Abstract
Peripheral blood contains two major particular infrequent dendritic cells (DC) subsets linking the innate and specific immune system, the myeloid DC and plasmacytoid DC equivalent to the natural interferon-producing cells (NIPC). The functional characterization of these cells demands large volumes of blood, making a large animal model more appropriate and beneficial for certain studies. Here, two subsets of porcine blood mononuclear cells expressing swine workshop cluster 3 (SWC3, a SIRP family member), are described and compared to monocytes. The blood DC specialized in T-cell stimulation were major histocompatibility complex (MHC) class II+, CD80/86+, CD1+/–, CD4−, and in contrast to monocytes CD14−. A CD16− and a CD16+ subset could be discriminated. Granulocyte–macrophage colony-stimulating factor and interleukin-3 were survival factors for this DC subset, and culture induced an up-regulation of MHC class II and CD80/86. The second subset described, are porcine NIPC, typically CD4++, MHC class IIlow, CD80/86low, CD1−, CD8−/low, CD16−/low and CD45RA−/low. Porcine NIPC had high interleukin-3 binding capacity, and survived in response to this cytokine. Their unique function was strong interferon type I secretion after virus stimulation. Both subsets were endocytically active when freshly isolated, and down-regulated this activity after in vitro maturation. Taken together, the present report has delineated porcine blood DC and NIPC, permitting a more detailed understanding of innate immune defences, particularly in response to infections.
Introduction
Dendritic cells (DC) are a heterogeneous group of potent antigen-presenting cells (APC), with the unique capacity to prime naive T-cell responses. 1 In order to fulfil their role as sentinels of the immune systems, they express specialized pattern recognition receptors, such as the Toll-like receptors (TLR), and a variety of antigen uptake receptors such as Fc receptors and certain C-type lectins.1,2 DC are the main cellular element controlling T lymphocyte activation and regulation. In addition, they are also involved in B-cell responses, through delivery of native antigen to B lymphocytes3 and induction of B-cell proliferation and isotype switching.4,5
In human blood, several populations of DC have been identified. The CD11c+ CD13+ CD33+ population, which is most likely of myeloid origin, contains a CD16− subset that can differentiate into Langerhans cells6 as well as a CD16+ subset were described.7 A second major human DC population – major histocompatibility complex (MHC) class II+ and of low-density – is negative for lineage markers (CD3, CD19, CD56 and CD14) and CD11c. It is phenotypically characterized by high CD4 and interleukin (IL)-3 receptor (CD123) expression. This population represents the ‘natural interferon-producing cells’ (NIPC), which was identified almost 20 years ago.8 Although phenotypic analysis of NIPC indicated that these specialized cells could be DC9 it was only recently demonstrated that NIPC can differentiate into DC in vitro, and correspond to the plasmacytoid DC.10 Nevertheless, whereas their contribution to antigen presentation in vivo is still unclear, their function as the major systemic source of interferon-α (IFN-α) during acute virus infection has been clarified in vivo.11,12 Because of the expression of certain genes and cell surface molecules related to the lymphoid lineage, it was speculated that pDC/NIPC should be of lymphoid origin. Recent studies have indicated that pDC/NIPC might be heterogeneous, containing cells with more lymphoid characteristics as well as cells with more myeloid characteristics.13
NIPC were identified in the pig based on their IFN-α production in response to transmissible gastroenteritis virus (TGEV), being non-adherent, non-T, non-B, CD4+, MHC-class-II-positive cells.14 The porcine model has permitted a number of early discoveries concerning NIPC, particularly with respect to their ontogeny15 and their migration to lymphoid tissue after viral challenge in vivo.12,16 Despite these early advances a detailed phenotypic and functional characterization of NIPC is still lacking. Their identification continues to rely on detection of IFN type I. In fact there are no reports on the identification and characterization of porcine blood DC in general. Consequently, it was to this end that the present work was designed, permitting the identification of two distinct subsets of porcine peripheral blood mononuclear cells (PBMC), expressing swine workshop cluster 3 (SWC3), a member of the signal-regulatory protein (SIRP) family.17 These were the CD4− CD14− blood DC subset and the CD4++ CD14− NIPC.
Materials and methods
Isolation and culture of cells
PBMC were isolated from citrated blood of specific pathogen-free pigs kept at the institute, using Ficoll-Paque (1·077 g/l, Pharmacia, Upsala, Sweden) density centrifugation.18 Cells were cultured in ‘growth medium’ phenol red-free Dulbecco's modified Eagle's medium (DMEM; Invitrogen, Basel, Switzerland), supplemented with 2 mm glutamine, 50 µm 2-mercaptoethanol and porcine serum (10% v/v, Sigma Chemicals, Buchs, Switzerland). For certain culture conditions, 100 U/ml recombinant porcine (rp) IL-3 (see below) or 25 ng/ml recombinant porcine granulocyte–macrophage colony-stimulating factor (rpGM-CSF) (kindly obtained from Shigeki Inumaru, Institute of Animal Health, Ibaraki, Japan) was added to the cultures.
Monoclonal antibodies (mAbs) and phenotyping
For phenotyping, mAbs against the following cell surface molecules were used (reviewed in 19 and 20): SWC3 (mAb 74-22-15A), CD1 (mAb 76-7-4), CD3 (mAbs 3E8 and PPT3), CD4 (mAbs 74-12-4 and PT90A), CD5 (mAb b53b7), CD6 (mAb a38b2), CD8α (mAbs PT81B and 76-2-11), CD11R2 (mAb S-Hcl3; cross-reactive antihuman CD11c, as defined by the 3rd Swine CD Workshop21), CD14 (mAbs MY4, and CAM36A), CD16 (mAb G7), CD21 (mAb CC51), CD45RA (mAb MIL13), MHC class II (mAb MSA3), surface immunoglobulin light chain (mAb K139 3E1: Serotec, Oxford, UK). CD80 and CD86 expression are detected using a human CTLA4-mouse-immunoglobulin fusion protein (Alexis Corporation, Lausen, Switzerland22). Hybridomas for the mAbs 11/8/1, MSA3, b53b7, a38b2, 74-22-15A, 76-7-4 and 74-12-4 were kindly donated by Dr A. Saalmüller (BFAV Tübingen, Germany), and for the mAbs PTT3, MIL2, MIL3, MIL4 and MIL13 by Dr K. Haverson, University of Bristol, UK. mAbs CAM36A, PT90A, PT81B and 3E8 were purchased from VMRD (Pullman, WA), G7 and S-Hcl3 from Becton Dickinson (San Jose, CA). Fluoroscein isothiocyanate (FITC), R-phycoerythrin (PE) or biotin-conjugated 74-12-4, 74-22-15, MY4 and MIL13 mAbs were obtained from Southern Biotechnology Associates, Inc. (Birminham, AL).
Multicolour immunofluorescence analyses by flow cytometry (FCM) were performed for the identification of leucocyte populations as previously described23 using isotype-specific conjugates: goat anti-mouse immunoglobulin G (IgG), F(ab′)2 fragments, conjugates with FITC, PE or biotin (Southern Biotechnology Associates), or with allophycocyanin (Caltag Laboratories, Life Technologies, Zürich, Switzerland). Biotinylated conjugates were detected using streptavidin–R-PE-Cy5.5 (Becton Dickinson). Acquisition of data used a FACSCalibur flow cytometer, and analysis was done with the CellQuest Pro program (both Becton Dickinson).
Cloning and expression of recombinant porcine IL-3
For cloning of rp IL-3, PBMC were stimulated for 18 hr with concanavalin A (ConA) (10 µg/ml), and total RNA isolated using RNAeasy-kit (Qiagen, Basel, Switzerland). After reverse transcription using oligo-dT primary and Omniscript RT (Qiagen), the porcine IL-3 gene24 was amplified by standard polymerase chain reaction (PCR). The PCR product was subsequently cloned into pEAK8-HIS expression vector (kindly provided by Dr Florian Wurm, EPFL Lausanne, Switzerland). This vector was derived from pEAK8 by replacing the multiple cloning site with a XbaI-6His-stop cassette, permitting the production of recombinant protein tagged with the 6-histidine epitope. The sequence of the cloned IL-3 corresponded to the published sequence.24 IL-3 was produced in HEK-293 EBNA cells using calcium-phosphate transfection.25 The supernatant was collected 3 days after transfection and contained IL-3 with 1000 U biological activity per ml, as tested on porcine bone marrow haematopoietic cells.24 For binding studies 1 × 106 PBMC were incubated with 100 µl of transfection supernatant containing IL-3 for 20 min at 4°. After washing with CellWash (Becton Dickinson), IL-3 was detected using a mAb against the histidine epitope (anti-His, IgG1; Roche Diagnostics).
Enrichment of blood DC
For isolation of blood DC, PBMC were cultured as described above for 18 hr, and non-adherent cells collected. These were enriched for low-density cells on a metrizamide gradient (14·5% w/v in RPMI 10% fetal bovine serum). To this end, 5 × 107 cells/ml were overlayed on the metrizamide and centrifuged at 450 g for 10 min at room temperature. Depletion of lymphocytes used a cocktail of anti-CD3 (T cells), anti-CD8 (T-cell subset and natural killer cells), anti-CD45RA (T-cell subset and B cells) and the Dynal magnetic separation system (Dynal, Oslo, Norway). The remaining population was further separated into a CD14+ and CD14− subset using the Miltenyi magnetic-activated cell sorting system (MACS; Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). Purity for SWC3 expression was 60–75% and for CD14 expression over 95%.
T-cell stimulation assays
The processing and presentation of antigen was assessed in a foot-and-mouth disease virus (FMDV)-specific restimulation assay.26 To this end, PBMC were isolated from specific pathogen-free pigs vaccinated against FMDV (three booster injections, serotype C1 Oberbayern; vaccine kindly provided by Dr P. Barnett, Institute for Animal Health, Pirbright, UK) and β-propiolactone-inactivated FMDV antigen.27 For superantigen staphylococcal enterotoxin B (SEB) presentation assays, CD6+ T cells were purified by MACS (Miltenyi Biotec;22 purity over 98%). The enriched APC population was incubated simultaneously with SEB (500 ng/ml; Toxin Technology, Sarasota, FL), and mitomycin C (10 µg/ml; Sigma) for 1 hr at 39°, followed by four washings. Triplicates of 2 × 105 T cells/well were seeded into 96-well plates together with titrated numbers of APC. After 3 days, cocultures were pulsed with 1 µCi/well [3H]methyl-thymidine (Moravek Biochemicals, Brea, CA) for 18 hr. The plates were harvested on to filter mats, and counted in a 1450 MicroBeta® TriLux counter (Wallac, Turku, Finland).
IFN-α induction and detection
TGEV (strain Perdue 115) was propagated in PD-5 or PK15 cells.28 For induction of IFN-α in PBMC populations, virus preparations were UV-inactivated under controlled conditions.29 Optimal concentrations of virus preparations for IFN induction were determined by titration on PBMC. Isolated subpopulations of PBMC were tested for IFN-α production by UV-inactivated TGEV (UV-TGEV) stimulation for 18 hr. The concentration of secreted IFN in the supernatants was quantified using a bioassay based on the antiviral effect of IFN against vesicular stomatitis virus (VSV). Reduction of VSV-induced cytopathic effect in PK-15 cells induced by the test samples, or by recombinant porcine IFN-α (R&D Systems, Barton Lane, UK) as a standard, was quantified as previously described. 30 The presence of IFN-α was controlled by addition of a neutralizing anti-IFN-α polyclonal antiserum (R&D Systems).
Detection of IFN-α producing cells by confocal microscopy and flow cytometry
SWC3+ PBMC, sorted using MACS (Miltenyi Biotec), were stimulated with UV-inactivated TGEV at concentrations giving optimal IFN responses, and cultured for 6 hr in growth medium. After staining of cell surface molecules, the cells were fixed and permeabilized (Fix&Perm, Caltag, CA) for staining with anti-IFN-α mAbs F17 and K9 (10 µg/ml; R&D Systems). For flow cytometric detection, isotype-specific conjugates – FITC, R-PE (SBA) and R-PE-Cy5 (Dako) – were used. For confocal microscopy, the isotype-specific conjugates carried the Alexa-488, Alexa-546 and Alexa-633 fluorochromes (Molecular Probes, Leiden, Netherlands).
Antigen uptake
Freshly isolated PBMC, as well as PBMC cultured for 24 hr in the presence of IL-3 (50 U/ml) and GM-CSF (50 U/ml), were labelled with SWC3, CD4 and CD14. The cells were then incubated at 37° for 15–30 min with DQ™ Green bovine serum albumin (BSA; 10 µg/ml, Molecular Probes), zymosan bioparticles Bodipy® FL (5 particles/cell in medium with 10% porcine serum, Molecular Probes) or dextran Bodipy® FL (0·1 mg/ml) (all Molecular Probes). DQ™ Green BSA acquires fluorescence after dequenching through proteolytic enzyme cleavage. As controls, the uptake at 4° was used. The geometric mean fluorescence intensities (MFI) values obtained at 4° were subtracted from the 37° MFI values.
Results
APC and NIPC are within the SWC3+ PBMC
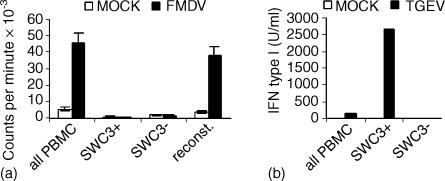
From previous work, it was known that the SWC3 molecule was dominant on myeloid cells.31–33 In addition, porcine monocyte-derived DC, bone marrow-derived DC,22 lamina propria DC,34 dermal DC35 and thymic DC36 all express SWC3. Consequently, it was assumed as a working hypothesis that blood DC should also express SWC3. This was confirmed by performing an antigen presentation assay with whole PBMC, PBMC depleted of SWC3+ cells, SWC3+ PBMC alone and SWC3− PBMC reconstituted with SWC3+ cells. It was only possible to obtain an anti-FMDV-specific proliferation when the SWC3+ cells were together with the responding lymphocytes. Neither SWC3+ cells alone nor SWC3− cells alone responded to the antigen recall (Fig. 1a).
Figure 1.
APC and NIPC are within SWC3+ PBMC. (a) FMDV-specific proliferation of unseparated PBMC, SWC3+ PBMC, SWC3− PBMC and PBMC reconstituted with the SWC3+ and SWC3− fractions. PBMC were isolated from pigs immune against FMDV and restimulated with inactivated FMDV. (b) IFN type I production of unseparated PBMC, SWC3+ PBMC, and SWC3− PBMC in response to UV-inactivated TGEV. IFN bioactivity was tested 18 hr post stimulation.
Using a similar approach, it was also demonstrated that cells producing IFN type I in response to UV-TGEV were also within the SWC3+ PBMC. While no IFN response was found in the SWC3− PBMC, IFN-producing cells were enriched 10–20-fold in the SWC3+ fraction (Fig. 1b). Because of the reported observation that UV-TGEV is a potent inducer of IFN-α in porcine NIPC,14 the results in Fig. 1(b) indicate that porcine NIPC are within the SWC3+ fraction of PBMC.
Phenotypic identification of putative DC and NIPC populations
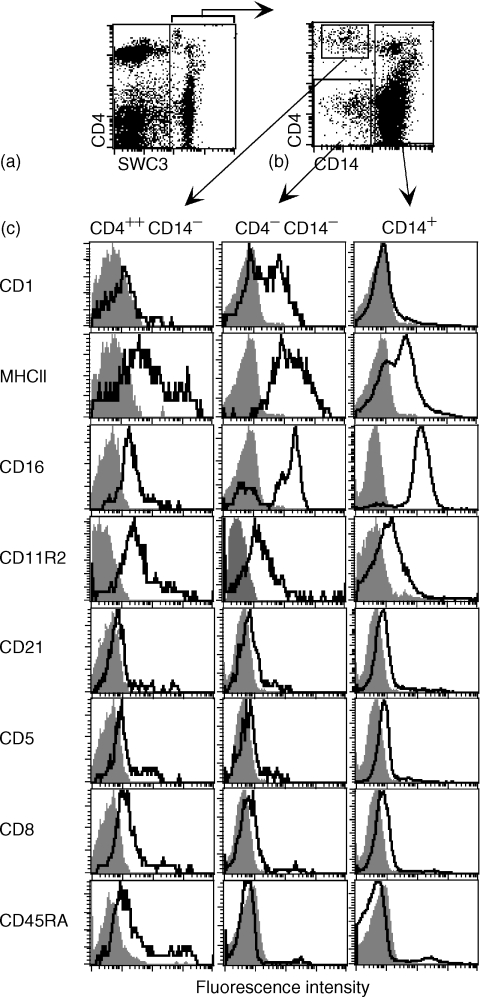
Using a SWC3/CD4/CD14 three-colour immunofluorescence analysis of freshly isolated PBMC, three subsets of SWC3+ cells were identified and defined by electronic gating on SWC3+ cells. This was performed in a SWC3/CD4 dot plot to ensure the inclusion the CD4++ cells, but the exclusion of SWC3low lymphocytes (Figs 2a, b). The major CD14+ population accounting 5–15% of all PBMC contains mostly or exclusively monocytes.23,31 Interestingly, a subset of these cells expressed high levels of CD4. These cells with high forward and side scatter were not further characterized in the present paper. Focusing on the CD14− subset within the SWC3+ PBMC, two populations, which have not yet been described in the pig, were detectable: a CD4++ CD14− representing 0·1–0·3% of all PBMC and a CD4− CD14− representing 0·2–0·6% of the PBMC. Further phenotypic characterization of these three SWC3+ mononuclear cell subsets was performed using four-colour immunofluorescence analysis (Fig. 2c). CD1 was expressed on 50–80% of CD4− CD14− cells, not on of CD14+ and on CD4++ CD14− cells. High MHC class II expression dominated the CD4− CD14− subset, whereas a more heterogeneous but clearly lower expression was observed on the CD14+ cells and on CD4++ CD14− cells. When several animals were tested, the MHC class II expression was often more homogeneous on the CD4++ CD14− subset, being seen as the MHC class II low peak in Fig. 2(c). CD16 expression was mostly high on the CD14+ cells, whereas the CD4− CD14− subset could be divided into a major CD16+ and a minor CD16− subpopulation. Analyses of CD11R2, a porcine integrin detected with a cross-reactive anti-human CD11c mAb, showed this to be expressed similarly on all subsets of SWC3+ cells. This mAb was shown to detect an integrin on porcine cells with a similar molecular weight but different cellular distribution to CD11c on human cells. 21
Figure 2.
Phenotype of SWC3+ CD4+ CD14−, SWC3+ CD4− CD14− and SWC3+ CD14+ PBMC. (a) SWC3/CD4 dot plot defining a region of SWC3+ cells. (b) Definition of three subsets by electronic gating based on CD14 and CD4 expression on SWC3+ cells defined in (a). (c) Expression of CD1, MHC class II, CD16, CD11R2, CD21, CD5, CD8, CD45RA on the CD4+ CD14− subset (histograms in left column), on the CD4− CD14− subset (histograms middle column) and on the CD14+ subset (histograms right column). A representative experiment out of five is shown.
None of the SWC3+ subsets defined in Fig. 2(a) expressed the T-cell marker CD3 (data not shown), CD5, nor markers for porcine B cells, such as surface immunoglobulin (data not shown) or CD21 (Fig. 2c). Another characteristic distinguishing all three SWC3+ subset defined in Fig. 2(a) from B cells was their high expression of SWC1, a marker absent on B cells (data not shown31). CD8α was expressed, but only weak and on a small subset of CD4++ CD14− cells, and not on the other CD14+ SWC3+ PBMC (Fig. 2c). CD45RA was expressed at low levels only on the CD4++ CD14− subset, but not on the majority of cells in the other subsets. Nevertheless, on all three defined subsets a small subpopulation of cells with higher levels of CD45RA was found (Fig. 2c).
Adherence properties of SWC3+ subsets
Monocytes are well known for their adherence and porcine cells are no exception.18 Consequently, PBMC were cultured for 18 hr on polystyrene plastic dishes separated into adherent and non-adherent cells. Within the adherent fraction only the CD14+ subset was found. In contrast, the non-adherent fraction contained all SWC3+ subsets, with an enrichment of the CD14− subsets (data not shown).
Culture-induced phenotypic maturation of SWC3+ subsets
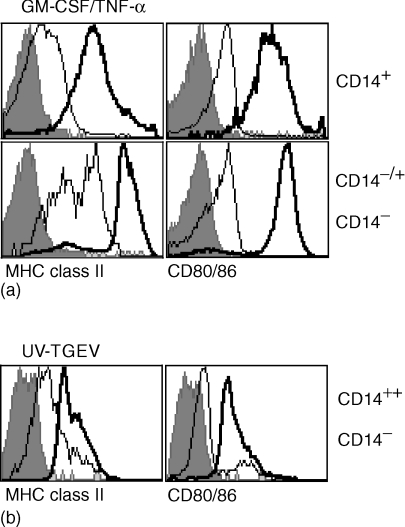
Human blood DC circulate in an immature state, and were thus also termed pre-DC. After culture, particularly in the presence of appropriate signals, a rapid maturation to fully active APC is induced.36,37 The existence of this phenomenon with putative porcine blood DC was tested by comparative analysis of MHC class II and CD80/86 as maturation markers. Interestingly, a culture-induced up-regulation of CD4 on CD1+ CD14− cells was found with some animals. This population now termed CD4−/+ CD14− corresponded phenotypically to the freshly isolated CD4− CD14− cells, and remained clearly distinguishable from the CD4++ CD1− CD14− subset (data not shown). Therefore, the phenotypic differentiation based on SWC3, CD4 and CD14 could still be employed for these analyses of in vitro cultured cells. After 24 hr culture, in the presence of TNF-α the strongest up-regulation of MHC class II and CD80/86 was observed on the CD4−/+ CD14− subset, followed by the non-adherent CD14+ subset (Fig. 3a). Only a minor up-regulation of MHC class II and CD80/86 was detectable after 24 hr on the CD4++ CD14− subset, even after stimulation with IFN-inducing UV-TGEV (data not shown). In contrast, when incubated for 3 days with UV-TGEV, an up-regulation of MHC class II and CD80/86 was now observed on the CD4++ CD14− subset (Fig. 3b), although the levels were still low compared to the CD4−/+ CD14− subset. These results demonstrated that all SWC3+ PBMC could express molecules related to antigen presentation.
Figure 3.
Culture-induced up-regulation of MHC class II and CD80/86. Freshly isolated or cultured PBMC were analysed by four-colour immunofluorescence labelling using mAbs against SWC3, CD4 and CD14 along with MHC class II or CD80/86. Using electronic gates as defined in Fig. 2, the MHC class II and CD80/86 expression on freshly isolated PBMC (light lines) and on cultured/stimulated PBMC (bold lines) was determined. The filled histograms represent the negative controls. (a) PBMC were cultured for 24 h in the presence of GM-CSF and TNF-α. Upper histograms: SWC3+CD14+ cells; lower histograms: SWC3+ CD14− CD4− cells. (b) PBMC were stimulated with UV-TGEV were cultured for 72 hr. The SWC3+ CD4++ CD14− subset is shown. A representative experiment out of three is shown.
Blood DC with high T-cell stimulatory activity are within the SWC3+ CD4− CD14− subset
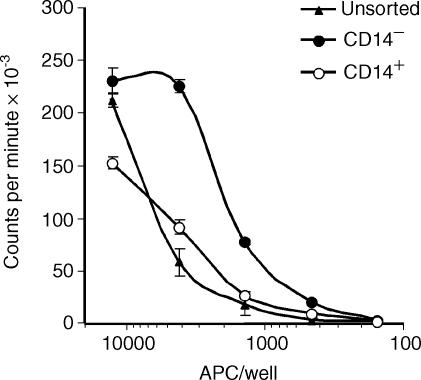
Based on the particularly high expression of MHC class II and CD80/86 of the CD4− CD14− subset after culture, it was assumed that their main function would be antigen presentation and the potent induction of T-cell activation. In order to verify this, PBMC were cultured for 18 hr to induce what should be the final differentiation of blood DC into potent APC. Low-density cells were then enriched using a metrizamide gradient, depleted of lymphocytes, and finally separated into a CD14− and a CD14+ fraction as indicated in materials and methods. As shown in Fig. 4, purified CD14− had the higher capacity to induce a T-cell proliferation, compared to the unsorted low density PBMC, or the cells enriched for CD14+ monocytes. Phase-contrast microscopic analysis of the fraction enriched for CD14− demonstrated the presence of cells with dendritic morphology (data not shown). Taken together, these results supported the conclusion that porcine blood DC with high T-cell stimulatory activity were within the CD14− subset.
Figure 4.
T-cell stimulatory capacity of CD14+ and CD14− PBMC. PBMC were cultured for 18 hr in the presence of GM-CSF, and low-density cells were enriched using a metrizamide gradient. These cells were further enriched for antigen presenting cells by depletion of CD3+, CD8+ and CD45RA+ lymphocytes, and then further separated into CD14+ and CD14− cells. The capacity of unsorted low-density cells (after metrizamide gradient, triangles), the CD14+ fraction (open circles) and the CD14− fraction (filled circles) loaded with SEB to stimulate purified T lymphocytes was determined by 3H-thymidine uptake.
NIPC are found within the SWC3+ CD4++ CD14− PBMC
Previous work has demonstrated that UV-TGEV is a potent inducer of IFN produced by porcine NIPC, and that these cells can be characterized as CD4+ non-T cells. Therefore, it was hypothesized that the phenotypically defined CD4++ CD14− subset of SWC3+ PBMC should represent or contain NIPC. In a first series of experiments, PBMC were separated by MACS sorting using mAbs against CD4, CD5, CD6, CD8, CD14, CD45RA, and MHC class II. Then the positive as well as the negative fraction and the unsorted PBMC were stimulated with UV-TGEV to induce type I IFN. The results summarized in Table 1 showed that anti-CD4 permitted a positive selection of IFN-producing cells, and a depletion of such cells from the negative fraction. Separation using anti-MHC class II mAb neither enriched nor depleted NIPC, while with all other mAbs NIPC were enriched or solely present in the negative fraction.
Table 1.
IFN type I production (U/ml) by PBMC sorted using the indicated mAbs*
| CD4 | CD5 | CD6 | CD8 | CD14 | CD45RA | MHCII | |
|---|---|---|---|---|---|---|---|
| Unseparated | 120 | 89 | 58 | 90 | 105 | 78 | 123 |
| Positive fraction | 651 | 0 | 0 | 0 | 0 | 0 | 150 |
| Negative fraction | 0 | 308 | 293 | 1650 | 186 | 2336 | 145 |
IFN type I was induced using UV-TGEV stimulation for 18 hr.
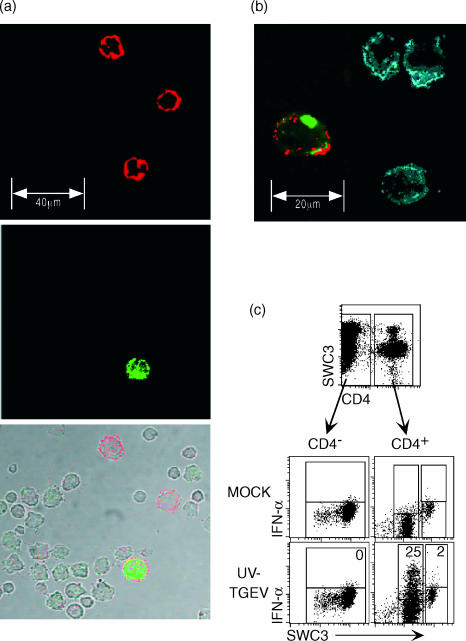
Using three-colour immunofluorescence confocal microscopy analysis of sorted SWC3+ PBMC, it was confirmed that all IFN-producing cells induced by UV-TGEV were CD4+, although some CD4+ did not stain for IFN-α (Fig. 5a,b). The SWC3+ CD4+ cells were large round cells with at least the size of the other CD4− monocytic cells (Fig. 5a,b). In a similar approach using FCM, it was demonstrated that intracellular IFN-α protein was only found within CD4+ SWC3+ (Fig. 5c, lower right plot). An important observation was that only the CD4+ cells with lower SWC3 expression produced IFN after UV-TGEV stimulation (Fig. 5b,c). These cells represented the CD14− subset described in Fig. 2(b), while the CD4+ cells with high levels of SWC3 also expressed high levels of CD14 (Fig. 2a), and did not produce IFN after UV-TGEV stimulation (Fig. 5c lower right plot). Nevertheless, the SWC3 expression on NIPC was still clearly higher than the low expression found on a subset of lymphocytes. The frequency of IFN-producing cells after UV-TGEV stimulation within this CD4++ SWC3low subset was 10–25%, dependent on the experiment. This was independent of the time points of analysis, which were 6, 8, or 16 hr post stimulation.
Figure 5.
Identification of NIPC. SWC3+ sorted PBMC were stimulated with mock or UV-inactivated TGEV and cultured for 6 hr. NIPC were identified using triple immunofluorescence analysis of SWC3/CD4/IFN-α expression. After cell surface labelling, the cells were fixed and permeabilized to detect intracellular cytokine. (a) Confocal laser scanning microscopy: CD4 (upper plot, red), IFN-α (middle plot, green) and Nomarski/CD4/IFN overlay (bottom plot). (b) SWC3/CD4/IFN-α expression a different selection with higher magnification. (c) Triple immunofluorescence of SWC3/CD4/IFN-α expression analysed by FCM: Using electronic gates CD4+ and CD4− fractions were defined (upper plot). The intracellular SWC3/IFN-α staining of these fractions after MOCK (middle plots) and UV-TGEV-stimulation (lower plots) is shown. The numbers shown in the latter plots represent the percentage of IFN-α positive cells relative to the SWC3+ CD4−, SWC3low CD4+ and the SWC3high CD4+ subsets as defined by electronic gates. A representative experiment out of five is shown.
Although CD5, CD8 and CD45RA can be expressed on a small CD4++ CD14− SWC3+ subset at high levels (Fig. 2c), double labelling with IFN demonstrated that the IFN-positive cells did not express high levels of CD5, CD8 or CD45RA (data not shown). This confirmed the results obtained using the MACS sorting described in Table 1, and clearly identified porcine NIPC as SWC3+/low cells with high expression of CD4 and little or no expression of CD5, CD8 and CD45RA.
Differential in vitro survival
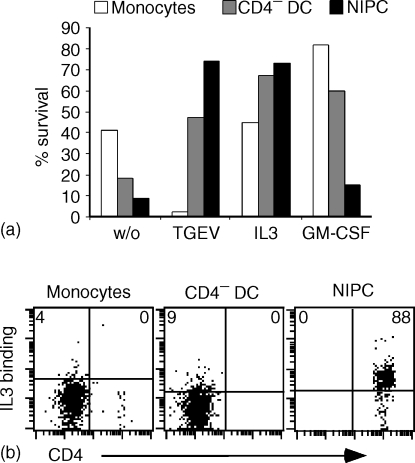
For these experiments unsorted PBMC were cultured for 24 hr in the presence of IL-3, GM-CSF or UV-TGEV, and their survival quantified by combining cell counting and multicolour FCM. As shown in Fig. 6(a), the three subsets – CD14+ monocytes, CD4− CD14− DC and CD4++ CD14− NIPC – had a different survival capacity after a short period of in vitro culture. Without addition of cytokines or virus, the monocytes had the highest survival rate. Addition of UV-TGEV reversed this situation to favour the CD4− CD14− blood DC and CD4++ CD14−NIPC.The UV-TGEV actually had a negative effect on the presence of non-adherent CD14+ cells. IL-3 also strongly promoted survival of the CD4− blood DC and the NIPC, but had no apparent influence on the CD14+ monocytes. In contrast, GM-CSF gave enhanced survival of the CD4− CD14− blood DC and CD14+ monocytes, but not of the CD4++ NIPC.
Figure 6.
Survival signals for and IL-3 binding to SWC3+ PBMC subsets. (a) PBMC were cultured for 24 hr in the presence of UV-TGEV, IL-3 or GM-CSF. Using an anti SWC3/CD4/CD14 triple labelling, the frequencies of SWC3+CD14+ monocytes (white bars), SWC3+ CD4− CD14− blood DC (grey bars) and of SWC3+ CD4+ CD14− NIPC (black bars) were determined before and after culture to calculate the survival of the defined subsets. (b) Binding capacity of recombinant porcine IL-3 to SWC3+ CD14+ monocytes, SWC3+ CD4− CD14− blood DC and to SWC3+ CD4+ CD14− NIPC. The populations were identified in four-colour immunofluorescence analysis. Supernatant from mock transfected cells were used as negative controls to set the quadrants giving percentages. For (a) and (b) representative experiments are shown.
The potent effect of IL-3 on NIPC survival has been documented for human NIPC/pDC and is related to high IL-3 receptor (CD123) expression on these cells. Binding studies with IL-3 on freshly isolated cells demonstrated that only the CD4++ CD14− subset of SWC3+ cells bound detectable levels of IL-3 (Fig. 6b). These results demonstrate that the CD4++ CD14− subset of SWC3+ PBMC represents a homogeneous population equivalent to the human NIPC/pDC population.
Antigen uptake of SWC3+ subsets
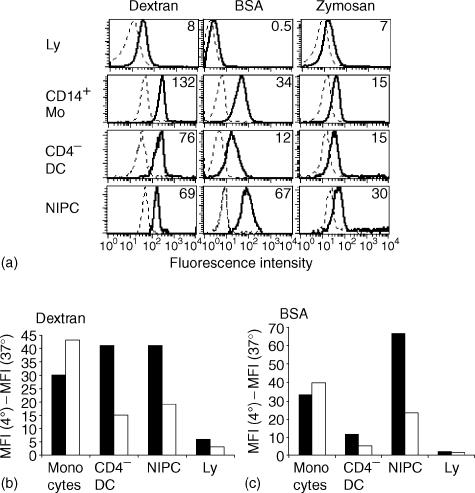
A key function of monocytic cells and DC is the uptake of foreign material from their environment. Consequently, the capacity of the three subsets of SWC3+ PBMC to endocytose BSA, dextran, and zymosan was determined. Initial analyses used freshly isolated cells. All SWC3 subsets possessed endocytic activity for BSA, dextran and zymosan. The differences in uptake of these antigens observed with the data shown in Fig. 7(a) was statistically not significant, when the MFI values from repeated experiments were analysed by Student's t-test (P > 0·05). Lymphocytes were also able to take up dextran, but always clearly at lower intensity compared to the SWC3+ subsets. An important characteristic for all subsets was the relative homogeneity in their endocytic activity, as seen by narrow peaks in the histograms (Fig. 7a).
Figure 7.
Endocytic activity of monocytes and blood DC subsets. (a) Comparative uptake of dextran, BSA and zymosan at 4° (dotted lines) and 37° (solid lines) by lymphocytes (Ly, defined as SWC3− cells), SWC3+ CD14+ monocytes, SWC3+ CD4− blood DC and SWC3+ CD4++ CD14− NIPC. The numbers shown represent the geometric mean fluorescence intensities (MFI) of the uptake at 4° subtracted from the uptake at 37°. (b) and (c) Influence of culture on uptake of dextran and BSA by monocytes, blood DC, NIPC and lymphocytes (Ly). The MFI of the uptake at 4° was subtracted from the uptake at 37°. Data shown for uncultured PBMC (filled bars) is compared with PBMC cultured for 24 hr (unfilled bars). A representative experiment out of three is shown.
The above analyses were extended by using in vitro culture for 24 hr to promote maturation of the DC from any pre-DC. This revealed a different regulation of the endocytic processes, dependent on the subset analysed (Fig. 7b,c). Both CD14− subsets – the CD4− and CD4++– down-regulated their capacity to endocytose dextran and BSA, whereas the CD14+ monocytes increased their dextran uptake and retained BSA endocytosis at high levels (Fig. 7b,c). These culture-induced differences were statistically significant (P > 0·05), and relate to the concept of rapid maturation of blood DC (including NIPC) after in vitro culture.
Discussion
The present report describes three subsets of SWC3+ PBMC. The CD4− CD14+ CD16+ monocytes were the dominant subset. These cells adhere to plastic and rapidly differentiate into macrophages26,32 or into monocyte-derived DC if cultured in the presence of IL-4 and GM-CSF.22 Nevertheless, even after overnight culture of PBMC, many CD14+ cells can still be found in the non-adherent fraction. These cells expressed relatively high levels of MHC class II and CD80/86 indicating the possibility that some may have differentiated into DC. In this respect, it is important to note that CD14 is retained on porcine monocyte-derived DC, whether immature or mature.22
The second subset of SWC3+ PBMC, which could be clearly characterized, is that containing the CD4− CD14− subset, which could be divided into a CD1+ and CD1− subset. The up-regulation of MHC class II and CD80/86 molecules following a short culture period along with the dendritic morphology and potent T-cell stimulatory capacity of the CD4− CD14− cells, led to the conclusion that these cells represents a blood DC subset. These blood DC are endocytically active for uptake of BSA and dextran, and the down-regulation of this activity after in vitro maturation is in accordance with the concept of DC maturation established for human DC.37,38 Another characteristic, which links to human blood DC is the heterogeneous expression of CD16. The porcine CD4− CD14− blood DC can be divided into a CD16+ and a CD16− subpopulation. Within the human CD11c+ blood DC a CD16− subset has been described to differentiate into Langerhans cells.6 The CD16+ subset of human blood DC is a potent producer of TNF-α, with antibody-dependent cellular cytotoxicity (ADCC) activity.7 Whether these two subpopulations of porcine blood DC separated on the basis of their CD16 expression can be distinguished similar to their human counterparts requires further investigation.
The third discernible subset is the SWC3+ CD1− CD4++ CD14− NIPC. Their most prominent feature is high IFN type I production, after stimulation with certain viruses. In contrast to the ‘classic’ IFN pathway induced in most nucleated cells following infection and replication of certain viruses, NIPC can be triggered to produce IFN by viral glycoprotein structures without requiring live virus or virus infection.40–42 This feature is unique to NIPC, and was the basis for their identification. Not all SWC3+ CD4++ CD14− PBMC produced IFN after stimulation with the virus employed in this study, TGEV. This was not because of a lack of sensitivity in the assay procedures; IFN-α enzyme-linked immunospot assays gave similar frequencies of response.29 It appeared that not all NIPC would respond to TGEV stimulation, at least with respect to IFN production. This was supported by the observation that stimulation of NIPC with TGEV and oligodeoxynucleotides containing certain CpG motifs induced up to 80% IFN-α secreting cells in the SWC3+ CD4++ phenotypically defined population (data not shown). Moreover, the capacity of all cells in the subset to bind IL-3 indicated a homogeneous population corresponding to human NIPC/pDC. It is important to note that also not all human NIPC/pDC defined on a phenotypic basis could be induced to produce IFN detectable by FCM. 44
In contrast to CD4− CD14− blood DC, porcine NIPC do not express high levels of MHC class II and CD80/86, and should be poor APC. NIPC can be induced to express MHC class II and CD80/86 by culture in the presence of IL-3. Nevertheless, the levels of expression do not reach those of the CD4− CD14− subset, continuing to argue against a major role of NIPC in antigen presentation. However, the endocytic activity of freshly isolated porcine NIPC was surprisingly potent for BSA and dextran uptake. This contrasts with a report on human pDC, being unable to take up dextran.45 However, these studies were performed with FITC-conjugated dextran incubated with the cells for 40 min The fluorescence of FITC is pH sensitive, and could have been lost within the acidic lysosomal compartment after such relatively long incubation. This endocytic activity of porcine NIPC would suggest an important role in the transport of antigen to the lymphoid tissue. Together with the reported active migration of NIPC into lymphoid tissue after viral challenge16,46,47 it would appear that NIPC function in antigen presentation.
Another open question in NIPC biology is the origin of these cells. The expression of SWC3 would imply a myeloid origin, because of the fact that SWC3 is typically expressed on myeloid cells including neutrophils, eosinophils, monocytes and macrophages but not on lymphocytes. However, SWC3 is expressed at low levels early in haematopoiesis, even on c-Kit+ lineage negative cells (Summerfield et al. unpublished results), and on a large proportion of primitive haematopoietic fetal liver cells.48 Consequently, NIPC may have differentiated directly from uncommitted progenitors independently of the lymphoid and myeloid lineage. Furthermore, not all NIPC located in porcine lymphoid tissue after virus infection expressed detectable levels of SWC3.12,16 This could reflect either several subsets of porcine NIPC, or a down-regulation of SWC3 after in vivo stimulation. Interestingly, phenotypic variability has also been described for the human pDC system.49
On the basis of comparative immunology, we propose that the SWC3+ CD1+ CD4− CD14− porcine blood DC correspond to the human CD11c+ myeloid blood DC subset. This is mainly based on their responsiveness to GM-CSF, their high expression of MHC class II and CD80/86 and their potent T-cell stimulatory activity. Porcine NIPC also relate to their human counterparts, in terms of high CD4 expression, which contrasts with the mouse pDC only expressing CD4 after culture.50 The converse is seen for CD11c expression. Human pDC are classically CD11c−, whereas the murine cells are CD11c+51 or CD11clow.52 Porcine NIPC appear to be CD11clow. However, the porcine integrin system differs significantly in cellular distribution from the human. This is why the detection with the anti-human CD11c was termed CD11R2.21 Porcine NIPC are also peculiar in their low expression of CD45RA, whereas mouse and human pDC express high levels of this molecule.49 A surprising finding was the low CD16 expression on freshly isolated porcine NIPC. Despite the phenotypic differences, porcine NIPC clearly relate to their human/murine counterparts, in that a main function is the rapid production of large amounts of IFN type I during viral infections. This IFN can be detected systemically as has been shown in vivo for the pig16 and the mouse.53,54
The present study has identified and characterized the porcine DC system of the peripheral blood. In this area the pig offers the possibility of sampling large volumes of blood, promoting it as an alternative animal model for clarification of many aspects of the biology of scarce cell populations, such as blood DC and NIPC. This is of critical importance for development of novel efficacious vaccines to combat infectious diseases.
Acknowledgments
This work was supported by the Swiss Federal Office for Education and Science (grants BBW 97·0423/EU project PL97-3732 and grant BBW 02·0093 project QLRT-2001-00825). We thank Heidi Gerber for the preparation of monoclonal antibodies, as well as Viviane Neuhaus and Marco Roos for preparation of recombinant cytokines.
Abbreviations
- NIPC
natural interferon-producing cells
- SWC
swine workshop cluster
- UV-TGEV
UV light-inactivated transmissible gastroenteritis virus
References
- 1.Kelsall BL, Biron CA, Sharma O, Kaye PM. Dendritic cells at the host–pathogen interface. Nat Immunol. 2002;3:699–702. doi: 10.1038/ni0802-699. [DOI] [PubMed] [Google Scholar]
- 2.Reis e Sousa C. Dendritic cells as sensors of infection. Immunity. 2001;14:495–8. doi: 10.1016/s1074-7613(01)00136-4. [DOI] [PubMed] [Google Scholar]
- 3.Balazs M, Martin F, Zhou T, Kearney J. Blood dendritic cells interact with splenic marginal zone B cells to initiate T-independent immune responses. Immunity. 2002;17:341–52. doi: 10.1016/s1074-7613(02)00389-8. [DOI] [PubMed] [Google Scholar]
- 4.Wykes M, MacPherson G. Dendritic cell–B-cell interaction. dendritic cells provide B cells with CD40-independent proliferation signals and CD40-dependent survival signals. Immunology. 2000;100:1–3. doi: 10.1046/j.1365-2567.2000.00044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johansson B, Ingvarsson S, Bjorck P, Borrebaeck CA. Human interdigitating dendritic cells induce isotype switching and IL-13-dependent IgM production in CD40-activated naive B cells. J Immunol. 2000;164:1847–54. doi: 10.4049/jimmunol.164.4.1847. [DOI] [PubMed] [Google Scholar]
- 6.Ito T, Inaba M, Inaba K, et al. A CD1a+/CD11c+ subset of human blood dendritic cells is a direct precursor of Langerhans cells. J Immunol. 1999;163:1409–19. [PubMed] [Google Scholar]
- 7.Schakel K, Kannagi R, Kniep B, et al. 6-Sulfo LacNAc, a novel carbohydrate modification of PSGL-1, defines an inflammatory type of human dendritic cells. Immunity. 2002;17:289–301. doi: 10.1016/s1074-7613(02)00393-x. [DOI] [PubMed] [Google Scholar]
- 8.Fitzgerald-Bocarsly P. Human natural interferon-alpha producing cells. Pharmacol Ther. 1993;60:39–62. doi: 10.1016/0163-7258(93)90021-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Svensson H, Johannisson A, Nikkila T, Alm GV, Cederblad B. The cell surface phenotype of human natural interferon-alpha producing cells as determined by flow cytometry. Scand J Immunol. 1996;44:164–72. doi: 10.1046/j.1365-3083.1996.d01-289.x. [DOI] [PubMed] [Google Scholar]
- 10.Siegal FP, Kadowaki N, Shodell M, et al. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–7. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 11.Dalod M, Salazar-Mather TP, Malmgaard L, et al. Interferon alpha/beta and interleukin 12 responses to viral infections: pathways regulating dendritic cell cytokine expression in vivo. J Exp Med. 2002;195:517–28. doi: 10.1084/jem.20011672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riffault S, Carrat C, Besnardeau L, La Bonnardiere C, Charley B. In vivo induction of interferon-alpha in pig by non-infectious coronavirus: tissue localization and in situ phenotypic characterization of interferon-alpha-producing cells. J Gen Virol. 1997;78(10):2483–7. doi: 10.1099/0022-1317-78-10-2483. [DOI] [PubMed] [Google Scholar]
- 13.Galibert L, Maliszewski CR, Vandenabeele S. Plasmacytoid monocytes/T cells: a dendritic cell lineage? Semin Immunol. 2001;13:283–9. doi: 10.1006/smim.2001.0324. [DOI] [PubMed] [Google Scholar]
- 14.Charley B, Lavenant L. Characterization of blood mononuclear cells producing IFN alpha following induction by coronavirus-infected cells (porcine transmissible gastroenteritis virus) Res Immunol. 1990;141:141–51. doi: 10.1016/0923-2494(90)90133-J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Splichal I, Rehakova Z, Sinkora M, Sinkora J, Trebichavsky I, Laude H, Charley B. In vivo study of interferon-alpha-secreting cells in pig foetal lymphohaematopoietic organs following in utero TGEV coronavirus injection. Res Immunol. 1997;148:247–56. doi: 10.1016/S0923-2494(97)80866-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riffault S, Carrat C, van Reeth K, Pensaert M, Charley B. Interferon-alpha-producing cells are localized in gut-associated lymphoid tissues in transmissible gastroenteritis virus (TGEV) infected piglets. Vet Res. 2001;32:71–9. doi: 10.1051/vetres:2001111. [DOI] [PubMed] [Google Scholar]
- 17.Alvarez B, Sanchez C, Bullido R, et al. A porcine cell surface receptor identified by monoclonal antibodies to SWC3 is a member of the signal regulatory protein family and associates with protein-tyrosine phosphatase SHP-1. Tissue Antigens. 2000;55:342–51. doi: 10.1034/j.1399-0039.2000.550408.x. [DOI] [PubMed] [Google Scholar]
- 18.McCullough KC, Schaffner R, Fraefel W, Kihm U. The relative density of CD44-positive porcine monocytic cell populations varies between isolations and upon culture and influences susceptibility to infection by African swine fever virus. Immunol Lett. 1993;37:83–90. doi: 10.1016/0165-2478(93)90136-p. [DOI] [PubMed] [Google Scholar]
- 19.Saalmuller A. Characterization of swine leukocyte differentiation antigens. Immunol Today. 1996;17:352–4. doi: 10.1016/S0167-5699(96)90273-X. [DOI] [PubMed] [Google Scholar]
- 20.Haverson K, Saalmuller A, Chen Z, et al. Summary of the first round analyses of the Third International Workshop on Swine Leukocyte Differentiation Antigens. Vet Immunol Immunopathol. 2001;80:25–34. doi: 10.1016/s0165-2427(01)00291-4. [DOI] [PubMed] [Google Scholar]
- 21.Dominguez J, Alvarez B, Alonso F, et al. Workshop studies on monoclonal antibodies in the myeloid panel with CD11 specificity. Vet Immunol Immunopathol. 2001;80:111–9. doi: 10.1016/s0165-2427(01)00286-0. [DOI] [PubMed] [Google Scholar]
- 22.Carrasco CP, Rigden RC, Schaffner R, et al. Porcine dendritic cells generated in vitro: morphological, phenotypic and functional properties. Immunology. 2001;104:175–84. doi: 10.1046/j.0019-2805.2001.01299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Summerfield A, McCullough KC. Porcine bone marrow myeloid cells: phenotype and adhesion molecule expression. J Leukoc Biol. 1997;62:176–85. doi: 10.1002/jlb.62.2.176. [DOI] [PubMed] [Google Scholar]
- 24.Hawley RJ, Abraham S, Akiyoshi DE, et al. Xenogeneic bone marrow transplantation: I. Cloning, expression, and species specificity of porcine IL-3 and granulocyte–macrophage colony-stimulating factor. Xenotransplantation. 1997;4::103–11. [Google Scholar]
- 25.Meissner P, Pick H, Kulangara A, Chatellard P, Friedrich K, Wurm FM. Transient gene expression: recombinant protein production with suspension-adapted HEK293-EBNA cells. Biotechnol Bioeng. 2001;75:197–203. doi: 10.1002/bit.1179. [DOI] [PubMed] [Google Scholar]
- 26.Basta S, Knoetig SM, Spagnuolo-Weaver M, Allan G, McCullough KC. Modulation of monocytic cell activity and virus susceptibility during differentiation into macrophages. J Immunol. 1999;162:3961–9. [PubMed] [Google Scholar]
- 27.Perrin P, Morgeaux S. Inactivation of DNA by beta-propiolactone. Biologicals. 1995;23:207–11. doi: 10.1006/biol.1995.0034. [DOI] [PubMed] [Google Scholar]
- 28.Riffault S, Grosclaude J, Vayssier M, Laude H, Charley B. Reconstituted coronavirus TGEV virosomes lose the virus ability to induce porcine interferon-alpha production. Vet Res. 1997;28:77–86. [PubMed] [Google Scholar]
- 29.Nowacki W, Charley B. Enrichment of coronavirus-induced interferon-producing blood leukocytes increases the interferon yield per cell: a study with pig leukocytes. Res Immunol. 1993;144:111–20. doi: 10.1016/0923-2494(93)80066-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knoetig SM, McCullough KC, Summerfield A. Lipopolysaccharide-induced impairment of classical swine fever virus infection in monocytic cells is sensitive to 2-aminopurine. Antiviral Res. 2002;53:75–81. doi: 10.1016/s0166-3542(01)00193-0. [DOI] [PubMed] [Google Scholar]
- 31.Saalmuller A, Reddehase MJ. Immune system of swine: dissection of mononuclear leucocyte subpopulations by means of two-colour cytofluorometric analysis. Res Vet Sci. 1988;45:311–6. [PubMed] [Google Scholar]
- 32.McCullough KC, Schaffner R, Natale V, Kim YB, Summerfield A. Phenotype of porcine monocytic cells: modulation of surface molecule expression upon monocyte differentiation into macrophages. Vet Immunol Immunopathol. 1997;58:265–75. doi: 10.1016/s0165-2427(97)00045-7. [DOI] [PubMed] [Google Scholar]
- 33.Blecha F, Kielian T, McVey DS, et al. Workshop studies on monoclonal antibodies reactive against porcine myeloid cells. Vet Immunol Immunopathol. 1994;43:269–72. doi: 10.1016/0165-2427(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 34.Haverson K, Singha S, Stokes CR, Bailey M. Professional and non-professional antigen-presenting cells in the porcine small intestine. Immunology. 2000;101:492–500. doi: 10.1046/j.1365-2567.2000.00128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bautista EM, Gregg D, Golde WT. Characterization and functional analysis of skin-derived dendritic cells from swine without a requirement for in vitro propagation. Vet Immunol Immunopathol. 2002;88:131–48. doi: 10.1016/s0165-2427(02)00152-6. [DOI] [PubMed] [Google Scholar]
- 36.Salmon H, Johnson I, Germana S, Haller GW, Sachs DH, Leguern C. Dendritic cells enriched from swine thymus co-express CD1, CD2 and major histocompatibility complex class II and actively stimulate alloreactive T lymphocytes. Scand J Immunol. 2000;52:164–72. doi: 10.1046/j.1365-3083.2000.00768.x. [DOI] [PubMed] [Google Scholar]
- 37.O'Doherty U, Steinman RM, Peng M, et al. Dendritic cells freshly isolated from human blood express CD4 and mature into typical immunostimulatory dendritic cells after culture in monocyte-conditioned medium. J Exp Med. 1993;178:1067–76. doi: 10.1084/jem.178.3.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou LJ, Tedder TF. A distinct pattern of cytokine gene expression by human CD83+ blood dendritic cells. Blood. 1995;86:3295–301. [PubMed] [Google Scholar]
- 39.Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–8. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 40.Feldman SB, Ferraro M, Zheng HM, Patel N, Gould-Fogerite S, Fitzgerald-Bocarsly P. Viral induction of low frequency interferon-alpha producing cells. Virology. 1994;204:1–7. doi: 10.1006/viro.1994.1504. [DOI] [PubMed] [Google Scholar]
- 41.Charley B, Lavenant L, Delmas B. Glycosylation is required for coronavirus TGEV to induce an efficient production of IFN alpha by blood mononuclear cells. Scand J Immunol. 1991;33:435–40. doi: 10.1111/j.1365-3083.1991.tb01792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baudoux P, Carrat C, Besnardeau L, Charley B, Laude H. Coronavirus pseudoparticles formed with recombinant M and E proteins induce alpha interferon synthesis by leukocytes. J Virol. 1998;72:8636–43. doi: 10.1128/jvi.72.11.8636-8643.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krug A, Rothenfusser S, Hornung V, et al. Identification of CpG oligonucleotide sequences with high induction of IFN-alpha/beta in plasmacytoid dendritic cells. Eur J Immunol. 2001;31:2154–63. doi: 10.1002/1521-4141(200107)31:7<2154::aid-immu2154>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 44.Feldman S, Stein D, Amrute S, et al. Decreased Interferon-alpha production in HIV-infected patients correlates with numerical and functional deficiencies in circulating type 2 dendritic cell precursors. Clin Immunol. 2001;101:201–10. doi: 10.1006/clim.2001.5111. [DOI] [PubMed] [Google Scholar]
- 45.Grouard G, Rissoan MC, Filgueira L, Durand I, Banchereau J, Liu YJ. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J Exp Med. 1997;185:1101–11. doi: 10.1084/jem.185.6.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Riffault S, Eloranta ML, Carrat C, Sandberg K, Charley B, Alm G. Herpes simplex virus induces appearance of interferon-alpha/beta-producing cells and partially interferon-alpha/beta-dependent accumulation of leukocytes in murine regional lymph nodes. J Interferon Cytokine Res. 1996;16:1007–14. doi: 10.1089/jir.1996.16.1007. [DOI] [PubMed] [Google Scholar]
- 47.Cella M, Jarrossay D, Facchetti F, Alebardi O, Nakajima H, Lanzavecchia A, Colonna M. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med. 1999;5:919–23. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- 48.Sinkora J, Rehakova Z, Sinkora M, Cukrowska B, Tlaskalova-Hogenova H. Early development of immune system in pigs. Vet Immunol Immunopathol. 2002;87:301–6. doi: 10.1016/s0165-2427(02)00056-9. [DOI] [PubMed] [Google Scholar]
- 49.Comeau MR, Van der Vuurst de Vries AR, Maliszewski CR, Galibert L. CD123bright plasmacytoid predendritic cells: progenitors undergoing cell fate conversion? J Immunol. 2002;169:75–83. doi: 10.4049/jimmunol.169.1.75. [DOI] [PubMed] [Google Scholar]
- 50.Hochrein H, O'Keeffe M, Wagner H. Human and mouse plasmacytoid dendritic cells. Hum Immunol. 2002;63:1103–10. doi: 10.1016/s0198-8859(02)00748-6. [DOI] [PubMed] [Google Scholar]
- 51.Nakano H, Yanagita M, Gunn MD. Cd11c (+) b220 (+) gr-1 (+) cells in mouse lymph nodes and spleen display characteristics of plasmacytoid dendritic cells. J Exp Med. 2001;194:1171–8. doi: 10.1084/jem.194.8.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O'Keeffe M, Hochrein H, Vremec D, Scott B, Hertzog P, Tatarczuch L, Shortman K. Dendritic cell precursor populations of mouse blood: identification of the murine homologues of human blood plasmacytoid pre-DC2 and CD11c+ DC1 precursors. Blood. 2003;101:1453–9. doi: 10.1182/blood-2002-03-0974. [DOI] [PubMed] [Google Scholar]
- 53.Asselin-Paturel C, Boonstra A, Dalod M, et al. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat Immunol. 2001;2:1144–50. doi: 10.1038/ni736. [DOI] [PubMed] [Google Scholar]
- 54.Barchet W, Cella M, Odermatt B, Asselin-Paturel C, Colonna M, Kalinke U. Virus-induced interferon alpha production by a dendritic cell subset in the absence of feedback signaling in vivo. J Exp Med. 2002;195:507–16. doi: 10.1084/jem.20011666. [DOI] [PMC free article] [PubMed] [Google Scholar]