Abstract
Signalling through the B-cell antigen receptor (BCR) is required throughout B-cell development and peripheral maturation. Targeted disruption of BCR components or downstream effectors indicates that specific signalling mechanisms are preferentially required for central B-cell development, peripheral maturation and repertoire selection. Additionally, the avidity and the context in which antigen is encountered determine both cell fate and differentiation in the periphery. Although the signalling and receptor components required at each stage have been largely elucidated, the molecular mechanisms through which specific signalling are evoked at each stage are still obscure. In particular, it is not known how the pre-BCR initiates the signals required for normal development or how immature B cells regulate the signalling pathways that determine cell fate. In this review, we will summarize the recent studies that have defined the molecules required for B-cell development and maturation as well as the theories on how signals may be regulated at each stage.
Introduction
First identified in 1970,1 the B-cell antigen receptor (BCR) is a multimeric complex consisting of an antigen-recognition structure and a membrane-bound immunoglobulin (mIg), associated non-covalently with a heterodimer of Igα and Igβ (Fig. 1). Except for immunoglobulin G (IgG), the cytoplasmic tails of the five types of mIg are all extremely short and lack signalling capacity.2–4 The 28 amino acid cytoplasmic tail of IgG does not have independent signalling capacity, but may serve to enhance peripheral immune responses.5,6 Signalling through the BCR is mediated by Igα and Igβ. Each mIg associates with a single Igα/Igβ heterodimer, and is, in turn, associated on the cell surface with several other mIg–Igα/Igβ complexes.7 Igα and Igβ each contain a large disulphide-linked extracellular domain (114 amino acids for murine Igα and 132 amino acids for murine Igβ), a transmembrane region and a cytoplasmic tail. Within each extracellular region are an immunoglobulin domain and a membrane-proximal stalk. The latter contains the cysteines that form the heterodimer-stabilizing disulphide bond. In addition, the extracellular region of Igβ contains a highly conserved N-terminal domain of 17 amino acids, the function of which is unknown. The transmembrane regions of Igα and Igβ are unremarkable, except for a polar patch in Igβ that probably associates with the transmembrane domain of the heavy chain.2,8 Although interactions in the transmembrane domains are dominant for most isotypes, including immunoglobulin D (IgD), other lower-affinity extracellular interactions may stabilize receptor complexes containing immunoglobulin M (IgM).9–11 The cytoplasmic tails of Igα and Igβ consist of 61 and 48 amino acids, respectively.12 Although these domains do not have any predicted secondary structure, they contain specific features that are required for initiating intracellular signalling pathways.
Figure 1.
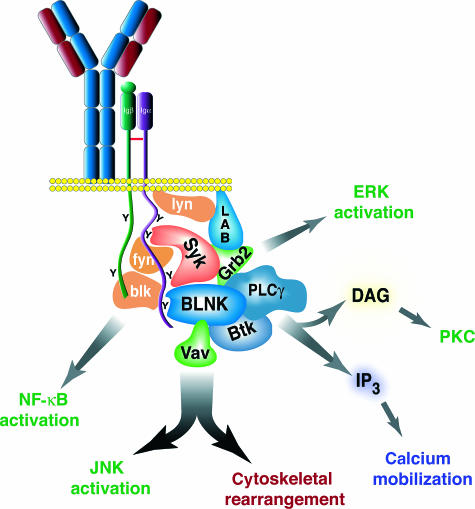
Proximal B-cell receptor-mediated signalling pathways. After binding to antigen, the immunoglobulin (Ig)α and Igβ cytoplasmic tails are phosphorylated on the immunoreceptor tyrosine-based activation motif (ITAM) tyrosines by Src-family tyrosine kinases (SFTKs) and/or Syk. Syk then binds to the Igα ITAM, and the B-cell linker protein (BLNK) binds to tyrosine 204 of Igα. This activates multiple signalling pathways, including: Btk, which activates phospholipase C (PLC)γ2 and leads to calcium flux (blue) and protein kinase C (PKC) activation (green); Grb2, which activates the Ras/Raf/mitogen-activated protein kinase (MEK) extracellular signal-regulated kinase (ERK) pathway (green); and Vav, which activates the Rac/Rho/Cdc42 pathway and results both in cytoskeletal rearrangement (maroon) and c-Jun N-terminal protein kinase (JNK) activation (green). The SFTKs themselves activate nuclear factor-κB (NF-κB) (green).
Initiation of receptor signalling
The signalling capacities of both Igα and Igβ are dependent upon a specific motif, found within each cytosolic tail, known as the immunoreceptor tyrosine-based activation motif (ITAM). Described by Reth in 1989,13,14 the core of this motif (D/E(X)7D/EXXYXXI/L(X)7YXX I/L) comprises two tyrosine residues separated by 11 residues, each followed by leucine or isoleucine at the +3 position. Other receptors involved in antigen responses, including the T-cell receptor (TCR) and many Fc receptors, also contain ITAMs.15,16 Mutational analysis has illustrated that the tyrosines, the 11 amino acid spacer between them17 and the +3 isoleucine/leucine residues,17,18 are all required for proper initiation of BCR-mediated signalling pathways.
Significant effort has been spent on determining how receptor aggregation induces phosphorylation of the ITAM tyrosines. The resting BCR is assembled with Src family tyrosine kinases (SFTKs), such as Blk, Lyn and Fyn, which become activated following receptor ligation.19 The tyrosine kinase Syk can also be detected in the resting receptor complex. The association of these tyrosine kinases with the receptor is mediated by unique tyrosine-independent motifs embedded within the cytosolic tail of Igα.20–22 These embedded motifs, in part, determine the signalling capacity of each chain and contribute to the preferred role of Igα as the primary activator of tyrosine kinases.17,23,24In vitro experiments, and reconstitutions in non-immune cells,25 indicated that Src kinases were the primary mediator of ITAM phosphorylation. However, recent studies have questioned this model. Stimulation of SFTK-deficient Drosophila cells reconstituted with Igα, Igβ and the non-Src family tyrosine kinase Syk (see below) resulted in tyrosine phosphorylation of Igα and Igβ,26 indicating that Syk may be a primary kinase. This conclusion is supported by studies of pro-B cells from mice lacking the Src-family kinases Lyn, Fyn and Blk, in which receptor cross-linking induces robust phosphorylation of Igα and Igβ.27
Tyrosine phosphorylation of the ITAM tyrosines enhances the recruitment and activation of Syk, which is the principal kinase that drives many signalling pathways, including the activation of phospholipase C γ2 (PLCγ2) and Ras. Syk is required for normal B-cell development. However, not all signalling pathways are dependent upon Syk, as the activation of nuclear factor κB (NF-κB) appears to be directly dependent on the activation of one or more Src-family kinases.27 These data indicate that the BCR independently activates both Syk and the Src-family kinases to initiate complementary downstream signalling pathways.
Coupling of receptor-associated kinases to downstream pathways is affected through a series of linker molecules, the most important of which is the B-cell adapter molecule BLNK (also referred to as SLP-65 or BASH). BLNK is a B-lymphocyte-specific 65 000 molecular weight analogue of the T-cell linker molecule SLP-76; it lacks intrinsic enzymatic activity yet contains several functional domains, including an SH2 domain, proline-rich domains and several potential tyrosine-phosphorylation sites. Recent evidence indicates that the SH2 domain of BLNK binds directly to a single, unique non-ITAM-phosphorylated tyrosine in the cytosolic tail of Igα.28,29 BLNK recruitment is required in vitro for coupling Igα to distal pathways, such as PLCγ2,28 and is important in vivo for pre-B-cell development, Dµ counterselection, tumour suppression, and peripheral selection30 (L. D. Wang, manuscript in preparation). Phosphorylation of the non-ITAM tyrosine and the recruitment of BLNK occur contemporaneously with phosphorylation of the ITAM tyrosines and the enhanced recruitment of Syk.28 Therefore, it is probable that BLNK recruitment to Igα is important in initiating signal activation.
BLNK interacts directly with PLCγ2 as well as with Btk,31–33 a tyrosine kinase required for normal PLCγ2 activation. It is probable that BLNK co-ordinates the approximation of both Syk and Btk with PLCγ2 to permit rapid and efficient activation.34 BLNK also recruits the Rho-family GTPase, Vav, and the linker protein, Nck,35–40 both of which are important in cytoskeleton remodelling. Recruitment of these molecules to the BCR brings them into close proximity with receptor-associated kinases necessary for their activation. BLNK is also constitutively associated with the linker molecule Grb2, coupling the BCR to the Ras pathway,33,38 as well as the negative regulatory co-receptor CD72 and the PTPase SHP-1.41 These results indicate that sequential protein–protein interactions nucleate a signalsome at the antigen receptor.
BLNK, like SLP-76, has been shown to interact indirectly with a lipid-raft associated linker, the linker of activated B cells (LAB), which may serve to recruit activated substrates into lipid rafts and augment distal signalling pathways.42 However, as with the TCR, it is unlikely that localization of substrates within lipid rafts is required for signal initiation in peripheral B cells. For example, the ligated receptor localizes to lipid rafts irrespective of receptor kinase activation (M. R. Clark, unpublished observation).43 However, as discussed below, lipid rafts may have an important function in initiating the pre-B-cell signals required for B-cell development, and may have additional roles in propagating signals.
Assembly of the signalsome on the cytosolic tail of Igα is dependent upon the phosphorylation status of the ITAM and non-ITAM tyrosines. From studies of receptor complexes containing just Igα or Igβ, it is apparent that Igβ can function as an amplifer of total Igα phosphorylation, serving to increase receptor sensitivity and lower receptor threshold.44 A similar function has been ascribed to the β chain of the Fcε receptor.45 Conversely, the C-terminal tyrosine in the Igα ITAM is negatively regulated by serine/threonine phosphorylation.46 As observed in growth factor receptor systems, serine/threonine kinases may provide feedback mechanisms to extinguish receptor signals and raise receptor threshold.
Signalling through the B-cell receptor is an intricate and complex process that has been predominantly studied in mature or activated B-cell lines. However, it is clear that BCR signalling is responsible for a wide variety of physiologically distinct responses through the course of B-cell development, implying that discrete signalling pathway subsets are developmentally co-ordinated as cells mature. Although the regulatory mechanisms that allow this co-ordination to occur are just starting to be elucidated, many of the developmental end-points are well characterized.
B-cell development
Pro-B cells
Pro-B cells represent the first irrevocably committed B-cell precursors,47,48 which can be distinguished from pre-pro-B cells by surface expression of CD19 and increased expression of HSA49 (Fig. 2). In these cells, the Igα–Igβ heterodimer is expressed on the cell surface in association with calnexin and perhaps other chaperone molecules.50 Rearrangement of the BCR heavy-chain locus (see below) is initiated during the pro-B-cell stage. However, the pre-BCR is not required for lineage commitment and the initiation of recombination. Rather, this is dependent upon the intrinsic expression of the E2A family transcription factors E12 and E47,51 and the transcription factor EBF,52 which have been shown to up-regulate expression of the B-cell-specific genes λ5, VpreB, Igα and Igβ, as well as the lymphoid-specific recombinase-activating genes RAG-1 and RAG-2, and the B-cell-specific transcription factor Pax5, or BSAP.53–55 Lineage commitment is enforced at the pro-B-cell stage by Pax5, which both activates B-cell-specific genes (including BLNK, CD19 and Igα) and represses the expression of non-B-lineage genes (including Notch1).47,56,57
Figure 2.
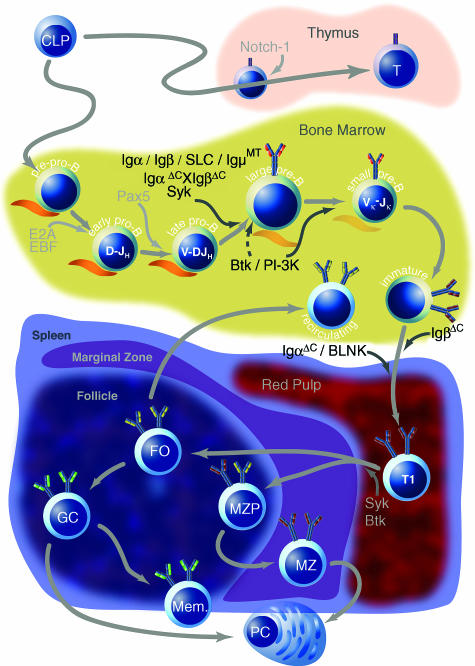
Overview of B-cell development in postnatal mammalian life. Common lymphoid progenitors (CLPs) can migrate to the thymus where they become T cells in response to signalling through Notch-1, or they can remain in the bone marrow where they enter the B lineage as pre-pro-B cells. Possibly in response to Notch-1 down-regulation by Pax5, transcription factors EBF and E2A are up-regulated to initiate a B-cell-specific programme of gene transcription. DH–JH rearrangements occur at the early pro-B stage, and subsequent V–DJ rearrangements commence at the late pro-B stage. These stages require direct contact with bone marrow stromal cells, depicted in orange. Signalling molecules, whose absence results in a developmental block, are listed in black; dashed arrows indicate incomplete impairment. Appropriate signalling through the pre-B-cell receptor (BCR) (the surrogate light chain is depicted in orange) mediates heavy-chain allelic exclusion and induces several rounds of proliferation. Subsequently, at the small pre-B stage, light-chain rearrangements begin. Successfully rearranged heavy and light chains appear on the cell surface at the immature B-cell stage, after which B cells emigrate to the periphery. Newly immigrated transitional immature B cells (T1) probably undergo further differentiation into T2 and possibly T3 transitional immature B cells, although this is controversial. Eventually, transitional cells enter splenic follicles, where they differentiate further along the marginal zone (MZP, MZ) or follicular (FO) pathways, probably in response to the strength of signals received through the BCR. Follicular cells differentiate either into plasma cells (PCs) or germinal centre B cells (GC) in response to primary antigen challenge, whereupon they undergo somatic hypermutation, affinity maturation and class switch before they differentiate into memory cells. Marginal zone cells, which leave the splenic follicle to reside in the marginal zone, are also capable of differentiating into plasma cells in response to primary antigen exposure.
Early B-cell development is not entirely intrinsic, as signalling through the interleukin-7 receptor (IL-7R) is required to generate CD19+ CD43+ B220+ pro-B cells.58 Interleukin-7 (IL-7) signalling also induces pro-B cells to proliferate and expand, and has been shown to up-regulate expression of CD19 and Pax5.59,60
The development of pre-B cells
Surface expression of a signalling-competent pre-BCR, containing an in-frame V–DJ rearrangement of the heavy chain, allows progression from the pro-B-cell to pre-B-cell stage; this is the earliest stage at which BCR signalling is required. Targeted gene mutations that eliminate surface expression of the pre-BCR result in developmental arrest at the pro- to pre-B-cell checkpoint. These include mutations in pre-BCR components, such as membrane-anchored heavy chain (µMT), λ5, VpreB, Igα and Igβ.61–64 Appropriate pre-BCR signalling results in allelic exclusion at the heavy-chain locus65,66 and also leads to changes in the phenotype of developing B cells (Fig. 2); cells become larger as they undergo a proliferative burst of two to five cycles67 and become more IL-7 responsive.68 After proliferation, cells enter the small pre-B stage, where they down-regulate HSA, CD43 and IL-7R, becoming IL-7 unresponsive. They then begin the process of light chain rearrangement, first at the kappa locus and then at the lambda locus.69,70
Although it is clear that a signal must be transduced through the pre-BCR in order for development to progress, the mechanisms responsible for initiating this signal are unclear. It has been proposed that simple assembly of the resting BCR complex is sufficient to mediate development. This model is supported by studies in which retroviral expression of a construct encoding the myristylation domain of Lck and the cytoplasmic domains of Igα and Igβ in RAG–/– pro-B cells is able to rescue progression past the pre-B stage.71 This model is also consistent with studies in which mice bearing a truncated µ chain that cannot associate with surrogate light chains, or a µ chain in which a majority of the extracellular region has been replaced with an irrelevant protein (CD8), are still competent to mediate later stages of B-cell development.72,73 In apparent contrast to these findings, a recent study suggests that a surrogate light chain is necessary for pre-BCR aggregation and signal initiation.74 Therefore, the mechanisms by which the pre-BCR mediates B-cell development are still unclear.
Even if surface expression is all that is required, it is probable that some aggregation of the receptors occurs at the cell surface. This could occur spontaneously, as has been hypothesized in the mature BCR,7 or through localization in cholesterol- and sphingolipid-rich lipid microdomains. In human pre-B-cell lines, 20–30% of resting pre-B-cell receptors accumulate in lipid rafts, whereas in mature B-cell lines, the BCR is excluded from the lipid rafts.75 Lipid rafts create microenvironments of skewed signalling molecule composition that may promote or prevent the activation of various proximal cascades.43
Some studies argue that pre-B-cell development is mediated by a selecting ligand in the bone marrow that aggregates the pre-B-cell receptor. Ligation of the Igα–Igβ heterodimer on pro-B cells using antibodies to the extracellular domain of Igβ induces cells to mature to the pre-B stage.50 Potential stromal ligands for the BCR,76 including galectin, have been postulated as a potential ligand for the human pre-B-cell receptor.77 However, the contribution of these ligands to selecting B-cell progenitors expressing competent pre-BCRs is unclear.
Beyond demonstrating a global requirement for the pre-BCR, several in vivo genetic studies over the last few years have revealed a requirement for specific functional domains of the pre-BCR in the development of pre-B cells. Deletion of the cytosolic tails of both Igα and Igβ (IgαΔc/Δc, IgβΔc/Δc) completely prevents the development of pre-B cells, despite normal surface expression of the pre-BCR.78 Mice bearing the same truncation in Igβ and a specific mutation in the ITAM tyrosines of Igα (IgαFF/FF, IgβΔc/Δc) have an identical developmental deficit, indicating that the non-ITAM portions of Igα are insufficient to mediate development.79 Interestingly, truncation of the tail of Igβ alone (IgβΔc/Δc) does not affect the development of pre-B cells,78 while deletion of the cytoplasmic tail of Igα alone (IgαΔc/Δc) results in an incomplete block.80 These results suggest that, during early development, Igβ is redundant to Igα, but the reverse is not true. Surprisingly, the ITAM of Igα is not required, as mice expressing IgαFF/FF have no early defect. The non-ITAM tyrosines may be important in early development, as mutations in molecules that are recruited to this motif, such as BLNK81,82 and Btk,83 result in a developmental block similar to that of IgαΔc/Δc. The role of the Igα non-ITAM tyrosines has not been directly addressed in vivo.
Deletion of Syk84 also results in a partial block in pre-B-cell development, although the severity of this block is somewhat ameliorated by expression of ZAP-70.85 Likewise, the deletion of individual Src-family kinases has no significant impact on pre-B-cell development, while depletion of the three principal Src-family kinases expressed in B cells (Lyn, Blk and Fyn) results in a complete block in pre-B-cell development.27 These data indicate that the pathways requisite for B-cell development utilize both Syk and the Src-family of tyrosine kinases.
Analysis of BLNK-deficient mice suggests that the development of pre-B cells may be mediated by the co-ordination of two or more proximal signalling pathways, each with a distinct function. Deletion of BLNK, like the deletion of other signalling molecules in the pre-BCR pathway, leads to a significant block in pre-B-cell development.81,82 However, not all aspects of B-cell development are equally affected. Deletion of BLNK inhibits the down-regulation of RAG, λ5, IL-7R and CD25 that normally occurs when pre-B cells mature.30 The pre-B-cell proliferative burst is also inhibited. Conversely, ectopic expression of BLNK in Pax5–/–µ+ cells, which are deficient in BLNK and arrest at a pre-B-like stage, rescues some aspects of pre-B-cell receptor signalling and initiates a proliferative and developmental programme.57In vitro, pre-B cells from BLNK–/– mice proliferate robustly in response to IL-7, indicating that the defect is proliferation is specific to the BCR.75 In contrast, heavy-chain allelic exclusion in BLNK–/– mice is intact.30 These findings imply that both BLNK-dependent and BLNK-independent pathways contribute to pre-B-cell development.
Dµ expression and signalling through Dµ
The pre-B-cell receptor is assembled with a V–DJ rearranged heavy chain. If DJH rearrangement occurs in reading frame 2, a protein termed Dµ can be translated.86 Signalling through Dµ leads to cellular deletion87 in a Syk- and BLNK-dependent manner.30,84 Because Dµ and pre-BCR signalling effect contrasting outcomes, it seems reasonable to conclude that they initiate qualitatively different signalling cascades. Indeed, examination of the Ras/Raf/mitogen-activated protein kinase (MAPK) pathway has demonstrated that activated Raf partially rescues development in RAG–/– mice,88 suggesting that Dµ activates a subset of Ras-dependent signalling pathways.
The development of immature b cells
Upon light-chain rearrangement, heavy and light chain are co-expressed on the cell surface, in association with Igα and Igβ, to form an antigen-specific surface receptor (Fig. 2). These IgM+ IgD– immature B cells undergo receptor-mediated negative selection, a process whereby autoreactive B-cell receptors are culled from the immune repertoire. Very few (≈20%) immature B cells survive this process; those that are not negatively selected leave the bone marrow and emigrate initially to the splenic red pulp, where they are known as transitional B cells.
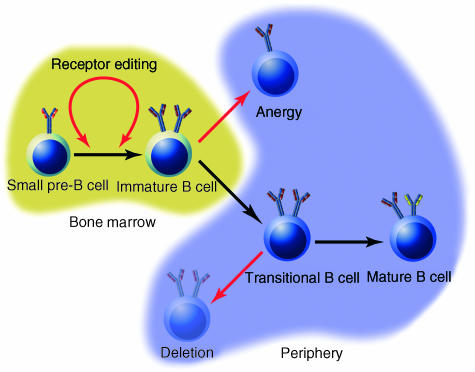
There are three known mechanisms of negative selection: deletion; anergy; and receptor editing (Fig. 3). Deletion in response to high-avidity ligands has been demonstrated in vivo in a number of BCR transgenic systems.89–91 Isolated immature B cells are also deleted in vitro when cultured with anti-BCR antibodies.92–94 In contrast, immature antigen-specific B cells, which encounter lower-avidity ligands, enter a state of anergy in which they are unresponsive to further antigen stimulation.95,96 These cells down-regulate surface IgM expression, are short-lived, and exhibit a characteristic signalling signature97,98 and pattern of gene expression.99 Self-reactive immature B cells can also induce secondary immunoglobulin gene rearrangements100–103 to replace their autoreactive receptors. Once this process of receptor editing is initiated, it presumably continues until a non-autoreactive receptor has been generated or, failing that, the cell is deleted.
Figure 3.
Mechanisms of negative selection during B-cell development. There are three described mechanisms whereby developing B cells can escape an autoreactive fate; all of these are mediated by signals transduced through the B-cell receptor (BCR) in response to BCR ligation and are indicated by red arrows. Which outcome results is largely a function of BCR signalling intensity, developmental stage and environmental milieu. Receptor editing is postulated to be a result either of retarded progression from the pre-B to immature B-cell stage, or of back-differentiation from the immature to pre-B-cell stage. Anergy is induced in immature bone marrow B cells as a response to receptor ligation, whereas deletion is induced in transitional B cells in response to the same stimulus.
Which of these three tolerance mechanisms is invoked depends on a myriad of factors, including receptor affinity, receptor expression level, developmental stage and site of encounter. As described above, ligand avidity is a primary determinant of whether a B cell is deleted or becomes anergic.91,95,96 The stage of maturation within the immature B-cell pool might also determine how a B cell responds to antigen. In vitro, ligation of the BCR on newly generated immature B cells, which are IgMlo, results in receptor editing, while ligating the receptor on more developed IgMhi B cells induces apoptosis.104 The location of receptor engagement may also determine cell fate; ligation of the immature BCR in a bone marrow environment results in receptor editing, whereas ligation in a splenic environment induces deletion. This difference was found to be caused by the protective effect of an unidentified Thy-1dull cell type in the bone marrow105 and was similar to the effect of introducing a bcl-2 or bcl-xL transgene into immature B cells.106,107 Finally, transport of the ligated BCR into lipid rafts may determine the biological outcome of receptor signalling.108,109
Signalling through the immature b-cell receptor
The replacement of surrogate light chains by Igκ or Igλ is required for the development of immature B cells.110 Truncation of the cytoplasmic tail of Igα (IgαΔc/Δc) causes a dramatic decrease in the numbers of immature B cells,80 highlighting the importance of BCR signalling at this stage. A similar truncation in Igβ (IgβΔc/Δc) has no effect on the generation of immature B cells,78 which implies that, as at the pro-B to pre-B transition, the functions of Igβ are redundant of Igα at this stage. Furthermore, truncation of the Igα cytosolic tail (IgαΔc/Δc) inhibits the generation of immature B cells to a greater degree than pre-B cells. This suggests that as B-cell progenitors progress through development, successively higher signalling capacities are required.
Although receptor editing occurs in the earlest populations of immature B cells, it is still unclear how the BCR initiates editing. One interesting hypothesis proposes that receptor editing is triggered by the absence of basal BCR signalling that occurs following ligand-induced downmodulation of the BCR. In this model, the lack of BCR signalling would allow immature B cells to back-differentate to an earlier stage in which RAG and other recombinase machinery genes are expressed. This hypothesis is based on the observation that conditional deletion of the BCR in immature B cells initiates new light-chain rearrangements (L. E. Tze et al., submitted) and the re-expression of pre-B-cell markers.111 Alternatively, editing may result from a BCR-dependent transient arrest in development at the pre-B/immature B-cell transition, a stage at which RAG is still expressed.112 This model is consistent with observations that once RAG expression has been turned off in splenic B cells, it cannot be reintroduced.113
Anergy, the outcome of low-affinity receptor crosslinking, results from the activation of calcineurin and extracellular signal-regulated kinase (ERK) signalling pathways and translocation of nuclear factor of activated T cells (NFAT) to the nucleus without the activation of NF-κB.114 This suggests that anergy may arise from the activation of a subset of the signalling pathways activated by the BCR. However, it is possible that anergy involves specific signalling effectors not requisite for normal activation. For example, B cells deficient in protein kinase Cδ, which have autoimmune nephritis, become activated in response to anergizing ligands.115
There are several possibilities for how the BCR on immature B cells may discriminate between activating and anergizing ligands. As recently demonstrated, one possibility is that anergizing ligands preferentially destabilize the BCR complex.116 Lowering the stoichiometry of mIg with Igα/Igβ may either play a role in initiating anergizing signals or in maintaining unresponsiveness to subsequent ligands. Anergy might also arise from a failure to phosphorylate fully the Igα ITAM tyrosines,20 leading to incomplete or abbreviated activation of Syk and other downstream effectors. It has also been proposed that anergy is maintained through chronic engagement of the BCR with low-avidity ligands (J. Cambier, personal communication).
Transitional b cells and signalling through their bcr
Bone marrow immature B cells progress to the periphery, where they continue to mature. These transitional cells can be subdivided on the basis of surface-marker expression into T1, T2 and, possibly, T3 populations. Most investigators agree that T1 cells are CD23– IgM+ IgDlo/–, whereas T2 cells are CD23+ IgM+ IgDlo/+. However, there is controversy concerning the functional significance of each population and the efficiency with which cells transit through each stage. Bromodeoxyuridine (BrdU) studies investigating the entry of cells into the T1, T2 and T3 populations indicate a 40–50% cell loss at the T1 to T2 transition.117 Other investigators contend that this can be accounted for by migration to other lymphoid organs and unexamined differentiative events.118 Regardless of the precise nomenclature, it is generally accepted that T1 cells migrate from the red pulp to the lymphoid follicles in the spleen, where they up-regulate IgD and CD23 as they mature.
Signalling through the BCR is important for B-cell maturation. Mice deficient in Btk have a profound block at, or immediately after, the T2 stage.117 As mentioned above, deletion of Syk or B-cell linker protein (BLNK), or truncation of the Igα or Igβ cytoplasmic tail, also impairs B-cell maturation (Fig. 2). The few Syk–/– cells that progress to the immature B-cell stage migrate to the splenic red pulp, but fail to enter B-cell follicles.119 Similarly, BLNK ablation allows transitional immature B cells to develop, but they fail to mature further.120 Truncation of the Igα cytoplasmic tail impairs pre-B-cell development; however, there is a much more severe block in export of bone marrow immature B cells to the periphery.121 Truncation of the Igβ tail, in contrast, allows the development of bone marrow immature B cells, but not their subsequent development to transitional immature B cells.78 The requirements for different signalling molecules or domains at each stage of B-cell maturation indicates that the BCR may be tested at each stage for a different functional capacity.
Aside from the BCR, it is becoming clear that the novel tumour necrosis factor (TNF) family member, B-cell activating factor (BAFF), is extremely important at the immature B-cell stage. BAFF-deficient B cells are developmentally arrested at the T1 stage.122 This is probably the result of a defect in survival rather than differentiation, however, as T2 cells die in vitro in the absence of BAFF signals123 and BAFF has been shown to stimulate NF-κB activation through a non-classical pathway124,125 and to modulate expression of bcl-2 family members.126,127
B-cell maturation and differentation in the periphery
As B cells mature, they down-regulate AA4·1 and start to express the δ heavy chain. Unlike other isotypes, IgD is expressed as a result of RNA, not DNA, splicing. This preserves the µ locus and allows mature B cells to co-express IgM and IgD. Some of these IgMhi IgDhi cells express intermediate levels of CD21, acquire the ability to recirculate, down-regulate IgM and become follicular/recirculating (IgMlo IgDhi CD23+) B cells. Follicular B cells are considered to be classical B2 cells and respond to T-dependent antigens, undergo germinal centre reactions, and give rise to memory cells. Other transitional cells express high levels of CD21 and give rise to marginal zone (MZ; IgMhi CD21hi CD23– IgDlo) B cells.128 MZ cells, which may also develop from follicular B cells,129 are long-lived and have a partially activated phenotype. They have some features in common with B1 cells in that they respond primarily to T-independent antigen and probably do not give rise to memory cells. However, they are CD5–. Additionally, the VH segments of their antigen receptors are largely germline in sequence130 and, despite their short CDR3 regions, some of these cells bear autoreactive receptors.131
Signalling through the mature b-cell receptor
The necessity of BCR signalling for the survival of mature peripheral B cells was demonstrated in an elegant set of experiments wherein the BCR was conditionally deleted following treatment with interferon-γ (IFN-γ).132 Deprived of BCR signals, peripheral B cells quickly died, conclusively demonstrating that at least a basal level of signalling is required to keep mature B cells alive. Whether BCR ligation is needed for peripheral B-cell maintenance is unknown. However, the magnitude of ligand-mediated BCR signalling influences the development of transitional cells into either MZ or follicular B cells; weak signals skew development towards MZ cells and strong ones induce follicular B-cell development.133 It has also been suggested that MZ cells require antigen-driven positive selection for development.134
Relatively little is known about the mechanisms underlying MZ B-cell development. The transcription factors NF-κB and c-Rel are important;135 deletion of the p50 subunit completely abrogates MZ-cell development, whereas cells lacking the p65 subunit or c-Rel develop into MZ cells at greatly reduced efficiency. Deletion of proline-rich tyrosine kinase 2 (Pyk2), a non-receptor tyrosine kinas, downstream of chemokine, cytokine and integrin receptors, also results in a complete loss of the MZ subtype.136 This suggests that factors distinct from BCR signalling may influence MZ B-cell development.
Conclusion
The primary purpose of B-cell development is to establish a diverse population of peripheral B cells that is both self-tolerant and reactive to foreign antigens. To ensure that these conditions are met, B-cell development is determined by the structural features and signalling capacities of the B-cell antigen receptor. Some BCR signals, such as those initiated by Syk and the cytoplasmic tail of Igα, are required for both B-cell development and peripheral maturation. Other signalling functions, such as those mediated by Btk and the cytosolic tail of Igβ, are required only at selected checkpoints. These stage-specific requirements reflect underlying differences in how the BCR signals in development and in the periphery, to decide cell fate.
Although BCR-mediated signals are the primary determinants of B-cell fate, environmental factors such as IL-7 and BAFF are also important. Understanding the molecular mechanisms by which the BCR determines cell fate, and how these decisions are influenced by the environments in which they take place, is one of the main challenges in B-lymphocyte biology.
Abbreviations
- BAFF
B-cell activating factor
- BCR
B-cell receptor
- BLNK
B-cell linker protein
- IL
interleukin
- ITAM
immunoreceptor tyrosine-based activation motif
- mIg
membrane-bound immunoglobulin
- MZ
marginal zone
- NF-κB
nuclear factor κB
- PLCγ2
phospholipase C γ2; RAG, recombinase-activating gene
- SFTK
Src family tyrosine kinases
- TCR
T-cell receptor
References
- 1.Raff MC, Steinberg J, Taylor R. Immunoglobulin determinants on the surface of mouse lymphoid cells. Nature. 1970;225:553. doi: 10.1038/225553a0. [DOI] [PubMed] [Google Scholar]
- 2.Grupp SA, Campbell K, Mitchell RN, Cambier JC, Abbas AK. Signaling-defective mutants of the B lymphocyte antigen receptor fail to associate with Ig-alpha and Ig-beta/gamma. J Biol Chem. 1993;268:25776. [PubMed] [Google Scholar]
- 3.Mitchell RN, Shaw AC, Weaver YK, Leder P, Abbas AK. Cytoplasmic tail deletion converts membrane immunoglobulin to a phosphatidylinositol-linked form lacking signaling and efficient antigen internalization functions. J Biol Chem. 1991;266:8856. [PubMed] [Google Scholar]
- 4.Blum JH, Stevens TL, Defranco AL. Role of the mu immunoglobulin heavy chain transmembrane and cytoplasmic domains in B-cell antigen receptor expression and signal transduction. J Biol Chem. 1993;268:27236. [PubMed] [Google Scholar]
- 5.Martin SW, Goodnow CC. Burst-enhancing role of the IgG membrane tail as a molecular determinant of memory. Nat Immunol. 2002;3:182. doi: 10.1038/ni752. [DOI] [PubMed] [Google Scholar]
- 6.Wakabayashi C, Adachi T, Wienands J, Tsubata T. A distinct signaling pathway used by the IgG-containing B cell antigen receptor. Science. 2002;298:2392. doi: 10.1126/science.1076963. [DOI] [PubMed] [Google Scholar]
- 7.Schamel WW, Reth M. Monomeric and oligomeric complexes of the B cell antigen receptor. Immunity. 2000;13:5. doi: 10.1016/s1074-7613(00)00003-0. [DOI] [PubMed] [Google Scholar]
- 8.Shaw AC, Mitchell RN, Weaver YK, Campos-Torres J, Abbas AK, Leder P. Mutations of immunoglobulin transmembrane and cytoplasmic domains: effects on intracellular signaling and antigen presentation. Cell. 1990;63:381. doi: 10.1016/0092-8674(90)90171-a. [DOI] [PubMed] [Google Scholar]
- 9.Schamel WW, Reth M. Stability of the B cell antigen receptor complex. Mol Immunol. 2000;37:253. doi: 10.1016/s0161-5890(00)00025-0. [DOI] [PubMed] [Google Scholar]
- 10.Hombach J, Lottspeich F, Reth M. Identification of the genes encoding the IgM-alpha and Ig-beta components of the IgM antigen receptor complex by amino-terminal sequencing. Eur J Immunol. 1990;20:2795. doi: 10.1002/eji.1830201239. [DOI] [PubMed] [Google Scholar]
- 11.Reth M, Wienands J, Tsubata T, Hombach J. Identification of components of the B cell antigen receptor complex. Adv Exp Med Biol. 1991;292:207. doi: 10.1007/978-1-4684-5943-2_23. [DOI] [PubMed] [Google Scholar]
- 12.Kurosaki T. Molecular mechanisms in B cell antigen receptor signaling. Curr Opin Immunol. 1997;9:309. doi: 10.1016/s0952-7915(97)80075-1. [DOI] [PubMed] [Google Scholar]
- 13.Reth M. Antigen receptor tail clue. Nature. 1989;338:383. [PubMed] [Google Scholar]
- 14.Cambier JC. New nomenclature for the Reth motif (or ARH1/TAM/ARAM/YXXL) Immunol Today. 1995;16:110. doi: 10.1016/0167-5699(95)80105-7. [DOI] [PubMed] [Google Scholar]
- 15.Samelson LE, Klausner RD. Tyrosine kinases and tyrosine-based activation motifs – current research on activation via the T cell antigen receptor. J Biol Chem. 1992;267:24913. [PubMed] [Google Scholar]
- 16.Weiss A, Littman DR. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez M, Misulovin Z, Burkhardt AL, Mahajan S, Costa T, Franke R, Bolen JB, Nussenzweig M. Signal transduction by immunoglobulin is mediated through Ig-alpha and Ig-beta. J Exp Med. 1993;178:1049. doi: 10.1084/jem.178.3.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flaswinkel H, Reth M. Dual role of the tyrosine activation motif of the Ig-α protein during signal transduction via the B cell antigen receptor. EMBO J. 1994;13:83. doi: 10.1002/j.1460-2075.1994.tb06237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burkhardt AL, Brunswick M, Bolen JB, Mond JJ. Anti-immunoglobulin stimulation of B lymphocytes activates src-related protein-tyrosine kinases. Proc Natl Acad Sci USA. 1991;88:7410. doi: 10.1073/pnas.88.16.7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson SA, Pleiman CM, Pao L, Schneringer J, Hippen K, Cambier JC. Phosphorylated immunoreceptor signalling motifs (ITAMs) exhibit unique abilities to bind and activate Lyn and Syk tyrosine kinases. J Immunol. 1995;155:4596. [PubMed] [Google Scholar]
- 21.Clark MR, Campbell KS, Kazlauskas A, Johnson SA, Hertz M, Potter TA, Pleiman C, Cambier JC. The B-cell antigen receptor complex – association of Ig-α and Ig-β with distinct cytoplasmic effectors. Science. 1992;258:123. doi: 10.1126/science.1439759. [DOI] [PubMed] [Google Scholar]
- 22.Clark MR, Johnson SA, Cambier JC. Analysis of Ig-alpha tyrosine kinase interaction reveals two levels of binding specificity and tyrosine phosphorylated Ig-alpha stimulation of Fyn activity. EMBO J. 1994;13:1911. doi: 10.1002/j.1460-2075.1994.tb06460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pracht C, Gimborn K, Reth M, Huber M. BCR mutants deficient in ligand-independent and more sensitive for ligand-dependent signaling. Eur J Immunol. 2002;32:1614. doi: 10.1002/1521-4141(200206)32:6<1614::AID-IMMU1614>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 24.Cassard S, Choquet D, Fridman WH, Bonnerot C. Regulation of ITAM signaling by specific sequences in Ig-β B cell antigen receptor subunit. J Biol Chem. 1996;271:23786. doi: 10.1074/jbc.271.39.23786. [DOI] [PubMed] [Google Scholar]
- 25.Iwashima M, Irving BA, Vanoers NSC, Chan AC, Weiss A. Sequential interactions of the TCR with 2 distinct cytoplasmic tyrosine kinases. Science. 1994;263:1136. doi: 10.1126/science.7509083. [DOI] [PubMed] [Google Scholar]
- 26.Rolli V, Gallwitz M, Wossning T, Flemming A, Schamel WW, Zurn C, Reth M. Amplification of B cell antigen receptor signaling by a Syk/ITAM positive feedback loop. Mol Cell. 2002;10:1057. doi: 10.1016/s1097-2765(02)00739-6. [DOI] [PubMed] [Google Scholar]
- 27.Saijo K, Schmedt C, Su IH, et al. Essential role of Src-family protein tyrosine kinases in NF-kappaB activation during B cell development. Nat Immunol. 2003;4:274. doi: 10.1038/ni893. [DOI] [PubMed] [Google Scholar]
- 28.Kabak S, Skaggs BJ, Gold MR, et al. The direct recruitment of BLNK to immunoglobulin alpha couples the B-cell antigen receptor to distal signaling pathways. Mol Cell Biol. 2002;22:2524. doi: 10.1128/MCB.22.8.2524-2535.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Engels N, Wollscheid B, Wienands J. Association of SLP-65/BLNK with the B cell antigen receptor through a non-ITAM tyrosine of Ig-α. Eur J Immunol. 2001;31:2126. doi: 10.1002/1521-4141(200107)31:7<2126::aid-immu2126>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 30.Hayashi K, Yamamoto M, Nojima T, Goitsuka R, Kitamura D. Distinct signaling requirements for Dmu selection, IgH allelic exclusion, pre-B cell transition, and tumor suppression in B cell progenitors. Immunity. 2003;18:825. doi: 10.1016/s1074-7613(03)00142-0. [DOI] [PubMed] [Google Scholar]
- 31.Hashimoto S, Iwamatsu A, Ishiai M, et al. Identification of the SH2 domain binding protein of Bruton's tyrosine kinase as BLNK-functional significance of Btk-SH2 domain in B-cell antigen receptor-coupled calcium signaling. Blood. 1999;94:2357. [PubMed] [Google Scholar]
- 32.Su Y-W, Zhang Y, Schweikert J, Koretzky GA, Reth M, Wienands J. Interaction of SLP adaptors with the SH2 domain of Tec family kinases. Eur J Immunol. 1999;29:3702. doi: 10.1002/(SICI)1521-4141(199911)29:11<3702::AID-IMMU3702>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 33.Ishiai M, Kurosaki M, Pappu R, et al. BLNK required for coupling Syk to PLCγ2 and Rac1-JNK in B cells. Immunity. 1999;10:117. doi: 10.1016/s1074-7613(00)80012-6. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki H, Matsuda S, Terauchi Y, et al. PI3K and Btk differentially regulate B cell antigen receptor-mediated signal transduction. Nat Immunol. 2003;4:280. doi: 10.1038/ni890. [DOI] [PubMed] [Google Scholar]
- 35.Johmura S, Oh-hora M, Inabe K, et al. Regulation of Vav localization in membrane rafts by adaptor molecules Grb2 and BLNK. Immunity. 2003;18:777. doi: 10.1016/s1074-7613(03)00139-0. [DOI] [PubMed] [Google Scholar]
- 36.Chiu CW, Dalton M, Ishiai M, Kurosaki T, Chan AC. BLNK: molecular scaffolding through ‘cis’-mediated organization of signaling proteins. EMBO J. 2002;21:6461. doi: 10.1093/emboj/cdf658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fu C, Chan AC. Identification of two tyrosine phosphoproteins, 70 and pp68, which interact with phospholipase Cγ, Grb2, and Vav after B cell antigen receptor activation. J Biol Chem. 1997;272:27362. doi: 10.1074/jbc.272.43.27362. [DOI] [PubMed] [Google Scholar]
- 38.Fu C, Turck CW, Kurosaki T, Chan AC. BLNK: a central linker protein in B cell activation. Immunity. 1998;9:93. doi: 10.1016/s1074-7613(00)80591-9. [DOI] [PubMed] [Google Scholar]
- 39.Goitsuka R, Fujimura Y-I, Mamada H, et al. Cutting edge. BASH, a novel signaling molecule preferentially expressed in B cells of the bursa of fabricius. J Immunol. 1998;161:5804. [PubMed] [Google Scholar]
- 40.Wienands J, Schweikert J, Wollscheid B, Jumaa H, Nielsen PJ, Reth M. SLP-65: a new signaling component in B lymphocytes which requires expression of the antigen receptor for phosphorylation. J Exp Med. 1998;188:791. doi: 10.1084/jem.188.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fusaki N, Tomita S, Wu Y, Okamoto N, Goitsuka R, Kitamura D, Hozumi N. BLNK is associated with the CD72/SHP-1/Grb2 complex in the WEHI231 cell line after membrane IgM cross-linking. Eur J Immunol. 2000;30:1326. doi: 10.1002/(SICI)1521-4141(200005)30:5<1326::AID-IMMU1326>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 42.Janssen E, Zhu M, Zhang W, Koonpaew S, Zhang W. LAB. A new membrane-associated adaptor molecule in B cell activation. Nat Immunol. 2003;4:117. doi: 10.1038/ni882. [DOI] [PubMed] [Google Scholar]
- 43.Dykstra M, Cherukuri A, Sohn HW, Tzeng SJ, Pierce SK. LOCATION IS EVERYTHING. Lipid rafts and immune cell signaling. Annu Rev Immunol. 2003;21:457. doi: 10.1146/annurev.immunol.21.120601.141021. [DOI] [PubMed] [Google Scholar]
- 44.Luisiri P, Lee YJ, Eisfelder BJ, Clark MR. Cooperativity and segregation of function within the Igα/β heterodimer of the B cell antigen receptor complex. J Biol Chem. 1996;271:5158. doi: 10.1074/jbc.271.9.5158. [DOI] [PubMed] [Google Scholar]
- 45.Lin S, Cicala C, Scharenberg AM, Kinet JP. The FcεRIβ subunit functions as an amplifier of FcεRIg-mediated cell activation signals. Cell. 1996;85:985. doi: 10.1016/s0092-8674(00)81300-8. [DOI] [PubMed] [Google Scholar]
- 46.Muller R, Wienands J, Reth M. The serine and threonine residues in the Ig-α cytoplasmic tail negatively regulate immunoreceptor tyrosine based activation motif-mediated signal transduction. Proc Natl Acad Sci USA. 2000;97:8451. doi: 10.1073/pnas.97.15.8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nutt SL, Heavey B, Rolink AG, Busslinger M. Commitment to the B-lymphoid lineage depends on the transcription factor Pax5. Nature. 1999;401:556. doi: 10.1038/44076. [DOI] [PubMed] [Google Scholar]
- 48.Allman D, Li J, Hardy RR. Commitment to the B lymphoid lineage occurs before DH-JH recombination. J Exp Med. 1999;189:735. doi: 10.1084/jem.189.4.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hardy RR, Carmack CE, Shinton SA, Kemp JD, Hayakawa K. Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. J Exp Med. 1991;173:1213. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nagata K, Nakamura T, Kitamura F, Kuramochi S, Taki S, Campbell KS, Karasuyama H. The Igα/Igβ heterodimer on µ-negative proB cells is competent for transducing signals to induce early B cell differentiation. Immunity. 1997;7:559. doi: 10.1016/s1074-7613(00)80377-5. [DOI] [PubMed] [Google Scholar]
- 51.Bain G, Maandag EC, Izon DJ, et al. E2A proteins are required for proper B cell development and initiation of immunoglobulin gene rearrangements [comment] Cell. 1994;79:885. doi: 10.1016/0092-8674(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 52.Lin H, Grosschedl R. Failure of B-cell differentiation in mice lacking the transcription factor EBF. Nature. 1995;376:263. doi: 10.1038/376263a0. [DOI] [PubMed] [Google Scholar]
- 53.Sigvardsson M, O'Riordan M, Grosschedl R. EBF and E47 collaborate to induce expression of the endogenous immunoglobulin surrogate light chain genes. Immunity. 1997;7:25. doi: 10.1016/s1074-7613(00)80507-5. [DOI] [PubMed] [Google Scholar]
- 54.Kee BL, Murre C. Induction of early B cell factor (EBF) and multiple B lineage genes by the basic helix-loop-helix transcription factor E12. J Exp Med. 1998;188:699. doi: 10.1084/jem.188.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O'Riordan M, Grosschedl R. Coordinate regulation of B cell differentiation by the transcription factors EBF and E2A. Immunity. 1999;11:21. doi: 10.1016/s1074-7613(00)80078-3. [DOI] [PubMed] [Google Scholar]
- 56.Souabni A, Cobaleda C, Schebesta M, Busslinger M. Pax5 promotes B lymphopoiesis and blocks T cell development by repressing Notch1. Immunity. 2002;17:781. doi: 10.1016/s1074-7613(02)00472-7. [DOI] [PubMed] [Google Scholar]
- 57.Schebesta M, Pfeffer PL, Busslinger M. Control of pre-BCR signaling by Pax5-dependent activation of the BLNK gene. Immunity. 2002;17:473. doi: 10.1016/s1074-7613(02)00418-1. [DOI] [PubMed] [Google Scholar]
- 58.Miller JP, Izon D, DeMuth W, Gerstein R, Bhandoola A, Allman D. The earliest step in B lineage differentiation from common lymphoid progenitors is critically dependent upon interleukin 7. J Exp Med. 2002;196:705. doi: 10.1084/jem.20020784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Corcoran AE, Riddell A, Krooshoop D, Venkitaraman AR. Impaired immunoglobulin gene rearrangement in mice lacking the IL-7 receptor. Nature. 1998;391:904. doi: 10.1038/36122. [DOI] [PubMed] [Google Scholar]
- 60.Billips LG, Nunez CA, Bertrand FE, III, Stankovic AK, Gartland GL, Burrows PD, Cooper MD. Immunoglobulin recombinase gene activity is modulated reciprocally by interleukin 7 and CD19 in B cell progenitors. J Exp Med. 1995;182:973. doi: 10.1084/jem.182.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350:423. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 62.Shimizu T, Mundt C, Licence S, Melchers F, Martensson IL. VpreB1/VpreB2/lambda 5 triple-deficient mice show impaired B cell development but functional allelic exclusion of the IgH locus. J Immunol. 2002;168:6286. doi: 10.4049/jimmunol.168.12.6286. [DOI] [PubMed] [Google Scholar]
- 63.Pelanda R, Braun U, Hobeika E, Nussenzweig MC, Reth M. B cell progenitors are arrested in maturation but have intact VDJ recombination in the absence of Ig-alpha and Ig-beta. J Immunol. 2002;169:865. doi: 10.4049/jimmunol.169.2.865. [DOI] [PubMed] [Google Scholar]
- 64.Gong S, Nussenzweig MC. Regulation of an early developmental checkpoint in the B cell pathway by Ig-beta. Science. 1996;272:411. doi: 10.1126/science.272.5260.411. [DOI] [PubMed] [Google Scholar]
- 65.Papavasiliou F, Misulovin Z, Suh H, Nussenzweig MC. The role of Ig-beta in precusor B cell transition and allelic exclusion. Science. 1995;268:408. doi: 10.1126/science.7716544. [DOI] [PubMed] [Google Scholar]
- 66.Kitamura D, Rajewsky K. Targeted disruption of mu chain membrane exon causes loss of heavy-chain allelic exclusion.[comment] Nature. 1992;356:154. doi: 10.1038/356154a0. [DOI] [PubMed] [Google Scholar]
- 67.Rolink A, Winkler T, Melchers F, Andersson J. Precursor B cell receptor-dependent B cell proliferation and differentiation does not require the bone marrow or fetal liver environment. J Exp Med. 2000;191:23. doi: 10.1084/jem.191.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marshall AJ, Fleming HE, Wu GE, Paige CJ. Modulation of the IL-7 dose–response threshold during pro-B cell differentiation is dependent on pre-B cell receptor expression. J Immunol. 1998;161:6038. [PubMed] [Google Scholar]
- 69.Stoddart A, Fleming HE, Paige CJ. The role of the preBCR, the interleukin-7 receptor, and homotypic interactions during B-cell development. Immunol Rev. 2000;175:47. [PubMed] [Google Scholar]
- 70.Yankee TM, Clark EA. Signaling through the B cell antigen receptor in developing B cells. Rev Immun. 2000;2:185. [PubMed] [Google Scholar]
- 71.Bannish G, Fuentes-Panana EM, Cambier JC, Pear WS, Monroe JG. Ligand-independent signaling functions for the B lymphocyte antigen receptor and their role in positive selection during B lymphopoiesis. J Exp Med. 2001;194:1583. doi: 10.1084/jem.194.11.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shaffer AL, Schlissel MS. A truncated heavy chain protein relieves the requirement for surrogate light chains in early B cell development. J Immunol. 1997;159:1265. [PubMed] [Google Scholar]
- 73.Muljo SA, Schlissel MS. The variable, C(H)1, C(H)2 and C(H)3 domains of Ig heavy chain are dispensable for pre-BCR function in transgenic mice. Int Immunol. 2002;14:577. doi: 10.1093/intimm/dxf023. [DOI] [PubMed] [Google Scholar]
- 74.Ohnishi K, Melchers F. The nonimmunoglobulin portion of lambda5 mediates cell-autonomous pre-B cell receptor signaling. Nat Immunol. 2003;4:849–56. doi: 10.1038/ni959. [DOI] [PubMed] [Google Scholar]
- 75.Guo B, Kato RM, Garcia-Lloret M, Wahl MI, Rawlings DJ. Engagement of the human pre-B cell receptor generates a lipid raft-dependent calcium signaling complex. Immunity. 2000;13:243. doi: 10.1016/s1074-7613(00)00024-8. [DOI] [PubMed] [Google Scholar]
- 76.Bradl H, Jack HM. Surrogate light chain-mediated interaction of a soluble pre-B cell receptor with adherent cell lines. J Immunol. 2001;167:6403. doi: 10.4049/jimmunol.167.11.6403. [DOI] [PubMed] [Google Scholar]
- 77.Gauthier L, Rossi B, Roux F, Termine E, Schiff C. Galectin-1 is a stromal cell ligand of the pre-B cell receptor (BCR) implicated in synapse formation between pre-B and stromal cells and in pre-BCR triggering. Proc Natl Acad Sci USA. 2002;99:13014. doi: 10.1073/pnas.202323999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reichlin A, Hu Y, Meffre E, Nagaoka H, Gong S, Kraus M, Rajewsky K, Nussenzweig MC. B cell development is arrested at the immature B cell stage in mice carrying a mutation in the cytoplasmic domain of immunoglobulin β. J Exp Med. 2001;193:13. doi: 10.1084/jem.193.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kraus M, Pao LI, Reichlin A, Hu Y, Canono B, Cambier JC, Nussenzweig MC, Rajewsky K. Interference with immunoglobulin (Ig) α immunoreceptor tyrosine-based activation motif (ITAM) phosphorylation modulates or blocks B cell development, depending on the availability of an Igβ cytoplasmic tail. J Exp Med. 2001;194:455. doi: 10.1084/jem.194.4.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Torres RM, Flaswinkel H, Reth M, Rajewsky K. Aberrant B cell development and immune response in mice with a compromised BCR complex. Science. 1996;272:1804. doi: 10.1126/science.272.5269.1804. [DOI] [PubMed] [Google Scholar]
- 81.Pappu R, Cheng AM, Li B, et al. Requirement for B cell linker protein (BLNK) in B cell development. Science. 1999;286:1949. doi: 10.1126/science.286.5446.1949. [DOI] [PubMed] [Google Scholar]
- 82.Jumaa H, Wollscheid B, Mitterer M, Wienands J, Reth M, Nielsen PJ. Abnormal development and function of B lymphocytes in mice deficient for the signaling adaptor protein SLP-65. Immunity. 1999;11:547. doi: 10.1016/s1074-7613(00)80130-2. [DOI] [PubMed] [Google Scholar]
- 83.Khan WN, Alt FW, Gerstein RM, Malynn BA, Larsson I, Rathbun G, Davidson L, Muller S, Kantor AB, Herzenberg LA. Defective B cell development and function in Btk-deficient mice. Immunity. 1995;3:283. doi: 10.1016/1074-7613(95)90114-0. [DOI] [PubMed] [Google Scholar]
- 84.Cheng AM, Rowley B, Pao W, Hayday A, Bolen JB, Pawson T. Syk tyrosine kinase required for mouse viability and B-cell development. Nature. 1995;378:303. doi: 10.1038/378303a0. [DOI] [PubMed] [Google Scholar]
- 85.Schweighoffer E, Vanes L, Mathiot A, Nakamura T, Tybulewicz VL. Unexpected requirement for ZAP-70 in pre-B cell development and allelic exclusion. Immunity. 2003;18:523. doi: 10.1016/s1074-7613(03)00082-7. [DOI] [PubMed] [Google Scholar]
- 86.Reth MG, Alt FW. Novel immunoglobulin heavy chains are produced from DJH gene segment rearrangements in lymphoid cells. Nature. 1984;312:418. doi: 10.1038/312418a0. [DOI] [PubMed] [Google Scholar]
- 87.Ehlich A, Martin V, Muller W, Rajewsky K. Analysis of the B-cell progenitor compartment at the level of single cells. Curr Biol. 1994;4:573. doi: 10.1016/s0960-9822(00)00129-9. [DOI] [PubMed] [Google Scholar]
- 88.Iritani BM, Alberola-IIa J, Forbush KA, Perlmutter RM. Distict signals mediate maturation and allelic exclusion in lymphocyte progenitors. Immunity. 1999;10:712. doi: 10.1016/s1074-7613(00)80070-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen C, Radic MZ, Erikson J, Camper SA, Litwin S, Hardy RR, Weigert M. Deletion and editing of B cells that express antibodies to DNA. J Immunol. 1994;152:1970. [PubMed] [Google Scholar]
- 90.Erikson J, Radic MZ, Camper SA, Hardy RR, Carmack C, Weigert M. Expression of anti-DNA immunoglobulin transgenes in non-autoimmune mice. Nature. 1991;349:331. doi: 10.1038/349331a0. [DOI] [PubMed] [Google Scholar]
- 91.Hartley SB, Crosbie J, Brink R, Kantor AB, Basten A, Goodnow CC. Elimination from peripheral lymphoid tissues of self-reactive B lymphocytes recognizing membrane-bound antigens. Nature. 1991;353:765. doi: 10.1038/353765a0. [DOI] [PubMed] [Google Scholar]
- 92.Yellen-Shaw A, Monroe JG. Differential responsiveness of immature- and mature-stage murine B cells to anti-IgM reflects both FcR-dependent and -independent mechanisms. Cell Immunol. 1992;145:339. doi: 10.1016/0008-8749(92)90336-n. [DOI] [PubMed] [Google Scholar]
- 93.Norvell A, Monroe JG. Acquisition of surface IgD fails to protect from tolerance-induction. Both surface IgM- and surface IgD-mediated signals induce apoptosis of immature murine B lymphocytes. J Immunol. 1996;156:1328. [PubMed] [Google Scholar]
- 94.Norvell A, Mandik L, Monroe JG. Engagement of the antigen-receptor on immature murine B lymphocytes results in death by apoptosis. J Immunol. 1995;154:4404. [PubMed] [Google Scholar]
- 95.Goodnow CC, Crosbie J, Adelstein S, et al. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988;334:676. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- 96.Goodnow CC, Crosbie J, Jorgensen H, Brink RA, Basten A. Induction of tolerance in mature peripheral B lymphocytes. Nature. 1989;342:385. doi: 10.1038/342385a0. [DOI] [PubMed] [Google Scholar]
- 97.Fulcher DA, Lyons AB, Korn SL, Cook MC, Koleda C, Parish C, Fazekas de St Groth B, Basten A. The fate of self-reactive B cells depends primarily on the degree of antigen receptor engagement and availability of T cell help [comment] J Exp Med. 1996;183:2313. doi: 10.1084/jem.183.5.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fulcher DA, Basten A. Reduced life span of anergic self-reactive B cells in a double-transgenic model. J Exp Med. 1994;179:125. doi: 10.1084/jem.179.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Glynne R, Ghandour G, Rayner J, Mack DH, Goodnow CC. B-lymphocyte quiescence, tolerance and activation as viewed by global gene expression profiling on microarrays. Immunol Rev. 2000;176:216. doi: 10.1034/j.1600-065x.2000.00614.x. [DOI] [PubMed] [Google Scholar]
- 100.Gay D, Saunders T, Camper S, Weigert M. Receptor editing: an approach by autoreactive B cells to escape tolerance. J Exp Med. 1993;177:999. doi: 10.1084/jem.177.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tiegs SL, Russell DM, Nemazee D. Receptor editing in self-reactive bone marrow B cells. J Exp Med. 1993;177:1009. doi: 10.1084/jem.177.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Prak EL, Weigert M. Light chain replacement: a new model for antibody gene rearrangement. J Exp Med. 1995;182:541. doi: 10.1084/jem.182.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hertz M, Nemazee D. BCR ligation induces receptor editing in IgM+ IgD– bone marrow B cells in vitro. Immunity. 1997;6:429. doi: 10.1016/s1074-7613(00)80286-1. [DOI] [PubMed] [Google Scholar]
- 104.Melamed D, Benschop RJ, Cambier JC, Nemazee D. Developemental regulation of B lymphocyte immune tolerance compartmentalizes clonal selection from receptor selection. Cell. 1998;92:173. doi: 10.1016/s0092-8674(00)80912-5. [DOI] [PubMed] [Google Scholar]
- 105.Sandel PC, Monroe JG. Negative selection of immature B cells by receptor editing or deletion is determined by site of antigen encounter. Immunity. 1999;10:289. doi: 10.1016/s1074-7613(00)80029-1. [DOI] [PubMed] [Google Scholar]
- 106.Lang J, Arnold B, Hammerling G, Harris AW, Korsmeyer S, Russell D, Strasser A, Nemazee D. Enforced Bcl-2 expression inhibits antigen-mediated clonal elimination of peripheral B cells in an antigen dose-dependent manner and promotes receptor editing in autoreactive, immature B cells. J Exp Med. 1997;186:1513. doi: 10.1084/jem.186.9.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fang W, Weintraub BC, Dunlap B, et al. Self-reactive B lymphocytes overexpressing Bcl-xL escape negative selection and are tolerized by clonal anergy and receptor editing. Immunity. 1998;9:35. doi: 10.1016/s1074-7613(00)80586-5. [DOI] [PubMed] [Google Scholar]
- 108.Chung JB, Baumeister MA, Monroe JG. Cutting edge: differential sequestration of plasma membrane-associated B cell antigen receptor in mature and immature B cells into glycosphingolipid-enriched domains. J Immunol. 2001;166:736. doi: 10.4049/jimmunol.166.2.736. [DOI] [PubMed] [Google Scholar]
- 109.Sproul TW, Malapati S, Kim J, Pierce SK. Cutting edge: B cell antigen receptor signaling occurs outside lipid rafts in immature B cells. J Immunol. 2000;165:6020. doi: 10.4049/jimmunol.165.11.6020. [DOI] [PubMed] [Google Scholar]
- 110.Zou X, Piper TA, Smith JA, Allen ND, Xian J, Bruggemann M. Block in development at the pre-B-II to immature B cell stage in mice without Igkappa and Iglambda light chain. J Immunol. 2003;170:1354. doi: 10.4049/jimmunol.170.3.1354. [DOI] [PubMed] [Google Scholar]
- 111.Tze LE, Baness EA, Hippen KL, Behrens TW. 2000 Ig light chain receptor editing in anergic B cells. J Immunol. 2003;165:6796. doi: 10.4049/jimmunol.165.12.6796. [DOI] [PubMed] [Google Scholar]
- 112.Casellas R, Shih TA, Kleinewietfeld M, Rakonjac J, Nemazee D, Rajewsky K, Nussenzweig MC. Contribution of receptor editing to the antibody repertoire [comment] Science. 2001;291:1541. doi: 10.1126/science.1056600. [DOI] [PubMed] [Google Scholar]
- 113.Yu W, Nagaoka H, Jankovic M, et al. Continued RAG expression in late stages of B cell development and no apparent re-induction after immunization [comment] Nature. 1999;400:682. doi: 10.1038/23287. [DOI] [PubMed] [Google Scholar]
- 114.Healy JI, Dolmetsch RE, Timmerman LA, Cyster JG, Thomas ML, Crabtree GR, Lewis RS, Goodnow CC. Different nuclear signals are activated by the B cell receptor during positive versus negative signaling. Immunity. 1997;6:419. doi: 10.1016/s1074-7613(00)80285-x. [DOI] [PubMed] [Google Scholar]
- 115.Mecklenbrauker I, Saijo K, Zheng NY, Leitges M, Tarakhovsky A. Protein kinase Cdelta controls self-antigen-induced B-cell tolerance. Nature. 2002;416:860. doi: 10.1038/416860a. [DOI] [PubMed] [Google Scholar]
- 116.Vilen BJ, Nakamura T, Cambier JC. Antigen-stimulated dissociation of BCR mlg from Ig-α/Ig-β: implications for receptor desensitizations. Immunity. 1999;10:239. doi: 10.1016/s1074-7613(00)80024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Allman D, Lindsley RC, DeMuth W, Rudd K, Shinton SA, Hardy RR. Resolution of three nonproliferative immature splenic B cell subsets reveals multiple selection points during peripheral B cell maturation. J Immunol. 2001;167:6834. doi: 10.4049/jimmunol.167.12.6834. [DOI] [PubMed] [Google Scholar]
- 118.Cariappa A, Pillai S. Antigen-dependent B-cell development. Curr Opin Immunol. 2002;14:241. doi: 10.1016/s0952-7915(02)00328-x. [DOI] [PubMed] [Google Scholar]
- 119.Turner M, Gulbranson-Judge A, Quinn ME, Walters AE, MacLennan ICM, Tybulewicz VL. Syk tyrosine kinase is required for the positive selection of immature B cells into the recirculating B cell pool. J Exp Med. 1997;186:2013. doi: 10.1084/jem.186.12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Xu S, Wong S-C, Lam K-P. B cell linker protein is dispensable for the allelic exclusion of immunoglobulin heavy chain locus but required for the persistence of CD5+ B cells. J Immunol. 2000;165:4153. doi: 10.4049/jimmunol.165.8.4153. [DOI] [PubMed] [Google Scholar]
- 121.Torres RM, Hafen K. A negative regulatory role for Ig-α during B cell development. Immunity. 1999;11:527. doi: 10.1016/s1074-7613(00)80128-4. [DOI] [PubMed] [Google Scholar]
- 122.Schiemann B, Gommerman JL, Vora K, Cachero TG, Shulga-Morskaya S, Dobles M, Frew E, Scott ML. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway [comment] Science. 2001;293:2111. doi: 10.1126/science.1061964. [DOI] [PubMed] [Google Scholar]
- 123.Batten M, Groom J, Cachero TG, Qian F, Schneider P, Tschopp J, Browning JL, Mackay F. BAFF mediates survival of peripheral immature B lymphocytes. J Exp Med. 2000;192:1453. doi: 10.1084/jem.192.10.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kayagaki N, Yan M, Seshasayee D, et al. BAFF/BLyS receptor 3 binds the B cell survival factor BAFF ligand through a discrete surface loop and promotes processing of NF-kappaB2. Immunity. 2002;17:515. doi: 10.1016/s1074-7613(02)00425-9. [DOI] [PubMed] [Google Scholar]
- 125.Claudio E, Brown K, Park S, Wang H, Siebenlist U. BAFF-induced NEMO-independent processing of NF-kappa B2 in maturing B cells [comment] Nat Immunol. 2002;3:958. doi: 10.1038/ni842. [DOI] [PubMed] [Google Scholar]
- 126.Hsu BL, Harless SM, Lindsley RC, Hilbert DM, Cancro MP. Cutting edge. BLyS enables survival of transitional and mature B cells through distinct mediators. J Immunol. 2002;168:5993. doi: 10.4049/jimmunol.168.12.5993. [DOI] [PubMed] [Google Scholar]
- 127.Do RK, Hatada E, Lee H, Tourigny MR, Hilbert D, Chen-Kiang S. Attenuation of apoptosis underlies B lymphocyte stimulator enhancement of humoral immune response. J Exp Med. 2000;192:953. doi: 10.1084/jem.192.7.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hao Z, Rajewsky K. Homeostasis of peripheral B cells in the absence of B cell influx from the bone marrow. J Exp Med. 2001;194:1151. doi: 10.1084/jem.194.8.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dammers PM, de Boer NK, Deenen GJ, Nieuwenhuis P, Kroese FG. The origin of marginal zone B cells in the rat. Eur J Immunol. 1999;29:1522. doi: 10.1002/(SICI)1521-4141(199905)29:05<1522::AID-IMMU1522>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 130.Dammers PM, Visser A, Popa ER, Nieuwenhuis P, Kroese FG. Most marginal zone B cells in rat express germline encoded Ig VH genes and are ligand selected. J Immunol. 2000;165:6156. doi: 10.4049/jimmunol.165.11.6156. [DOI] [PubMed] [Google Scholar]
- 131.Li Y, Li H, Weigert M. Autoreactive B cells in the marginal zone that express dual receptors. J Exp Med. 2002;195:181. doi: 10.1084/jem.20011453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lam K-P, Kuhn R, Rajewsky K. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell. 1997;90:1073. doi: 10.1016/s0092-8674(00)80373-6. [DOI] [PubMed] [Google Scholar]
- 133.Cariappa A, Tang M, Parng C, Nebelitskiy E, Carroll M, Georgopoulos K, Pillai S. The follicular versus marginal zone B lymphocyte cell fate decision is regulated by Aiolos, Btk, and CD21. Immunity. 2001;14:603. doi: 10.1016/s1074-7613(01)00135-2. [DOI] [PubMed] [Google Scholar]
- 134.Martin F, Kearney JF. Positive selection from newly formed to marginal zone B cells depends on the rate of clonal production, CD19, and btk. Immunity. 2000;12:39. doi: 10.1016/s1074-7613(00)80157-0. [DOI] [PubMed] [Google Scholar]
- 135.Cariappa A, Liou HC, Horwitz BH, Pillai S. Nuclear factor kappa B is required for the development of marginal zone B lymphocytes. J Exp Med. 2000;192:1175. doi: 10.1084/jem.192.8.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Guinamard R, Okigaki M, Schlessinger J, Ravetch JV. Absence of marginal zone B cells in Pyk-2-deficient mice defines their role in the humoral response. Nat Immunol. 2000;1:31. doi: 10.1038/76882. [DOI] [PubMed] [Google Scholar]