1169:280–90. doi: 10.1016/0005-2760(93)90252-5. [DOI] [PubMed] [Google Scholar]
- 26.Baldie G, Kaimakamis D, Rotondo D. Fatty acid modulation of cytokine release from human monocytic cells. Biochim Biophys Acta. 1993;1179:125–33. doi: 10.1016/0167-4889(93)90133-a. [DOI] [PubMed] [Google Scholar]
- 27.Calder PC, Bond JA, Harvey DJ, Gordon S, Newsholme EA. Uptake and incorporation of saturated and unsaturated fatty acids into macrophage lipids and their effect upon macrophage adhesion and phagocytosis. Biochem J. 1990;269:807–14. doi: 10.1042/bj2690807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joulain C, Guichardant M, Lagarde M, Prigent AF. Influence of polyunsaturated fatty acids on lipid metabolism in human blood mononuclear cells and early biochemical events associated with lymphocyte activation. J Lipid Med Cell Signal. 1995;11:63–79. doi: 10.1016/0929-7855(94)00028-b. [DOI] [PubMed] [Google Scholar]
- 29.Karsten S, Schafer G, Schauder P. Cytokine production and DNA synthesis by human peripheral lymphocytes in response to palmitic, stearic, oleic, and linoleic acid. J Cell Physiol. 1994;161:15–22. doi: 10.1002/jcp.1041610103. [DOI] [PubMed] [Google Scholar]
- 30.Ferrante JV, Huang ZH, Nandoskar M, et al. Altered responses of human macrophages to lipopolysaccharide by hydroperoxy eicosatetraenoic acid, hydroxy eicosatetraenoic acid, and arachidonic acid: inhibition of tumor necrosis factor production. J Clin Invest. 1997;99:1445–52. doi: 10.1172/JCI119303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calder PC, Yaqoob P, Harvey DJ, Watts A, Newsholme EA. Incorporation of fatty acids by concanavalin A-stimulated lymphocytes and the effect on fatty acid composition and membrane fluidity. Biochem J. 1994;300:509–18. doi: 10.1042/bj3000509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khalfoun B, Thibault G, Lacord M, Gruel Y, Bardos P, Lebranchu Y. Docosahexaenoic and eicosapentaenoic acids inhibit human lymphoproliferative responses in vitro but not the expression of T cell surface activation markers. Scand J Immunol. 1996;43:248–56. doi: 10.1046/j.1365-3083.1996.d01-42.x. [DOI] [PubMed] [Google Scholar]
- 33.Purasiri P, McKechnie A, Heys SD, Eremin O. Modulation in vitro of human natural cytotoxicity, lymphocyte proliferative response to mitogens and cytokine production by essential fatty acids. Immunology. 1997;92:166–72. doi: 10.1046/j.1365-2567.1997.d01-2308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jenski LJ, Scherer JM, Caldwell LD, Ney VA, Stillwell W. The triggering signal dictates the effect of docosahexaenoic acid on lymphocyte function in vitro. Lipids. 1998;33:869–78. doi: 10.1007/s11745-998-0283-x. [DOI] [PubMed] [Google Scholar]
- 35.Soyland E, Nenseter MS, Braathen L, Drevon CA. Very long chain n-3 and n-6 polyunsaturated fatty acids inhibit proliferation of human T-lymphocytes in vitro. Eur J Clin Invest. 1993;23:112–21. doi: 10.1111/j.1365-2362.1993.tb00750.x. [DOI] [PubMed] [Google Scholar]
- 36.Calder PC, Newsholme EA. Polyunsaturated fatty acids suppress human peripheral blood lymphocyte proliferation and interleukin-2 production. Clin Sci (Colch) 1992;82:695–700. doi: 10.1042/cs0820695. [DOI] [PubMed] [Google Scholar]
- 37.Calder PC, Bond JA, Bevan SJ, Hunt SV, Newsholme EA. Effect of fatty acids on the proliferation of concanavalin A-stimulated rat lymph node lymphocytes. Int J Biochem. 1991;23:579–88. doi: 10.1016/0020-711x(87)90052-8. [DOI] [PubMed] [Google Scholar]
- 38.Santoli D, Zurier RB. Prostaglandin E precursor fatty acids inhibit human IL-2 production by a prostaglandin E-independent mechanism. J Immunol. 1989;143:1303–9. [PubMed] [Google Scholar]
- 39.DeMarco DM, Santoli D, Zurier RB. Effects of fatty acids on proliferation and activation of human synovial compartment lymphocytes. J Leukoc Biol. 1994;56:612–5. doi: 10.1002/jlb.56.5.612. [DOI] [PubMed] [Google Scholar]
- 40.Zurier RB, Rossetti RG, Seiler CM, Laposata M. Human peripheral blood T lymphocyte proliferation after activation of the T cell receptor: effects of unsaturated fatty acids. Prostaglandins Leukot Essent Fatty Acids. 1999;60:371–5. doi: 10.1016/s0952-3278(99)80015-5. [DOI] [PubMed] [Google Scholar]
- 41.Borofsky MA, Zurier RB, Rosenbaum H, Weiner DB, Williams WV. Effects of polyunsaturated fatty acids on interleukin-2-dependent T cell growth. Immunol Res. 1992;11:154–64. doi: 10.1007/BF02918620. [DOI] [PubMed] [Google Scholar]
- 42.Iversen L, Fogh K, Kragballe K. Effect of dihomogammalinolenic acid and its 15-lipoxygenase metabolite on eicosanoid metabolism by human mononuclear leukocytes in vitro: selective inhibition of the 5-lipoxygenase pathway. Arch Dermatol Res. 1992;284:222–6. doi: 10.1007/BF00375798. [DOI] [PubMed] [Google Scholar]
- 43.Miller CC, McCreedy CA, Jones AD, Ziboh VA. Oxidative metabolism of dihomogammalinolenic acid by guinea pig epidermis: evidence of generation of anti-inflammatory products. Prostaglandins. 1988;35:917–38. doi: 10.1016/0090-6980(88)90116-5. [DOI] [PubMed] [Google Scholar]
- 44.Rola-Pleszczynski M, Chavaillaz PA, Lemaire I. Stimulation of interleukin 2 and interferon gamma production by leukotriene B4 in human lymphocyte cultures. Prostaglandins Leukot Med. 1986;23:207–10. doi: 10.1016/0262-1746(86)90187-3. [DOI] [PubMed] [Google Scholar]
- 45.Gagnon L, Filion LG, Dubois C, Rola-Pleszczynski M. Leukotrienes and macrophage activation. augmented cytotoxic activity and enhanced interleukin-1, tumor necrosis factor and hydrogen peroxide production. Agents Actions. 1989;26:141–7. doi: 10.1007/BF02126587. [DOI] [PubMed] [Google Scholar]
- 46.Goldyne ME. Leukotrienes: clinical significance. J Am Acad Dermatol. 1984;10:659–68. doi: 10.1016/s0190-9622(84)80274-1. [DOI] [PubMed] [Google Scholar]
- 47.Johnson HM, Torres BA. Leukotrienes: positive signals for regulation of gamma-interferon production. J Immunol. 1984;132:413–6. [PubMed] [Google Scholar]
- 48.Rola-Pleszczynski M. Differential effects of leukotriene B4 on T4+ and T8+ lymphocyte phenotype and immunoregulatory functions. J Immunol. 1985;135:1357–60. [PubMed] [Google Scholar]
- 49.Payan DG, Missirian-Bastian A, Goetzl EJ. Human T-lymphocyte subset specificity of the regulatory effects of leukotriene B4. Proc Natl Acad Sci USA. 1984;81:3501–5. doi: 10.1073/pnas.81.11.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jump DB, Clarke SD. Regulation of gene expression by dietary fat. Annu Rev Nutr. 1999;19:63–90. doi: 10.1146/annurev.nutr.19.1.63. [DOI] [PubMed] [Google Scholar]
- 51.Wanidworanun C, Strober W. Predominant role of tumor necrosis factor-alpha in human monocyte IL-10 synthesis. J Immunol. 1993;151:6853–61. [PubMed] [Google Scholar]
- 52.Kelley DS, Taylor PC, Nelson GJ, et al. Docosahexaenoic acid ingestion inhibits natural killer cell activity and production of inflammatory mediators in young healthy men. Lipids. 1999;34:317–24. doi: 10.1007/s11745-999-0369-5. [DOI] [PubMed] [Google Scholar]
- 53.Meydani SN, Endres S, Woods MM, Goldin BR, Soo C, Morrill-Labrode A, Dinarello CA, Gorbach SL. Oral (n-3) fatty acid supplementation suppresses cytokine production and lymphocyte proliferation: comparison between young and older women. J Nutr. 1991;121:547–55. doi: 10.1093/jn/121.4.547. [DOI] [PubMed] [Google Scholar]