Abstract
Increasing evidence suggests that parental allergic status, especially that of the mother, may play a unique and important role in influencing the development of fetal infant immune responses to inhaled allergens, independently of genetic predisposition. We have developed an experimental model in dogs where the offspring from allergic parents, when exposed to inhaled allergen, develop allergic sensitization and an asthmatic phenotype, whereas the offspring from non-allergic parents do not. Offspring from ragweed-sensitized (two litters, n = 10) or non-sensitized (two litters, n = 11) Beagle dogs were exposed repeatedly, by inhalation, to ragweed or filtered air (negative control) beginning within 1 week after birth. Serum levels of total immunoglobulin (Ig)E, and ragweed-specific IgE and IgG, were measured at specific time-points up to 40 weeks after birth. Cell differentials in the bronchoalveolar lavage were determined on days 1 and 4 following ragweed instillation into the offspring's lungs at 26 weeks of age. Changes in pulmonary resistance following challenge with histamine and ragweed (five breaths) were measured at 40 weeks after birth. Offspring from sensitized parents exposed to ragweed developed elevated serum total IgE and ragweed-specific IgE and IgG, and showed an increased pulmonary resistance to histamine and ragweed, and increased numbers of eosinophils in bronchoalveolar lavage. In contrast, offspring from non-sensitized parents did not exhibit this immune response. These results suggest that parental allergic sensitivity is important in the development of allergic sensitization and an asthmatic phenotype in the offspring.
Introduction
Several studies have reported that children from allergic mothers are more likely to develop allergies/asthma than children from allergic fathers.1–7 Mothers of children with eczema have a more T helper 2 (Th2)-biased immune response than mothers of children without eczema.8 This suggests that children from an allergic mother are exposed to a unique biological environment that may increase their risk of developing asthma. Whether such a maternal influence is mediated by factors present during pregnancy and/or nursing is unknown. Although controversial, recent data suggest that the rate of allergic children is higher in mothers who breast-feed than in allergic mothers who do not breast-feed.9 In contrast, others have reported a significantly reduced risk of childhood asthma if breast-feeding is continued for at least 4 months after birth.10
The fetus and newborn may be exposed to various maternal factors (e.g. antibodies, hormones, cytokines, etc.) that may influence the development of the fetal/infant immune system. Offspring from allergic mothers are exposed to maternal allergen-specific antibodies during pregnancy and nursing. It is well known that maternal immunoglobulin G (IgG) crosses the placenta, or is passed through breast milk, to provide the fetus/infant with specific immunity at a stage when its own immune responses are still developing.11,12 Additionally, immunoglobulin E (IgE) is detectable in amniotic fluid at 16–17 weeks of gestation, as well as at term, in levels that correlate with maternal levels, suggesting that IgE passes from the vascularized deciduas to amniotic fluid.13 Allergic mothers have higher concentrations of interleukin (IL)-4, IL-8 and RANTES (regulated on activation, normal T cell expressed and secreted) in their breast milk.14,15 Whether such factors passed from an allergic mother impart a greater risk for developing allergic sensitization and/or asthma in her children is not known. Other studies suggest that allergens inhaled by the mother, independent of allergic status, might cross the placenta during a critical period of fetal development and lead to priming of infant T cells (e.g. in utero allergic sensitization).16–22
Using our canine model of allergic disease we sought to confirm and further explore these human studies which indicate that parental/maternal allergic status may influence the development of allergic sensitization and asthma in offspring. We have previously developed a canine model of asthma in which high IgE-responder Beagle dogs were sensitized to ragweed (RW) by injection.23 These dogs develop serum RW-specific IgE and IgG, increased airway hyper-responsiveness to inhaled RW and histamine challenge, increased lung eosinophilia and increased mucous in the lung. Herein, we bred RW-sensitized dogs and exposed their offspring to RW by inhalation to determine whether they were at a greater risk for developing allergic sensitization and an asthmatic phenotype. As the offspring matured, total and RW-specific antibody levels, lung inflammation and airway hyper-responsiveness were periodically measured to assess the development of an asthmatic phenotype. The results indicate that the offspring from RW-sensitized parents develop allergic sensitization and an asthmatic phenotype, whereas offspring from non-sensitized parents do not.
Materials and methods
Animals and exposure to allergen
Beagles were born and maintained in the Lovelace Respiratory Research Institute (LRRI) dog colony. Dogs were housed in temperature-controlled kennel buildings with access to indoor and outdoor runs. The dogs were fed dry dog food once per day (Wayne Mini Laboratory Dog Diet 8759; Teklad Premier Laboratory Diets, Madison, WI); water was available at all times. All animal procedures were carried out in accordance with protocols approved by the LRRI Institutional Animal Care and Use Committee. The LRRI is fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care.
Four (two female and two male) RW-sensitized adult dogs, and four age-matched non-sensitized adult dogs, served as breeders for this study. The procedure for sensitizing the breeder dogs to RW has been described previously.23 Briefly, Beagle puppies were sensitized by intraperitoneal (i.p.) injection of RW extract (RW short, Ambrosia artemisifolia; Bayer Corporation, Elkhart, IN) combined with aluminium hydroxide (Accurate Chemical and Scientific, Westbury, NY) within 24 hr after birth and then weekly for 4 weeks. The dogs received additional subcutaneous (s.c.) injections weekly during weeks 5–8, and then biweekly during weeks 10–22. These breeder dogs developed serum RW-specific IgE and IgG, increased airway hyper-responsiveness to inhaled RW and histamine challenge, and increased lung eosinophilia.23
Breeder pairs (e.g. sensitized female–male or non-sensitized female–male) were paired, and their offspring were exposed by inhalation, within 24 hr of birth, to filtered air (control) or RW. Offspring were allowed to begin nursing immediately after birth. Exposures were carried out by a modification of a method used previously in our laboratory.23 Briefly, inhalation exposure was carried out using a dynamic six-port canine exposure system that allows continuous, muzzle-only (adult) or head-only (newborn; <5 weeks of age) exposure. The system consists of an exhaust plenum and an inlet plenum located centrally and internal to the exhaust plenum. Six exposure ports are located symmetrically and equidistantly in pairs, three on each side of the exhaust plenum. An exposure cone is placed into each side of the exhaust plenum and sealed with o-rings at the exhaust and inlet interfaces. A constant supply of fresh aerosol is delivered to the inlet of the exposure cones and drawn past the dog's breathing zone by the exhaust plenum. Three sampling ports at the junction of each pair of exposure ports and the inlet plenum were used to collect filter samples for determining aerosol concentration by gravimetric analysis. Offspring were exposed repeatedly, for 20 min each time, to aerosolized RW extract (short, A. artemisifolia; Hollister-Stier, Spokane, WA) according to the schedule in Fig. 1. Aerosolization of the ragweed extract resulted in a mass median diameter particle of 1·06 ± 0·17 µm. The average total lung deposition of RW protein for the first four exposures (weeks 1–4) was 123·85 ± 16·87 µg (offspring from non-sensitized parents) and 135·48 ± 19·47 µg (offspring from sensitized parents). The average total lung deposition of RW protein for the last four exposures (weeks 8–20) was 448·77 ± 39·11 µg (offspring from non-sensitized parents) and 414 ± 48·82 µg (offspring from sensitized parents). Lung deposition was calculated as follows: [concentration (mg/m3) × minute ventilation (l/min) × exposure time (min) × deposition fraction], where minute ventilation was calculated based on body weight, and the deposition fraction was estimated to be 10%.24,25
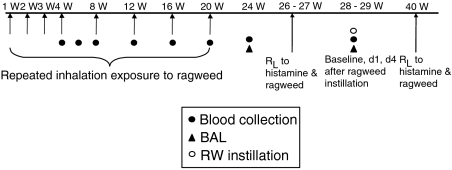
Figure 1.
Offspring were exposed to ragweed (RW) by inhalation, repeatedly after birth. Blood was collected periodically to measure serum antibody levels. At 24 weeks of age, a baseline bronchoalveolar lavage (BAL) was performed. Approximately 4 weeks later, RW was instilled into six different lung lobes (100 µg each), and BAL was performed 1 and 4 days afterwards. Pulmonary resistance (RL) to histamine, and RW challenge (five breaths), was measured in offspring when ≈26 and 40 weeks of age.
Serum collection
Serum was obtained from whole blood collected from the jugular vein at 4, 6, 8, 12, 16, 20, 24 and 28 weeks after birth (Fig. 1). Frozen aliquots were stored at −80° for subsequent evaluation of total IgE and RW-specific IgE and IgG.
Bronchoalveolar lavage
At 24 weeks of age, the offspring had their right and left cardiac lung lobes lavaged (baseline 1). At 28–29 weeks of age, the offspring were again lavaged (baseline 2). Five days later they were challenged with RW, by lung instillation using a fibreoptic bronchoscope (5·5 mm OD, Model BF-4B2; Olympus, Lake Success, NY), to assess the pulmonary immune response, as described previously.23 Briefly, the dogs were sedated with acepromazine (Phoenix Pharmaceutical, St Joseph, MO; 2 mg in 200 µl) and then anaesthetized with isoflurane. The bronchoscope was advanced into six different lung lobes, wedged in a specific segmental bronchus and 100 µg of RW was instilled into each segment. The right and left cardiac lobes were then lavaged individually by instillation and withdrawal of 5 × 10-ml aliquots of normal saline (0·9% NaCl) solution on days 1 and 4 after instillation. Cells were separated from bronchoalveolar lavage fluid (BALF) by centrifugation, washed with phosphate-buffered saline (PBS), resuspended in 5 ml of 10% fetal calf serum (FCS) (Sigma, St Louis, MO) in RPMI-1640 (Hyclone, Logan, UT), and counted using a cell counter (Model ZBI; Coulter Electronics, Hialeah, FL). Cells were used to prepare cytocentrifuge slides for evaluation of cell differentials. Cytocentrifuge slides were stained using a modified Wright-Giemsa stain (Diff-Quik; Baxter Healthcare, McGraw Park, IL). The numbers of eosinophils, neutrophils, macrophages, lymphocytes and mast cells were determined according to standard morphological criteria.26 A final average was determined by combining numbers from the left and right cardiac lobes.
Pulmonary resistance
Airways reactivity was evaluated at 26 and 40 weeks of age by a method used routinely in the laboratory.23 Briefly, dogs were sedated with acepromazine and then anaesthetized with isoflurane. The dogs were intubated, ventilated on a Harvard pump (Harvard Apparatus, Holliston, MA), and maintained on isoflurane throughout the procedure. Ventilation was maintained with 2% isoflurane at a tidal volume of ≈20–25 ml/kg body weight. The respiratory rate was set at 18 breaths/min, and the end-tidal CO2 ranges from 4 to 5% (LB2, medical gas analyser; Beckman Instruments, Palo Alto, CA). An oesophageal balloon catheter was positioned in the caudal thoracic portion of the oesophagus. Transpulmonary pressure was measured by connecting the oesophageal catheter to one side of a differential pressure transducer (model MP45; Validyne, Northridge, CA); the other side was connected to a port at the proximal end of the endotracheal tube. Respiratory flow was measured using a pneumotachograph (Fleisch no. 1) connected to the endotracheal tube. Custom-designed computer software (LabView 5·1; National Instruments, Austin, TX) was used to facilitate integration of the flow signal to yield volume and to derive the total pulmonary resistance.
After the mechanical indices were stabilized, airway responsiveness was assessed by obtaining dose–response curves of responses to histamine and RW aerosols. Histamine diphosphate was prepared at concentrations of 0·3, 1·0, 3·0 and 10·0 mg/ml in sterile water. Similarly, RW was prepared at concentrations of 0·01, 0·03, 0·10, 0·30, 1·00 and 3·00 mg/ml in water. Aerosols were generated using a jet nebulizer (No. 950; Hospitak, Lindenhurst, NJ) connected directly to the end of the endotracheal tube. Five breaths of nebulized vehicle (water), histamine, or RW solutions, standardized to an end-inspiratory transpulmonary pressure of 15 cm H2O, were allowed, and the response was assessed until peak resistance was reached. Pulmonary resistance values were allowed to recover to within 10% of baseline values prior to subsequent challenge or until a plateau was reached. The response was evaluated by calculating the percentage change in peak resistance following histamine or RW inhalation compared with baseline.
Immunoglobulin measurement
Serum total IgE and RW-specific IgE and IgG levels were measured by enzyme-linked immunosorbent assay (ELISA), as previously described in detail.23 Briefly, for total IgE, 96-well microtitre plates were coated with 100 µl of dog serum diluted 1 : 1000 in 0·1 m sodium carbonate buffer (pH 9·6) and incubated overnight at 4°. Plates were washed and blocked for 2 hr at room temperature with 0·4% bovine serum albumin (BSA) in PBS. A monoclonal mouse anti-dog IgE (antibody D9 provided by Dr Doug DeBoer, University of Wisconsin School of Veterinary Medicine, Madison, WI) was then added (at a dilution of 1 : 2000) to each well and incubated for 2 hr at 37°. After washing, 100 µl of donkey anti-mouse IgG horseradish peroxidase-conjugated antibody (Jackson Laboratories, Westgrove, PA) was added (at a dilution of 1 : 2000) to each well and incubated for 1 hr at 37°. Plates were washed, then peroxidase substrate (ABTS peroxidase substrate system; Kirkegaard and Perry Laboratories, Gaithersburg, MD) was added to each well and incubated for 30–60 min at 37°. Colour development was stopped by adding 5% sodium dodecyl sulphate (SDS) in water to each well. Plates were read at an absorbance (A) of 405 nm on a microtitre plate reader (Molecular Devices, Sunnyvale, CA). Results are expressed in terms of the actual A values measured.
To measure RW-specific IgG and IgE, 96-well plates were coated with 100 µl of 5 µg/ml RW extract in PBS (pH 7·2) and incubated overnight at 4°. Plates were washed with PBS containing 0·1% Tween-20 (Sigma) and blocked with 2·5% skimmed milk at room temperature for 1 hr. Samples (100 µl per well) were diluted in 2·5% skimmed milk and incubated for 1 hr at 37°. After washing, 100 µl of goat anti-dog IgG (Kirkegaard and Perry Laboratories), diluted 1 : 1000, or D9 mouse anti-dog IgE monoclonal antibody, diluted 1 : 2000 in 2·5% skimmed milk, was added to each well and the plates were incubated for 1 hr at 37°. Plates were washed, and either 100 µl of rabbit anti-goat IgG conjugated to horseradish peroxidase (for dog RW-specific IgG (Kirkegaard and Perry Laboratories) or donkey anti-mouse IgG conjugated to horseradish peroxidase (for dog RW-specific IgE, Jackson Laboratories), diluted 1 : 2000 in 2·5% skimmed milk, was added to each well. Antibodies for IgG1, IgG2, IgG3, and IgG4 were provided by Dr Michael Day, University of Bristol School of Veterinary Medicine (Bristol, UK).27,28
Results
Systemic immunity
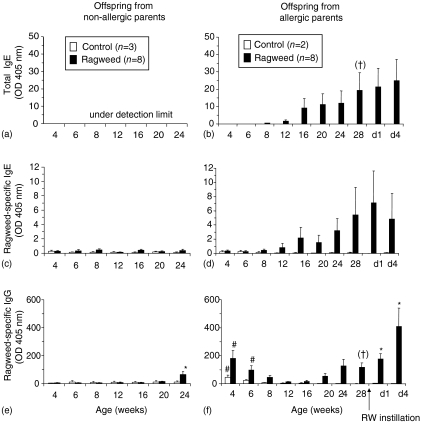
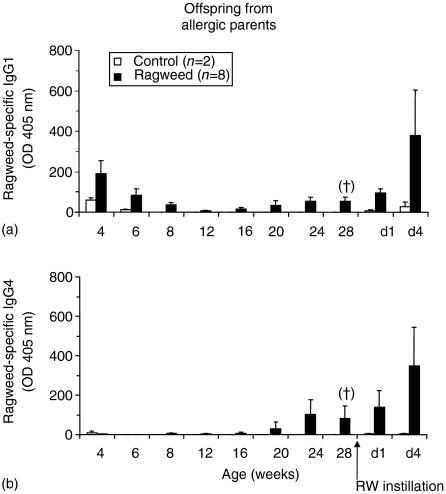
Serum antibody responses were measured in offspring from RW-sensitized (allergic) and non-sensitized (non-allergic) parents, beginning 4 weeks after birth and continuing up to 28 weeks of age. None of the offspring exposed to filtered air (control) alone developed total or RW-specific IgE (Fig. 2a–2d). Only the offspring from allergic parents (OAP) that were exposed to RW developed significant levels of total and RW-specific IgE, beginning at ≈12–16 weeks of age (Fig. 2b, 2d). Serum levels of RW-specific IgG were elevated in the OAP, independently of RW exposure, at 4 weeks of age and decreased to levels, similar to those found in non-allergic offspring, by 12 weeks of age (Fig. 2f). Subsequently, those OAP that were exposed to RW developed increasing levels of RW-specific IgG beginning at 16–20 weeks of age. The levels of RW-specific IgG in the OAP at 4–8 weeks of age were primarily of the IgG1 subtype (Fig. 3a, 3b). Levels of IgG4 were minimal, or below the level of detection of the assay, in OAP of 4 to 16 weeks of age. At 16–20 weeks of age, the levels of RW-specific IgG1 and IgG4 increased in OAP. Serum antibody levels of IgG2 and IgG3 were negligible and/or below the level of detection of the assay (data not shown). Subtypes of IgG were characterized only for the offspring from allergic parents.
Figure 2.
Serum total immunoglobulin E (IgE) (a, b), ragweed (RW)-specific IgE (c, d) and immunoglobulin G (IgG) (e, f) levels in offspring, exposed to either RW or air (control), from non-allergic (a, c, e) or allergic (b, d, f) parents. After the collection of blood at 28 weeks of age, dogs were challenged by instillation with RW, and further blood samples were collected on day 1 (d1) and day 4 (d4) after challenge. *P < 0·05 versus control. #P < 0·05 versus non-allergic [one-way analysis of variance (anova) with Tukey multiple comparison post-test]. Linear trend post-test analysis using 12–28 week samples (†P < 0·05). Linear trend analysis for RW-specific IgE did not reach statistical significance (P = 0·08). All error bars are expressed as mean + standard error of the mean (SEM).
Figure 3.
Serum ragweed (RW)-specific immunoglobulin G1 (IgG1) (a) and immunoglobulin G4 (IgG4) (b) levels in offspring (from allergic parents) exposed to either RW or air (control). After the collection of blood at 28 weeks of age, dogs were challenged by instillation with RW, and further blood samples were collected on day 1 (d1) and day 4 (d4) after challenge. No statistically significant differences were determined by one-way one-way analysis of variance (anova) with Tukey multiple comparison post-test. Linear trend post-test analysis using 12–28 week samples (†P < 0·05).
Pulmonary inflammation
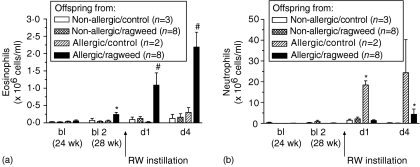
Baseline numbers of eosinophils were significantly elevated in the BALF of 28-week-old OAP exposed to RW by inhalation (Fig. 4a). Furthermore, OAP that were exposed to inhaled RW had higher numbers of eosinophils in the BALF on days 1 and 4 following RW challenge by instillation than any other treatment group (Fig. 4a). Neutrophil numbers in BALF on day 1 following RW challenge by instillation tended to be higher for all offspring treatment-groups (Fig. 4b). However, OAP that did not receive RW exposure by inhalation had neutrophil numbers significantly higher than all other groups. By day 4 after RW challenge, OAP exposed to inhaled RW had significantly increased numbers of neutrophils in the BALF. The number of macrophages or lymphocytes in BALF did not change significantly for any offspring exposure-group (results not shown).
Figure 4.
Numbers of eosinophils (a) and neutrophils (b) in bronchoalveolar lavage (BAL) from offspring following ragweed (RW) instillation into the lung at 28 weeks of age. *P < 0·05 versus non-sensitized and baseline values. #P < 0·05 versus all treatment groups [one-way analysis of variance (anova) with Tukey multiple comparison post-test].
Airways responses
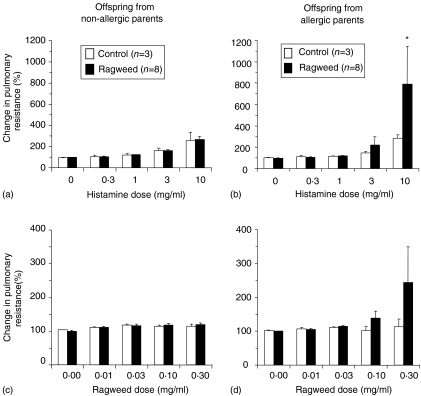
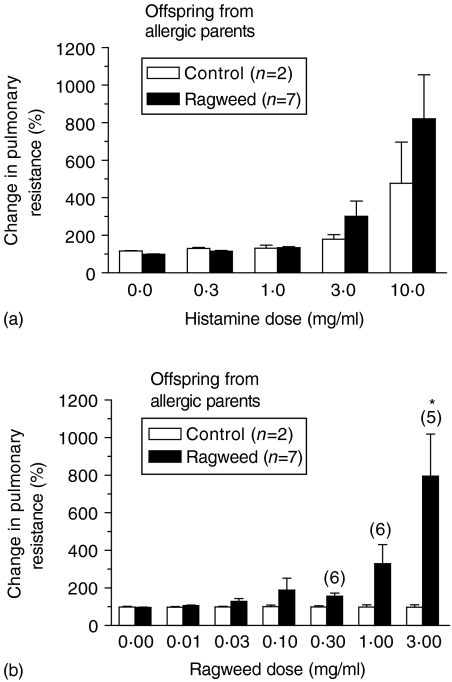
At 26 weeks of age, OAP exposed to inhaled RW had increased pulmonary resistance in response to histamine and RW challenge (Fig. 5b, 5d). This response was dose dependent; however, the increase was not significant for RW at the doses tested. The other offspring exposure-groups had a slight dose-dependent increase in histamine-induced pulmonary resistance, but they did not respond to RW challenge (Fig. 5a, 5c). At 40 weeks of age, OAP exposed to inhaled RW maintained a dose-dependent increase in pulmonary resistance following challenge with histamine and RW (Fig. 6a, 6b). However, this response was only significant for RW challenge. OAP exposed by inhalation to filtered air only, tended to have higher responses to histamine at 40 weeks of age, but still did not respond to RW challenge.
Figure 5.
Changes in pulmonary resistance after histamine (a, b) and ragweed (RW) (c, d) challenge in offspring (26 weeks of age) from non-allergic (a, c) or allergic (b, d) parents. *P < 0·05 versus control group [one-way analysis of variance (anova) with Tukey multiple comparison post-test].
Figure 6.
Changes in pulmonary resistance after histamine (a) and ragweed (RW) (b) challenge in offspring (40 weeks of age) from allergic parents. Numbers in parentheses above the bars indicate the number of offspring challenged at that dose. Some offspring were too reactive to safely challenge at the higher doses. Thus, these results probably underestimate the magnitude of the true average response at the higher doses. *P < 0·05 versus the control group [one-way analysis of variance (anova) with Tukey multiple comparison post-test].
Discussion
The aim of the present study was to determine whether the OAP were more susceptible to developing allergic sensitivity and an asthmatic phenotype than offspring from non-allergic parents. Offspring from RW-sensitized Beagle dogs that were exposed to inhaled RW, beginning within 1 week after birth, developed pulmonary eosinophilia, elevated levels of IgE and airway hyper-responsiveness when challenged with RW. Offspring from non-allergic parents that were exposed to RW by inhalation did eventually develop serum RW-specific IgG, but in the absence of IgE-mediated allergic sensitivity or an asthmatic phenotype. This response is supported by recent human studies.29 Interestingly, OAP (n = 2) that were not repeatedly exposed to RW by inhalation did show increased levels of neutrophils in the BALF when RW was instilled into the lung. We have shown previously that non-allergic animals can develop a transient increase in neutrophils following exposure to RW.23 Additionally, repeated exposure to aerosolized RW in the absence of an adjuvant does not lead to allergic sensitization in adult non-sensitized dogs (Barrett Lab, unpublished).23 These results indicate that parental allergic status, combined with inhalation exposure to allergen shortly after birth, are important risk factors for the development of an asthmatic phenotype in offspring.
Both the male and female breeders in this study were from the LRRI dog colony that has been maintained for the past 40 years; thus, we consider that the genetic contribution from the RW-sensitized and non-sensitized parents are very similar. Furthermore, while both allergic parents were sensitized to RW in the present study, we speculate that the maternal component is the primary parental contributor to the development of allergic sensitization and the asthmatic phenotype in the offspring. Independently of genetic factors, fetal and newborn exposure to various maternal factors (e.g. antibodies, hormones, cytokines, etc.) may influence the development of the fetal/infant immune system.
The transmission of antibody from the mother across the placenta or through breast milk to the fetus and the infant is well known.11,12 Serum RW-specific IgG, present in the OAP, of the current study, at up to 8 weeks of age, is believed to be from the mother. The decrease from 4 to 8 weeks corresponds with the offspring being weaned from their mothers at ≈6 weeks of age. Interestingly, only IgG1 appears to be present in the offspring at significant levels up to 8 weeks of age. Human data show that allergen-specific IgG1 is preferentially (IgG1 > IgG3 > IgG4 > IgG2) passed to the infant through breast milk during nursing or through the placenta before birth.11,12 In humans, maternal IgG2 reaches the fetal capillary in far lower amounts than does IgG1 as a result of the selective properties of the fetal capillary endothelia.30 Whether the same processes mediate the transfer of IgG in dogs is unknown. The lower levels of RW-specific IgG observed in the air-exposed (control; n = 2) offspring at 4 weeks of age are probably a result of the offspring being from the same litter, whereas the RW-exposed offspring were the average of two litters. In other words, the control offspring were from a mother with lower serum RW-specific IgG levels.
The role that maternal allergen-specific IgG may play in the sensitization of the offspring is unclear. At birth, there is a positive correlation between house dust mite (HDM)-specific IgG subclass levels in cord blood, maternal atopy and the magnitude of lymphoproliferative responses to HDM.31 Passive sensitization of mice with antigen-specific IgG1, but not IgG2a or IgG3, causes immediate cutaneous hypersensitivity and increased airway hyper-responsiveness after antigen challenge.32 In contrast, others suggest that maternal IgG may protect offspring from later development of allergic disease. Studies in rodents suggest that maternal IgG actually suppresses IgE responses in the neonate.33 Proliferation responses to HDM by umbilical cord blood cells are inversely correlated with levels of cord blood HDM-specific IgG.12 In addition, elevated maternal anti-Bet v 1 IgG at birth is associated with a reduced prevalence of allergic disease at 18 months of age.34 The presence of maternal RW-specific IgG1, in the present study, does correlate with the development of allergic sensitization and an asthmatic phenotype, but only in those OAP exposed to inhaled RW beginning within 1 week after birth.
Exposure of the offspring to RW, starting shortly after birth, appears to be an important component in the present model. Several studies suggest that HDM exposure during the first year of life can influence immune responses.35–38 However, as definitive results about human immune responses (e.g. skin test or allergen-specific IgE or IgG) cannot be determined until children are >3 years of age, it is currently not possible to ascertain whether exposure during the first 6 months, or the cumulative exposure over 3 years, is important. Thus, the ability to collect samples shortly after birth, and to detect changes in antibody levels, lung inflammation and airway function within a relatively short time after birth, could make this canine model a useful tool for understanding the effects of cumulative allergen exposure over time and exposure to allergen during nursing.
Several studies have suggested that maternal exposure to an allergen during pregnancy may lead to fetal exposure to allergens and subsequent in utero sensitization.16–22 Jones et al. found that infants of mothers who were exposed to birch pollen at >22 weeks of gestation had significantly higher cord blood mononuclear cell proliferative responses to birch pollen at birth than infants of mothers who had either not been exposed during their pregnancy or had been exposed prior to 22 weeks.17 In the present study, maternal exposure to allergen during pregnancy was not required for the development of allergic sensitization and an asthmatic phenotype in her offspring. These results support previous findings of no association between maternal exposure to HDM, cord blood mononuclear cell proliferation, and the incidence of an asthmatic phenotype and rhinitis at 12 and 24 months after birth.39,40
The data presented here demonstrate an increased risk for developing allergic sensitization and an asthmatic phenotype in OAP exposed to inhaled RW. While we speculate that this response is primarily mediated by maternally derived factors, further experiments are required to confirm this hypothesis (e.g. by pairing a non-sensitized male with a sensitized female). Development of this canine model will provide a unique experimental platform to further explore the early events that may shape a child's lifelong immune response to allergens. We hypothesize that Th2 cytokine production by the mother/placenta/fetus at critical time-points during the development of the fetal/infant immune system is an important factor driving the development of allergic sensitization and an asthmatic phenotype in the canine offspring from the allergic mother. Other important questions can also be pursued using this model. These include:
How long does this maternal influence persist into the life of the child?
Does breast-feeding by the allergic mother increase or decrease the risk?
Is the risk only for those allergens to which the mother is allergic?
Is this influence mediated by maternal cytokines, antibodies, hormones, etc.?
Will other environmental exposures (e.g. bacterial, viral, pollutants, etc.) alter the response?
Could treatment of the mother or newborn prevent this maternal influence?
Acknowledgments
The authors wish to thank Monique Nysus for her technical assistance with the animals and assays.
Abbreviations
- BALF
bronchoalveolar lavage fluid
- HDM
house dust mite
- IgE
immunoglobulin E
- IgG
immunoglobulin G
- IL
interleukin
- LRRI
Lovelace Respiratory Research Institute
- OAP
offspring from allergic parents
- PBS
phosphate-buffered saline
- RW
ragweed
- Th2
T helper 2
References
- 1.Ruiz RG, Richards D, Kemeny DM, Price JF. Neonatal IgE: a poor screen for atopic disease. Clin Exp Allergy. 1991;21:467. doi: 10.1111/j.1365-2222.1991.tb01687.x. [DOI] [PubMed] [Google Scholar]
- 2.Aberg N. Familial occurrence of atopic disease: genetic versus environmental factors. Clin Exp Allergy. 1993;23:829. doi: 10.1111/j.1365-2222.1993.tb00260.x. [DOI] [PubMed] [Google Scholar]
- 3.Omran M, Russell G. Continuing increase in respiratory symptoms and atopy in Aberdeen schoolchildren. Br Med J. 1996;312:34. doi: 10.1136/bmj.312.7022.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinez FD. Maternal risk factors in asthma. Ciba Found Symp. 1997;206:233. [PubMed] [Google Scholar]
- 5.Holberg CJ, Morgan WJ, Wright AL, Martinez FD. Differences in familial segregation of FEV1 between asthmatic and nonasthmatic families. Role of a maternal component. Am J Respir Crit Care Med. 1998;158:162. doi: 10.1164/ajrccm.158.1.9706117. [DOI] [PubMed] [Google Scholar]
- 6.Litonjua AA, Carey VJ, Burge HA, Weiss ST, Gold DR. Parental history and the risk for childhood asthma. Does mother confer more risk than father? Am J Respir Crit Care Med. 1998;158:176. doi: 10.1164/ajrccm.158.1.9710014. [DOI] [PubMed] [Google Scholar]
- 7.Tariq SM, Matthews SM, Hakim EA, Stevens M, Arshad SH, Hide DW. The prevalence of and risk factors for atopy in early childhood: a whole population birth cohort study. J Allergy Clin Immunol. 1998;101:587. doi: 10.1016/S0091-6749(98)70164-2. [DOI] [PubMed] [Google Scholar]
- 8.Kurzius-Spencer M, Halonen M, Holberg CJ, Lohman IC, Martinez FD, Wright AL. Prenatal influences on the development of eczema in the first year of life. Am J Respir Crit Care Med. 2002;165:A127. [Google Scholar]
- 9.Wright AL, Holberg CJ, Taussig LM, Martinez F. Material asthma status alters relation of infant feeding to asthma in childhood. Adv Exp Med Biol. 2000;478:131. doi: 10.1007/0-306-46830-1_11. [DOI] [PubMed] [Google Scholar]
- 10.Oddy WH, Holt PG, Sly PD, Read AW, Landau LI, Stanley FJ, Kendall GE, Burton PR. Association between breast feeding and asthma in 6 year old children: findings of a prospective birth cohort study. Br Med J. 1999;319:815. doi: 10.1136/bmj.319.7213.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palfi M, Selbing A. Placental transport of maternal immunoglobulin G. Am J Reprod Immunol. 1998;39:24. doi: 10.1111/j.1600-0897.1998.tb00329.x. [DOI] [PubMed] [Google Scholar]
- 12.Warner JA, Jones CA, Jones AC, Miles EA, Francis T, Warner JO. Immune responses during pregnancy and the development of allergic disease. Pediatr Allergy Immunol. 1997;8–10:5. [PubMed] [Google Scholar]
- 13.Jones CA, Warner JA, Warner JO. Fetal swallowing of IgE [letter] Lancet. 1998;351:1859. doi: 10.1016/S0140-6736(05)78805-X. [DOI] [PubMed] [Google Scholar]
- 14.Bottcher MF, Jenmalm MC, Bjorksten B, Garofalo RP. Chemoattractant factors in breast milk from allergic and nonallergic mothers. Pediatr Res. 2000;47:592. doi: 10.1203/00006450-200005000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Bottcher MF, Jenmalm MC, Garofalo RP, Bjorksten B. Cytokines in breast milk from allergic and nonallergic mothers. Pediatr Res. 2000;47:157. doi: 10.1203/00006450-200001000-00026. [DOI] [PubMed] [Google Scholar]
- 16.Holt PG, O'Keeffe P, Holt BJ, et al. T-cell ‘priming’ against environmental allergens in human neonates: sequential deletion of food antigen reactivity during infancy with concomitant expansion of responses to ubiquitous inhalant allergens. Pediatr Allergy Immunol. 1995;6:85. doi: 10.1111/j.1399-3038.1995.tb00264.x. [DOI] [PubMed] [Google Scholar]
- 17.Jones AC, Miles EA, Warner JO, Colwell BM, Bryant TN, Warner JA. Fetal peripheral blood mononuclear cell proliferative responses to mitogenic and allergenic stimuli during gestation. Pediatr Allergy Immunol. 1996;7:109. doi: 10.1111/j.1399-3038.1996.tb00117.x. [DOI] [PubMed] [Google Scholar]
- 18.Miles EA, Warner JA, Jones AC, Colwell BM, Bryant TN, Warner JO. Peripheral blood mononuclear cell proliferative responses in the first year of life in babies born to allergic parents. Clin Exp Allergy. 1996;26:780. [PubMed] [Google Scholar]
- 19.Miller RL, Chew GL, Bell CA, et al. Prenatal exposure, maternal sensitization, and sensitization in utero to indoor allergens in an inner-city cohort. Am J Respir Crit Care Med. 2001;164:995. doi: 10.1164/ajrccm.164.6.2011107. [DOI] [PubMed] [Google Scholar]
- 20.Piccinni MP, Mecacci F, Sampognaro S, Manetti R, Parronchi P, Maggi E, Romagnani S. Aeroallergen sensitization can occur during fetal life. Int Arch Allergy Immunol. 1993;102:301. doi: 10.1159/000236541. [DOI] [PubMed] [Google Scholar]
- 21.Prescott SL, Macaubes C, Yabuhara A, et al. Developing patterns of T cell memory to environmental allergens in the first two years of life. Int Arch Allergy Immunol. 1997;113:75. doi: 10.1159/000237512. [DOI] [PubMed] [Google Scholar]
- 22.Szepfalusi Z, Pichler J, Elsasser S, van Duren K, Ebner C, Bernaschek G, Urbanek R. Transplacental priming of the human immune system with environmental allergens can occur early in gestation. J Allergy Clin Immunol. 2000;106:530. doi: 10.1067/mai.2000.108710. [DOI] [PubMed] [Google Scholar]
- 23.Redman TK, Rudolph K, Barr EB, Bowen LE, Muggenburg BA, Bice DE. Pulmonary immunity to ragweed in a beagle dog model of allerigc asthma. Exp Lung Res. 2001;27:433. doi: 10.1080/019021401300317143. [DOI] [PubMed] [Google Scholar]
- 24.Stahl WR. Scaling of respiratory variables in mammals. J Appl Physiol. 1967;22:453. doi: 10.1152/jappl.1967.22.3.453. [DOI] [PubMed] [Google Scholar]
- 25.Schlesinger RB. Comparative deposition of inhaled aerosols in experimental animals and humans: a review. J Toxicol Environ Health. 1985;15:197. doi: 10.1080/15287398509530647. [DOI] [PubMed] [Google Scholar]
- 26.Rebar AH, DeNicola DB, Muggenburg BA. Bronchopulmonary lavage cytology in the dog: normal findings. Vet Pathol. 1980;17:294. doi: 10.1177/030098588001700303. [DOI] [PubMed] [Google Scholar]
- 27.Mazza G, Whiting AH, Day MJ, Duffus WP. Development of an enzyme-linked immunosorbent assay for the detection of IgG subclasses in the serum of normal and diseased dogs. Res Vet Sci. 1994;57:133. doi: 10.1016/0034-5288(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 28.Mazza G, Whiting AH, Day MJ, Duffus WP. Preparation of monoclonal antibodies specific for the subclasses of canine IgG. Res Vet Sci. 1994;57:140. doi: 10.1016/0034-5288(94)90049-3. [DOI] [PubMed] [Google Scholar]
- 29.Platts-Mills TA, Erwin EA, Allison AB, Blumenthal K, Barr M, Sredl D, Burge H, Gold D. The relevance of maternal immune responses to inhalant allergens to maternal symptoms, passive transfer to the infant, and development of antibodies in the first 2 years of life. J Allergy Clin Immunol. 2003;111:123. doi: 10.1067/mai.2003.10. [DOI] [PubMed] [Google Scholar]
- 30.Mongan LC, Ockleford CD. Behaviour of two IgG subclasses in transport of immunoglobulin across the human placenta. J Anat. 1996;188:43. [PMC free article] [PubMed] [Google Scholar]
- 31.Prescott SL, Holt PG, Jenmalm M, Bjorksten B. Effects of maternal allergen-specific IgG in cord blood on early postnatal development of allergen-specific T-cell immunity. Allergy. 2000;55:470. doi: 10.1034/j.1398-9995.2000.00286.x. [DOI] [PubMed] [Google Scholar]
- 32.Oshiba A, Hamelmann E, Takeda K, Bradley KL, Loader JE, Larsen GL, Gelfand EW. Passive transfer of immediate hypersensitivity and airway hyperresponsiveness by allergen-specific immunoglobulin (Ig) E and IgG1 in mice. J Clin Invest. 1996;97:1398. doi: 10.1172/JCI118560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jarrett EE, Hall E. IgE suppression by maternal IgG. Immunology. 1983;48:49. [PMC free article] [PubMed] [Google Scholar]
- 34.Jenmalm MC, Holt PG, Bjorksten B. Maternal influence on IgG subclass antibodies to Bet v, 1 during the first 18 months of life as detected with a sensitive ELISA. Int Arch Allergy Immunol. 1997;114:175. doi: 10.1159/000237664. [DOI] [PubMed] [Google Scholar]
- 35.Arshad SH, Matthews S, Gant C, Hide DW. Effect of allergen avoidance on development of allergic disorders in infancy. Lancet. 1992;339:1493. doi: 10.1016/0140-6736(92)91260-f. [DOI] [PubMed] [Google Scholar]
- 36.Peat JK, Tovey E, Gray EJ, Mellis CM, Woolcock AJ. Asthma severity and morbidity in a population sample of Sydney schoolchildren: part II – importance of house dust mite allergens. Aust N Z J Med. 1994;24:270. doi: 10.1111/j.1445-5994.1994.tb02171.x. [DOI] [PubMed] [Google Scholar]
- 37.Peat JK, Tovey E, Toelle BG, Haby MM, Gray EJ, Mahmic A, Woolcock AJ. House dust mite allergens. A major risk factor for childhood asthma in Australia. Am J Respir Crit Care Med. 1996;153:141. doi: 10.1164/ajrccm.153.1.8542107. [DOI] [PubMed] [Google Scholar]
- 38.Sporik R, Holgate ST, Platts-Mills TA, Cogswell JJ. Exposure to house-dust mite allergen (Der p I) and the development of asthma in childhood. A prospective study. N Engl J Med. 1990;323:502. doi: 10.1056/NEJM199008233230802. [DOI] [PubMed] [Google Scholar]
- 39.Chan-Yeung M, Ferguson A, Chan H, Dimich-Ward H, Watson W, Manfreda J, Becker A. Umbilical cord blood mononuclear cell proliferative response to house dust mite does not predict the development of allergic rhinitis and asthma. J Allergy Clin Immunol. 1999;104:317. doi: 10.1016/s0091-6749(99)70373-8. [DOI] [PubMed] [Google Scholar]
- 40.Smillie FI, Elderfield AJ, Patel F, et al. Lymphoproliferative responses in cord blood and at one year: no evidence for the effect of in utero exposure to dust mite allergens. Clin Exp Allergy. 2001;31:1194. doi: 10.1046/j.1365-2222.2001.01173.x. [DOI] [PubMed] [Google Scholar]