Abstract
Antigenic encounter by T cells induces immunological synapse formation and T-cell activation. Using different concentrations of toxic shock syndrome toxin-1 (TSST-1) as stimulus, we examined the capacities of dendritic cells (DC) and macrophages (Mφ) to prime syngeneic naive T cells. DCs were, under all experimental settings, more efficient than Mφ at clustering T cells. Translocation of the T-cell receptor (TCR) to the contact area was found to be induced by DCs, as well as by Mφ, in an antigen-dependent manner, although Mφ were less efficient at inducing TCR translocation. Capping of protein kinase C θ (PKCθ) was also antigen dependent but induced exclusively by DCs. Likewise, DCs were found to be more potent inducers of interleukin-2 (IL-2) production and proliferation of naive T cells than Mφ. After 3 days of culture, DCs presenting 100 ng/ml TSST-1 induced interferon-γ (IFN-γ)-secreting cells, whereas Mφ did not. After 7 days of culture, DCs presenting 0·1 ng/ml TSST-1, and Mφ presenting high (as well as low) doses of TSST-1, induced IL-4-producing cells. We therefore provide evidence to show that antigen dose, type of antigen-presenting cell and time of differentiation can contribute to T-cell differentiation.
Introduction
T helper 1 (Th1) and T helper 2 (Th2) are differentiated effector cells that produce different patterns of cytokines. Cells of either type can confer protection against pathogens or lead to immunopathology. Factors contributing to Th1/Th2 differentiation are the dose of antigen, strength of antigenic stimulation, duration of T-cell receptor (TCR) ligation, the nature of costimulation and the type of antigen-presenting cell (APC).1 The immune response is initiated when T lymphocytes recognize antigenic peptide bound to major histocompatibility complex (MHC) molecules on the surface of APCs. The major APCs are dendritic cells (DCs), which efficiently present antigen to naive T cells and thus initiate the primary immune response. Other APCs, such as activated B cells and macrophages (Mφ), may also stimulate naive T cells but at much lower response rates.2,3 During this activation process, T cells polarize towards the APC4,5 and a specific large-scale molecular complex is built at the APC–T-cell interface. This specialized junction is called the immunological synapse; here, several surface and signalling molecules segregate at the interface.6,7 Superantigens (sAg) provide a means to pursue the activation process of naive T cells. These molecules, in this case the staphylococcal exotoxin toxic shock syndrome toxin 1 (TSST-1), do not require cellular processing, and clonal activation can be traced by staining for the TSST-1-cognate Vβ2-chain of theTCR. TSST-1, used at different concentrations, evokes different Th cell responses,8,9 a phenomenon well described for conventional antigens.10–12 In this study we used Mφ and DCs, derived from CD34+ haematopoietic progenitor cells (HPC) presenting different amounts of TSST-1 to naive T cells, to gain information on the different immune responses elicited when applying different levels of stimulation on naive T cells. We demonstrate how naive T cells, depending on the strength of the stimulus and the type of the APC, selectively differentiate into a Th1 or a Th2 phenotype. Th1 induction only occurs when T cells are exposed to high TSST-1 doses presented by DCs, whereas Mφ elicit a Th2 reaction in this setting. At low sAg doses, DCs, as well as Mφ, induce a Th2 phenotype, but Mφ evoke lower response rates.
Materials and methods
Reagents and antibodies
The medium used was RPMI-1640 supplemented with 2 mm l-glutamine, 1 mm sodium pyruvate, non-essential amino acids, 50 IU/ml penicillin, 50 µg/ml streptomycin and 10% heat-inactivated fetal calf serum (FCS) (all from Biochrom, Berlin, Germany). Ficoll–Hypaque (1·077 g/ml) was also obtained from Biochrom. Recombinant human growth factors used in this study were granulocyte–macrophage colony-stimulating factor (GM-CSF) (Novartis, Basel, Switzerland), tumour necrosis factor-α (TNF-α), stem cell factor (SCF), macrophage colony-stimulating factor (M-CSF) and interleukin (IL)-4 (all from PeproTech, Rocky Hill, NJ). TSST-1 was obtained from Alexis Biochemicals (Grünberg, Germany). Brefeldin A was purchased from Sigma (Deisenhofen, Germany). The anti-Vβ2 fluorescein isothiocyanate (FITC) conjugate, MPB2D5, was purchased from Beckman Coulter (Unterschleissheim, Germany) and the polyclonal goat anti-PKCθ (C-18) was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit anti-FITC Alexa 488 and donkey anti-goat Alexa 594 secondary antibodies were purchased from Molecular Probes (Leiden, the Netherlands). The anti-CD3 was obtained from Janssen-Cilag (Neuss, Germany). Anti-CD28 (CD28.2), anti-CD1a (HI149), anti-CD11a (HI111), anti-CD18 (6.7), anti-CD14 (Leu-M3), anti-CD34 (8G12), anti-CD40 (5C3), anti-CD45RA (L48), anti-CD54 (HA58), anti-CD58 (1C3), anti-CD80 (L307.4), anti-CD86 (IT2.2), anti-CD209 (DCN46), anti-human leucocyte antigen (HLA)-DR (L243), anti-interferon-γ (IFN-γ) (4S.B3) and anti-IL-4 (8D4-8) were all obtained from Becton-Dickinson (Heidelberg, Germany). Streptavidin-Tricolor was from Medac (Hamburg, Germany). Paraformaldehyde was obtained from Riedel-de-Haën (Seelze, Germany). Citrate–phosphate–dextrose solution, poly-l-lysine, saponin, fish skin gelatin, HEPES, Hanks' balanced salt solution (HBSS) and mitomycin C were purchased from Sigma-Aldrich (Deisenhofen, Germany). Vectashield was obtained from Vector Laboratories (Burlingame, CA). The CD34 multisort kit and the bead-coated antibodies (anti-CD14, anti-CD8, anti-CD34, anti-CD45RO, anti-CD56, anti-HLA-DR and anti-glycophorine A) were all obtained from Miltenyi Biotec (Bergisch-Gladbach, Germany). [3H]Thymidine was purchased from Amersham Life Science (Bucks., UK).
Cord blood mononuclear cells
Cord blood (CB) was obtained following institutional guidelines and stored in 50 ml Falcon tubes containing a citrate–phosphate–dextrose solution supplemented with 20 mm HEPES, 50 IU/ml penicillin, 50 µg/ml streptomycin and 5 µg/ml amphotericin B until required for the isolation of cord blood mononuclear cells (CBMCs). CBMCs were prepared by density centrifugation of CB.
Isolation of HPC
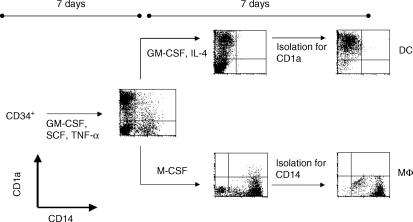
CD34+ cells were separated from CBMCs by using the CD34 multisort kit. The kit was used according to the guidelines of the manufacturer. Purity of isolations always exceeded 95%, as measured by flow cytometry. Following the modified protocols of Caux and co-workers,13 purified HPCs were seeded at a density of 2 × 104 cells/ml in 24-well tissue-culture plates in fresh complete medium supplemented with 250 U/ml TNF-α, 500 U/ml GM-CSF and 10 U/ml SCF at 37° in a humidified atmosphere in the presence of 5% CO2. Cells were analysed for the expression of CD1a and CD14 by flow cytometry and counted in the improved Neubauer-chamber.
Isolation and culture of CD1a+ DCs
Cells were cultured for the first 7 days as described. On day 7, the medium was exchanged and cultures were supplemented with 100 U/ml GM-CSF, 250 U/ml TNF-α and 50 U/ml IL-4. CD1a+ cells were isolated on day 14 of culture in an AutoMACS, as described above (isolation mode: POSSEL_S). Analysis of the sorted cells showed a purity of >95%.
Isolation and culture of CD14+ Mφ
HPCs were cultured as described above. From day 7 onwards, cells were cultured in medium containing 25 U/ml M-CSF. On day 14, cells were sorted for the expression of CD14 using anti-CD14 MicroBeads (Miltenyi Biotec) as described above using an AutoMACS (isolation mode: POSSEL). Purity of isolation was always >95%.
Purification of CB naive T cells
Naive T cells were obtained from CBMCs by immunomagnetic depletion. CBMCs were labelled by a cocktail of antibody-coated beads directed to CD8, CD34, CD45RO, CD56, HLA-DR and glycophorine A (all from Miltenyi Biotec). Thereafter, the cells were applied to an LD column and labelled cells were depleted using a magnet. The cells running through the column were CD45RA+, at a percentage of >98%, and considered to be naive T cells.
Cytofluorometric cell phenotyping
Cells (2 × 105) were incubated with the appropriate concentration of the indicated fluorochrome-conjugated Abs (15 min at 4°): anti-CD1a, anti-CD11a, anti-CD18, anti-CD14, anti-CD34, anti-CD40, anti-CD45RA, anti-CD54, anti-CD58, anti-CD80, anti-CD86, anti-CD209 and anti-HLA-DR. Unbound excess antibodies were removed by washing in HBSS/0·1% sodium azide.
Fluorescence-activated cell sorter (FACS)-based conjugation assay
For conjugation, 2·5 × 105 APCs were combined with 2·5 × 105 Vβ2+ T cells in a total volume of 0·5 ml containing 0·1 ng/ml or 100 ng/ml TSST-1, or left unstimulated. To maximize conjugate formation, cells were centrifuged for 5 min at 100 g and incubated at 37° for 60 min in a humidified atmosphere in the presence of CO2. Cells were then gently resuspended by inversion and fixed in phosphate-buffered saline (PBS) containing paraformaldehyde. After one wash, Vβ2 and CD1a or CD14 were stained according to the manufacturer's instructions. Cells were again gently resuspended and, after another wash, immediately analysed on a FACScan flow cytometer (Becton-Dickinson). The relative proportion of red (APCs), green (Vβ2+) and red/green (conjugates) events was determined by two-colour flow cytometric analysis. Percentage of conjugation was calculated as the number of red/green events divided by the sum of green events.
Conjugation for fluorescence microscopy
A total of 2·5 × 105 APCs per well were placed in 250 µl of RPMI-1640 on poly-l-lysine-coated chamber slides (Nunc, Roskilde, Denmark) and allowed to adhere for 30 min. The APCs were then co-cultured with an equal number of naive T cells in a final volume of 500 µl of RPMI-1640 for 60 min. Cells were cultured without antigen or were stimulated with TSST-1 at a concentration of 0·1 or 100 ng/ml. Samples were fixed in 4% paraformaldehyde for 10 min at room temperature, permeabilized for 10 min with PBS containing 0·1% saponin (0·1% saponin/PBS) and blocked with cold water fish skin gelatin in 0·1% saponin/PBS for 30 min at 37°. Samples were then incubated with primary Abs (mouse anti-Vβ2 and goat anti-PKCθ) for 45 min at 37°. After washing in 0·1% saponin/PBS, cells were incubated with secondary Abs (rabbit anti-FITC Alexa 488 and donkey anti-goat Alexa 594) for 45 min at 37°. Slides were then mounted in Vectashield antifade reagent. Cell conjugate formation was analysed quantitatively by fluorescence microscopy. Slides were viewed using an Olympus BH-2 microscope (Olympus, Hamburg, Germany), equipped with a 100-W mercury lamp and a charge-coupled device camera with a 100 × 1·7 oil-immersion objective. Conjugates were first identified by directly observing cell morphology using phase-contrast microsopy and green-fluorescent Vβ2+ T cells. The proportion of conjugates with Vβ2 and PKCθ redistributed to the contact areas was calculated by counting 100 different Vβ2-expressing cells and identifying which of these had an accumulation of fluorescent signal at the contact region. Image capture and processing was performed using Image Compact (Matrix Vision, Oppenweiler, Germany). Three independent repetitions were performed of each experimental condition.
Conjugation for electron microscopy
Cells were co-cultured on poly-l-lysine-coated chamber slides, as described above. At the end of the culture period, slides were briefly rinsed with PBS and clusters were fixed in a karnovsky-picric acid solution (for 15 min at 4°). After washing, cells were dehydrated in a series of graded ethanol, coated with gold by sputtering and observed using a DSM 950 scanning electron microscope (Zeiss, Oberkochen, Germany).
T-cell proliferation assay
APC were incubated with mitomycin C, at a concentration of 25 µg/ml, to avoid proliferation. Graded numbers of day-14 DC or Mφ were then dispensed into individual wells of 96-well flat-bottom microtitre plates and co-cultured with 3 × 105 syngeneic naive T cells for 3 days in culture medium. Cells were stimulated with 0·1 ng/ml or 100 ng/ml TSST-1, or left unstimulated. During the last 18 hr of co-culture, DNA synthesis was monitored by adding 0·5 µCi [3H]thymidine. Tests were carried out in quadruplicate and results expressed as mean counts per minute (c.p.m.).
Intracellular cytokine staining
In parallel cultures, varying numbers of APCs and 3 × 105 syngeneic naive T cells/well were stimulated with 0·1– 10 µg/ml TSST-1, as indicated. After 3 or 7 days of incubation, cells were restimulated with plate-bound anti-CD3 (5 µg/ml) and soluble CD28 (0·5 µg/ml) for 16 hr. Cytokine secretion was inhibited by 1 µm brefeldin A. The cytokines were measured as described previously.14,15 Cells were washed in HBSS and then fixed, for 10 min, in ice-cold HBSS containing 4% paraformaldehyde. Cells were washed twice. Permeabilization of the cell membrane was achieved by resuspension in 100 µl of HBSS containing 0·1% saponin and 0·01 m HEPES buffer (saponin buffer). The permeabilized cells were incubated with FITC-labelled antibodies against the TCR-Vβ2 chain, biotinylated anti-IFN-ã and phycoerythrin (PE)-labelled anti-IL-4 for 20 min at 4°, washed and then incubated with streptavidin-Tricolor for 20 min at 4°. After washing with saponin buffer, the cells were resuspended in 200 µl of HBSS for flow-cytometric analysis.
Results
Characterization of the cells
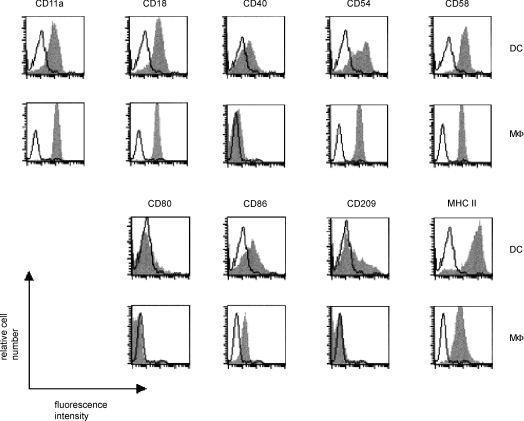
APC used in this study were CD1a+ DCs and CD14+ Mφ generated from CD34+ CB HPCs cultured in the presence of IL-4 + GM-CSF or M-CSF, respectively. Cells were purified by autoMACS on the basis of CD1a or CD14 expression (Fig. 1). Figure 2 shows the phenotype profiles of representative populations of DCs and Mφ. Cells were stained for the adhesion molecules lymphocyte function-associated antigen-1 (LFA-1) (CD11a, CD18), CD54, CD58 and CD209. Costimulatory molecules CD40, CD80 and CD86, as well as MHC class II were also stained. DCs were of an immature phenotype showing high levels of the adhesion molecules CD11a, CD18, CD54, CD58 and CD209. MHC class II molecules and the costimulatory molecule, CD86, were also highly expressed, whereas CD80 and CD40 were expressed at trace levels. Both Mφ and DCs expressed the adhesion molecules LFA-1 (CD11a, CD18), CD54 and CD58, and the costimulatory molecule, CD86, at similar levels. Expression of the costimulatory molecules CD40 and CD80, as well as of the adhesion molecule CD209, was weak or at background levels. MHC class II staining was present on Mφ at a lower level than on DCs. Preliminary experiments showed that the expression of surface molecules did not change following exposure to TSST-1 for up to 72 hr. Responding T cells were syngeneic naive CD4+ T cells purified from CB by immunomagnetic depletion of all non-T cells, CD8+ T cells and HLA-DR+ cells. Such isolated T cells were CD45RA+ at 98%. About 8% of these cells expressed the TSST-1 cognate Vβ2-chain of the TCR.
Figure 1.
Antigen-presenting cells used in this study were CD1a+ dendritic cells (DC) and CD14+ macrophages (Mφ) generated from CD34+ cord-blood haematopoietic progenitor cells (HPCs). CD34+ HPCs were cultured for 7 days in the presence of granulocyte–macrophage colony-stimulating factor (GM-CSF), stem cell factor (SCF) and tumour necrosis factor-α (TNF-α). Cells were then seeded for 7 additional days in the presence of either M-CSF, or GM-CSF + IL-4. On day 14, cells were sorted for the expression of CD1a or CD14 in an AutoMACS. Results are representative of more than 30 experiments.
Figure 2.
CD34+ haematopoietic progenitor cells (HPCs) differentiate into CD1a+ dendritic cells (DCs) or CD14+ macrophage (Mφ), depending on which cytokines are added to the culture. On day 14, cells were sorted for the expression of either CD1a or CD14. The phenotype of the cells was determined by single-colour flow cytometry. Open histograms show the isotype match controls. Data shown are representative of four experiments.
Clustering of naive T cells by APCs
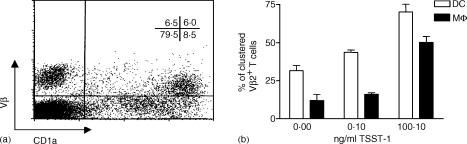
Antigen-dependent and -independent APC–T-cell conjugate formation was studied using two methods. Adhesion of Vβ2+ naive T cells was assessed by flow cytometry and fluorescence microscopy. Similar results were obtained by both methods. Antigen-independent contacts were established by DCs, as well as by Mφ, although DCs were much more potent at forming clusters with naive T cells. In the absence of antigen, DCs clustered about 30% of Vβ2+ T cells whereas Mφ bound only ≈12%. When stimulated with TSST-1, DCs clustered up to 75% of Vβ2+ T cells at high sAg doses. In DCs, as well as in Mφ, the ability to bind naive T cells increased with the amount of TSST-1 added to the culture (Fig. 3).
Figure 3.
Adhesion of naive CD4+ T cells to antigen-presenting cells (APCs). Conjugate formation was studied by flow cytometry. T cells were mixed with dendritic cells (DCs) or macrophage (Mφ), at a ratio of 1 : 1, in the presence or absence of toxic shock syndrome toxin-1 (TSST-1). Conjugate formation was observed after 60 min. T cells were stained for Vβ2 expression, whereas APCs were stained for CD1a or CD14, respectively. The relative proportion of red (APCs), green (Vβ2+) and red/green (conjugates) events was determined by two-colour flow cytometric analysis. Percentage conjugation was calculated as the number of red/green events divided by the sum of green events. (a) Dot-plot representing conjugate formation between naive T cells and DCs at a TSST-1 concentration of 100 ng/ml. (b) Results are the mean of four independent experiments ± standard deviation (SD).
Analysis of T cell–APC contacts by scanning electron microscopy
The structure of the contact zone was examined by scanning electron microscopy. DCs and Mφ were easily distinguished from T cells by the smaller size of the latter. In the absence of TSST-1, APCs interacted with T cells only by forming bundles of filopodia. These filopodia were spread out from the T cells as well as from the APCs, as judged by their morphology. In the presence of sAg, contacts again either consisted of a bundle of filopodia or were broad with T cells engulfed by the APCs (Fig. 4).
Figure 4.
Scanning electron microscope analysis of conjugates between naive T cells and antigen-presenting cells (APCs) in the presence or absence of 100 ng/ml toxic shock syndrome toxin-1 (TSST-1). Cells were co-cultured as described in the legend to Fig. 3.
Analysis of T cell–APC contacts by fluorescence microscopy
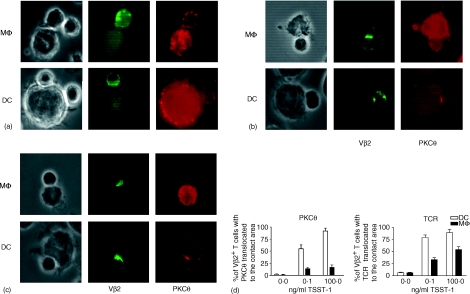
On examination by fluorescence microscopy, APCs varied in their capacity to promote the early phases of naïve T-cell activation, which was dependent on the sAg dose used, as judged by marked differences in TCR and PKCθ distribution along the contact areas. Polarization of T cells towards the APCs was not seen in antigen-independent contacts (Fig. 5a). Vβ2 translocated to the contact area only in the presence of TSST-1 (Fig. 5b, 5c). This translocation was seen in contacts with DCs as well as in contacts with Mφ, although Mφ generally induced fewer T cells to translocate Vβ2 to the contact zones. The percentage of T cells with Vβ2 clustered at the contact areas increased with the amount of TSST-1 added to the culture. PKCθ translocation to the contact zone was also antigen dependent and almost exclusively seen in contacts with DCs. Again, the percentage of T cells with PKCθ capped at the contact areas increased with higher doses of TSST-1. Mφ induce T cells to translocate PKCθ to the cell contact areas to a substantially lesser degree (Fig. 5d).
Figure 5.
Immunofluorescence microscopy of antigen-presenting cell (APC)–T-cell conjugates. Cells were mixed, as described in the legend to Fig. 3, in the absence of antigen (a), or were stimulated with 0·1 ng/ml toxic shock syndrome toxin-1 (TSST-1) (b) or 100 ng/ml TSST-1 (c) The cells were labelled with goat anti-PKCθ (right column) and mouse anti-Vβ2 fluorescein isothiocyanate (FITC) (middle column). Secondary antibodies were donkey anti-goat and rabbit anti-FITC labelled with Alexa488 and Alexa594, respectively. The left column shows the phase-contrast image of the same cells. (d) Effect of the type of APC and antigen dose on the translocation of the T-cell receptor (TCR) and protein kinase C θ (PKCθ) towards the contact area. Results are given as the mean of three independent experiments ± standard deviation (SD).
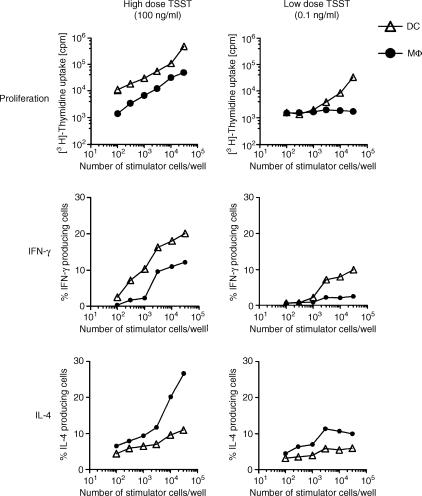
APC number influences proliferation and Th1/Th2 effector development
Proliferation assays of naive T cells were performed with increasing numbers of DCs and Mφ loaded with either a high (100 ng/ml) or a low (0·1 ng/ml) dose of TSST-1. Mφ were much less efficient stimulators of T-cell proliferation than DCs, even at a high dose of antigen and high APC–T-cell ratios. At 0·1 ng/ml TSST-1, Mφ induced only a modest degree of T-cell proliferation. Comparable results were obtained for the production of IFN-γ measured on day 3 of the culture. In contrast, IL-4, determined in parallel, was induced by Mφ and only to a modest degree by DCs.
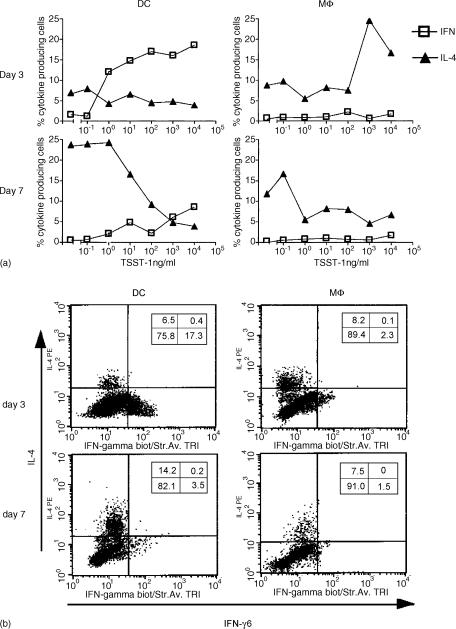
APCs promote Th1/Th2 effector development in sAg dose and a time-dependent manner
In an attempt to survey the ability of APCs, in conjunction with various doses of sAg, to modulate the development of Th1 and Th2 phenotypes, we used intracellular cytokine staining on T cells cultured for 3 or 7 days. High doses of TSST-1 in cultures of DCs were required to generate IFN-γ-producing Th1 cells. At a low sAg dose, IFN-γ induction by DCs in Vβ2+ naive T cells was significantly reduced. However, this Th1 phenotype was restricted to an early time-point in the culture, given that few were detected in day-7 cultures. IL-4-secreting Th2 cells were also present early in the culture (day 3) but only in small numbers. This population expanded over time, reaching a maximum on day 7. T cells were skewed towards this phenotype by stimulation with a high dose of TSST-1 in the presence of Mφ. In the presence of 0·1 ng of TSST-1, Mφ also induced an IL-4-producing phenotype, but very few naive cells were stimulated. At a low TSST-1 dose in the presence of DCs, a Th2 phenotype was induced in the long term, whereas in the presence of 100 ng of TSST-1, DCs completely suppressed the development of IL-4-producing cells (Fig. 6).
Figure 6.
Antigen dose, type of antigen-presenting cell (APC) and time of differentiation contribute to T helper 1 (Th1)/T helper 2 (Th2) cytokine production. A total of 3 × 105 syngeneic naive T cells/well were incubated with dendritic cells (DCs) or macrophage (Mφ) (3 × 103/well), pretreated with mitomycin C and different doses of toxic shock syndrome toxin-1 (TSST-1). After 3 or 7 days, cells were assayed for the production of interferon-γ (IFN-γ) and interleukin-4 (IL-4). Data shown are the average from four experiments (a) or dot-plots of intracellular IL-4 and IFN-γ expressed by Vβ2+ cells stimulated with a TSST-1 concentration of 10 ng/ml (b).
Discussion
In this study, we show that the type of APC and the dose of sAg regulate T-cell differentiation. APCs generated from CD34+ haematopoietic progenitor cells in the presence of GM-CSF + IL-4 or M-CSF had the morphology, phenotype and functions either of immature DCs or of Mφ. DCs had a strong stimulatory capacity on allogeneic CD4+ naive T cells, whereas Mφ had no stimulatory effect on those cells.16 Cells were 95% pure, as shown by staining for CD1a and CD14. The sAg used was TSST-1, a staphylococcal exotoxin bridging MHC class II molecules on APCs and Vβ2 chains of the TCR on the respective Vβ2+ T cells, resulting in clonal activation and expansion. TSST-1 is used in vitro as an inducer of Th1 immune responses,17,18 but is also described as polarizing naive T cells towards a Th2 phenotype.8,9,19 DCs were, at all experimental settings, more effective at clustering naive T cells than Mφ, especially in an antigen-independent manner.3,20,21 Many receptors involved in the APC–T-cell interaction, including LFA-1 (CD11a, CD18), CD54 and CD58, were expressed at the same level on both cell types. CD209, also known as the DC-specific intracellular adhesion molecule-3 (ICAM-3) grabbing non-integrin (DC-SIGN), was expressed exclusively on DCs. This molecule binds with high affinity to CD50 (ICAM-3),22 a molecule described to be involved in establishing the initial antigen-independent adhesion between APCs and T cells.23,24 CD50 is expressed on the surface of naive T cells and exerts a costimulatory effect on these cells.24,25 Both types of APCs also expressed the adhesion molecule, CD54, which binds to LFA-1 expressed on naive T cells. However, LFA-1 on resting T cells is in a low-avidity state and has to be activated by crosslinking of the TCR,26,27 or binding of CD50 to one of its ligands,25,28 before mediating adhesion to APCs. The avidity up-regulation of LFA-1 by TCR triggering may explain the strong binding ability of Mφ seen at high sAg doses, as Lee and co-workers29 reported TCR signalling in naive murine T cells in initial contacts before forming a mature immunological synapse. Differences in the ability of APCs to bind naive T cells in an antigen-independent manner, and at low sAg doses, may therefore be linked to differences in CD209 ligand expression on APCs. Once initial adhesion has been established, morphological differences between DC–T cell and Mφ–T-cell clusters are not very pronounced. As judged by scanning electron morphology, antigen-independent contacts are rather small. Antigen-dependent contacts are marked by a widening of the contact zones and an engulfment of the T cells by the APCs.30,31 Marked differences between APCs occurred with regard to the molecular reorganization at the T cell–APC interface. TCRs, as shown by staining of the TSST-1-cognate Vβ2 chain, accumulated at the contact region in an antigen-dependent manner. Revy and co-workers32 found antigen-independent DC–T-cell synapses in which TCRs, as well as PKCθ, were translocated to the contact areas. These differences from our findings may be explained by our use of rather immature DCs, as DCs at different maturational stages display different abilities of binding to T cells.33 Furthermore, mature DCs secrete chemokines that stimulate T-cell adhesion and modify contact formation.34 Mφ were far less effective than DCs at inducing a reorientation of TCRs towards the cell–cell interface. Redistribution of PKCθ was also antigen dependent, and enrichment at contact areas increased in a dose-dependent manner.6 Capping of PKCθ could only be detected in clusters with DCs. Translocation of PKCθ, a protein kinase involved in key positions in the activation of T cells,35,36 is mediated by crosslinking of the TCR and CD28 costimulation.37,38 CD86, the ligand for CD28, is expressed at an equal level by DCs and Mφ. However, DCs express ≈10-fold more MHC class II, the ligand for the TCR. When high concentrations of sAg were used, the dose–response curve for proliferation versus DC number was shifted to the left by a factor of 10 compared with the curve versus Mφ number (Fig. 7). The dose–response curves for IFN-γ generation behaved similarly (Fig. 7). Thus, in this setting, the expression of MHC class II might be the limiting factor for cell activation. At a low concentration of sAg, however, Mφ, in contrast to DCs, induced neither proliferation nor production of IFN-γ (Fig. 7). In this situation, the additional expression of costimulatory molecules, such as CD209, might be a critical factor for inducing the T-cell response. Interestingly, Mφ were superior to DCs in inducing IL-4 generation on day 3 of culture (Fig.7). Thus, suboptimal antigen presentation via MHC class II, and suboptimal costimulation, might contribute to the preferential induction of IL-4. DCs and Mφ induced different immune responses, depending on the amount of TSST-1 used, a phenomenon previously described in detail for conventional Ags.10–12 DCs in co-cultures stimulated with high sAg doses induced a Th1 phenotype, whereas low sAg doses elicited a Th2 response. Mφ induced a Th2 phenotype, regardless of the amount of TSST-1 used. Similar results have been already reported by other groups.8,9 Brandt and co-workers,9 however, also observed a Th1 response in co-cultures with Mφ. This difference may result from the addition of exogenous IL-2 to the culture medium (which favours a Th1 response)39,40 and restimulation of the T cells with phorbol 12-myristate 13-acetate (PMA) and calcium-ionophore. These stimuli induce IFN-γ production,14,41 probably via activation of the PKCθ pathway.42–46 Differentiation of naive T cells was also affected by the length of the stimulation period. Development towards a Th1 phenotype occurred early in the culture, with only very few Th1 cells found in the culture after 7 days. This may be attributed to the activation-induced cell death of Th1 effectors via the Fas/FasL pathway following stimulation with high doses of Ag.47,48 The number of Th2 cells increased over time, a phenomenon perhaps explained by the need of a prolonged stimulation period to induce IL-4 production.49 Thus, a high TSST-1-dose favoured the increased production of IFN-γ early in the culture,50 and may be detrimental to T-cell survival, whereas a low sAg dose induced a Th2 phenotype later during the culture.50–52 However, in addition to the strength of the antigenic signal, other factors, such as microbial stimuli,53 cytokines54 and endogenous danger signals,55 have been shown to be of key importance in determining the ability of APC to induce T-cell differentiation.
Figure 7.
Proliferation and cytokine production of naive T cells upon stimulation with different doses of toxic shock syndrome toxin-1 (TSST-1), presented on dendritic cells (DCs) or macrophage (Mφ). A total of 3 × 105 syngeneic naive T cells/well were incubated with various numbers (1 × 102−3 × 104/well) of DCs or Mφ pretreated with mitomycin C. Measurements were performed after 72 hr. During the last 18 hr of co-culture, cells were pulsed with 0·5 µCi [3H]thymidine or restimulated with plate-bound anti-CD3 and anti-CD28 + brefeldin A. Cytokine production was assessed by intracellular staining in Vβ2+ cells. Data are shown as the average from quadruplicate cultures.
Acknowledgments
We acknowledge the expert technical assistance of Angelika Michel and Veronika Baumeister. This work was supported by FoRUM grant AZ 328/2002
Abbreviations
- APC
antigen-recepter cell
- CB
cord blood
- CBMC
cord blood mononuclear cell
- DC
dendritic cell
- FCS
fetal calf serum
- FITCC
fluorescein isothiocyanate
- GM-CSF
granulocyte–macrophage colony-stimulating factor
- HBSS
Hanks' balanced salt solution
- HLA
human leucocyte antigen
- HPCs
haematopoietic progenitor cells
- IL
interleukin
- MACS
magnetic associated cell sorting
- M-CSF
macrophage colony-stimulating factor
- Mφ
macrophage
- MHC
major histocompatibility complex
- PBS
phosphate-buffered saline
- sAg
superantigen
- SCF
stem cell factor
- TCR
T-cell receptor
- Th1
T helper 1
- Th2
T helper 2
- TNF-α
tumour necrosis factor-α
- TSST-1
toxic shock syndrome toxin-1
References
- 1.Constant SL, Bottomly K. Induction of Th1 and Th2 CD4+ T cell responses: the alternative approaches. Annu Rev Immunol. 1997;15:297. doi: 10.1146/annurev.immunol.15.1.297. [DOI] [PubMed] [Google Scholar]
- 2.Croft M, Duncan DD, Swain SL. Response of naive antigen-specific CD4+ T cells in vitro: characteristics and antigen-presenting cell requirements. J Exp Med. 1992;176:1431. doi: 10.1084/jem.176.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhardwaj N, Young JW, Nisanian AJ, Baggers J, Steinman RM. Small amounts of superantigen, when presented on dendritic cells, are sufficient to initiate T cell responses. J Exp Med. 1993;178:633. doi: 10.1084/jem.178.2.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kupfer A, Swain SL, Singer SJ. The specific direct interaction of helper T cells and antigen-presenting B cells. II. Reorientation of the microtubule organizing center and reorganization of the membrane-associated cytoskeleton inside the bound helper T cells. J Exp Med. 1987;165:1565. doi: 10.1084/jem.165.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kupfer A, Mosmann TR, Kupfer H. Polarized expression of cytokines in cell conjugates of helper T cells and splenic B cells. Proc Natl Acad Sci USA. 1991;88:775. doi: 10.1073/pnas.88.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 7.Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 8.Langenkamp A, Messi M, Lanzavecchia A, Sallusto F. Kinetics of dendritic cell activation: impact on priming of TH1, TH2 and nonpolarized T cells. Nat Immunol. 2000;1:311. doi: 10.1038/79758. [DOI] [PubMed] [Google Scholar]
- 9.Brandt K, van der Bosch J, Fliegert R, Gehring S. TSST-1 induces Th1 or Th2 differentiation in naive CD4+ T cells in a dose- and APC-dependent manner. Scand J Immunol. 2002;56:572. doi: 10.1046/j.1365-3083.2002.01170.x. [DOI] [PubMed] [Google Scholar]
- 10.Constant S, Pfeiffer C, Woodard A, Pasqualini T, Bottomly K. Extent of T cell receptor ligation can determine the functional differentiation of naive CD4+ T cells. J Exp Med. 1995;182:1591. doi: 10.1084/jem.182.5.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hosken NA, Shibuya K, Heath AW, Murphy KM, O'Garra A. The effect of antigen dose on CD4+ T helper cell phenotype development in a T cell receptor-alpha beta-transgenic model. J Exp Med. 1995;182:1579. doi: 10.1084/jem.182.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Secrist H, DeKruyff RH, Umetsu DT. Interleukin 4 production by CD4+ T cells from allergic individuals is modulated by antigen concentration and antigen-presenting cell type. J Exp Med. 1995;181:1081. doi: 10.1084/jem.181.3.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caux C, Vanbervliet B, Massacrier C, et al. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to GM-CSF + TNF alpha. J Exp Med. 1996;184:695. doi: 10.1084/jem.184.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jung T, Schauer U, Heusser C, Neumann C, Rieger C. Detection of intracellular cytokines by flow cytometry. J Immunol Methods. 1993;159:197. doi: 10.1016/0022-1759(93)90158-4. [DOI] [PubMed] [Google Scholar]
- 15.Bartz H, Turkel O, Hoffjan S, Rothoeft T, Gonschorek A, Schauer U. Respiratory syncytial virus decreases the capacity of myeloid dendritic cells to induce interferon-gamma in naive T cells. Immunology. 2003;109:49. doi: 10.1046/j.1365-2567.2003.01629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bartz H, Rothoeft T, Anhenn O, Bunse D, Schauer U. Large-scale isolation of immature dendritic cells with features of Langerhans' cells by sorting CD34+ cord blood stem cells cultured in the presence of TGF-beta1 for cutaneous leukocyte antigen (CLA) J Immunol Methods. 2003;275:137. doi: 10.1016/s0022-1759(03)00018-8. [DOI] [PubMed] [Google Scholar]
- 17.Cameron SB, Nawijn MC, Kum WW, Savelkoul HF, Chow AW. Regulation of helper T cell responses to staphylococcal superantigens. Eur Cytokine Netw. 2001;12:210. [PubMed] [Google Scholar]
- 18.Kum WW, Hung RW, Cameron SB, Chow AW. Temporal sequence and functional implications of V beta-specific T cell receptor down-regulation and costimulatory molecule expression following in vitro stimulation with the staphylococcal superantigen toxic shock syndrome toxin-1. J Infect Dis. 2002;185:555. doi: 10.1086/338634. [DOI] [PubMed] [Google Scholar]
- 19.Gehring S, Schlaak M, van der Bosch J. A new in vitro model for studying human T cell differentiation: T(H1)/T(H2) induction following activation by superantigens. J Immunol Methods. 1998;219:85. doi: 10.1016/s0022-1759(98)00142-2. [DOI] [PubMed] [Google Scholar]
- 20.Inaba K, Steinman RM. Accessory cell–T lymphocyte interactions. Antigen-dependent and -independent clustering. J Exp Med. 1986;163:247. doi: 10.1084/jem.163.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inaba K, Romani N, Steinman RM. An antigen-independent contact mechanism as an early step in T cell-proliferative responses to dendritic cells. J Exp Med. 1989;170:527. doi: 10.1084/jem.170.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geijtenbeek TB, Torensma R, van Vliet SJ, van Duijnhoven GC, Adema GJ, van Kooyk Y, Figdor CG. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell. 2000;100:575. doi: 10.1016/s0092-8674(00)80693-5. [DOI] [PubMed] [Google Scholar]
- 23.de Fougerolles AR, Springer TA. Intercellular adhesion molecule 3, a third adhesion counter-receptor for lymphocyte function-associated molecule 1 on resting lymphocytes. J Exp Med. 1992;175:185. doi: 10.1084/jem.175.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montoya MC, Sancho D, Bonello G, et al. Role of ICAM-3 in the initial interaction of T lymphocytes and APCs. Nat Immunol. 2002;3:159. doi: 10.1038/ni753. [DOI] [PubMed] [Google Scholar]
- 25.Campanero MR, del Pozo MA, Arroyo AG, Sanchez-Mateos P, Hernandez-Caselles T, Craig A, Pulido R, Sanchez-Madrid F. ICAM-3 interacts with LFA-1 and regulates the LFA-1/ICAM-1 cell adhesion pathway. J Cell Biol. 1993;123:1007. doi: 10.1083/jcb.123.4.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dustin ML, Springer TA. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 1989;341:619. doi: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- 27.van Kooyk Y, van de Wiel-van Kemenade P, Weder P, Kuijpers TW, Figdor CG. Enhancement of LFA-1-mediated cell adhesion by triggering through CD2 or CD3 on T lymphocytes. Nature. 1989;342:811. doi: 10.1038/342811a0. [DOI] [PubMed] [Google Scholar]
- 28.Bleijs DA, Binnerts ME, van Vliet SJ, Figdor CG, van Kooyk Y. Low-affinity LFA-1/ICAM-3 interactions augment LFA-1/ICAM-1-mediated T cell adhesion and signaling by redistribution of LFA-1. J Cell Sci. 2000;113:391. doi: 10.1242/jcs.113.3.391. [DOI] [PubMed] [Google Scholar]
- 29.Lee KH, Holdorf AD, Dustin ML, Chan AC, Allen PM, Shaw AS. T cell receptor signaling precedes immunological synapse formation. Science. 2002;295:1539. doi: 10.1126/science.1067710. [DOI] [PubMed] [Google Scholar]
- 30.Delon J, Bercovici N, Raposo G, Liblau R, Trautmann A. Antigen-dependent and -independent Ca2+ responses triggered in T cells by dendritic cells compared with B cells. J Exp Med. 1998;188:1473. doi: 10.1084/jem.188.8.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Donnadieu E, Lang V, Bismuth G, Ellmeier W, Acuto O, Michel F, Trautmann A. Differential roles of Lck and Itk in T cell response to antigen recognition revealed by calcium imaging and electron microscopy. J Immunol. 2001;166:5540. doi: 10.4049/jimmunol.166.9.5540. [DOI] [PubMed] [Google Scholar]
- 32.Revy P, Sospedra M, Barbour B, Trautmann A. Functional antigen-independent synapses formed between T cells and dendritic cells. Nat Immunol. 2001;2:925. doi: 10.1038/ni713. [DOI] [PubMed] [Google Scholar]
- 33.Blanchard N, Hivroz C. The immunological synapse: the more you look the less you know. Biol Cell. 2002;94:345. doi: 10.1016/s0248-4900(02)00007-2. [DOI] [PubMed] [Google Scholar]
- 34.Bromley SK, Dustin ML. Stimulation of naive T-cell adhesion and immunological synapse formation by chemokine-dependent and -independent mechanisms. Immunology. 2002;106:289. doi: 10.1046/j.1365-2567.2002.01441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coudronniere N, Villalba M, Englund N, Altman A. NF-kappa B activation induced by T cell receptor/CD28 costimulation is mediated by protein kinase C-theta. Proc Natl Acad Sci USA. 2000;97:3394. doi: 10.1073/pnas.060028097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khoshnan A, Bae D, Tindell CA, Nel AE. The physical association of protein kinase C theta with a lipid raft-associated inhibitor of kappa B factor kinase (IKK) complex plays a role in the activation of the NF-kappa B cascade by TCR and CD28. J Immunol. 2000;165:6933. doi: 10.4049/jimmunol.165.12.6933. [DOI] [PubMed] [Google Scholar]
- 37.Bi K, Tanaka Y, Coudronniere N, Sugie K, Hong S, van Stipdonk MJ, Altman A. Antigen-induced translocation of PKC-theta to membrane rafts is required for T cell activation. Nat Immunol. 2001;2:556. doi: 10.1038/88765. [DOI] [PubMed] [Google Scholar]
- 38.Huang J, Lo PF, Zal T, Gascoigne NR, Smith BA, Levin SD, Grey HM. CD28 plays a critical role in the segregation of PKC theta within the immunologic synapse. Proc Natl Acad Sci USA. 2002;99:9369. doi: 10.1073/pnas.142298399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rogers PR, Huston G, Swain SL. High antigen density and IL-2 are required for generation of CD4 effectors secreting Th1 rather than Th0 cytokines. J Immunol. 1998;161:3844. [PubMed] [Google Scholar]
- 40.Tao X, Constant S, Jorritsma P, Bottomly K. Strength of TCR signal determines the costimulatory requirements for Th1 and Th2 CD4+ T cell differentiation. J Immunol. 1997;159:5956. [PubMed] [Google Scholar]
- 41.Sander B, Andersson J, Andersson U. Assessment of cytokines by immunofluorescence and the paraformaldehyde-saponin procedure. Immunol Rev. 1991;119:65. doi: 10.1111/j.1600-065x.1991.tb00578.x. [DOI] [PubMed] [Google Scholar]
- 42.Werlen G, Jacinto E, Xia Y, Karin M. Calcineurin preferentially synergizes with PKC-theta to activate JNK and IL-2 promoter in T lymphocytes. EMBO J. 1998;17:3101. doi: 10.1093/emboj/17.11.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ghaffari-Tabrizi N, Bauer B, Villunger A, Baier-Bitterlich G, Altman A, Utermann G, Uberall F, Baier G. Protein kinase Ctheta, a selective upstream regulator of JNK/SAPK and IL-2 promoter activation in Jurkat T cells. Eur J Immunol. 1999;29:132. doi: 10.1002/(SICI)1521-4141(199901)29:01<132::AID-IMMU132>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 44.Bauer B, Krumbock N, Ghaffari-Tabrizi N, et al. T cell expressed PKCtheta demonstrates cell-type selective function. Eur J Immunol. 2000;30:3645. doi: 10.1002/1521-4141(200012)30:12<3645::AID-IMMU3645>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 45.Avraham A, Jung S, Samuels Y, Seger R, Ben-Neriah Y. Co-stimulation-dependent activation of a JNK-kinase in T lymphocytes. Eur J Immunol. 1998;28:2320. doi: 10.1002/(SICI)1521-4141(199808)28:08<2320::AID-IMMU2320>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 46.Su B, Jacinto E, Hibi M, Kallunki T, Karin M, Ben-Neriah Y. JNK is involved in signal integration during costimulation of T lymphocytes. Cell. 1994;77:727. doi: 10.1016/0092-8674(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 47.Zhang X, Brunner T, Carter L, et al. Unequal death in T helper cell (Th) 1 and Th2 effectors: Th1, but not Th2, effectors undergo rapid Fas/FasL-mediated apoptosis. J Exp Med. 1997;185:1837. doi: 10.1084/jem.185.10.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ise W, Totsuka M, Sogawa Y, et al. Naive CD4+ T cells exhibit distinct expression patterns of cytokines and cell surface molecules on their primary responses to varying doses of antigen. J Immunol. 2002;168:3242. doi: 10.4049/jimmunol.168.7.3242. [DOI] [PubMed] [Google Scholar]
- 49.Iezzi G, Scotet E, Scheidegger D, Lanzavecchia A. The interplay between the duration of TCR and cytokine signaling determines T cell polarization. Eur J Immunol. 1999;29:4092. doi: 10.1002/(SICI)1521-4141(199912)29:12<4092::AID-IMMU4092>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 50.Rogers PR, Croft M. Peptide dose, affinity, and time of differentiation can contribute to the Th1/Th2 cytokine balance. J Immunol. 1999;163:1205. [PubMed] [Google Scholar]
- 51.Croft M, Swain SL. Recently activated naive CD4 T cells can help resting B cells, and can produce sufficient autocrine IL-4 to drive differentiation to secretion of T helper 2-type cytokines. J Immunol. 1995;154:4269. [PubMed] [Google Scholar]
- 52.Nakamura T, Kamogawa Y, Bottomly K, Flavell RA. Polarization of IL-4- and IFN-gamma-producing CD4+ T cells following activation of naive CD4+ T cells. J Immunol. 1997;158:1085. [PubMed] [Google Scholar]
- 53.de Jong EC, Vieira PL, Kalinski P, Schuitemaker JH, Tanaka Y, Wierenga EA, Yazdanbakhsh M, Kapsenberg ML. Microbial compounds selectively induce Th1 cell-promoting or Th2 cell-promoting dendritic cells in vitro with diverse Th cell-polarizing signals. J Immunol. 2002;168:1704. doi: 10.4049/jimmunol.168.4.1704. [DOI] [PubMed] [Google Scholar]
- 54.Hilkens CM, Kalinski P, de Boer M, Kapsenberg ML. Human dendritic cells require exogenous interleukin-12-inducing factors to direct the development of naive T-helper cells toward the Th1 phenotype. Blood. 1997;90:1920. [PubMed] [Google Scholar]
- 55.Aliberti J, Viola JP, Vieira-de-Abreu A, Bozza PT, Sher A, Scharfstein J. Cutting edge: bradykinin induces IL-12 production by dendritic cells: a danger signal that drives Th1 polarization. J Immunol. 2003;170:5349. doi: 10.4049/jimmunol.170.11.5349. [DOI] [PubMed] [Google Scholar]