Abstract
The expression of the C5a-receptor (C5aR) on dendritic cells, its regulation and function have not been well established thus far. We show that the C5aR is expressed on human monocyte-derived dendritic cells (DC) and can be down-regulated by maturation stimuli such as tumour necrosis factor-α (TNF-α), lipopolysaccharide (LPS) or CD40L and by the T helper 1-cytokine interferon-γ (INF-γ). Prostaglandin E2 (PGE2), a proinflammatory mediator supporting dendritic cell activation and necessary for adequate DC migration, leads to the up-regulation of C5aR expression when incubated alone and prevents down-regulation when given in combination with TNF-α or LPS. Stimulation of C5aR on DC triggered F-actin polymerization, indicating the chemotactic potential of DC elicited by C5a. C5a induced F-actin polymerization was increased when C5aR was up-regulated by PGE2. Stimulation of DC with C5a resulted in interleukin-10 production which was significantly increased after C5aR up-regulation with TNF-α and PGE2. Therefore, up-regulation of the C5aR on human DC alters their chemotactic and immunologic response to C5a.
Introduction
The C5a-receptor mediates proinflammatory and immunomodulatory effects of the anaphylatoxin C5a and its natural catabolite C5adesArg.1 It belongs to the superfamily of G-protein coupled receptors with seven hydrophobic domains and a serpentine transmembrane structure2,3 and is expressed on cells of myeloid origin such as granulocytes and monocytes/macrophages.4,5 Its ligand, C5a, is generated by cleavage of the fifth component of complement (C5) upon activation of the classical or the alternative pathway of the complement system. It has several proinflammatory effects which may lead to changes in blood flow and impairment of vascular integrity associated with oedema.6 These effects are exerted by deliberation of enzymes, activation of effector mechanisms such as production of reactive oxygen species and rapid changes in expression of membrane molecules.7–10
By inducing the synthesis of inflammatory mediators such as interleukin-1 (IL-1), IL-6, IL-8 and tumour necrosis factor-α (TNF-α) and suppressing the production of IL-12 in monocytes, C5a modulates the course of inflammation.11–15 Recently, C5a has been shown to play an important role in allergy by initiating T-cell recruitment in vivo in a murine model of contact hypersensitivity.16,17 Furthermore, in humans the gene locus of C5a has been shown to play a relevant role in the susceptibility to allergic bronchial hyperreactivity.18 Therefore, C5a appears to have a significant role in allergic reactions; however, its exact immunological role has not been elucidated yet. In particular the target cells of C5a are not known. Myeloid dendritic cells (DC) are a possible target for C5aR signalling in allergic reactions, because (1) they are present in allergic inflammation;19–22 (2) they express C5aR; and (3) they show a chemotactic migration towards a C5a gradient.23–26
Myeloid dendritic cells are crucial in initiating the primary immune response.27 They reside in an immature state in many non-lymphoid tissues which are under high pathogen exposure like skin or airways mucosa as sentinels of the immune system.28,29 After receiving activation signals such as bacterial products, cytokines like TNF-α and IL-1β, or cognate signals like CD40 ligation, they migrate to the draining lymph nodes where they encounter naive T cells. After antigen capture and during their migration DC convert from an antigen-capturing to an antigen-presenting mode, which is termed ‘maturation’. They up-regulate major histocompatibility complex (MHC) class II and costimulatory molecules such as CD80, CD86 and CD40. During this process, the anaphylatoxin C5a might influence immune or chemotactic functions of DC.
Therefore, we investigated the regulation of C5aR expression on human DC and immunomodulatory effects of C5a stimulation with regard to receptor expression.24,25 We demonstrate that the C5a receptor is differentially expressed on DC depending on their mode of maturation. DC matured in the absence of prostaglandin E2 (PGE2) down-regulated the C5aR, whereas PGE2 up-regulated the receptor. This up-regulation was associated with an increased response of DC to C5a stimulation with regard to F-actin polymerization and IL-10 production.
Materials and methods
Preparation and fluorescence-activated cell sorting (FACS) analysis of monocyte-derived dendritic cells
Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll-Hypaque density gradient centrifugation of heparinized leucocyte-enriched buffy-coats and allowed to adhere in cell culture flasks in humidified atmosphere at 37° and 5% CO2. After 2 hr, non-adherent cells were removed by five vigorous washes with phosphate-buffered saline (PBS). The remaining adherent cells were cultured in Iscove's modified Dulbecco's medium (IMDM) supplemented with 4% heat-inactivated human serum, 250 U/ml IL-4 (R&D Systems, Wiesbaden, Germany), and 1000 U/ml granulocyte–macrophage colony-stimulating factro (GM-CSF; Novartis Pharma, Nürnberg, Germany). The cultures were fed with fresh medium and cytokines on day 3 of culture. Non-adherent cells, thereafter called immature dendritic cells (DC), were harvested at day 7. Before initiation of further experiments, cells were analysed by double colour flow cytometry for contaminating CD3+ T cells, CD20+ B cells, CD56+ natural killer (NK) cells and CD16+ NK cells or granulocytes, respectively. Only preparations with ≤5% contaminating T, B, or NK cells were used in subsequent experiments (for reverse transcriptase–polymerase chain reaction (RT–PCR) experiments the contamination of T, B or NK cells was required to be <2%) as reported previously.30 For investigation of cell surface marker expression, cells were incubated for 24 hr with C5a (1 µg/ml) with and without 24-hr preincubation with TNF-α and PGE2 (to up-regulate the C5aR) or TNF-α and IFN-γ (to down-regulate the C5aR), respectively. Next, cells were stained with the following antibodies or isotype-matched controls: HLA-DR, CD86, CD80, CD54, CD40, CD83 (all from Immunotech).
Preparation of C5aR antibody
C5aR (CD88) P12/1 monoclonal antibody was generated by intraperitoneal immunization of BALB/c mice, with the peptide EX1, consisting out of the first 31 amino acids of the aminoterminal domain of the C5aR as reported previously.31
Maturation of DC and analysis of C5aR expression by FACS
Immature DC after 7 days of culture in GM-CSF and IL-4 containing media, were matured with LPS (50 ng/ml, Sigma, Deisenhofen, Germany, Escherichia coli serotype 055:B55), CD40L (2 µg/ml, Alexis Biochemicals, San Diego, CA), IFNγ (200 U/ml, R&D Systems), TNF-α (200 U/ml, BioSource, Solingen, Germany), PGE2 (1 µg/ml, Sigma), either alone or in combinations as indicated. After 48 hr, cells were harvested and stained as previously reported.24 In brief, 1 × 105 cells were washed and resuspended in PBS containing 0·2% gelatine (Sigma), 20 mm sodium-azide (Merck, Darmstadt, Germany) and 10 µg/ml heat-aggregated human immunoglobulin G (IgG; Sigma). Subsequently, cells were incubated with saturating doses of anti-C5aR monoclonal antibody P12/1 for 45 min on ice. In a second step, cells were incubated with a fluoroscein isothiocyanate (FITC)-conjugated goat anti-mouse IgG antibody (Dianova, Hamburg, Germany) for 30 min on ice. In double staining experiments, cells were further treated with 50 µg mouse IgG (Sigma) to completely saturate all binding sites of the secondary antibody. After an additional washing step cells were incubated with phycoerythrin-labelled anti-CD83 monoclonal antibody (Immunotech, Hamburg, Germany). Stained cells were washed three times and subsequently analysed using a FACScan (Becton Dickinson, Heidelberg, Germany).
mRNA isolation and RT
mRNA was isolated from 1 × 105 enriched DC using a mRNA isolation kit (Roche Molecular Biochemicals, Mannheim, Germany) according to the suppliers' instructions. The resulting Poly(A)+ RNA was stored at −80°. RNA was then subjected to first strand cDNA synthesis using Oligo(dT)15 for full length cDNA synthesis. The RT reaction mixture contained a final concentration of 50 U Expand-RT (Roche Molecular Biochemicals), 20 U of RNase inhibitor (RNase out, Life Technologies, Eggenheim, Germany), 10 mm dithiothreitol, 1 × first-strand RT buffer for Expand-RT, 0·5 mm of each dNTP (Roche Molecular Biochemicals) and 80 pmol Oligo(dT)15 (Roche Molecular Biochemicals). To control for genomic DNA contamination, cDNA synthesis was performed in the absence of RT. First strand cDNA was stored at −20°.
LightCycler real-time fluorescence PCR
The following primers were used for PCR amplification: C5aR sense: 5′-GAG CCC AGG AGA CCA GAA CAT G, and C5aR antisense: 5′-TAC ATG TTG AGC AGG ATG AGG GA, β-actin sense: 5′-AAG GCC AAC CGC GAG AAG ATG A, and β-actin antisense: 5′-GGA AGA GTG CCT CAG GGC AGC G.32
PCR was performed on a LightCycler (Roche Molecular Biochemicals) in LightCycler capillaries using a commercially available master mix containing Taq DNA polymerase, SYBR-Green I, dNTPs (LightCycler DNA master SYBR-Green I, Roche Molecular Biochemicals). After addition of primers (final concentration: 0·25 pm), MgCl2 (3·5 mm) and template DNA to the master mix, 37 cycles of denaturation (94° for 1 s), annealing (55° for 5 s) and extension (72° for 12 s) were performed. All ramp-rates were set to 20°/s. After the final PCR cycle, the PCR products were denatured at 95°, annealed at 68°, and gradually heated to 95° while the melting curve was recorded by measuring the fluorescence stepwise every 0·1°. After completion of LightCycler analysis, PCR products were subjected to electrophoresis on a 2% agarose gel (Qualex Gold, AGS, Heidelberg, Germany), stained with ethidium bromide, visualized, and photographed under ultraviolet illumination. Expected band lengths were 381 bp for C5aR and 451 bp for β-actin, respectively.
Assessment of F-actin polymerization
Nitrobenzoxadiazole (NBD)-phallacidin (Molecular Probes, Eugene, OR) staining of DC was carried out by modification of the method described by Howard and Meyer.33 Briefly, cells were resuspended at a concentration of 2 × 105 cells/ml in PBS-buffer lacking Ca2+ and stimulated with C5a (Sigma) at room temperature. 1 µg/ml and 0·1 µg/ml C5a were used to stimulate the DC. This corresponds to 8·9 × 10−8 and 8·9 × 10−9 molar concentrations, which are in the range of C5a concentrations considered as ‘chemotactic’.26,34
Following stimulation, cells were fixed using 3·7% formaldehyde for 60 min. Lysophosphatidylcholine (20 µg/ml, Sigma) and 3·7 × 10−7m NBD-phallacidin were added to the sample and incubated for a period of 60 min in the dark. NBD-phallacidin-stained cells were analysed on a Becton Dickinson FACScan with a linear fluorescence channel (FL1) where the fluorescence is proportional to F-actin content. Relative F-actin content is expressed as the ratio of the mean channel fluorescence (= integrated fluorescence) between stimulated and non-stimulated cells.
Cytokine assessment
Immature DC (1 × 105) and DC preincubated with TNF-α and PGE2 or TNF-α and IFN-γ were stimulated with 1 µg/ml and 0·1 µg/ml C5a, respectively. To exclude LPS-induced effects 20 µg/ml polymyxin B (Sigma) was added. Supernatants were harvested after 2 days and analysed for IL-10 content using a commercially available high sensitive enzyme-linked immunosorbent assay (ELISA; R&D Systems).
Statistical analysis
Statistical analyses were performed with the paired t-test. Results with P < 0·05 were considered significant.
Results
Immature DC express the C5aR
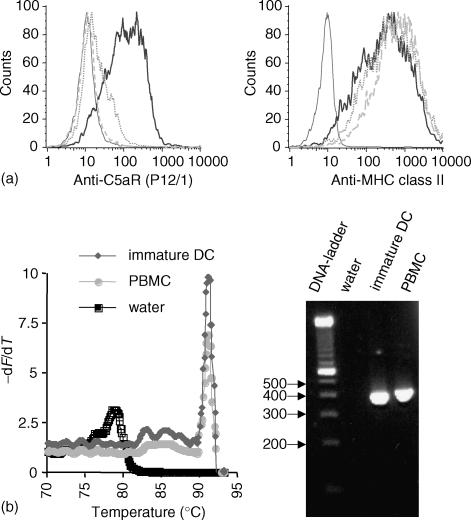
Expression of C5aR on immature DC was demonstrated on the protein and mRNA level. Surface protein expression of C5aR could be detected by binding of monoclonal anti-C5aR antibody P12/1 on immature DC. Specificity of this binding was shown by preincubation of anti-C5aR antibody P12/1 with the specific peptide (EX1) used to generate the antibody that completely abrogated binding of P12/1 to immature DC (Fig. 1a). Moreover, preincubation of DC with C5a diminished binding of P12/1 since the binding site of P12/1 is located in the C5a binding region. In contrast, binding of MHC class II antibody was neither impaired by preincubation of the antibody with EX1 nor by preincubation of cells with C5a (Fig. 1a).
Figure 1.
Immature monocyte derived dendritic cells express the C5aR on the protein level (a) and mRNA level (b). (a) Binding of anti C5aR monoclonal antibody P12/1 to immature DC as determined by fluorescence cytometry. The histogram of the isotype control (thin line) and C5aR monoclonal antibody P12/1 binding (thick line) is shown. The specifity of binding was tested by preincubation of the antibody P12/1 with a 20fold weight excess of the peptide EX1 (dotted line), which had been used for generating the antibody, and by preincubation of the cells with C5a (dashed line). The preincubation prevented the binding of P12/1 to the C5aR. Anti-MHC class II antibody staining was not altered by preincubation of the antibody with EX1 peptide or preincubation of cells with C5a. (b). Detection of C5aR in immature DC by LightCycler RT-PCR. LightCycler melting curve analysis showed the specific peak for C5aR, which is clearly distinct from the peak caused by primer-dimer formation visible in the water (= negative) control. PBMC were used as positive control. Analysis of LightCycler PCR products by agarose gel electrophoresis revealed bands of the expected size (381 bp).
cDNA of immature DC was amplified with a LightCycler System using primers specific for the C5aR. The PCR reaction was analysed by agarose gel electrophoresis (Fig. 1b), demonstrating bands of expected sizes (381 bp). LightCycler melting curve analysis yielded a melting peak for C5aR at 91° (Fig. 1b). This peak was clearly separated from the peak caused by primer–dimer formation as shown with the ‘water’ control without template DNA.
C5aR is up-regulated by PGE2 and down-regulated upon maturation of DC
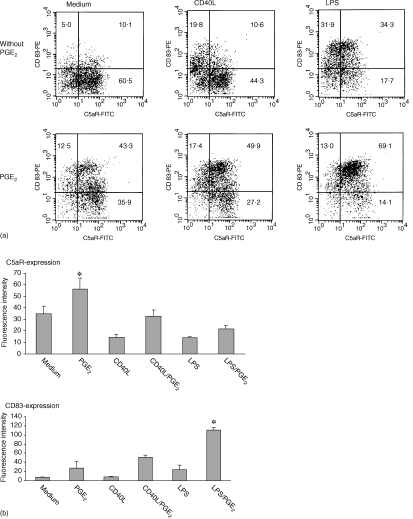
Immature DC were incubated with CD40L or LPS, different stimuli known to induce maturation of dendritic cells. After 48 hr of incubation with LPS or CD40L the DC down-regulated the C5aR and up-regulated the maturation marker CD83 (Fig. 2). Treatment of DC with TNF-α also down-regulated the C5aR (not shown). Addition of PGE2 significantly increased C5aR expression of DC or prevented the down-regulation of C5aR during DC maturation with CD40L or LPS (Fig. 2). PGE2 also significantly increased CD83 expression of LPS (Fig. 2) or TNF-α (data not shown) treated DC.
Figure 2.
PGE2 up-regulates C5aR on DC and prevents its down-regulation upon maturation. Immature DC were incubated with CD40L or LPS, respectively, with or without the presence of PGE2 for 48 hr. The expression of C5aR and CD83 was analysed by fluorescence cytometry. Presence of PGE2 led to up-regulation of C5aR, while LPS and CD40L led to down-regulation. (a) One representative experiment (numbers are percentage of cells in the corresponding quadrants), (b) mean fluorescence intensity ± SEM (of all cells acquired) of five independent experiments (*, significant difference, P < 0.05).
PGE2 promotes the C5aR expression of DC, while an INF-γ-containing-milieu leads to its down-regulation
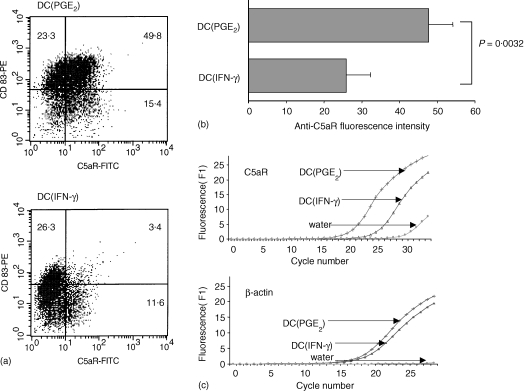
DC were matured with TNF-α in the presence of INF-γ (DC(INF-γ)) or PGE2 (DC(PGE2)). While the maturation marker CD83 was up-regulated on DC(INF-γ) and DC(PGE2), we found a down-regulation of C5aR on DC(INF-γ) and an up-regulation on DC(PGE2). The difference was statistically significant and demonstrated on the protein level by flow cytometry and on the RNA level by real time LightCycler PCR (Fig. 3).
Figure 3.
DC(INF-γ) (matured with TNF-α+ IFN-γ) and DC(PGE2) (matured with TNF-α+ PGE2 show significant differences in C5aR expression, while CD83 is up-regulated on both DC(INF-γ) and DC(PGE2). One representative experiment is shown in (a) (numbers are percentage of cells in the corresponding quadrants), and the mean ± SEM of seven independent experiments is shown in (b). (c) The difference of C5aR expression on DC(INF-γ) and DC(PGE2) on the mRNA level by LightCycler real time PCR.
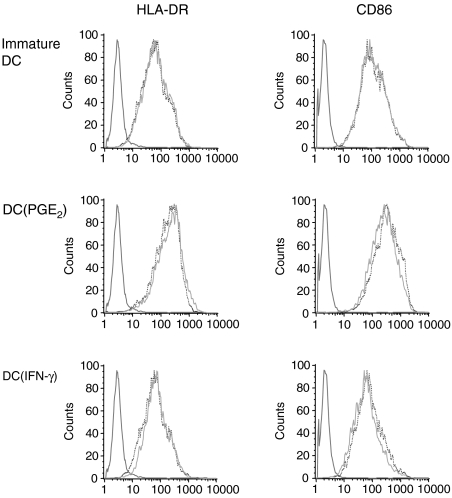
C5a stimulation does not alter expression of surface molecules on DC
DC were either immature or preincubated with TNF-α and PGE2 to up-regulate the C5aR (DC(PGE2)) or TNF-α and IFN-γ to down-regulate the C5aR (DC(IFN-γ)). These pretreated cells were either unstimulated or stimulated for 24 hr with C5a (1 µg/ml). Next, surface molecule expression was assessed by fluorescence cytometry using antibodies binding to CD80, CD86, CD83, CD54, CD40 and HLA-DR. Eleven independent experiments were performed but did not reveal a significant effect of C5a on any of these makers, whereas TNF-α and PGE2 and less TNF-α and IFN-γ (known maturation stimuli) led to an up-regulation of all markers. Figure 4 shows one representative experiment for CD86 and human leucocyte antigen (HLA)-DR, results of the other markers are not shown.
Figure 4.
C5a has no effect of surface molecule expression of DC. Immature DC, DC(PGE2) and DC(IFN-γ) stimulated with C5a did not change the expression of surface molecules as shown here in one representative experiment (out of 11) for HLA-DR and CD86. Note that mature DC(PGE2) have a considerable increase in HLA-DR and CD86 expression as compared to the unstimulated controls.
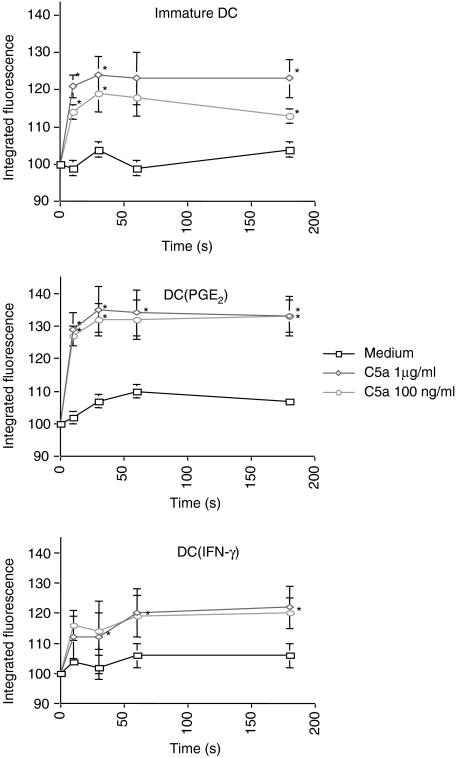
DC(PGE2) show increased F-actin polymerization upon C5a stimulation
F-actin polymerization as an indicator for chemotactic activity was determined in unstimulated DC, DC(INF-γ) and DC(PGE2) in response to C5a stimulation. DC(PGE2) showed an enhanced response to C5a stimulation compared to DC(INF-γ) (Fig. 5), which correlated to the level of receptor expression.
Figure 5.
C5a induces F-actin polymerization in DC correlating to C5aR expression. F-actin polymerization, as an indicator of chemotactic activity, of immature DC, DC(PGE2) and DC(IFN-γ) to stimulation with 1 µg/ml or 0·1 µg/ml C5a was determined. The response of DC(PGE2) was markedly higher than of DC(IFN-γ), corresponding to the C5aR expression level. The mean ± SEM out of n = 5 independent experiments is shown (*, significant difference, P < 0.05).
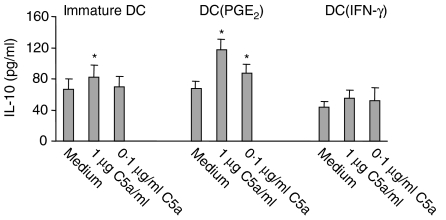
DC(PGE2) produce increased amounts of IL-10 upon C5a stimulation
Immature DC were cultured in medium, DC(PGE2) or DC(IFN-γ) milieu for 24 hr and stimulated with C5a for another 24 hr. The resulting supernatants were subsequently assayed for IL-10 contents. C5a could significantly up-regulate IL-10 production. This effect was increased in DC(PGE2) and decreased in DC(IFN-γ) (Fig. 6).
Figure 6.
C5a induces IL-10 production in DC correlating to C5aR expression.Stimulation of DC(PGE2) resulted in significantly increased IL-10 production upon either 1 µg/ml or 0·1 µg/ml C5a as determined by ELISA. Immature DC showed only after 1 µg/ml C5a stimulation significant increased IL-10 production, whereas DC(IFN-γ) did not significantly alter their IL-10 production after C5a stimulation. The mean value ± SEM of five different experiments is shown (*, significant difference, P < 0.05).
Discussion
In the present study, we investigated the dependence of C5aR expression on monocyte derived DC with regard to the surrounding microenvironment. Immature DC expressed the C5aR receptor, as reported before by Yang et al.26 and by ourselves.24 In our previous study we observed a diminished C5aR expression after TNF-α incubation. Here we show that C5a receptor expression is also decreased by maturation with the cognate stimulus CD40L and the bacterial product LPS. PGE2 could be demonstrated as important factor preventing the down-regulation or even up-regulating C5aR expression on DC.
Yang and coworkers showed the expression of C5aR on monocyte derived dendritic cells and a chemotactic effect of C5a on DC, but no influence of dendritic cell maturation on C5aR expression.26 The difference to our results might be explained by different culture conditions of DC. Yang et al. used media containing fetal calf serum to generate their DC, while we used media supplemented with human serum for our experiments. Using fetal calf serum containing media, we observed only little influence of PGE2 on C5aR expression (data not shown). An important effect of the type of sera used on dendritic cells has also been reported for the IL-12 production by DC. DC differentiated in media containing fetal calf serum produced much higher IL-12 levels upon stimulation as compared to cells cultured in medium containing human serum.35 Moreover, Chang et al. demonstrated the importance of the cell culture medium as well: The use of RPMI resulted in different subsets of DC with regard to surface protein expression and cytokine profile as compared to DC generated in media based on IMDM.36 Here we used IMDM supplemented with human AB serum which could be shown to lead to a reproducible DC-specific phenotype.
Similar to other groups26,37,38 we were able to show an effect of C5a on the cytoskeleton of DC, indicated by F-actin polymerization, as indirect measurement for chemotaxis. This effect was markedly increased after up-regulation of the C5aR with TNF-α and PGE2. Therefore, PGE2 can maintain and increase the migratory potential of DC to C5a despite the maturation process which is induced by the stimulation with TNF-α and PGE2. The migratory effect of C5a on mature DC has also been shown in a recent study in DC matured with TNF-α alone.26 PGE2 has recently been demonstrated to represent a crucial stimulus for the migration of human monocyte derived DC. Scandella et al. showed PGE2 to be obligatory for the up-regulation of the CCR7 and migration of DC towards CCR7-ligands, in particular CCL19 and CCL21.39 The Maraskovsky's group defined ‘migratory’ type DC, migrating towards a gradient of the CCR7 ligands CCL19 and CCL21 and to proinflammatory chemokines, such as CXCL12, CCL3, CCL7 and CXCL9, which were obtained after maturation with PGE2. In contrast, DC matured in the absence of PGE2 did not migrate towards these chemokines and were termed ‘non-migratory’, located, cytokine-secreting DC.40 Both types of DC expressed similar levels of CCR7, therefore the authors speculate on a possible activation of CCR7 signalling pathways by PGE2.
Our findings of PGE2 as a C5aR up-regulating factor highlight another facet of its important role concerning the complex mechanisms of DC migration. This might play a role in diseases such as psoriasis, where increased numbers of mature dendritic cells were found.41,42 C5a has been demonstrated as main attractor for DC in psoriatic scales, and PGE2 is also significantly increased intralesionally.43 It is tempting to speculate that PGE2 augments the DC attracting capabilities of C5a in this disease.
C5a also exerts immunomodulatory effects, for example our laboratory and others could demonstrate that C5a suppresses the production of IL-12 of prestimulated monocytes.15,23 Here, we show an induction of IL-10 production by C5a, which was increased after up-regulation of the C5aR with PGE2 and decreased after down-regulation with IFN-γ (Fig. 6). This is interesting, since IL-10 has been shown to result in uncoupling of chemokine receptors such as CCR1, CCR2 and CCR5 in DC, leading to the inability of these chemokine receptors to elicit migration.26,44 With regard to C5aR signalling, F-actin polymerization was maintained and even increased despite an increase of IL-10 production. Therefore, C5aR function might not be affected by IL-10 in contrast to chemokine receptors. However, since the maximal increase of IL-10 from 67 pg/ml to 117 pg/ml was only about twofold, the biological significance of C5a induced IL-10 production remains to be studied.
In summary, the C5aR is increased on MoDC by PGE2, which results in increased chemotactic and immunomodulatory effects of C5a on MoDC. This could be relevant in particular in diseases such as psoriasis, where dendritic cells, PGE2 and C5a have been demonstrated to play important pathogenetic roles.37,41,42,45,46
Acknowledgments
O.W. and R.G. contributed equally to this study. Supported by DFG grant WE 1289/2–1.
References
- 1.Goldstein IM. Complement-biologically active products. In: Gallin JI, Goldstein IM, Snyderman R, editors. Inflammation: Basic Principles and Clinical Correlates. 2. New York: Raven Press; 1992. pp. 63–80. [Google Scholar]
- 2.Gerard C, Gerard NP. C5A anaphylatoxin and its seven transmembrane-segment receptor. Annu Rev Immunol. 1994;12:775–808. doi: 10.1146/annurev.iy.12.040194.004015. [DOI] [PubMed] [Google Scholar]
- 3.Boulay F, Mery L, Tardif M, Brouchon L, Vignais P. Expression cloning of a receptor for C5a anaphylatoxin on differentiated HL-60 cells. Biochemistry. 1991;30:2993–9. doi: 10.1021/bi00226a002. [DOI] [PubMed] [Google Scholar]
- 4.Zwirner J, Fayyazi A, Gotze O. Expression of the anaphylatoxin C5a receptor in non-myeloid cells. Mol Immunol. 1999;36:877–84. doi: 10.1016/s0161-5890(99)00109-1. [DOI] [PubMed] [Google Scholar]
- 5.Werfel T, Oppermann M, Schulze M, Krieger G, Weber M, Gotze O. Binding of fluorescein-labeled anaphylatoxin C5a to human peripheral blood, spleen, and bone marrow leukocytes. Blood. 1992;79:152–60. [PubMed] [Google Scholar]
- 6.Frank MM, Fries LF. The role of complement in inflammation and phagocytosis. Immunol Today. 1991;12:322–6. doi: 10.1016/0167-5699(91)90009-I. [DOI] [PubMed] [Google Scholar]
- 7.Werfel T, Sonntag G, Weber MH, Gotze O. Rapid increases in the membrane expression of neutral endopeptidase (CD10), aminopeptidase N (CD13), tyrosine phosphatase (CD45), and Fc gamma-RIII (CD16) upon stimulation of human peripheral leukocytes with human C5a. J Immunol. 1991;147:3909–14. [PubMed] [Google Scholar]
- 8.Elsner J, Dichmann S, Kapp A. Activation of the respiratory burst in human eosinophils by chemotaxins requires intracellular calcium fluxes. J Invest Dermatol. 1995;105:231–6. doi: 10.1111/1523-1747.ep12317519. [DOI] [PubMed] [Google Scholar]
- 9.Fayyazi A, Sandau R, Duong LQ, Gotze O, Radzun HJ, Schweyer S, Soruri A, Zwirner J. C5a receptor and interleukin-6 are expressed in tissue macrophages and stimulated keratinocytes but not in pulmonary and intestinal epithelial cells. Am J Pathol. 1999;154:495–501. doi: 10.1016/S0002-9440(10)65295-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris JA, Hyde DM, Wang QJ, Stovall MY, Giri SN. Repeated episodes of C5a-induced neutrophil influx do not result in pulmonary fibrosis. Inflammation. 1991;15:233–50. doi: 10.1007/BF00918649. [DOI] [PubMed] [Google Scholar]
- 11.Ember JA, Sanderson SD, Hugli TE, Morgan EL. Induction of interleukin-8 synthesis from monocytes by human C5a anaphylatoxin. Am J Pathol. 1994;144:393–403. [PMC free article] [PubMed] [Google Scholar]
- 12.Montz H, Koch KC, Zierz R, Gotze O. The role of C5a in interleukin-6 production induced by lipopolysaccharide or interleukin-1. Immunology. 1991;74:373–9. [PMC free article] [PubMed] [Google Scholar]
- 13.Morin M, Schindler R, Wakabayashi G, Daumy G, Dinarello CA, Gelfand JA. Picogram concentrations of endotoxin stimulate synthesis of IL-1 beta and TNF alpha by human peripheral blood mononuclear cells exposed to recombinant human C5a. Eur Cytokine Netw. 1991;2:27–30. [PubMed] [Google Scholar]
- 14.Schindler R, Lonnemann G, Shaldon S, Koch KM, Dinarello CA. Transcription, not synthesis, of interleukin-1 and tumor necrosis factor by complement. Kidney Int. 1990;37:85–93. doi: 10.1038/ki.1990.12. [DOI] [PubMed] [Google Scholar]
- 15.Wittmann M, Zwirner J, Larsson VA, Kirchhoff K, Begemann G, Kapp A, Gotze O, Werfel T. C5a suppresses the production of IL-12 by IFN-gamma-primed and lipopolysaccharide-challenged human monocytes. J Immunol. 1999;162:6763–9. [PubMed] [Google Scholar]
- 16.Tsuji RF, Geba GP, Wang Y, Kawamoto K, Matis LA, Askenase PW. Required early complement activation in contact sensitivity with generation of local C5-dependent chemotactic activity, and late T cell interferon gamma: a possible initiating role of B cells. J Exp Med. 1997;186:1015–26. doi: 10.1084/jem.186.7.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsuji RF, Kawikova I, Ramabhadran R, Akahira-Azuma M, Taub D, Hugli TE, Gerard C, Askenase PW. Early local generation of C5a initiates the elicitation of contact sensitivity by leading to early T cell recruitment. J Immunol. 2000;165:1588–98. doi: 10.4049/jimmunol.165.3.1588. [DOI] [PubMed] [Google Scholar]
- 18.Karp CL, Grupe A, Schadt E, et al. Identification of complement factor 5 as a susceptibility locus for experimental allergic asthma. Nat Immunol. 2000;1:221–6. doi: 10.1038/79759. [DOI] [PubMed] [Google Scholar]
- 19.Banfield CC, Callard RE, Harper JI. The role of cutaneous dendritic cells in the immunopathogenesis of atopic dermatitis. Br J Dermatol. 2001;144:940–6. doi: 10.1046/j.1365-2133.2001.04179.x. [DOI] [PubMed] [Google Scholar]
- 20.Till SJ, Jacobson MR, O'Brien F, Durham SR, KleinJan A, Fokkens WJ, Juliusson S, Lowhagen O. Recruitment of CD1a+ Langerhans cells to the nasal mucosa in seasonal allergic rhinitis and effects of topical corticosteroid therapy. Allergy. 2001;56:126–31. doi: 10.1034/j.1398-9995.2001.056002126.x. [DOI] [PubMed] [Google Scholar]
- 21.Lambrecht BN, De Veerman M, Coyle AJ, Gutierrez-Ramos JC, Thielemans K, Pauwels RA. Myeloid dendritic cells induce Th2 responses to inhaled antigen, leading to eosinophilic airway inflammation. J Clin Invest. 2000;106:551–9. doi: 10.1172/JCI8107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bertorelli G, Bocchino V, Zhou X, Zanini A, Bernini MV, Di Damia RCV, Grima P, Olivieri D. Dendritic cell number is related to IL-4 expression in the airways of atopic asthmatic subjects. Allergy. 2000;55:449–54. doi: 10.1034/j.1398-9995.2000.055005449.x. [DOI] [PubMed] [Google Scholar]
- 23.Braun MC, Lahey E, Kelsall BL. Selective suppression of IL-12 production by chemoattractants. J Immunol. 2000;164:3009–17. doi: 10.4049/jimmunol.164.6.3009. [DOI] [PubMed] [Google Scholar]
- 24.Kirchhoff K, Weinmann O, Zwirner J, Begemann G, Gotze O, Kapp A, Werfel T. Detection of anaphylatoxin receptors on CD83+ dendritic cells derived from human skin. Immunology. 2001;103:210–7. doi: 10.1046/j.1365-2567.2001.01197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morelli A, Larregina A, Chuluyan I, Kolkowski E, Fainboim L. Expression and modulation of C5a receptor (CD88) on skin dendritic cells. Chemotactic effect of C5a on skin migratory dendritic cells. Immunology. 1996;89:126–34. doi: 10.1046/j.1365-2567.1996.d01-701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang D, Chen Q, Stoll S, Chen X, Howard OM, Oppenheim JJ. Differential regulation of responsiveness to fMLP and C5a upon dendritic cell maturation: correlation with receptor expression. J Immunol. 2000;165:2694–702. doi: 10.4049/jimmunol.165.5.2694. [DOI] [PubMed] [Google Scholar]
- 27.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 28.Gong JL, McCarthy KM, Telford J, Tamatani T, Miyasaka M, Schneeberger EE. Intraepithelial airway dendritic cells: a distinct subset of pulmonary dendritic cells obtained by microdissection. J Exp Med. 1992;175:797–807. doi: 10.1084/jem.175.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nestle FO, Zheng XG, Thompson CB, Turka LA, Nickoloff BJ. Characterization of dermal dendritic cells obtained from normal human skin reveals phenotypic and functionally distinctive subsets[published erratum appears. J Immunol. 1994;152(1):376. [PubMed] [Google Scholar]; J Immunol. 1993. pp. 6535–45. [PubMed]
- 30.Gutzmer R, Langer K, Lisewski M, Mommert S, Rieckborn D, Kapp A, Werfel T. Expression and function of histamine receptors 1 and 2 on human monocyte-derived dendritic cells. J Allergy Clin Immunol. 2002;109:524–31. doi: 10.1067/mai.2002.121944. [DOI] [PubMed] [Google Scholar]
- 31.Oppermann M, Raedt U, Hebell T, Schmidt B, Zimmermann B, Gotze O. Probing the human receptor for C5a anaphylatoxin with site-directed antibodies. Identification of a potential ligand binding site on the NH2-terminal domain. J Immunol. 1993;151:3785–94. [PubMed] [Google Scholar]
- 32.Werfel T, Zwirner J, Oppermann M, Sieber A, Begemann G, Drommer W, Kapp A, Gotze O. CD88 antibodies specifically bind to C5aR on dermal CD117+ and CD14+ cells and react with a desmosomal antigen in human skin. J Immunol. 1996;157:1729–35. [PubMed] [Google Scholar]
- 33.Howard TH, Meyer WH. Chemotactic peptide modulation of actin assembly and locomotion in neutrophils. J Cell Biol. 1984;98:1265–71. doi: 10.1083/jcb.98.4.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monsinjon T, Gasque P, Chan P, Ischenko A, Brady JJ, Fontaine MC. Regulation by complement C3a and C5a anaphylatoxins of cytokine production in human umbilical vein endothelial cells. FASEB J. 2003;17:1003–14. doi: 10.1096/fj.02-0737com. [DOI] [PubMed] [Google Scholar]
- 35.Ebner S, Ratzinger G, Krosbacher B, et al. Production of IL-12 by human monocyte-derived dendritic cells is optimal when the stimulus is given at the onset of maturation, and is further enhanced by IL-4. J Immunol. 2001;166:633–41. doi: 10.4049/jimmunol.166.1.633. [DOI] [PubMed] [Google Scholar]
- 36.Chang CC, Wright A, Punnonen J. Monocyte-derived CD1a+ and CD1a- dendritic cell subsets differ in their cytokine production profiles, susceptibilities to transfection, and capacities to direct Th cell differentiation. J Immunol. 2000;165:3584–91. doi: 10.4049/jimmunol.165.7.3584. [DOI] [PubMed] [Google Scholar]
- 37.Mrowietz U, Koch WA, Zhu K, Wiedow O, Bartels J, Christophers E, Schroder JM. Psoriasis scales contain C5a as the predominant chemotaxin for monocyte-derived dendritic cells. Exp Dermatol. 2001;10:238–45. doi: 10.1034/j.1600-0625.2001.100403.x. [DOI] [PubMed] [Google Scholar]
- 38.Sozzani S, Sallusto F, Luini W, et al. Migration of dendritic cells in response to formyl peptides, C5a, and a distinct set of chemokines. J Immunol. 1995;155:3292–5. [PubMed] [Google Scholar]
- 39.Scandella E, Men Y, Gillessen S, Forster R, Groettrup M. Prostaglandin E2 is a key factor for CCR7 surface expression and migration of monocyte-derived dendritic cells. Blood. 2002;100:1354–61. doi: 10.1182/blood-2001-11-0017. [DOI] [PubMed] [Google Scholar]
- 40.Luft T, Jefford M, Luetjens P, et al. Functionally distinct dendritic cell (DC) populations induced by physiologic stimuli. prostaglandin E (2) regulates the migratory capacity of specific DC subsets. Blood. 2002;100:1362–72. doi: 10.1182/blood-2001-12-0360. [DOI] [PubMed] [Google Scholar]
- 41.Nestle FO, Turka LA, Nickoloff BJ. Characterization of dermal dendritic cells in psoriasis. Autostimulation of T lymphocytes and induction of Th1 type cytokines. J Clin Invest. 1994;94:202–9. doi: 10.1172/JCI117308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koga T, Duan H, Urabe K, Furue M. In situ localization of CD83-positive dendritic cells in psoriatic lesions. Dermatology. 2002;204:100–3. doi: 10.1159/000051825. [DOI] [PubMed] [Google Scholar]
- 43.Reilly DM, Parslew R, Sharpe GR, Powell S, Green MR. Inflammatory mediators in normal, sensitive and diseased skin types. Acta Derm Venereol. 2000;80:171–4. doi: 10.1080/000155500750042907. [DOI] [PubMed] [Google Scholar]
- 44.D'Amico G, Frascaroli G, Bianchi G, et al. Uncoupling of inflammatory chemokine receptors by IL-10: generation of functional decoys. Nat Immunol. 2000;1:387–91. doi: 10.1038/80819. [DOI] [PubMed] [Google Scholar]
- 45.Bergh K, Iversen OJ, Lysvand H. Surprisingly high levels of anaphylatoxin C5a des Arg are extractable from psoriatic scales. Arch Dermatol Res. 1993;285:131–4. doi: 10.1007/BF01112914. [DOI] [PubMed] [Google Scholar]
- 46.Takematsu H, Tagami H. Quantification of chemotactic peptides (C5a anaphylatoxin and IL-8) in psoriatic lesional skin. Arch Dermatol. 1993;129:74–80. [PubMed] [Google Scholar]