Abstract
Human FcγRIII (CD16) is a low-affinity receptor for immunoglobulin G (IgG). There are two different isoforms of this protein: CD16a (transmembranous, expressed on natural killer cells and on macrophages) and CD16b (glycosylphosphatidylinositol-linked, expressed on neutrophilic granulocytes in two allelic forms NA1 and NA2). Both forms of the protein have a variable glycosylation pattern. The NA1 allele of CD16B has four asparagine (N)-linked glycosylation sites. One of them (N163) is localized in the ligand-binding site of domain II. This site is shared by the NA2 allele and CD16A. To examine the functional role of the glycosylation we mutated the four glycosylation sites of the NA1 allele (N39, N75, N163, N170) into glutamine (Q). HEK293 cells were stably transfected with the single mutants and wild-type CD16 as control. We determined binding of human IgG to transfected cells using immunofluorescence studies with anti-human IgG antibody. Monomeric IgG bound to N163Q transfectants with higher affinity than to other transfectants, showing that glycosylation in N163 influences the affinity of CD16 to its ligand. In addition, preincubation of WT-CD16-transfected cells with Tunicamycin (an inhibitor of N-glycosylation) resulted in an increased binding of monomeric IgG whereas N163Q-CD16-transfected cells remained unaffected. Therefore, glycosylation in N163 is a mechanism of regulating affinity of FcγRIII to its ligand IgG.
Introduction
Receptors for the Fc part of immunoglobulins are expressed on the surface of many different cell types. They mediate phagocytosis, endocytosis, antibody-dependent cellular cytotoxicity and the release of inflammatory mediators to provide a link between the humoral and antibody-triggered cellular immune responses.1,2 Three classes of Fcγ receptors have been identified so far: a high-affinity receptor FcγRI (CD64) and two low-affinity receptors FcγRII (CD32) and FcγRIII (CD16).
Human CD16 is encoded by two genes, FcγRIIIA and FcγRIIIB. FcγRIIIa is a transmembranous isoform of the receptor3 expressed on the surface of natural killer (NK) cells, macrophages, a subset of monocytes and of T cells in association with dimers of the γ-chain of FcεRI4–8 and/or ζ-chain of T-cell receptors.9 FcγRIIIb is a glycosylphosphatidylinositol-anchored isoform restricted to neutrophils.9–12 FcγRIII plays a significant role in the clearance of immune complexes,13 antibody-dependent cellular cytotoxicity of K/NK cells,14–16 phagocytosis17 and antigen presentation.18
The immunoglobulin G (IgG)-binding site of FcγRIII has been well characterized as a discontinuous area on the membrane proximal extracellular domain of the receptor including the amino acids lysine162 and valine164.19 Co-crystallization experiments with soluble FcγRIII and the Fc-portion of human IgG confirmed this localization of the IgG-binding site.20,21
The NA1 allele of CD16B has four asparagine-linked glycosylation sites.4 The glycosylation pattern of all four sites is highly variable. One of the sites (N163) shared by the NA2 allele and CD16A is localized in the ligand-binding region of domain II of the receptor. This site is shared by the NA2 allele of CD16B and CD16A but not by the other Fc receptors. It has been shown that FcγIII receptors of natural killer cells and macrophages differ in their affinity to IgG because of cell type-specific glycosylation of the receptor.22
Since the glycosylation pattern of CD16 is highly variable, we hypothesized that the degree of glycosylation at the site N163 affects the affinity of CD16 to its ligand IgG. We mutated the four glycosylation sites of CD16B NA1 (N39, N75, N163 and N170) to glutamine (Q) to examine the functional role of these sites. HEK293 cells were stably transfected with the mutated cDNAs and wild-type CD16 as control. The transfected cells were used to determine the effect of the mutated sites on receptor function by analysing the binding of monomeric IgG to the cells.
Materials and methods
Mutagenesis
To mutate the asparagine (N)-linked glycosylation sites of CD16B NA1 into glutamine, a site-directed polymerase chain reaction (PCR) mutagenesis was used. The template cDNA originally described by Simmons et al.11 was provided by Dr A. Tamm (Tartu University Hospital, Tartu, Estonia), so the numbering of the amino acid sequence described by Tamm et al. 199619 was also used in this study. For each site two reactions with primers overlapping the desired amino acid exchange were performed: CD16 5′-ATG TGG CAG CTG CTC C, CD16 3′-GGG AGA TCT TTA ATG ATG ATG ATG ATG ATG TTG GTA CCC AGG TGG AG (introducing a His-Tag and a BglII restriction site), N39Q 5′-TCC CCT GAG GAC CAG TCC ACA CAG TGG and 3′-CCA CTG TGT GGA CTG GTC CTC AGG GGA, N75Q 5′-GGT GCC AGA CAC AGC TCT CCA CCC TC and 3′-GAG GGT GGA GAG CTG TGT CTG GCA CC, N163Q 5′-GGT GGG AGT AAA CAG GTG TCT TCA GAG and 3′-CTC TGA AGA CAC CTG TTT ACT CCC AAC, N170Q 5′-CAG AGA CTG TGC AGA TCA CCA TCA CTC and 3′-GAG TGA TGG TGA TCT GCA CAG TCT CTG. In a second PCR step both products were fused and amplified using the flanking CD16 primers. The PCR reactions (50 μl) contained 100 ng of each primer, dNTP-mix (20 nmol each) and 4 Units of Taq-polymerase (Boehringer Mannheim, Germany) in the recommended buffer conditions. We used 30 cycles of 1 min at 94°, 1 min at 56°, 2 min at 72° with a final elongation step of 5 min at 72°. The PCR products were ligated into the pCR2.1 vector (Invitrogen, Groningen, the Netherlands) and sequenced using the M13 reverse primer. The BamHI–KpnI fragment was cloned in front of the sequence coding for the GPI anchor in the pcDNA3.1 vector (Invitrogen).
Stable transfection
The mutated DNAs and wild-type CD16B NA1 were used to transfect HEK293 cells (transformed human kidney cell line). The SuperFect Transfection Reagent (QIAGEN, Hilden, Germany) is an activated dendrimer that assembles the DNA into compact structures to allow the entry of DNA into the cell. The transfected cells were selected by adding 500 μg/ml Geneticin sulphate (PAA Laboratories, Linz, Austria) to the culture medium. For each cell line at least 10 clones expressing the respective CD16-mutant were pooled.
Flow cytometry
For immunofluorescence studies various murine monoclonal antibodies against CD16 were used: DJ130c [fluorescein isothiocyanate (FITC)-conjugated; DAKO, Hamburg, Germany] and the antibodies of the Leucocyte Typing Workshop VI: BL-LGL.2, CLB-Gran1, CLB-Gran11, Leu11c, BW209, YFC120.5, GRM1, 3G8, G7E11 (unconjugated). These antibodies were detected with a FITC-labelled goat anti-mouse serum (Pharmingen, Hamburg, Germany). Levels of fluorescence were quantified with a fluorescence-activated cell sorter (FACS) Calibur cytometer (Becton Dickinson, Heidelberg, Germany).
IgG-binding assay
To determine the binding of IgG-transfected cells, 105 cells/well were suspended in 100 μl phosphate-buffered saline (PBS) with different amounts of monomeric human IgG (Octagam, Octapharma GmbH). The cells were incubated for 1 hr at 4° and washed with PBS afterwards. Binding of IgG to cells was detected using a FITC-conjugated rabbit anti-human IgG antibody (DAKO) in FACS analysis.
Tunicamycin assay
Tunicamycin (isolated from different strains of Streptomyces) blocks the synthesis of N-glycosylations by inhibiting the transfer of N-acetylglucosamine-1-phosphate to dolichol-monophosphate. Washed cells were resuspended in culture medium containing 1 μg tunicamycin/ml (Sigma-Aldrich Chemie, Steinheim, Germany) and incubated for 30 hr at 37°. Cells were washed again and the expression of CD16 and binding of IgG was determined using FACS analysis.
Results
Characterization of CD16 expression of transfected cells
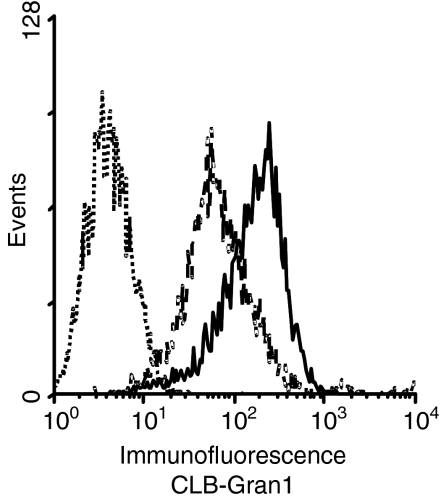
Each of the four asparagine-linked glycosylation sites (N39, N75, N163 and N170) of the NA1 allele of CD16b was mutated into glutamine using site-directed PCR mutagenesis resulting in four mutants each lacking one single glycosylation site. The mutated cDNAs and wild-type CD16B NA1 were stably transfected into HEK293 cells. More than 95% of each of five transfected cell lines expressed CD16 (Fig. 1). Epitope mapping was performed to exclude severe structural alterations by the introduced amino acid exchanges. Binding of nine different CD16 antibodies was compared to reference antibody CLB-Gran1 using flow cytometry (Fig. 2). These data showed that all CD16 constructs were expressed with an almost identical mean fluorescence intensity (MFI). Using antibody CLB-Gran1 as a reference for CD16 expression, eight of nine antibodies revealed similar binding levels on all cell lines. Only binding of G7E11 to CD16-N163Q was significantly reduced.
Figure 1.
Expression of CD16b mutants and wild-type CD16 on transfected cell lines. Indirect immunofluorescence was performed with either unspecific antibody 5E5 as negative control (dotted line) or CLB-Gran1 as CD16-specific antibody (solid line).
Figure 2.
Epitope mapping of nine CD16 antibodies using the transfectants WT-CD16, N39Q, N75Q, N163Q and N170Q. The relative MFIs of the CD16 antibodies indicated on the x-axis in comparison to the MFI of the reference antibody CLB-Gran1 are shown on the y-axis.
Functional analysis of mutated FcγRIII
Binding of different concentrations of monomeric IgG to CD16 transfectants was analysed and MFI were determined. Comparison of FACS histograms revealed an enhanced binding of IgG to cells expressing the mutated receptor CD16-N163Q. Figure 3(a) shows representative flow cytometry data of an IgG-binding assay with two cell lines expressing CD16-N163Q and wild-type CD16 as control. Binding of IgG to CD16-N163Q was increased by up to 150% of the wild-type CD16 level. Comparison of all five cell lines and standardization with reference antibody CLB-Gran1 is shown in Fig. 3(b). There was increased binding of IgG to cells expressing the mutated receptor N163Q. This significant effect (P < 0·0001) was observed with each IgG concentration used.
Figure 3.
IgG-binding assay with CD16-transfected cells. The binding of human monomeric IgG to the mutated receptors on the cell surface was determined using an αIgG antibody in FACS analysis. (a) The histogram shows the direct comparison of the cell lines transfected with CD16-WT (dashed line), CD16-N163Q (solid line) and untransfected HEK293 (dotted line). The cells were incubated with 500 μg/ml of human monomeric IgG and subsequently with a FITC-labelled monoclonal antibody directed against human IgG. (b) All five transfectants were incubated with the indicated concentrations of human monomeric IgG. To take into account slightly different expressions of CD16, the MFI measured for each cell line and concentration of IgG (MFI X) was divided by the MFI of the reference antibody CLB-Gran1 on the respective cell line. All assays were performed in triplicate.
Effect of blockade of glycosylation on receptor function
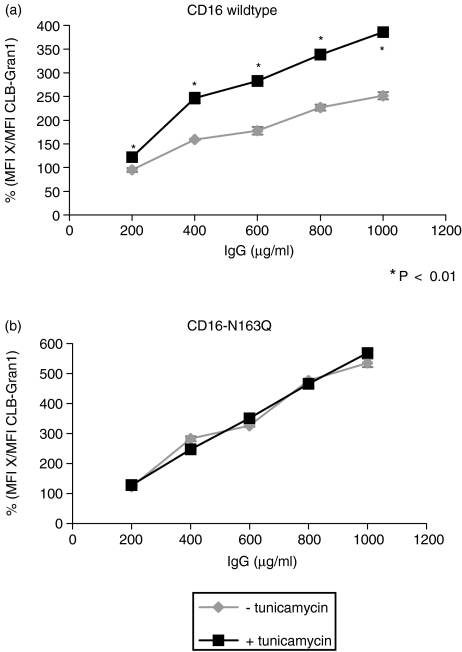
To determine the effect of blockade of glycosylation of CD16 on IgG binding, transfected cells (WT-CD16 and CD16-N163Q) were incubated with the glycosylation inhibitor tunicamycin. Then expression of CD16 and binding of IgG were analysed. Figure 4 shows direct comparison of cells transfected with WT-CD16 after incubation with or without tunicamycin. There was a significant reduction of receptor density on the cell surface after incubation with tunicamycin compared to control cells. Binding of different concentrations of monomeric IgG to transfected cells with WT-CD16 and CD16-N163Q was analysed and MFI were determined. MFIs of both cell lines with and without tunicamycin were standardized using reference antibody CLB-Gran1 and were compared with each other (Fig. 5). Diagrams show binding of IgG to transfected cells with WT-CD16 (Fig. 5a) and CD16-N163Q (Fig. 5b) after the incubation with tunicamycin compared to control cells. WT-CD16-transfected cells showed a significant increase of IgG binding after inhibition of glycosylation, in contrast, there was no difference in tests with CD16-N163Q. After incubation with tunicamycin IgG binding to cells with unmutated receptor WT-CD16 was increased almost up to the binding level of mutated receptor N163Q.
Figure 4.
Influence of tunicamycin on the expression of CD16. Cells transfected with WT-CD16B were incubated for 30 hr with or without tunicamycin (0·5 μg/ml) and the expression of CD16B was determined using the monoclonal antibody CLB-Gran1. This figure shows the comparison of CD16 expression on untransfected cells (dotted line) and transfected cells after incubation with (dashed line) or without tunicamycin (solid line).
Figure 5.
IgG-binding assay with CD16-transfected cells after blockade of glycosylation with tunicamycin. Cells transfected with WT-CD16B (a) and CD16-N163Q (b) were incubated for 30 hr with or without tunicamycin (0·5 μg/ml) and used for an IgG-binding assay afterwards. The cells were incubated with the indicated concentrations of human monomeric IgG (μg/ml) and subsequently with a FITC-labelled monoclonal antibody against human IgG. To take into account slightly different expressions of CD16, the MFI measured for each cell line and concentration of IgG (MFI X) was divided by the MFI of the reference antibody CLB-Gran1 on the respective cell line. All assays were performed in triplicate.
Discussion
Human Fc receptors for IgG (FcγR) play an important role in triggering immune responses because they link the humoral system with the cellular effectors. In the present study, the effect of glycosylation on the binding of IgG to its receptor was analysed. The results showed binding of IgG to FcγRIII to be inhibited by glycosylation of N163. This amino acid is located in the binding region of the membrane proximal domain,19 which has been demonstrated previously to be essential for binding IgG.4 The localization of the glycosylation site in the centre of the binding region was confirmed by cocrystallization of soluble FcγRIII in complex with the Fc part of IgG.20 Although glycosylation of CD16 could not be analysed in the cocrystal, generated using deglycosylated proteins, a steric influence of the degree of glycosylation in N163 on interaction between the receptor and its ligand might be possible.
In addition, experiments comparing the affinity of eukaryotic (glycosylated) and prokaryotic (non-glycosylated) soluble FcγRIIIb showed higher affinity of the non-glycosylated molecules.23 It was concluded that glycosylation plays an important role in the regulation of receptor affinity. Furthermore, it has recently been reported that addition or subtraction of one sugar residue to the IgG-Fc markedly influences its affinity for FcγRIII,24–26 implying that glycosylated residues of both FcRs and IgG influence the affinity of their interaction.
Attempts with human cells expressing FcγRIIIa in IgG-binding assays revealed higher IgG-binding affinity by NK cells compared to monocytes. It was shown that receptors of both cell types had identical protein cores but undergo cell-type specific glycosylation differing in the level of integrated mannose molecules. FcγRIIIa of NK cells is glycosylated with high-mannose oligosaccharides forming a compact structure and has a higher affinity compared to the low-mannose receptor type expressed on monocytes and macrophages.22 These results confirm the effect of glycosylation of FcγRIII on IgG binding.
These mutation studies have shown for the first time the exact localization of a glycosylation site involved in the fine tuning of receptor affinity. The site N163 is conserved in FcγRIII of all species examined so far27,28 but it is not present in other Fc receptors. Although direct proof from mutation studies is lacking, there is considerable evidence, that glycosylation influences the affinity of other receptors as well, such as CD22,29 CD4330 and CD8231.
N-linked glycosylation appears to be affected by host-stress in inflammation or infection.32 Experiments with mice after induction of inflammation revealed general changes in the carbohydrate-derived profile of serum glycoproteins like α1-acid glycoprotein, α1-esterase and α1-protease inhibitor.33 It was shown that inflammation has an effect on the glycosylation of four rat acute-phase glycoproteins (α1-anti-trypsin, ceruloplasmin, α1-acid glycoprotein and haptoglobin). Inflammation resulted in a two- to five-fold increased secretion of concanavalin-A-reactive forms and altered reactivity of proteins.34 This modulation appears to be regulated by different inflammatory cytokines. It was shown that interleukin-6-type cytokines induced changes in acute-phase protein glycosylation.35,36 Other experiments indicated an influence of other cytokines, such as interleukin-1, tumour necrosis factor-α, interferon-γ and transforming growth factor-β, on N-glycosylation.37 These cytokines triggered changes in the degree of branching of oligosaccharides. Therefore it is likely, that chronic inflammation may alter the glycosylation of CD16 as well. Thus, immune activation via CD16 may be reduced in inflammation. The glycosylation of proteins does not merely serve purposes in stabilizing the structure, but may be more important for protein–protein interactions than previously anticipated.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft DFG, Sonderforschungsbereiche 244 Project A09 and 566 Project A02.
References
- 1.Unkeless JC, Scigliano E, Freedman VH. Structure and function of human and murine receptors for IgG. Annu Rev Immunol. 1988;6:251. doi: 10.1146/annurev.iy.06.040188.001343. [DOI] [PubMed] [Google Scholar]
- 2.Van de Winkel JG, Capel PJ. Human IgG Fc receptor heterogeneity: molecular aspects and clinical implications. Immunol Today. 1993;14:215. doi: 10.1016/0167-5699(93)90166-I. [DOI] [PubMed] [Google Scholar]
- 3.Ravetch JV. Fc receptors: rubor redux. Cell. 1994;78:553. doi: 10.1016/0092-8674(94)90521-5. [DOI] [PubMed] [Google Scholar]
- 4.Hulett MD, Hogarth PM. Molecular basis of Fc receptor function. Adv Immunol. 1994;57:127. doi: 10.1016/s0065-2776(08)60671-9. [DOI] [PubMed] [Google Scholar]
- 5.Van de Winkel JG, Anderson CL. Biology of human immunoglobulin G Fc receptors. J Leukoc Biol. 1991;49:511. doi: 10.1002/jlb.49.5.511. [DOI] [PubMed] [Google Scholar]
- 6.Peltz GA, Grundy HO, Lebo RV, Yssel H, Barsh GS, Moore KW. Human Fc gamma RIII. Clining, expression, and identification of the chromosomal locus of two Fc receptors for IgG. Proc Natl Acad Sci USA. 1989;86:1013. doi: 10.1073/pnas.86.3.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurosaki T, Ravetch JV. A single amino acid in the glycosyl-phosphatidylinositol attachment domain determines the membrane topology of Fc gamma RIII. Nature. 1989;342:805. doi: 10.1038/342805a0. [DOI] [PubMed] [Google Scholar]
- 8.Edberg JC, Kimberly RP. Modulation of Fc gamma and complement receptor function by the glycosyl-phosphatidylinositol-anchored form of Fc gamma RIII. J Immunol. 1994;152:5826. [PubMed] [Google Scholar]
- 9.Lanier LL, Yu G, Phillips JH. Coassociation of CD3 zeta with a receptor (CD16) for IgG Fc on human natural killer cells. Nature. 1989;342:803. doi: 10.1038/342803a0. [DOI] [PubMed] [Google Scholar]
- 10.Uciechowski P, Gessner JE, Schindler R, Schmidt RE. Fcgamma RIII activation is different in CD16+ cytotoxic T lymphocytes and natural killer cells. Eur J Immunol. 1992;22:1635. doi: 10.1002/eji.1830220643. [DOI] [PubMed] [Google Scholar]
- 11.Simmons D, Seed B. The Fc gamma receptor of natural killer cells is a phospholipid-linked membrane protein. Nature. 1988;333:568. doi: 10.1038/333568a0. [DOI] [PubMed] [Google Scholar]
- 12.Hundt M, Schmidt RE. The glycosylphosphatidylinositol-linked Fc gamma receptor III represents the dominant receptor structure for immune complex activation of neutrophils. Eur J Immunol. 1992;22:811. doi: 10.1002/eji.1830220327. [DOI] [PubMed] [Google Scholar]
- 13.Frank MM, Hamburger MI, Lawley TJ, Kimberly RP, Plotz PH. Defective reticuloendothelial system Fc-receptor function in systemic lupus erythematosus. N Engl J Med. 1979;300:518. doi: 10.1056/NEJM197903083001002. [DOI] [PubMed] [Google Scholar]
- 14.Werfel T, Uciechowski P, Tetteroo PA, Kurrle R, Deicher H, Schmidt RE. Activation of cloned human natural killer cells via Fc gamma RIII. J Immunol. 1989;142:1102. [PubMed] [Google Scholar]
- 15.Anasetti C, Martin PJ, Morishita Y, Badger CC, Bernstein ID, Hansen JA. Human large granular lymphocytes express high affinity receptors for murine monoclonal antibodies of the IgG3 subclass. J Immunol. 1987;138:2979. [PubMed] [Google Scholar]
- 16.Kipps TJ, Parham P, Punt J, Herzenberg LA. Importance of immunoglobulin isotype in human antibody-dependent, cell-mediated cytotoxicity directed by murine monoclonal antibodies. J Exp Med. 1985;161:1. doi: 10.1084/jem.161.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gresham HD, Clement LT, Lehmeyer JE, Griffin FM, Jr, Volonakis JE. Stimulation of human neutrophil Fc receptor-mediated phagocytosis by a low molecular weight cytokine. J Immunol. 1986;137:868. [PubMed] [Google Scholar]
- 18.Amigorena S, Lankar D, Briken V, Gapin L, Viguier M, Bonnerot C. Type II and III receptors for immunoglobulin G (IgG) control the presentation of different T cell epitopes from single IgG-complexed antigens. J Exp Med. 1998;187:505. doi: 10.1084/jem.187.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tamm A, Kister A, Nolte KU, Gessner JE, Schmidt RE. The IgG binding site of human Fc gamma RIIIB involves CC'and FG loops of the membrane-proximal domain. J Biol Chem. 1996;271:3659. doi: 10.1074/jbc.271.7.3659. [DOI] [PubMed] [Google Scholar]
- 20.Sondermann P, Huber R, Oosthuizen V, Jacob U. The 3.2-Å crystal structure of the human IgG1 Fc fragment-FcγRIII complex. Nature. 2000;406:267. doi: 10.1038/35018508. [DOI] [PubMed] [Google Scholar]
- 21.Radaev S, Motyka S, Fridman W-H, Sautes-Fridman C, Sun PD. The structure of a human type III Fcgamma receptor in complex with Fc. J Biol Chem. 2001;276:16469. doi: 10.1074/jbc.M100350200. [DOI] [PubMed] [Google Scholar]
- 22.Edberg JC, Kimberly RP. Cell type-specific glycoforms of Fc gamma RIIIa (CD16): differential ligand binding. J Immunol. 1997;159:3849. [PubMed] [Google Scholar]
- 23.Galon J, Robertson MW, Galinha A, Mazières N, Spagnoli R, Fridman W-H, Sautès C. Affinity of the interaction between Fc gamma receptor type III (FcγRIII) and monomeric human IgG subclasses. Role of FcγRIII glycosylation. Eur J Immunol. 1997;27:1928–32. doi: 10.1002/eji.1830270816. [DOI] [PubMed] [Google Scholar]
- 24.Davies J, Jiang L, Pan LZ, LaBarre MJ, Anderson D, Reff M. Expression of GnTIII in a recombinant anti-CD20 CHO production cell line: expression of antibodies with altered glycoforms leads to an increase in ADCC trough higher affinity for Fc gamma RIII. Biotechnol Bioeng. 2001;74:288–94. [PubMed] [Google Scholar]
- 25.Shields RL, Lai J, Keck R, et al. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J Biol Chem. 2002;277:26733–40. doi: 10.1074/jbc.M202069200. [DOI] [PubMed] [Google Scholar]
- 26.Shinkawa T, Nakamura K, Yamane N, et al. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J Biol Chem. 2003;278:3466–73. doi: 10.1074/jbc.M210665200. [DOI] [PubMed] [Google Scholar]
- 27.Nishimura Y, Miyazawa T, Ikeda Y, et al. Molecular cloning and sequencing of the cDNA encoding the feline FcgammaRIIIA (CD16) homologue. Vet Immunol Immunopathol. 2000;73:353–9. doi: 10.1016/s0165-2427(00)00156-2. [DOI] [PubMed] [Google Scholar]
- 28.Yan Y, Zhang G, Chen C, Li X, Li Q. Bovine FcgammaRIII with a single extracellular domain. Res Vet Sci. 2000;68:115–18. doi: 10.1053/rvsc.1999.0343. [DOI] [PubMed] [Google Scholar]
- 29.Carlow DA, Ardman B, Ziltener HJ. A novel CD8 T cell-restricted CD45RB epitope shared by CD43 is differentially affected by glycosylation. J Immunol. 1999;163:1441–8. [PubMed] [Google Scholar]
- 30.Sgroi D, Nocks A, Stamenkovic I. A single N-linked glycosylation site is implicated in the regulation of ligand recognition by the I-type lectins CD22 and CD33. J Biol Chem. 1996;271:18803–9. doi: 10.1074/jbc.271.31.18803. [DOI] [PubMed] [Google Scholar]
- 31.Ono M, Handa K, Withers DA, Hakomori S. Motility inhibition and apoptosis are induced by metastasis-suppressing gene product CD82 and its analogue CD9, with concurrent glycosylation. Cancer Res. 1999;59:2335–9. [PubMed] [Google Scholar]
- 32.Van den Steen P, Rudd PM, Dwek RA, Van Damme J, Opdenakker G. Cytokine and protease glycosylation as a regulatory mechanism in inflammation and autoimmunity. Adv Exp Med Biol. 1998;435:133–43. doi: 10.1007/978-1-4615-5383-0_13. [DOI] [PubMed] [Google Scholar]
- 33.Heegaard PM. Changes in serum glycoprotein glycosylation during experimental inflammation in mice are general, unrelated to protein type, and opposite changes in man and rat: studies on mouse serum α 1-acid glycoprotein, α 1-esterase, and α 1-protease inhibitor. Inflammation. 1992;16:631–44. doi: 10.1007/BF00919346. [DOI] [PubMed] [Google Scholar]
- 34.Pos O, Van Dijk W, Ladiges N, Linthorst C, Sala M, Van Tiel D, Boers W. Glycosylation of four acute-phase glycoproteins secreted by rat liver cells in vivo and in vitro. Effects of inflammation and dexamethasone. Eur J Cell Biol. 1988;46:121–8. [PubMed] [Google Scholar]
- 35.Van Dijk W, Mackiewicz A. Interleukin-6-type cytokine-induced changes in acute phase protein glycosylation. Ann N Y Acad Sci. 1995;762:319–30. doi: 10.1111/j.1749-6632.1995.tb32336.x. [DOI] [PubMed] [Google Scholar]
- 36.Gryska K, Slupianek A, Laciak M, Baumann H, Mackiewicz A. Interleukin-6-type cytokines affect glycosylation of acute phase proteins in vitro. Ann N Y Acad Sci. 1995;762:413–15. doi: 10.1111/j.1749-6632.1995.tb32351.x. [DOI] [PubMed] [Google Scholar]
- 37.Mackiewicz A, Laciak M, Gorny A, Baumann H. Leukemia inhibitory factor, interferon gamma and dexamethasone regulate N-glycosylation of alpha 1-protease inhibitor in human hepatoma cells. Eur J Cell Biol. 1993;60:331–6. [PubMed] [Google Scholar]