Abstract
Thymocyte responses to functional activation are of relevance to the evaluation of the efficacy of in ovo immunotherapies and vaccines in chickens. In this study we have demonstrated differences in chicken thymocyte responses according to developmental age. RNA samples from stimulated and unstimulated chicken thymocytes were assayed for messenger RNA encoding the cytokines interleukin-1β (IL-1β), IL-2, interferon-α (IFN-α), IFN-β, IFN-γ and transforming growth factor-β4 (TGF-β4), and also components of the major histocompatibility complex (MHC), β2-microglobulin (β2M) and the MHC class I α-chain (MHC IA). At embryonic day 14 thymocytes were least responsive to functional activation and differences existed even between thymocyte populations at embryonic day 18 and day 1 post-hatch. The duration of proliferation in response to stimulation was found to increase with increasing embryonic age. Mitogen stimulation of embryonic day 18 and day 1 post-hatch thymocytes induced up-regulation of IFN-γ, IL-1β and TGF-β transcripts, and down-regulation of IFN-α, IFN-β and IL-2 transcripts, with a higher induction of IFN-γ, IL-1β and TGF-β transcripts in more immature T-cell-receptor-negative (TCR−) than TCR+ (TCR1+, TCR2+, or TCR3+) subsets. In contrast, in the mouse and human, both mature and immature thymocytes respond to mitogen stimulation with up-regulation of IL-2. Thymocytes from embryonic day 14 chicks responded to mitogen with a short burst of unsustained proliferation, and transcriptional down-regulation of the cytokines IL-2, IL-1β, IFN-α, IFN-β and IFN-γ. These results suggest that embryonic day 14 thymocytes are largely unresponsive to mitogen. Transcripts encoding TGF-β and type I interferons (IFN-α and IFN-β) were constitutively expressed at high levels in very early thymocytes at embryonic day 14. Thymocytes at embryonic days 14 and 18 and day 1 post-hatch responded to mitogen stimulation with up-regulation of MHC IA transcript. The pattern of β2M transcription following mitogen stimulation was distinct from that of the globally up-regulated MHC IA transcript, with up-regulation of β2M transcription observed at embryonic day 18 and day 1 post-hatch but not at embryonic day 14. In thymocyte subsets, up-regulation of β2M transcription was found to be specific to the CD8+ TCR+ population. The balance of responses in the embryonic thymus suggests that at all stages thymocytes have a reduced capacity for activation in comparison to mature thymocyte populations.
Introduction
The accessibility of the chick embryo has established the chicken as a model system for studying embryonic development. However, a lack of avian-specific assays has limited understanding of the ontogeny of the avian immune system. Increasing awareness and emphasis by the poultry industry on the vertical transmission of microbial pathogens and the cost associated with immunosuppressive infections arising in very young chicks have increased the importance of understanding the development of the chick thymus. Research into vaccine delivery by the in ovo route, and the potential of cytokines to act as adjuvants in such a system, necessitates an understanding of the capacity of the embryonic thymus to respond to stimulation.
The embryonic thymus contains distinct thymocyte subtypes, both functionally and in terms of developmental progression, at different embryonic ages. At embryonic day 14 (E14) the predominant component of the population is early T-cell receptor (TCR) 1+ (γδ) CD3+ CD4− CD8− thymocytes derived from the first wave of stem-cell progenitors to seed the thymus at E7.1–4 On entry into the thymus there is rapid cell surface expression of TCR1+ and cells remain CD4− and CD8− until they enter the peripheral tissues.1–4 The thymic stromal environment encountered by the first wave of progenitors is unique relative to all subsequent clonal lineages, as it lacks mature selected T cells.1–4 Development of thymocytes occurs in a diffuse distribution in the cortex and there is rapid progression into the medulla with thymic emigrants appearing at E15. These cells give rise to the mature TCR1+ population which becomes predominantly CD8+ in the periphery. Two-thirds of these cells home to the spleen and intestine, and the chicken is unusual in that 20–50% of circulating T cells are TCR1+.1–4 As mature γδ T cells they are functionally distinct, with poor responses to mitogen stimulation relative to αβ T cells.1–4 They have a diffuse distribution in the spleen and intestine, rather than forming nodular associations. Previous studies have demonstrated a high background proliferative rate, and consequently a limited response to mitogen stimulation, in E14 thymocytes.
The predominant component of the thymocyte population at E18 is TCR2+ (αβ1) CD3+ thymocytes, with a smaller population of TCR3+ (αβ2) CD3+ thymocytes and an even smaller population of γδ T cells, as most have emigrated from the thymus to the periphery.1–4 The αβ T cells are also derived from the first wave of stem-cell progenitors, which persist in the thymus for 3 weeks.1–4 For the αβ T cells the developmental pattern, thymic stromal environment and functional capacity of the mature T-cell populations are distinctly different from the earlier γδ T cells. The αβ thymocytes have an extended development in the thymic cortex before progressing to the corticomedullary junction after 5 days. In contrast to the γδ T cells, these early αβ T cells initially express both CD4 and CD8 and there is a delay in the full rearrangement of the TCR β-chain and the subsequent surface expression of TCR-αβ.1–4
At 1 day post-hatch (D1) there is a change in the ratio of TCR2+ to TCR3+ thymocytes.1–4 TCR3+ cell numbers peak in the thymus and TCR2+ cell numbers are declining as a result of emigration. The second wave of stem-cell progenitors seeding the thymus commences at E20.1–4 In addition, the newly hatched chick has been subjected to the physiological stress of hatching.
The thymocyte population at E14 is of interest not only in defining the capacity of the embryonic thymus to respond to stimulation, but also in establishing the phenotype of the progenitor population of γδ T cells. Little is known about their regulation or the capacity of these unique early thymocytes for functional activation. At E18 thymic populations include more mature CD4+ TCR+ and CD8+ TCR+, earlier CD4+ CD8+ TCR− and very early CD4+ TCR− and CD8+ TCR− thymocytes because of overlap in the development of thymocytes. Thus it would be informative to compare the function at E18 of the more mature CD4+ TCR+ and CD8+ TCR+ cells and very early CD4+ TCR− and CD8+ TCR− thymocytes. Because D1 chicks are more frequently studied than embryos and also because of the physiological and developmental differences between E18 and D1 chickens, it would also be interesting to compare the function of thymocytes at D1 to those at E18.
The objective of this study was an investigation of the development of proliferative and cytokine activation responses to mitogen stimulation in the embryonic thymus. Thymocyte activation, proliferation and cytokine induction were examined in chicks at E14 and E18, and at D1, and in thymocyte subsets at E18. RNA samples from stimulated and control thymocytes were assayed for messenger RNA (mRNA) encoding the cytokines interleukin-1β (IL-1β), IL-2, interferon-α (IFN-α), IFN-β, IFN-γ and transforming growth factor-β4 (TGF-β4), and also components of the major histocompatibility complex (MHC), β2 microglobulin (β2M) and the MHC class I α-chain (MHC IA). The only group of cytokines not represented in our studies were the T helper type 2 (Th2) cytokines, for which there is, as yet, no evidence in any non-mammalian species.
Materials and methods
Experimental animals
Embryonated eggs and day-old chicks were obtained from a specific-pathogen-free Rhode Island Red flock maintained at the Institute for Animal Health. UK Home Office Guidelines were followed for the humane killing of the chick embryos by refrigeration and of the day-old chicks by cervical dislocation.
Thymocyte preparations
Thymuses were placed into chilled Dulbecco's Modified Eagle's Medium (DMEM) (Invitrogen Life Technologies, Paisley, UK), supplemented with bovine serum albumin (BSA) (Sigma, Poole, UK) at 2 mg/ml, penicillin at 1 U/ml, streptomycin at 1 μg/ml and 1%l-glutamine. The tissue was roughly macerated and filtered through a 50-μm-pore nylon mesh. Thymic lymphocytes were purified by centrifugation for 5 min at 1000 g over a Ficoll-Paque (Amersham Pharmacia Biotech, Buckinghamshire, UK) gradient and the collected cells were washed twice in culture medium. Triplicate haemocytometer readings were performed and the cells were suspended in DMEM at a final concentration of 2 × 107 lymphocytes/ml.
Proliferation assays
Thymocytes were isolated, resuspended as above and added (100 μl/well) to 96-well plates. Assays were generally carried out in triplicate. Thymocytes were unstimulated or stimulated in the presence of 1 μg/ml murine anti-chicken CD28 monoclonal antibody (mAb) AV7 (Southern Biotechnology, Birmingham, AL), 50 ng/ml ionomycin and phorbol 12-myristate 13-acetate (PMA) at either 200, 500, 800, or 1500 ng/ml. Incubations were for 24 or 48 hr at 41° in 5% CO2. Samples were pulsed 6 hr prior to collection with 37 kBq [3H]thymidine (specific activity 185 GBq/mmol) (Amersham Pharmacia Biotech). Cells were harvested using a Skatron cell harvester onto glass fibre filter mats (PerkinElmer Wallac, Frieberg, Germany) and air-dried. Filter mats were treated with scintillation liquid (Wallac Betaplate Scint, PerkinElmer Wallac) and counted in a 1205 Betaplate counter (PerkinElmer Wallac). The mean counts per minute (c.p.m.) were determined for all triplicate samples of stimulated and unstimulated cells and the stimulation indices were calculated from the ratio mean c.p.m. stimulated : mean c.p.m. unstimulated.
In addition, the experiment was replicated in 24-well plates with a final volume per well of 2 ml. Cell pellets were collected, for RNA isolation, after 24 and 48 hr of incubation by centrifugation at 1000 g for 5 min.
Fluorescence-activated cell sorting (FACS) of thymic lymphocytes
Thymocytes were isolated from the thymuses of 15 E18 embryos and sorted into four populations; CD4+ TCR+ (TCR1+, TCR2+, or TCR3+), CD4+ TCR−, CD8+ TCR+ (TCR1+, TCR2+, or TCR3+) and CD8+ TCR−. Two staining treatments were performed on duplicate samples of 109 lymphocytes. All mAb were diluted 1/100 in FACS buffer (phosphate-buffered saline and 1% BSA). Cells were incubated for 30 min at 4° with murine mAb, conjugated to phycoerythrin, to the following chicken cell surface markers: TCR1, TCR2 and TCR3 (all Southern Biotechnology), and then for 30 min with murine mAb, conjugated to fluorescein isothiocyanate (FITC), to either chicken CD4 or CD8 (both Southern Biotechnology).
CD4- and CD8-positive populations were purified using a MiniMACS system (Miltenyi Biotech, Surrey, UK) according to the manufacturer's instructions. Briefly, stained cells were incubated for 10 min at room temperature with 200 μl of anti-FITC magnetic antibody cell sorting (MACS) beads. The cells and beads were washed twice in FACS buffer and applied to a prepared MiniMACS column mounted in a magnetic field. Following two washes the column was removed from the magnetic field and the cells were rapidly eluted from the column under pressure. Purified CD4+ and CD8+ populations were then further sorted into TCR+ and TCR− populations using a fluorocytometer (FACScalibur; Becton Dickson, Oxford, UK).
Proliferation and stimulation assays for RNA extraction were carried out on the CD4+ TCR+ and CD8+ TCR+ thymocyte populations. Stimulation assays were carried out on the CD4+ TCR− and CD8+ TCR− thymocyte populations, but there were insufficient cells in the sorted populations to perform proliferation assays.
Double-positive CD4+ CD8+ thymocytes were not removed from the CD4+ and CD8+ thymocyte subsets because the thymus of the chick embryo is extremely small, the cell numbers are limited and the sorting of such small thymocytes subsets to the purity required to make meaningful analyses is therefore time limiting. The ability to represent thymocyte function accurately was balanced against the detrimental effects of prolonged repeated staining and multiple sorting steps as a third sorting of thymocytes would have necessitated handling of the cells for a further 8–12 hr prior to assay and the recovery of cells would have been too low to perform all the assays in this study. However, when the sorted cells were assayed CD4+ CD8+ double-positive cells comprised, on average, 34% of both subsets (data not shown). As this population was detected at an equivalent ratio within both the CD4+ and CD8+ thymocyte subsets, the presence of double-positive cells was not expected to influence the comparisons of the fold changes in cytokine transcripts in the CD4+ and CD8+ populations.
RNA extraction
Total cellular RNA was prepared from the stimulated thymocyte samples using the RNeasy mini kit (Qiagen, Crawley, UK) following the manufacturer's instructions. Purified RNA was eluted in a final volume of 50 μl RNase-free water and stored at − 70°.
Real-time quantitative reverse transcription–polymerase chain reaction (RT-PCR)
All mRNA levels were quantified by real-time quantitative RT-PCR based on the method described by Kaiser et al.5 RNA samples from stimulated and unstimulated thymocytes were assayed for mRNA encoding β2M and MHC IA, and the cytokines IL-1β, IL-2, IFN-α, IFN-β, IFN-γ and TGF-β4.
Primers and probes were designed using the primer express software program (PE Applied Biosystems, Foster City, CA). Details of the probes and primers are given in Table 1. As far as possible, all probes were designed, from the sequence of the relevant genes, to lie across intron : exon boundaries. All probes were labelled with the fluorescent reporter dye 5-carboxyfluorescein (FAM) at the 5′ end and the quencher N,N,N,N′-tetramethyl-6-carboxyrhodamine (TAMRA) at the 3′ end.
Table 1.
Real-time quantitative RT-PCR probes and primers
| RNA target | Probe/primer sequence | Accession number | Exon boundaries* | |
|---|---|---|---|---|
| 28S | Probe | (FAM)-AGGACCGCTACGGACCTCCACCA-(TAMRA) | ||
| F | GGCGAAGCCAGAGGAAACT | |||
| R | GACGACCGATTTGCACGTC | |||
| IFN-α | Probe | (FAM)-CTCAACCGGATCCACCGCTACACC-(TAMRA) | U07868 | – |
| F | GACAGCCAACGCCAAAGC | |||
| R | GTCGCTGCTGTCCAAGCATT | |||
| IFN-β | Probe | (FAM)-TTAGCAGCCCACACACTCCAAAACACTG-(TAMRA) | X92479 | – |
| F | CCTCCAACACCTCTTCAACATG | |||
| R | TGGCGTGCGGTCAAT | |||
| IFN-γ | Probe | (FAM)-TGGCCAAGCTCCCGATGAACGA-(TAMRA) | Y07922 | 3/4 |
| F | GTGAAGAAGGTGAAAGATATCATGGA | |||
| R | GCTTTGCGCTGGATTCTCA | |||
| IL-1β | Probe | (FAM)-CCACACTGCAGCTGGAGGAAGCC-(TAMRA) | AJ245728 | 5/6 |
| F | GCTCTACATGTCGTGTGTGATGAG | |||
| R | TGTCGATGTCCCGCATGA | |||
| IL-2 | Probe | (FAM)-ACTGAGACCCAGGAGTGCACCCAGC-(TAMRA) | AJ009800 | 2/3 |
| F | TTGGAAAATATCAAGAACAAGATTCATC | |||
| R | TCCCAGGTAACACTGCAGAGTTT | |||
| TGF-β4 | Probe | (FAM)-ACCCAAAGGTTATATGGCCAACTTCTGCAT-(TAMRA) | M31160 | 6/7 |
| F | AGGATCTGCAGTGGAAGTGGAT | |||
| R | CCCCGGGTTGTGTTGGT | |||
| β2M | Probe | (FAM)-TCTTCAGACCGTGCTCATCCCGG-(TAMRA) | Z48921 | 2/3/4 |
| F | CTACAAGTGGGATCCCGAGTTC | |||
| R | TCATTTCAACTTGGAATGCAGAA | |||
| MHC IA | Probe | (FAM)-ACCGGCGGGTCTCACACAGTGC-(TAMRA) | M84766 | 2/3 |
| F | CAGCGGCGCTACAACCA | |||
| R | GATGTCACAGCCGTACATCCA |
There are 10 intronless IFN-α genes, with a high level of sequence identity, and a single intronless IFN-β gene.
The RT-PCR assay used the TaqMan EZ RT-PCR kit (PE Applied Biosystems). Amplification and detection of specific products was achieved with the ABI PRISM 7700 Sequence Detection System (PE Applied Biosystems) with the following cycle profile: 50° for 2 min, 96° for 5 min, 60° for 30 min and 95° for 5 min, and 40 cycles of 94° for 20 s and 59° for 1 min.
Quantification was based on the increased fluorescence detected by the ABI PRISM 7700 Sequence Detection System as a result of hydrolysis of the target-specific probes by the 5′ nuclease activity of the rTth DNA polymerase during PCR amplification. The passive reference dye ROX, which was not involved in amplification, was used to correct for fluctuations in fluorescence resulting from changes in the reaction conditions and for normalization of the reporter signal. Results were expressed in terms of the threshold cycle value (Ct), the cycle at which the change in the reporter dye (ΔRn) passed a significance threshold. In this study, the threshold values of ΔRn for all reactions described were as shown in Table 2.
Table 2.
Optimized primer concentrations, slope of the regression line and R2 statistics for all real-time, quantitative RT-PCR assays
| Target | Primer concn (μm) | ΔRn significance threshold | Ct | R2 | Gradient |
|---|---|---|---|---|---|
| IFN-α | 0·1 | 0·03 | 15–40 | 0·9898 | 3·95 |
| IFN-β | 1·0 | 0·02 | 15–40 | 0·9941 | 3·016 |
| IFN-γ | 0·6 | 0·03 | 15–40 | 0·9995 | 3·135 |
| IL-1β | 0·4 | 0·02 | 15–40 | 0·9999 | 3·408 |
| IL-2 | 0·6 | 0·02 | 15–40 | 0·989 | 2·748 |
| TGF-β4 | 0·1 | 0·04 | 15–40 | 0·9893 | 3·589 |
| β2M | 1·0 | 0·01 | 15–40 | 0·9959 | 3·442 |
| MHC IA | 1·0 | 0·01 | 15–40 | 0·9916 | 3·213 |
ΔRn = change in the reporter dye; Ct = threshold cycle value, the cycle at which the change in the reporter dye levels detected passes the ΔRn; R2 = coefficient of regression.
Standard curves were established for all RT-PCR assays using a 10-fold dilution series of an RNA standard. With the exception of IFN-β, the RNA standard for all assays was total RNA extracted from stimulated chicken splenocytes. For IFN-β, RNA was extracted from COS-7 cells transiently transfected with a pcDNA.1::chIFN-β clone.6 Each RT-PCR experiment contained three controls without template, test samples and a 10-fold dilution series of RNA standards. Each experiment was performed in triplicate, with replicates performed on different days. Regression analysis of the mean values of six replicate RT-PCRs for the log10 diluted RNA was used to generate standard curves.
Statistical analysis
To control for variation in cell numbers, sampling and RNA preparation the Ct values were standardized against the Ct value for the internal standard 28S rRNA. For each sample a constant calculated from the mean 28S Ct value was divided by the overall mean of the 28S Ct value. Then, 40 − Ct values for each sample were divided by this constant and multiplied by a ratio of the gradients of the assay and 28S standard curves; i.e. quantification of sample cytokine transcript = (40 − Ct) × [m(cytokine standard)/m(28S standard)]/[μ Ct(sample 28S)/[μ Ct(overall 28S)], where m = gradient and μ = mean.
Fold changes in cytokine transcription were then calculated from the difference between stimulated and unstimulated thymocyte populations. Analysis of difference between means was carried out using a one-way analysis of variance (anova). Where a level of significance was found, a Tukey's test was then conducted on the sample data to determine the relative difference between the means within each set of data. Where there were only two groups of data, means were compared using a paired Student's t-test.
Results
Thymocyte populations
The mean number of cells obtained from the thymus was 1·5 × 106 for E14 embryos, 3·8 × 109 for E18 embryos and 1·4 × 1010 for D1 chicks. A total of 3 × 107 thymocytes were recovered from 15 E14 embryos, and 5 × 1010 and 6 × 1010 thymocytes were recovered from five E18 embryos and five D1 chicks, respectively. Lymphocytes constituted 98–99% of all thymocyte preparations and 99% of these were viable as assessed by dye exclusion. The mean proportion of CD4+ thymocytes at E14 was 52% and the mean proportion of CD8+ thymocytes was 44%. The mean proportion of CD4+ thymocytes at E18 was 50% and the mean proportion of CD8+ thymocytes was 46%. The mean proportion of CD4+ thymocytes at D1 was 51% and the mean proportion of CD8+ thymocytes was 46%.
The CD4+ lymphocytes from E18 chicks constituted 96% of the preparations sorted on the basis of CD4+ using MiniMACS, and CD8+ lymphocytes constituted 97% of the preparations sorted on the basis of CD8+ using MiniMACS. These populations were then sorted by FACS to obtain 99–100% pure populations. The proportion of CD4+ and CD8+ lymphocytes in the thymus preparations that were TCR− was 3–5%.
Proliferation assays
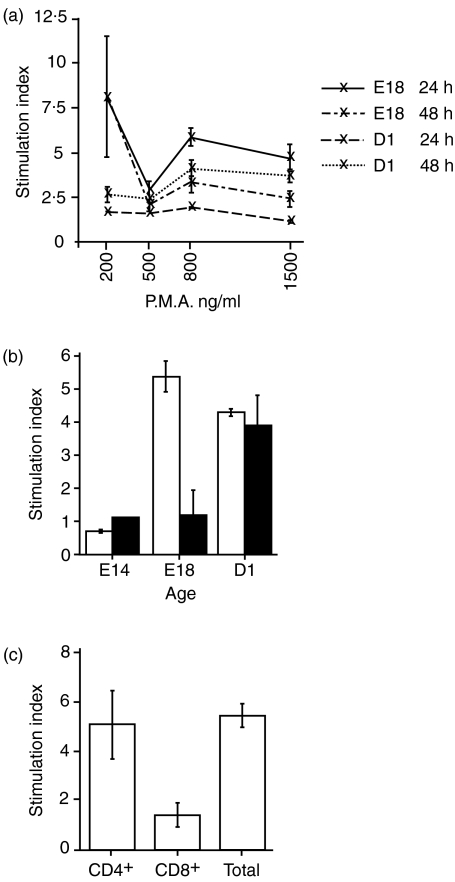
Mitogen concentrations and incubation periods were optimized using thymocytes from E18 embryos and D1 chicks. Optimal stimulation conditions for E18 thymocytes were for 24 hr in the presence of PMA at 200 ng/ml, AV7 at 1 μg/ml and ionomycin at 50 ng/ml (Fig. 1a), and stimulation for 48 hr in the presence of PMA at 800 ng/ml, AV7 at 1 μg/ml and ionomycin at 50 ng/ml for D1 thymocytes. Using optimized mitogen concentrations and 24 hr of stimulation, the effect of embryonic age on the ability of thymocytes to proliferate was investigated (Fig. 1b). In stimulated E14 thymocytes the stimulation index was 0·7 at 24 hr. Unstimulated thymocytes from E14 embryos had a very high background rate of proliferation based on incorporation of radiolabel. Thymocytes from E14 embryos responded to mitogen stimulation with a rapid burst of proliferation over 12–18 hr (data not shown) which was not sustained and was followed by slowed growth from 18 to 48 hr (Fig. 1b). The rapid response to stimulation over the initial 18-hr period was indicated by an acidic change in the culture medium and a sharp increase in cell numbers. Proliferation following stimulation, as measured by a [3H]thymidine pulse between 18 and 24 hr, was lower than the background proliferation in unstimulated controls. Stimulation caused increased proliferation in thymocytes from E18 embryos and D1 chicks relative to unstimulated controls, significantly higher than in thymocytes from E14 embryos.
Figure 1.
Proliferation of thymocytes in response to stimulation with anti-CD28 mAb, ionomycin and phorbol 12-myristate 13-acetate (PMA). (a) Proliferation of thymocytes from E18 embryos and D1 chicks following stimulation with different PMA concentrations for 24 and 48 hr. Mean counts per minute (c.p.m.) for thymocytes from the E18 embyros at 24 hr were 177 c.p.m. (unstimulated), 1839 c.p.m. (200 ng PMA/ml), 504 c.p.m. (500 ng PMA/ml), 957 c.p.m. (800 ng PMA/ml) and 741 c.p.m. (1500 ng PMA/ml), and from the E18 embyros at 48 hr were 48 c.p.m. (unstimulated), 384 c.p.m. (200 ng PMA/ml), 92 c.p.m. (500 ng PMA/ml), 143 c.p.m. (800 ng PMA/ml) and 121 c.p.m. (1500 ng PMA/ml). Mean c.p.m. for thymocytes from the D1 chicks at 24 hr were 1758 c.p.m. (unstimulated), 2080 c.p.m. (200 ng PMA/ml), 2429 c.p.m. (500 ng PMA/ml), 2243 c.p.m. (800 ng PMA/ml) and 1977 c.p.m. (1500 ng PMA/ml), and from the D1 chicks at 48 hr were 182 c.p.m. (unstimulated), 516 c.p.m. (200 ng PMA/ml), 391 c.p.m. (500 ng PMA/ml), 821 c.p.m. (800 ng PMA/ml) and 594 c.p.m. (1500 ng PMA/ml). (b) Effect of developmental stage on the ability of thymocytes to proliferate following stimulation with 200 ng PMA/ml for 24 and 48 hr. Mean c.p.m. for thymocytes from the E14 embyros at 24 hr were 9722 c.p.m. (unstimulated) and 6815 c.p.m. (200 ng PMA/ml), and from the E14 embyros at 48 hr were 5146 c.p.m. (unstimulated) and 5774 c.p.m. (200 ng PMA/ml). Mean c.p.m. for thymocytes from the E18 embyros at 24 hr were 139 c.p.m. (unstimulated), 780 c.p.m. (200 ng PMA/ml), and from the E18 embyros at 48 hr were 875 c.p.m. (unstimulated) and 981 c.p.m. (200 ng PMA/ml). Mean c.p.m. for thymocytes from the D1 chicks at 24 hr were 163 c.p.m. (unstimulated) and 780 c.p.m. (200 ng PMA/ml), and from the D1 chicks at 48 hr were 875 c.p.m. (unstimulated) and 981 c.p.m. (200 ng PMA/ml). Proliferation at 24 hr is shown as unshaded columns, and at 48 hr as shaded columns. (c) Proliferation of CD4+ TCR+ and CD8+ TCR+ thymocyte subsets from E18 embryos stimulated with 200 ng PMA/ml for 24 hr. Thymocytes with a double-positive phenotype are present in both populations. Mean c.p.m. for the CD4+ TCR+ thymocytes were 148 c.p.m. (unstimulated) and 736 c.p.m. (200 ng PMA/ml), for the CD4+ TCR+ thymocytes were 403 cpm (unstimulated) and 607 c.p.m. (200 ng PMA/ml), and for unsorted thymocytes were 150 c.p.m. (unstimulated) and 828 c.p.m. (200 ng PMA/ml).
Proliferation was rapidly induced in thymocytes from E18 embryos and was sustained for a 24-hr period (Fig. 1b). The proliferative response was reduced to marginally higher than non-stimulated cells by 48 hr after stimulation (Fig. 1b). Rapid induction of proliferation was seen in thymocytes from D1 chicks and the response was sustained for the entire 48 hr incubation period (Fig. 1b).
The proliferative response of sorted CD4+ TCR+ thymocytes was significantly greater than that seen for sorted CD8+ TCR+ thymocytes, and was not significantly different from the proliferation observed for the total mixed thymocyte population from E18 embryos (Fig. 1c).
Standardization of real-time quantitative RT-PCR assays
Real-time quantitative RT-PCR assays were developed for IFN-α, IFN-β, TGF-β4, MHC IA and β2M probes. A linear relationship between RNA concentration and signal was established for 10-fold dilution series of RNA standards for each assay. Correlation coefficients and gradients for regression lines for each assay are given in Table 2.
Constitutive expression of cytokine, MHC IA and β2M mRNA in unstimulated thymocytes
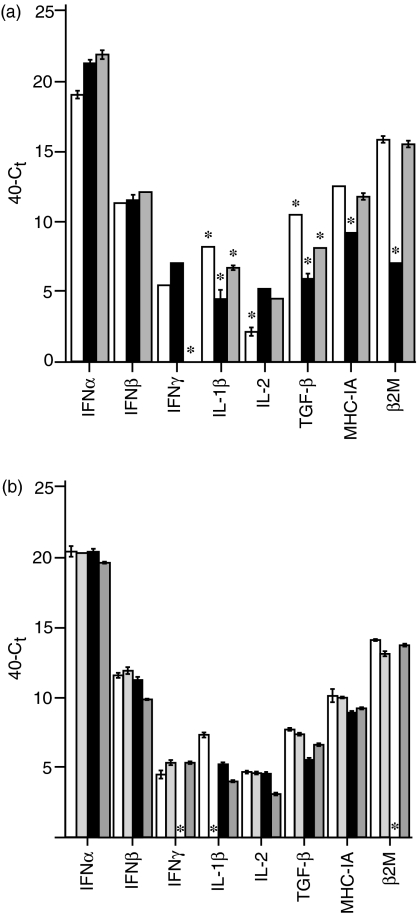
IFN-α, IFN-β, IFN-γ, IL-1β, IL-2, TGF-β4, MHC IA and β2M transcripts were constitutively expressed in all thymocyte samples tested, with the exception of IFN-γ mRNA which was not detected in thymocytes from D1 chicks (Fig. 2a). Unstimulated thymocytes from E14 embryos expressed significantly lower levels of IL-2, and higher levels of IL-1β and TGF-β4, than unstimulated thymocytes from E18 embryos or D1 chicks. Expression of both MHC IA and β2M transcripts was significantly lower in thymocytes from E18 embryos than in thymocytes from E14 embryos or D1 chicks.
Figure 2.
Constitutive transcription of IFN-α, IFN-β, IFN-γ, IL-1β, IL-2, TGF-β4, MHC IA and β2M in unstimulated thymocytes. Transcript is quantified as 40–Ct and standardized against a 28S RNA standard. Significant differences in measured transcript are indicated by a star above the sample. (a) Transcript levels measured at E14 (unshaded), E18 (black shading) and D1 (grey shading). (b) Transcript levels in CD4+ TCR+ (unshaded), CD8+ TCR+ (light grey), CD4+ TCR− (black) and CD8+ TCR− (dark grey) subsets at E18.
Thymocyte subset populations from E18 embryos did not differ significantly in their relative levels of cytokine or MHC expression (Fig. 2b), with the exception of the lack of detectable IFN-γ or β2M mRNA in the CD4+ TCR− population, and of IL-1β mRNA in the CD8+ TCR+ population.
Induction of MHC IA and β2M transcription in response to mitogen activation
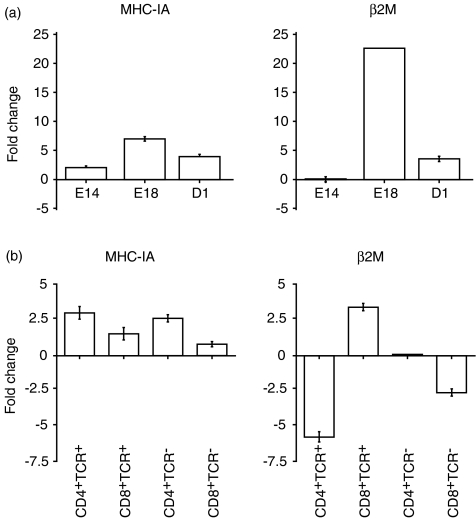
MHC IA expression was up-regulated in thymocytes from all developmental stages studied (E14, E18 and D1) following mitogen stimulation, with maximal up-regulation in thymocytes from E18 embryos (Fig. 3a). A similar pattern was seen for β2M, in that its expression was also up-regulated following mitogen stimulation in thymocytes from E18 embryos and D1 chicks, again with maximal up-regulation at E18. However, mitogen stimulation of thymocytes from E14 embryos had no effect on β2M transcription, compared to unstimulated controls.
Figure 3.
Fold change in MHC IA and β2M transcript levels following stimulation of thymocytes with anti-CD28 mAb, ionomycin and PMA. (a) Fold change in thymus at E14, E18 and D1. (b) Fold change in CD4+ TCR+, CD4+ TCR−, CD8+ TCR+ and CD8+ TCR− thymocyte subsets at E18.
Thymocyte subset populations from E18 embryos all up-regulated MHC IA expression following stimulation (Fig. 3b), to a greater degree in the CD4+ subsets than the CD8+ subsets. In the TCR+ subsets, β2M expression was up-regulated in CD8+ thymocytes, but down-regulated in CD4+ thymocytes, compared to unstimulated controls. In the TCR− subsets, β2M expression was down-regulated in CD8+ thymocytes compared to unstimulated controls, but was not detected in CD4+ thymocytes (either stimulated or unstimulated).
Induction of cytokine gene transcription in response to mitogen activation
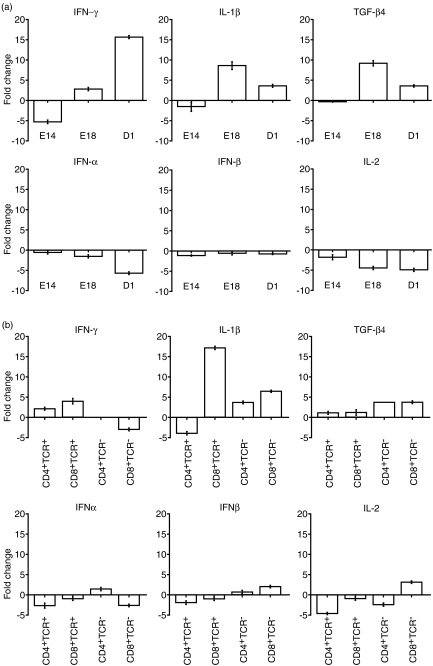
The cytokine transcripts IFN-γ, IL-1β and TGF-β4 were up-regulated in E18 and D1, stimulated, unsorted thymocytes. The cytokine transcripts IFN-α, IFN-β and IL-2 were down-regulated in stimulated, unsorted thymocytes in all age groups.
IFN-γ expression was down-regulated in stimulated thymocytes from E14 embryos, and up-regulated in stimulated thymocytes from E18 embryos and D1 chicks, with maximal up-regulation in the latter. IL-1β and TGF-β4 transcript was essentially unchanged in stimulated thymocytes from E14 embryos, was up-regulated in stimulated thymocytes from E18 embryos and D1 chicks, with maximal up-regulation in stimulated E18 thymocytes.
The degrees of down-regulation of IFN-α and IL-2 expression after stimulation increased with increasing embryonic age. In contrast, the down-regulation of IFN-β expression was of a similar degree at all developmental stages.
The pattern of changes in cytokine gene expression after stimulation in thymocyte subset populations from E18 embryos was more complex (Fig. 4b). IFN-γ expression was up-regulated in the TCR+ subsets and down-regulated in the CD8+ TCR− subset following mitogen stimulation, compared to unstimulated controls, but was not detected in the CD4+ TCR− subset (either stimulated or unstimulated). IL-1β expression was up-regulated in both CD8+ subsets, and in the CD4+ TCR− subset, but down-regulated in the CD4+ TCR+ subset. TGF-β4 expression was up-regulated in all subsets, but to a greater degree in the TCR− subsets than in the TCR+ subsets.
Figure 4.
Fold change in IFN-α, IFN-β, IFN-γ, IL-1β, IL-2 and TGF-β4 transcript levels following stimulation of thymocytes with anti-CD28 mAb, ionomycin and PMA. (a) Fold changes in thymus at E14, E18 and D1. (b) Fold changes in CD4+ TCR+, CD4+ TCR−, CD8+ TCR+ and CD8+ TCR− thymocyte subsets at E18.
IFN-α expression was down-regulated in both CD8+ subsets, and in the CD4+ TCR+ subset, but was up-regulated in the CD4+ TCR− subset. IFN-β expression was down-regulated in both TCR+ subsets, but was up-regulated in both TCR− subsets. IL-2 expression was down-regulated in both CD4+ subsets, and in the CD8+ TCR+ subset, but was up-regulated in the CD8+ TCR− subset.
Discussion
In this study we have demonstrated differences in chicken thymocyte responses related to developmental stage. Whilst the use of cell surface markers to characterize the sequential waves of thymocyte populations in the developing thymus has been described previously in the literature, the functional capacity of these cells has not been established. Our studies have identified differences in proliferation and cytokine induction in response to mitogen stimulation in the thymus of embryonic chickens at E14, E18 and D1. Only the group of Th2 cytokines was not examined, but there is, as yet, no evidence for these in any non-mammalian species.
The approach used did not remove CD4+ CD8+ double-positive thymocytes from the CD4+ and CD8+ subsets. However, as there are equal numbers of CD4+ CD8+ double-positive thymocytes in each of these subsets, the background contribution of double-positives would not be expected to influence the direct comparison of the CD4+ and CD8+ subsets. Therefore the conclusions drawn about the behaviour of single-positive cells are valid. The CD4+ CD8+ double-positive thymocytes are an intermediate stage in development. Thus, although our study was unable to assay these double-positive thymocytes, by comparing the TCR− cells, which are the very early thymocytes, and the single positives, which are the more mature thymocytes, our study has examined the responses of cells at either end of thymocyte development.
Thymocytes at different stages of embryonic development vary to a greater extent in the duration over which the proliferative response is maintained, rather than the absolute magnitude of the proliferative response. Proliferation in vitro at 24 hr in response to mitogen was not significantly different in magnitude between thymocyte populations at E18 and D1. However, the duration of the mitogen-induced stimulation was found to differ with developmental age. Thymocytes at D1 were induced to proliferate over the 48 hr duration of the assay, whereas at E18 thymocyte proliferation was only sustained for 24 hr. The earliest thymocytes, at E14, had a high background proliferation rate and, in response to mitogen, a rapid response to stimulation that was only maintained over a limited 12–18-hr period. The poor proliferative responses in E14 thymocytes may be associated with either thymocyte immaturity or, alternatively, may be a characteristic of the dominant subpopulation of progenitor TCR1+ (γδ) thymocytes populating the thymus at this stage, as mature γδ cells also exhibit low proliferation in response to mitogen.7 Proliferation in vitro at 24 hr in response to mitogen in sorted CD4+ thymocytes was significantly greater than in CD8+ thymocytes and was not significantly different to mixed total thymocytes. These findings are consistent with a requirement of CD8+ thymocytes for CD4+ thymocyte helper function.
Thymocytes were stimulated with a combination of PMA, ionomycin and an anti-CD28 mAb. PMA remains active in culture medium over an extended period, providing the prolonged mitogenic stimulus required for sustained T-lymphocyte proliferation.8–10 PMA combined with ionomycin and an anti-CD28 mAb induces proliferation in mature avian and mammalian T lymphocytes, leading in mammals to IL-2 and IL-2 receptor expression, and subsequent commitment to activation and clonal expansion through the autocrine actions of IL-2.8–15 In mammals (and presumably in chickens) PMA directly activates the protein kinase C second messenger system, bypassing the TCR. The observed variations in stimulation are therefore not attributable to differences in TCR cell surface expression between immature and mature thymocytes, or between TCR subpopulations. The short duration of the response to mitogen in thymocytes at E14 suggests initial activation of the protein kinase C signalling cascade, with either inhibition of the response by 12–18 hr, or failure of a second signal required for sustained proliferation beyond 12 hr. The down-regulation of IL-2, by an as yet unknown mechanism, in all embryonic thymocyte subsets is consistent with the absence of an autocrine signal, believed to be required for sustained activation and proliferation in mature thymocytes.16
By using highly sensitive real-time quantitative RT-PCR assays constitutive expression of transcripts was measured. Constitutive expression of transcripts was demonstrated for all cytokines tested in unsorted thymocytes at all developmental stages (with the exception of IFN-γ in D1 thymocytes), and in most thymic subsets at E18 (Fig. 2). The role of constitutive expression may be to maintain a thymic milieu that promotes a normal developmental progression. High levels of expression of TGF-β in the mammalian embryonic thymus has prompted the suggestion that it is a key modulator of developmental progression.17 TGF-β was also constitutively expressed at high levels in the chicken thymus in all embryonic populations (Fig. 2), and the magnitude of the expression was significantly greater in the very early thymocyte population at E14. As in mammals the type I interferons, IFN-α and IFN-β, were also found to have high constitutive expression levels.18
In the human and mouse, both mature and immature thymocyte populations respond to mitogen stimulation with the transcriptional up-regulation of IL-2 and IL-2 receptor.19 We found that immature TCR− chicken thymocytes at E14, E18 and D1, and all thymocyte subsets at E18, respond to mitogen stimulation with transcriptional down-regulation of IL-2. Down-regulation of transcript production indicates a functional down-regulation of IL-2 activity, as IL-2 transcription and translation are co-ordinated.20 In addition, mitogen activation stimulated transcriptional up-regulation of TGF-β and IFN-γ in thymocytes at E18 and D1. These findings therefore point to a fundamental difference in the capacity for thymocyte activation during development between chickens and mammals.
TGF-β is secreted as an inactive propeptide and transcription does not necessarily correlate with active protein.21 TGF-β has an immunomodulatory influence on lymphocyte cell cycle progression and its effects are extremely contextual21,22 with inhibitory effects on mature lymphocytes and stimulatory effects on the growth of immature lymphocytes. It prolongs the S phase and delays progression into the G1 phase of the cell cycle, thereby modulating proliferation.23,24 Previous studies in the chicken have demonstrated that TGF-β can enhance growth of CD4+ CD8+ thymocytes.25 However, these thymocytes, although possessing an immature phenotype, were isolated from 4-week-old-chickens. This study is the first to examine the embryonic thymus itself. Consistent with an enhanced role for TGF-β in immature thymocytes, its induction in response to mitogen stimulation in TCR− populations at E18 was 3·5-fold compared to a 1·1-fold induction in TCR+ E18 thymocytes (Fig. 4b). IFN-γ transcription was induced in stimulated thymocytes at E18 and D1 but not at E14. IFN-γ can also slow the progress of the cell cycle and modulate proliferation.18 In mammals, both TGF-β and IFN-γ can lead to down-regulation of IL-2 transcription. The observed IL-2 down-regulation in stimulated cells in this study is therefore consistent with the observed up-regulation of IFN-γ and TGF-β in stimulated cells.
Interestingly IL-1β is up-regulated in unsorted thymocytes at E18 and D1, and in all TCR− and CD8+ TCR+ thymocytes, but not CD4+ TCR+ thymocytes, at E18. IL-1β is translated as a propeptide requiring proteolytic cleavage by caspase 1 for activation.26,27 Therefore, as with TGF-β, IL-1β transcript does not necessarily correlate with functional protein. IL-1β mediates transcriptional up-regulation of IFN-β in mammalian fibroblast lines.28,29 IFN-β expression was concurrently up-regulated in the TCR− populations alone. This perhaps suggests that IFN-β up-regulation is specific to very early immature TCR− thymocytes, and, in common with fibroblasts, is a downstream effect of IL-1β.
The cytokine gene transcript profile in thymocytes at E14 was markedly different to those for E18 and D1. IL-2, IL-1β, IFN-α, IFN-β and IFN-γ were transcriptionally down-regulated in response to mitogen (Fig. 4a). In combination with the short duration of the response to stimulation that was observed, these results suggest E14 thymocytes are largely unresponsive to mitogen.
Mature CD8+ MHC class I restricted lymphocytes respond to activation by up-regulating cell surface expression of MHC class I. Thymocytes at E14, E18 and D1 responded to mitogen with an up-regulation of MHC class I α-chain transcript. The magnitude of this response at E18 was greater in CD4+ as opposed to CD8+ thymocytes. IFN-γ and TGF-β up-regulate MHC class I expression in mammals.30 It is doubtful that MHC IA up-regulation was the result of the observed up-regulation of IFN-γ and TGF-β at E18 and D1, as MHC IA was also up-regulated at E14 where these cytokines were down-regulated. The pattern of β2M transcription following mitogen stimulation is quite distinct from that of the globally up-regulated MHC IA transcript. Up-regulation of β2M transcription was observed at E18 and D1 but not at E14, and, when examined in thymocyte subsets, up-regulation was found to be specific to the CD8+ TCR+ thymocyte population. These findings suggest that at the level of transcription it is the tight control of β2M in the embryonic thymocyte, rather than of MHC IA, that regulate MHCI expression. However, all embryonic thymocytes constitutively expressed MHC IA and β2M transcripts.
Our studies did not examine the signalling pathways that might control cytokine, MHC IA and β2M expression. Such studies are currently not possible in chickens as there are no published reports of chicken signal transducers and activators of transcription (STATs), and only a couple of expressed sequence tags in the databases with homology to mammalian STATs and only one complete chicken Jak sequence in the databases. The reports of STAT activity in chicken cells in vitro have relied on cross-reactive anti-mammalian STAT antibodies, which have not been definitively shown to recognize the same molecules in the chicken.
The balance of responses in the embryonic thymus suggests that at all stages thymocytes have a reduced capacity for activation in comparison to mature thymocyte populations. At E14 thymocytes are least responsive to functional activation and differences exist even between thymocyte populations at E18 and D1. The results of this study indicate that the pathways stimulated by mitogen are tightly regulated in the embryonic thymus, and the cytokine data point to TGF-β and the type I interferons as likely mediators of this regulation. These results are of particular relevance to evaluation of the efficacy of in ovo immunotherapies and vaccines in chickens, as in ovo vaccinations are typically given at E18. The administration of vaccine antigens in ovo is unlikely to induce a strong anamnestic immune response, and the thymus may be refractory to adjuvant stimulation because of the tight regulation of proliferation during development. Further studies are required to examine the capacity of IL-2 administered as a cytokine adjuvant to override the down-regulation of IL-2 autocrine activity observed in this study.
Acknowledgments
The pcDNA.1::IFN-β clone was kindly supplied by Professor Peter Staeheli. This work was funded in part by a grant from the Rural Industries Research and Development Corporation. M.P. was supported by an Australian Postgraduate Award, a Rural Industries Research and Development Corporation Scholarship, a Barrenger Scholarship and a Melbourne University Postgraduate Overseas Research Experience Scholarship.
References
- 1.Cooper MD, Chen CL, Bucy RP, Thompson CB. Avian T cell ontogeny. Adv Immunol. 1991;50:87–117. doi: 10.1016/s0065-2776(08)60823-8. [DOI] [PubMed] [Google Scholar]
- 2.Lampisuo M, Liippo J, Vainio O, McNagny KM, Kulmala J, Lassila O. Characterization of prethymic progenitors within the chicken embryo. Int Immunol. 1999;11:63–9. doi: 10.1093/intimm/11.1.63. [DOI] [PubMed] [Google Scholar]
- 3.Marth JD, Ong CJ, Chui D. Specific CD45 isoforms regulate T cell ontogeny and are functionally distinct in modifying immune activation. Adv Exp Med Biol. 1994;365:149–66. doi: 10.1007/978-1-4899-0987-9_16. [DOI] [PubMed] [Google Scholar]
- 4.Vainio O, Imhof BA. The immunology and developmental biology of the chicken. Immunol Today. 1995;16:365–70. doi: 10.1016/0167-5699(95)80002-6. [DOI] [PubMed] [Google Scholar]
- 5.Kaiser P, Rothwell L, Galyov EE, Barrow PA, Burnside J, Wigley P. Differential cytokine expression in avian cells in response to invasion by Salmonella typhimurium, Salmonella enteritidis and Salmonella gallinarum. Microbiology. 2000;146:3217–26. doi: 10.1099/00221287-146-12-3217. [DOI] [PubMed] [Google Scholar]
- 6.Sick C, Schultz U, Staeheli P. A family of genes coding for two serologically distinct chicken interferons. J Biol Chem. 1996;271:7635–9. doi: 10.1074/jbc.271.13.7635. [DOI] [PubMed] [Google Scholar]
- 7.Arstila TP, Toivanen P, Lassila O. Helper activity of CD4+ alpha beta T cells is required for the avian gamma delta T cell response. Eur J Immunol. 1993;23:2034–7. doi: 10.1002/eji.1830230848. [DOI] [PubMed] [Google Scholar]
- 8.Berry N, Ase K, Kishimoto A, Nishizuka Y. Activation of resting human T cells requires prolonged stimulation of protein kinase C. Proc Natl Acad Sci U S A. 1990;87:2294–8. doi: 10.1073/pnas.87.6.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kraft AS, Anderson WB. Phorbol esters increase the amount of Ca2+, phospholipid-dependent protein kinase associated with plasma membrane. Nature. 1983;301:621–3. doi: 10.1038/301621a0. [DOI] [PubMed] [Google Scholar]
- 10.Quest AF, Bardes ES, Bell RM. A phorbol ester binding domain of protein kinase C gamma. High affinity binding to a glutathione-S-transferase/Cys2 fusion protein. J Biol Chem. 1994;269:2953–60. [PubMed] [Google Scholar]
- 11.Asaoka Y, Oka M, Yoshida K, Nishizuka Y. Metabolic rate of membrane-permeant diacylglycerol and its relation to human resting T-lymphocyte activation. Proc Natl Acad Sci USA. 1991;88:8681–5. doi: 10.1073/pnas.88.19.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bell RM, Burns DJ. Lipid activation of protein kinase C. J Biol Chem. 1991;266:4661–4. [PubMed] [Google Scholar]
- 13.Beverly B, Kang SM, Lenardo MJ, Schwartz RH. Reversal of in vitro T cell clonal anergy by IL-2 stimulation. Int Immunol. 1992;4:661–71. doi: 10.1093/intimm/4.6.661. [DOI] [PubMed] [Google Scholar]
- 14.De la Hera A, Toribio ML, Marcos MA, Marquez C, Martinez C. Interleukin 2 pathway is autonomously activated in human T11+3–4−6–8– thymocytes. Eur J Immunol. 1987;17:683–7. doi: 10.1002/eji.1830170516. [DOI] [PubMed] [Google Scholar]
- 15.Schorle H, Holtschke T, Hunig T, Schimpl A, Horak I. Development and function of T cells in mice rendered interleukin-2 deficient by gene targeting. Nature. 1991;352:621–4. doi: 10.1038/352621a0. [DOI] [PubMed] [Google Scholar]
- 16.Wilson A, Corthesy P, Reichenbach P, MacDonald HR, Nabholz M. Interleukins (IL) -1 and IL-2 control IL-2 receptor alpha and beta expression in immature thymocytes. Eur J Immunol. 1994;24:1729–35. doi: 10.1002/eji.1830240802. [DOI] [PubMed] [Google Scholar]
- 17.Schluns KS, Grutkoski PS, Cook JE, Engelmann GL, Le PT. Human thymic epithelial cells produce TGF-beta 3 and express TGF-beta receptors. Int Immunol. 1995;7:1681–90. doi: 10.1093/intimm/7.10.1681. [DOI] [PubMed] [Google Scholar]
- 18.Montgomery RA, Dallman MJ. Semi-quantitative polymerase chain reaction analysis of cytokine and cytokine receptor gene expression during thymic ontogeny. Cytokine. 1997;9:717–26. doi: 10.1006/cyto.1997.0227. [DOI] [PubMed] [Google Scholar]
- 19.Willerford DM, Chen J, Ferry JA, Davidson L, Ma A, Alt FW. Interleukin-2 receptor alpha chain regulates the size and content of the peripheral lymphoid compartment. Immunity. 1995;3:521–30. doi: 10.1016/1074-7613(95)90180-9. [DOI] [PubMed] [Google Scholar]
- 20.Rothwell L, Hamblin A, Kaiser P. Production and characterisation of monoclonal antibodies specific for chicken interleukin-2. Vet Immunol Immunopathol. 2001;83:149–60. doi: 10.1016/s0165-2427(01)00391-9. [DOI] [PubMed] [Google Scholar]
- 21.Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Annu Rev Immunol. 1998;16:137–61. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- 22.Burt DW, Jakowlew SB. Correction: a new interpretation of a chicken transforming growth factor-beta 4 complementary DNA. Mol Endocrinol. 1992;6:989–92. doi: 10.1210/mend.6.6.1353860. [DOI] [PubMed] [Google Scholar]
- 23.Schluns KS, Cook JE, Le PT. TGF-beta differentially modulates epidermal growth factor-mediated increases in leukemia-inhibitory factor, IL-6, IL-1 alpha, and IL-1 beta in human thymic epithelial cells. J Immunol. 1997;158:2704–12. [PubMed] [Google Scholar]
- 24.Dupuy d'Angeac A, Reme T, Monier S, Gao Q, Duperray C, Jullien P, Dornand J. Contrasting effect of transforming growth factor type beta 1 (TGF-beta 1) on proliferation and interleukin-2 receptor expression in activated and rapidly cycling immature (CD3–CD4–CD8–) thymocytes. J Cell Physiol. 1993;154:44–52. doi: 10.1002/jcp.1041540107. [DOI] [PubMed] [Google Scholar]
- 25.Mukamoto M, Kodama H. Regulation of early chicken thymocyte proliferation by transforming growth factor-beta from thymic stromal cells and thymocytes. Vet Immunol Immunopathol. 2000;77:121–32. doi: 10.1016/s0165-2427(00)00223-3. [DOI] [PubMed] [Google Scholar]
- 26.Dinarello CA. Interleukin-1 beta, interleukin-18, and the interleukin-1 beta converting enzyme. Ann N Y Acad Sci. 1998;856:1–11. doi: 10.1111/j.1749-6632.1998.tb08307.x. [DOI] [PubMed] [Google Scholar]
- 27.Fantuzzi G, Dinarello CA. Interleukin-18 and interleukin-1 beta: two cytokine substrates for ICE (caspase-1) J Clin Immunol. 1999;19:1–11. doi: 10.1023/a:1020506300324. [DOI] [PubMed] [Google Scholar]
- 28.Hissong BD, Carlin JM. Potentiation of interferon-induced indoleamine 2,3-dioxygenase mRNA in human mononuclear phagocytes by lipopolysaccharide and interleukin-1. J Interferon Cytokine Res. 1997;17:387–93. doi: 10.1089/jir.1997.17.387. [DOI] [PubMed] [Google Scholar]
- 29.Struyf S, Van Collie E, Paemen L, Put W, Lenaerts JP, Proost P, Opdenakker G, Van Damme J. Synergistic induction of MCP-1 and -2 by IL-1beta and interferons in fibroblasts and epithelial cells. J Leukoc Biol. 1998;63:364–72. doi: 10.1002/jlb.63.3.364. [DOI] [PubMed] [Google Scholar]
- 30.Giacomini P, Fisher PB, Duigou GJ, Gambari R, Natali PG. Regulation of class II MHC gene expression by interferons. insights into the mechanism of action of interferon (review) Anticancer Res. 1988;8:1153–61. [PubMed] [Google Scholar]