Abstract
CD1d-reactive natural killer T (NKT) cells can rapidly produce T helper type 1 (Th1) and/or Th2 cytokines, can activate antigen-presenting cell (APC) interleukin-12 (IL-12) production, and are implicated in the regulation of adaptive immune responses. The role of the CD1d system was assessed during infection with encephalomyocarditis virus (EMCV-D), a picornavirus that causes acute diabetes, paralysis and myocarditis. EMCV-D resistance depends on IL-12-mediated interferon-γ (IFN-γ) production. CD1d-deficient mice, which also lack CD1d-reactive NKT cells, were substantially more sensitive to infection with EMCV-D. Infected CD1d knockout mice had decreased IL-12 levels in vitro and in vivo, and indeed were protected by treatment with exogenous IL-12. IFN-γ production in CD1d knockout mice was decreased compared with that in wild-type (WT) mice in response to EMCV-D in vitro, although differences were not detected in vivo. Treatment with anti-asialo-GM1 antibody, to deplete NK cells, caused a marked increase in susceptibility of WT mice to EMCV-D infection, whereas CD1d knockout mice were little affected, suggesting that NK-cell-mediated protection is CD1d-dependent. Therefore, these data indicate that CD1d is essential for optimal responses to acute picornaviral infection. We propose that CD1d-reactive T cells respond to early immune signals and function in the innate immune response to a physiological viral infection by rapidly augmenting APC IL-12 production and activating NK cells.
Introduction
Critical elements of successful immune responses to acute challenges are activation of the innate immune system and the subsequent adaptive immune responses, the latter mediated by antigen-specific T and B cells. Mammals have populations of T cells that specifically recognize non-polymorphic major histocompatibility complex class I-like CD1d, constitutively expressed on antigen-presenting cells (APC) and inducible on other cells, and specialized for lipid antigen presentation.1–8 Many CD1d-reactive T cells express natural killer (NK) cell markers such as CD161 (‘NKT cells’), and a major subset uses an invariant T-cell receptor (TCR) α-chain and limited TCRβ-chain repertoire (‘invariant NKT cells’). CD1d-reactive NKT cells have been implicated as regulators of adaptive immune responses based on their unique ability to rapidly produce high levels of T helper type 1- (Th1) and Th2-type cytokines, including interferon-γ (IFN-γ) and interleukin-4 (IL-4), respectively, in response to CD1d.9–13 In support of this hypothesis, decreased numbers of invariant NKT cells and decreased IL-4 relative to IFN-γ production are associated with progression in human and murine Th1-like autoimmune diseases, including diabetes.14,15 Additional functions for CD1d-reactive T cells consistent with a protective role in autoimmune disease are the suppression of responses to antigen in an immune privileged site, tolerance to solid organ transplants, and suppression of tumour surveillance.16–19 Finally, repeated doses of a specific activating lipid antigen for CD1d-reactive invariant NKT cells, α-galactosylceramide (αGalCer), can stimulate Th2-type antigen-specific responses.20–23
In contrast to the potential roles of CD1d-reactive T cells in Th2 responses and immune suppression, these cells are also reported to augment Th1 responses including IL-12-mediated anti-tumour responses and IFN-γ production in response to exogenous IL-12.24–28 Cancer progression in humans is associated with reduced numbers of invariant NKT cells and a marked Th2 bias in residual cells.29,30 Evidence for involvement of CD1d-reactive T cells in Th1-like immune responses to pathogens is contradictory, presumably reflecting specific resistance mechanisms.31–43 Pharmacological activation of invariant NKT cells by αGalCer induces rapid systemic cytokine production and transient stimulation of both the innate and adaptive immune systems, including NK cells.38,39 Consistent with these activities, αGalCer inhibits the replication of hepatitis B virus in a transgenic model system40 and is protective in a malaria model.41 However, CD1d-reactive T cells are not essential for physiological protection from malaria.42
CD1d-reactive T cells appear to contribute selectively to resistance to certain viruses. We recently reported that αGalCer treatment could protect BALB/c mice from infection with the diabetogenic strain of the encephalomyocarditis virus (EMCV-D), and that BALB/c CD1d-deficient mice appeared to be more susceptible.43 CD1d-reactive T cells appear to stimulate CD8 T-cell responses against respiratory syncytial virus, but the reverse has been found in the case of lymphocytic choriomeningitis virus.35,36 Finally, optimal resistance to herpes simplex virus also requires CD1d-reactive T cells.37
EMCV-D is a picornavirus that causes acute paralysis, diabetes and myocarditis.43–46 The virus infects pancreatic islet cells, the nervous system, and heart muscle in vivo but clearance of the virus results in the resolution of acute disease.44–49 T lymphocytes, macrophages, NK cells, and NK-like spleen cells have been reported to contribute to EMCV-D resistance, and wild-type (WT) mice can be protected by IL-12 treatment and consequent IFN-γ production.47–51 Resistance to EMCV-D correlates with early IFN-γ production and IFN-γ-receptor-deficient mice are highly susceptible, even when treated with IL-12.51–54 This study demonstrates in independently derived CD1d-deficient mice that CD1d is essential for optimal EMCV-D resistance and assesses the mechanism of resistance. The results show that EMCV-D infection results in rapid induction of an innate immune response with IL-12 production and requirement for NK cells, and that these responses are absent in CD1d-deficient mice, but can be bypassed with exogenous IL-12. Together with results showing that invariant NKT can directly activate NK cells38,39 these findings demonstrate a critical physiological function for CD1d and provide evidence for a role of CD1d-reactive T cells in the innate immune response to a viral infection.
Materials and methods
EMCV-D infection, treatments and disease measurement
Mice deficient in both CD1 genes55,56 were prepared as described previously.57,58 The mice used were (129 × C57BL/6)F2 CD1d knockout (KO) mice (Fig. 1) and CD1d KO mice back-crossed for six generations (F6) in the C57BL/6 J background. Mice were infected by intraperitoneal (i.p.) injection with 800 plaque-forming units of EMCV-D.44 Glucose tolerance tests (GTT) were performed by i.p. injection of 2 g/kg glucose and blood was collected at 1 hr with a glucosidase inhibitor.44 Encephalitis was assessed by paralysis: 1 = no paralysis, 2 = weakness in one limb, 3 = one completely paralysed limb, 4 = weakness in two limbs, 5 = paralysis of two or more limbs. The mice indicated were given murine IL-12 (Wyeth Research, Cambridge, MA, 2·7 × 106 U/mg) at 1·3 μg (3500 U) i.p. daily from 3 days prior to infection to day 6, or were treated i.p. with 250 μg rabbit anti-asialo GM1 antibody (anti-ASGM1) (Wako Chemicals Inc, Richmond, VA) or control rabbit immunoglobulin G (IgG) 24 hr prior to infection to deplete NK cells.16 Data were analysed by paired two-tailed t-test and one-way analysis of variance (anova), and standard deviations are shown.
Figure 1.
Generation of CD1d-deficient mice. (a) Map of murine CD1d.1 and CD1d.2 loci and gene targeting construct. (b) (left panel) Southern blot of ScaI-digested ES cells with the 5′-SacI–HindIII probe. Lane 1, WT with arrow indicating WT band; lane 2, homologous recombinant with 0·5 kb smaller band indicated with arrowhead; (right panel) Southern blot of NheI-digested ES cells with the 3′-BglII probe, lanes 1 and 2 as above. (c) Flow cytometry of (129 × C57BL/6)F2 WT or CD1d KO spleen cells stained with anti-CD1d (bold) or isotype control (ISO) antibodies.
Measurement of cytokines in vivo and in vitro
Serum cytokine levels were determined by enzyme-linked immunosorbent assay (ELISA; R & D, Minneapolis, MN) and limits of detection were <10 pg/ml for serum (measured at 1 : 10 dilution).
Splenocytes (1 × 106 per well in 24-well plates) were infected with EMCV-D (multiplicity of Infection 0·1/cell to ensure viable cells for cytokine production) as previously described50–54 or treated with 1 μg/ml anti-CD3 monoclonal antibody (145-2C11) bound to 24-well plates. Murine IL-12 was added where indicated at 1 ng/ml. Plates were incubated for 24 hr and cytokine levels were determined in triplicate by ELISA as described above. Limit of detection was 0·1 pg/ml for splenocyte supernatants, which were assayed undiluted, and are shown with standard deviations.
Results
Increased susceptibility of CD1d KO mice to EMCV-D infection
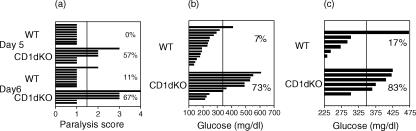
To assess the role of CD1d-reactive T cells in the response to EMCV-D, CD1d KO mice lacking both CD1d genes (Fig. 1) were used. These mice have diminished numbers of NKT cells and no CD1d-reactive NKT cells.16,33 EMCV-D infection of male (129 × C57BL/6)F2 CD1d KO versus WT mice demonstrated a markedly increased incidence of paralysis in the CD1d KO mice (Fig. 2a). Severity of the paralysis was also substantially greater in CD1d KO mice, with half of the animals having at least complete paralysis of one limb. A marked difference between EMCV-D-infected CD1d KO and WT mice was similarly found by glucose tolerance testing (Fig. 2b). In a representative experiment, hyperglycaemia was observed in 73% (8/11) of infected (129 × C57BL/6)F2 CD1d KO mice, but in only 7% (1/15) of the infected WT controls.
Figure 2.
EMCV-D infection of WT and CD1d KO mice. (a) Eight-week-old (129 × C57BL/6)F2 WT and CD1d KO male mice were infected with EMCV-D and assessed for paralysis on days 5 and 6, differences between the WT and CD1d KO were significant (P < 0·01). (b) Eight-week-old (129 × C57BL/6)F2 WT and CD1d KO male mice infected with EMCV-D and analysed by glucose tolerance testing at 7 days post-infection. Hyperglycaemia was defined as values >3 times the SD over the mean value of phosphate-buffered-saline-injected uninfected controls, differences between the WT and CD1d KO were significant (P < 0·03). (c) Six-week-old-male C57BL/6 CD1d KO and WT mice infected with EMCV-D and analysed by glucose tolerance testing, differences between WT and CD1d KO were significant (P < 0·05).
Results from a series of EMCV-D infections are summarized in Table 1. Overall, only 8% of (129 × C57BL/6)F2 WT males analysed at 5 days post-EMCV-D infection showed paralysis, with a cumulative incidence of 11% paralysis at 7 days. In contrast, 41% of the (129 × C57BL/6)F2 CD1d KO males were paralysed at day 5 and 56% at days 6–7, with the paralysis also being more severe in this group. The differences between WT and CD1d KO mice at both day 5 and days 6–7 were significant (P < 0·05). Similar results were obtained by glucose tolerance testing, with 77% of CD1d KO animals hyperglycemic at day 7 vs. 17% of WT mice (P < 0·01). The CD1d KO mice also had higher absolute levels of blood glucose than the WT mice, indicative of more severe disease (Fig. 2b). These findings confirmed the markedly increased EMCV-D sensitivity of (129 × C57BL/6)F2 CD1d KO males. In a smaller number of infections in female mice, which are more resistant to EMCV-D than males,52–54 hyperglycaemia was observed in 33% of CD1d KO mice (3/9), but in none (0/10) of the WT mice (not shown).
Table 1.
EMCV-D-induced paralysis and hyperglycemia in WT and CD1d KO mice
| Paralysis | |||
|---|---|---|---|
| lncidence | Score | Hyperglycemia | |
| Day 5 | |||
| WT | 1/13 (8%)* | 2 | ND |
| KO | 12/29 (41%)* | 2–4 | 5/7 (71%) |
| Day 6 or 7 | |||
| WT | 4/38 (11%)* | 2 | 5/30 (17%)* |
| KO | 23/41 (56%)* | 2–4 | 17/22 (77%)* |
(129 × C57BL/6)F2 WT or CD1d KO mice were infected i.p. with EMCV-D. Cumulative results at days 5–7 post-infection were pooled from five independent experiments, including those in Fig. 2 (in two of five experiments CD1d KO mouse disease was judged too severe to leave until day 7, so all mice were killed at day 6). The fractions of hyperglycaemic WT and CD1d KO mice are shown. Ranges of glucose values were 150–500 mg/dl for WT mice and 150 to >850 mg/dl for CD1d KO animals. Control mice injected with phosphate-buffered saline were all normoglycaemic (range: 160–260 mg/dl).
Significant difference between CD1d KO and WT (paralysis P < 0·05, hyperglycaemia P < 0·01).
The significant difference in EMCV-D susceptibility observed between CD1d KO and WT mice on the mixed (129 × C57BL/6)F2 background indicated a major effect on disease resistance. To assess further the relative importance of CD1d in EMCV-D responses, the CD1d KO allele was back-crossed onto C57BL/6 mice, a strain with strong Th1-like and NK-cell responses.59 Six-week-old C57BL/6 WT male mice were resistant to EMCV-D infection, based upon glucose tolerance testing (Fig. 2c) and paralysis (not shown). In contrast, C57BL/6 CD1d KO mice were highly sensitive to EMCV-D. Taken together, these findings confirmed a major role for CD1d in mediating resistance to EMCV-D infection.
Cytokine responses of CD1d KO mice to EMCV-D
Exogenous IL-12 is protective against EMCV through an IFN-γ-dependent pathway51 and there is a correlation between the resistance of female mice of particular strains and IFN-γ production in vitro in EMCV-D-infected splenocyte cultures52–54 as with other viruses.59 Previous reports have shown that anti-tumour responses and IFN-γ production stimulated by IL-12 are decreased in CD1d KO mice,24,25,27,28 raising the possibility that the susceptibility of CD1d KO mice to EMCV-D infection could be due primarily to decreased IFN-γ production in response to IL-12. Consistent with this hypothesis, EMCV-D-infected splenocyte cultures from (129 × C57BL/6)F2 CD1d KO mice produced lower levels of IFN-γ compared to WT controls at both early and late time-points (Fig. 3a). However, in contrast to these cultured splenocyte results, measurements of serum IFN-γ in infected CD1d KO and WT control mice yielded highly variable results, with comparable mean values in both groups. This indicated that decreased systemic IFN-γ was not the major factor responsible for the increased susceptibility of CD1d KO mice (not shown).
Figure 3.
Cytokine production in vivo and in splenocytes cultures. (a) Splenocyte IFN-γ production from replicate cultures following EMCV-D infection in vitro of cells from 8-week-old WT and CD1d KO male (129 × C57BL/6)F2 mice. (b) Splenocyte p70 IL-12 production following EMCV-D infection as in Fig. 2(a). (c) Endogenous serum p70 IL-12 levels prior to and during EMCV-D infection of 8-week-old WT and CD1d KO male (129 × C57BL/6)F2 mice (n > 5). (d) Individual 6-week-old-male C57BL/6 WT and CD1d KO serum p70 IL-12 levels at 6 days after EMCV-D infection.
Splenocyte cultures and mouse sera were next tested for bioactive p70 IL-12 production in response to EMCV-D infection. EMCV infects lymphocytes and monocytic cells in vitro.50–54 Splenocytes from (129 × C57BL/6)F2 WT mice produced IL-12 at 14 hr after infection and the levels increased over 5 days (Fig. 3b). In contrast, cultures from CD1d KO mice did not produce IL-12 levels above background at 14 hr and had approximately four-fold lower levels 5 days after infection. Importantly, IL-12 was similarly decreased in infected CD1d KO mice in vivo, with (129 × C57BL/6) CD1d KO mice having three-fold less IL-12 by day 7 (Fig. 3c). Similarly, IL-12 levels in infected C57BL/6 CD1d KO mice were markedly reduced, with a mean of <30 pg/ml versus 175 pg/ml in the WT mice at day 6 (Fig. 3d). Therefore, these studies in different genetic backgrounds demonstrated early and persistent reductions in systemic IL-12 levels in EMCV-D-infected CD1d KO mice.
IL-12 protects against EMCV-D infection in CD1d KO mice
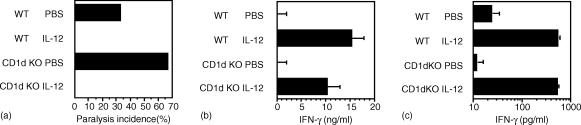
The above data indicated that decreased production of IL-12, rather than a decreased response to IL-12, contributed to increased susceptibility of the CD1d KO mice. Therefore, it was next determined whether exogenous IL-12 could elicit IFN-γ and protect (129 × C57BL/6)F2 CD1d KO male mice against EMCV-D infection. IL-12 treatment provided complete protection against EMCV-D infection in both the WT and CD1d KO mice (Fig. 4a). Protection correlated with high levels of IFN-γ in both the WT and CD1d KO mice, with systemic IFN-γ levels in the 10–15 ng/ml range at day 7 after infection (Fig. 4b). IFN-γ production in vitro in EMCV-D-infected splenocyte cultures from both WT and CD1d KO mice were also markedly augmented by IL-12 (Fig. 4c). Taken together with the IFN-γ data, these results indicated a primary defect in IL-12 production in the infected CD1d KO mice, rather than in the IL-12-stimulated IFN-γ response.
Figure 4.
Effects of exogenous IL-12 on EMCV-D infection of WT and CD1d KO mice. (a) WT and CD1d KO (129 × C57BL/6)F2 mice treated with IL-12 or phosphate-buffered saline (PBS) were infected with EMCV-D and assessed for paralysis at day 7 (n = 6/group). (b) Serum IFN-γ from the experiment shown in (a) determined 7 days post-infection. (c) Splenocyte IFN-γ production on log scale following EMCV-D infection ±IL-12 (1 ng/ml). Uninfected cultures contained no detectable cytokine.
Effect of NK-cell depletion on protection against EMCV-D infection in WT and CD1d KO mice
Previous data indicated that NK cells were important effectors in the protective immune response to EMCV-D.47 The contribution of NK cells to EMCV-D resistance in (129 × C57BL/6)F2 WT male mice was assessed by in vivo treatment with anti-asialo GM1 antibody, which has been shown to deplete NK cells selectively and not NKT cells.16 A single treatment 24 hr before EMCV-D infection of (129 × C57BL/6)F2 WT mice resulted in a marked increase in susceptibility compared to control IgG-treated mice (Fig. 5). In contrast, anti-asialo GM1 antibody did not markedly increase the susceptibility of the CD1d KO mice. These findings confirmed the importance of NK cells in EMCV-D resistance and suggested that the NK-cell response to EMCV-D infection was impaired in the CD1d KO mice.
Figure 5.
NK cell depletion in EMCV-D-infected CD1d KO and WT mice. WT and CD1d KO (129 × C57BL/6)F2 mice were pretreated with anti-ASGM1 (CD1d KO n = 9; WT n = 6) or IgG control (CD1d KO, n = 8; WT, n = 4) 24 hr prior to EMCV-D infection and glucose tolerance testing was performed on day 7.
Discussion
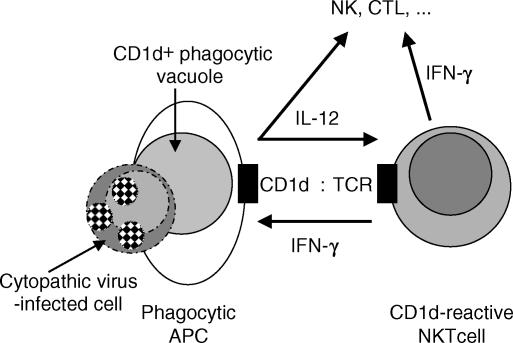
The increased susceptibility of (129 × C57BL/6)F2 and C57BL/6 CD1d KO mice to EMCV-D infection, in conjunction with the increased susceptibility of independently derived BALB/c CD1d KO mice,43 confirmed a role for CD1d in the immune response to this virus in vivo. Previous reports have shown that the increased resistance to EMCV-D in WT female mice correlates with IFN-γ production and that WT mice can be protected with IL-12 treatment.51–54 The findings that endogenous IL-12 levels were decreased in infected CD1d KO mice in vivo and in spleen cell cultures in vitro support a central role for this cytokine, and implicate a critical in vivo effector function of CD1d-reactive T cells in EMCV-D infection as augmentation of dendritic cell IL-12 production (Fig. 6). Consistent with this hypothesis, in vitro studies with αGalCer have shown that IL-12 production by dendritic cells is stimulated by invariant NKT-cell IFN-γ production (which may not be reflected in systemic levels) and by direct NKT : APC contacts involving CD154 : CD40 interactions.60,61
Figure 6.
Model for surveillance for viral infection by CD1d-reactive NKT cells. Dying virus-infected cells are phagocytosed and digested in CD1d+ phagocytic vacuoles. CD1d presents glycoplipids from the infected cell to local CD1d-reactive NKT cells. CD1d-reactive NKT cell IFN-γ induces APC IL-12 production, amplifying CD1d-reactive NKT cell and APC activation. IL-12 and/or IFN-γ rapidly activate innate NK cells and subsequently contribute to the activation of adaptive cytotoxic T-cell responses downstream.
The conclusion that CD1d KO mice had a defect in IL-12 production, rather in their response to IL-12, was supported by the finding that IL-12 treatment rendered the CD1d KO mice fully resistant to viral infection. These results indicating intact responses to IL-12 are in contrast to previous reports showing lack of anti-tumour effects and decreased IFN-γ production in response to exogenous IL-12 in CD1d KO mice and in Jα281 KO mice, which lack invariant NKT cells.24,25 Subsequent reports have shown that responses to pharmacological doses of IL-12, mediated by NK cells, remained intact in CD1d KO mice.27,28 However, these tumour models still suggest a primary role for CD1d-reactive T cells in enhancing the responses to IL-12, rather than in stimulating IL-12 production. Taken together, these observations suggest that the rapid activation of dendritic cells to produce IL-12 in response to an acute viral infection may be particularly dependent on CD1d-reactive T cells, while IL-12 production in other settings may be more strongly stimulated by other mechanisms.
A critical role for CD1d in rapid antiviral immune responses suggests that CD1d-reactive T cells respond to innate immune signals triggered by viral infection. Potential mediators in viral infections include IFN-α/β, but also include IL-12 and IL-18 produced by dendritic cells in response to cytopathic virus infection of tissues.59 Indeed, IL-12 and IL-18 can act synergistically directly to activate CD1d-reactive invariant NKT cells62 as well as NK cells.59 Alternatively, although endogenous or pathogen-derived lipid antigens recognized by CD1d-reactive T cells remain to be identified, increased CD1d expression by dendritic cells or presentation of endogenous lipid antigens from cytopathic virus-infected cells could mediate local activation of CD1d-reactive T cells (Fig. 6).
NK cells can be activated by IL-12 and have been shown to function as critical effector cells in the innate immune response to EMCV-D infection47 and other antiviral responses.59 This study suggests that NK cells are rapidly activated in response to EMCV-D infection and that this activation is CD1d dependent, implicating a critical role for CD1d in the innate NK-cell response to this virus. CD1d dependency of NK-cell activation may be mediated by IL-12, as production of IL-12 in response to infection was also rapid. However, additional CD1d-reactive T-cell or APC-derived cytokines, such as IL-18, or possibly direct cell–cell interactions may also contribute to the NK cell activation.
EMCV and related picornaviruses such as coxsackie viruses also induce a Th1-type myocarditis. Unlike direct viral-mediated diabetes and encephalitis, the myocarditis is predominantly autoimmune in nature, and indeed reflects robust protective responses in other organs.44–46 We have found that myocarditis is reduced in CD1d KO mice infected with EMCV or coxsackie virus43,63,64 and that CD1d-reactive T cells can contribute to antiviral inflammatory responses.63–65 However, αGalCer activation of CD1d-reactive T cells is also protective against myocarditis.43
While the focus of this work has been on antiviral innate and Th1-like immune responses, other data indicate that CD1d-reactive T cells contribute to Th2 responses and immune suppression. To reconcile these diverse and potentially opposing functions, we propose that the default pathway for CD1d-reactive T cells is to suppress Th1 responses and that one or more signals from the innate immune system indicative of an intracellular infection are required to shift these cells toward a Th1-promoting response. The physiological role for CD1d-reactive T cells would then be to integrate signals from CD1d, cytokines, and the innate immune system rapidly and to influence both the innate response and the decision for Th1, Th2, or Th0-type adaptive responses based upon the nature of the antigen challenge. CD1d-reactive T cells are clearly not the only mediators of Th1 versus Th2 responses, and defects in CD1d KO mice may only be demonstrable when other redundant pathways are inadequate, compromised, or too slow, as is apparently the case in acute EMCV-D infection. Nonetheless, this model predicts that therapeutic modulation of CD1d-reactive T cells in the context of the appropriate innate immune signals may be an approach to optimize natural and vaccine-induced anti-pathogen immune responses, whilst minimizing inflammatory sequelae.
Acknowledgments
We thank Dr D. Giron for developing the EMCV-D model, Dr J. Lawitts and the BIDMC Transgenic Core for blastocyst injection and implantations, Drs M. Brenner, I. N. Crispe, J. Leonard, G. Sunshine, J. Stein-Streilein and S. B. Wilson for advice, comments on the results, and/or reagents, the Wyeth Research for murine IL-12, and T. Frye, M. Hamilton, J. Rogers and A. Fertig for technical support. The work was supported by NIH CA89567 (M.A.E), AI42955 (S.P.B) and the American Heart Association, Ohio Affiliate grant MV-97–01-S (N.J.B).
References
- 1.Bendelac A, Lantz O, Quimby ME, Yewdell JW, Bennink JR, Brutkiewicz RR. CD1 recognition by mouse NK1+ T lymphocytes. Science. 1995;268:863–5. doi: 10.1126/science.7538697. [DOI] [PubMed] [Google Scholar]
- 2.Exley M, Garcia J, Balk SP, Porcelli S. Requirements for CD1d recognition by human invariant Valpha24+ CD4–CD8– T cells. J Exp Med. 1997;186:109–20. doi: 10.1084/jem.186.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Porcelli SA, Modlin RL. The CD1 system. antigen-presenting molecules for T cell recognition of lipids and glycolipids. Annu Rev Immunol. 1999;17:297–329. doi: 10.1146/annurev.immunol.17.1.297. [DOI] [PubMed] [Google Scholar]
- 4.Brossay L, Tangri S, Bix M, Cardell S, Locksley R, Kronenberg M. Mouse CD1-autoreactive T cells have diverse patterns of reactivity to CD1+ targets. J Immunol. 1998;160:3681–8. [PubMed] [Google Scholar]
- 5.Behar SM, Podrebarac TA, Roy CJ, Wang CR, Brenner MB. Diverse TCRs recognize murine CD1. J Immunol. 1999;162:161–7. [PubMed] [Google Scholar]
- 6.Eberl G, Lees R, Smiley ST, Taniguchi M, Grusby MJ, MacDonald HR. Tissue-specific segregation of CD1d-dependent and CD1d-independent NKT cells. J Immunol. 1999;162:6410–19. [PubMed] [Google Scholar]
- 7.Chiu YH, Jayawardena J, Weiss A, Lee D, Park SH, Dautry-Varsat A, Bendelac A. Distinct subsets of CD1d-restricted T cells recognize self-antigens loaded in different cellular compartments. J Exp Med. 1999;189:103–10. doi: 10.1084/jem.189.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Exley MA, Tahir SM, Cheng O, Shaulov A, Joyce R, Avigan D, Sackstein R, Balk SP. Cutting edge: a major fraction of human bone marrow lymphocytes are Th2-like CD1d-reactive T cells that can suppress mixed lymphocyte responses. J Immunol. 2001;167:5531–4. doi: 10.4049/jimmunol.167.10.5531. [DOI] [PubMed] [Google Scholar]
- 9.Yoshimoto T, Bendelac A, Watson C, Hu-Li J, Paul WE. Role of NK1.1+ T cells in a TH2 response and in immunoglobulin E production. Science. 1995;270:1845–7. doi: 10.1126/science.270.5243.1845. [DOI] [PubMed] [Google Scholar]
- 10.Joyce S. Natural T cells: cranking up the immune system by prompt cytokine secretion. Proc Natl Acad Sci USA. 2000;97:6933–5. doi: 10.1073/pnas.97.13.6933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smiley ST, Kaplan MH, Grusby MJ. Immunoglobulin E production in the absence of interleukin-4-secreting CD1-dependent cells. Science. 1997;275:977–9. doi: 10.1126/science.275.5302.977. [DOI] [PubMed] [Google Scholar]
- 12.Chen YH, Chiu NM, Mandal M, Wang N, Wang CR. Impaired NK1+ T cell development and early IL-4 production in CD1-deficient mice. Immunity. 1997;6:459–67. doi: 10.1016/s1074-7613(00)80289-7. [DOI] [PubMed] [Google Scholar]
- 13.Mendiratta SK, Martin WD, Hong S, Boesteanu A, Joyce S, Van Kaer L. CD1d1 mutant mice are deficient in natural T cells that promptly produce IL-4. Immunity. 1997;6:469–77. doi: 10.1016/s1074-7613(00)80290-3. [DOI] [PubMed] [Google Scholar]
- 14.Gombert JM, Herbelin A, Tancrede-Bohin E, Dy M, Carnaud C, Bach JF. Early quantitative and functional deficiency of NK1+-like thymocytes in the NOD mouse. Eur J Immunol. 1996;26:2989–98. doi: 10.1002/eji.1830261226. [DOI] [PubMed] [Google Scholar]
- 15.Wilson SB, Kent SC, Patton KT, et al. Extreme Th1 bias of invariant Valpha24JalphaQ T cells in type 1 diabetes. Nature 1998. 1998;391:177–81. doi: 10.1038/34419. [DOI] [PubMed] [Google Scholar]
- 16.Sonoda KH, Exley M, Snapper S, Balk SP, Stein-Streilein J. CD1-reactive natural killer T cells are required for development of systemic tolerance through an immune-privileged site. J Exp Med. 1999;190:1215–26. doi: 10.1084/jem.190.9.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seino KK, Fukao K, Muramoto K, et al. Requirement for natural killer T (NKT) cells in the induction of allograft tolerance. Proc Natl Acad Sci U S A. 2001;98:2577–81. doi: 10.1073/pnas.041608298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Terabe M, Matsui S, Noben-Trauth N, et al. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nat Immunol. 2000;1:515–20. doi: 10.1038/82771. [DOI] [PubMed] [Google Scholar]
- 19.Moodycliffe AM, Nghiem D, Clydesdale G, Ullrich SE. Immune suppression and skin cancer development: regulation by NKT cells. Nat Immunol. 2000;1:521–5. doi: 10.1038/82782. [DOI] [PubMed] [Google Scholar]
- 20.Kawano T, Cui J, Koezuka Y, et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278:1626–9. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 21.Burdin N, Brossay L, Koezuka Y, et al. Selective ability of mouse CD1 to present glycolipids: alpha-galactosylceramide specifically stimulates V alpha 14+ NKT lymphocytes. J Immunol. 1998;161:3271–81. [PubMed] [Google Scholar]
- 22.Burdin N, Brossay L, Kronenberg M. Immunization with alpha-galactosylceramide polarizes CD1-reactive NKT cells towards Th2 cytokine synthesis. Eur J Immunol. 1999;29:2014–25. doi: 10.1002/(SICI)1521-4141(199906)29:06<2014::AID-IMMU2014>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 23.Singh N, Hong S, Scherer DC, Serizawa I, Burdin N, Kronenberg M, Koezuka Y, Van Kaer L. Cutting edge. activation of NKT cells by CD1d and alpha-galactosylceramide directs conventional T cells to the acquisition of a Th2 phenotype. J Immunol. 1999;163:2373–7. [PubMed] [Google Scholar]
- 24.Cui J, Shin T, Kawano T, et al. Requirement for Valpha14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623–6. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 25.Kawamura T, Takeda K, Mendiratta SK, Kawamura H, Van Kaer L, Yagita H, Abo T, Okumura K. Critical role of NK1+ T cells in IL-12-induced immune responses in vivo. J Immunol. 1998;160:16–9. [PubMed] [Google Scholar]
- 26.Smyth MJ, Thia KY, Street SE, et al. Differential tumor surveillance by natural killer (NK) and NKT cells. J Exp Med. 2000;191:661–8. doi: 10.1084/jem.191.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takeda K, Hayakawa Y, Atsuta M, et al. Relative contribution of NK and NKT cells to the anti-metastatic activities of IL-12. Int Immunol. 2000;12:909–14. doi: 10.1093/intimm/12.6.909. [DOI] [PubMed] [Google Scholar]
- 28.Smyth MJ, Taniguchi M, Street SE. The anti-tumor activity of IL-12: mechanisms of innate immunity that are model and dose dependent. J Immunol. 2000;165:2665–70. doi: 10.4049/jimmunol.165.5.2665. [DOI] [PubMed] [Google Scholar]
- 29.Kawano T, Nakayama T, Kamada N, et al. Antitumor cytotoxicity mediated by ligand-activated human V alpha24 NKT cells. Cancer Res. 1999;59:5102–5. [PubMed] [Google Scholar]
- 30.Tahir SM, Cheng O, Shaulov A, Koezuka Y, Bubley GJ, Wilson SB, Balk SP, Exley MA. Loss of IFN-gamma production by invariant NKT cells in advanced cancer. J Immunol. 2001;167:4046–50. doi: 10.4049/jimmunol.167.7.4046. [DOI] [PubMed] [Google Scholar]
- 31.Behar SM, Dascher CC, Grusby MJ, Wang CR, Brenner MB. Susceptibility of mice deficient in CD1D or TAP1 to infection with Mycobacterium tuberculosis. J Exp Med. 1999;189:1973–80. doi: 10.1084/jem.189.12.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szalay G, Ladel CH, Blum C, Brossay L, Kronenberg M, Kaufmann SH. Cutting edge. Anti-CD1 monoclonal antibody treatment reverses the production patterns of TGF-beta 2 and Th1 cytokines and ameliorates listeriosis in mice. J Immunol. 1999;162:6955–8. [PubMed] [Google Scholar]
- 33.Nieuwenhuis EE, Matsumoto T, Exley M, et al. CD1d-dependent macrophage-mediated clearance of Pseudomonas aeruginosa from lung. Nat Med. 2002;8:588–93. doi: 10.1038/nm0602-588. [DOI] [PubMed] [Google Scholar]
- 34.Spence PM, Sriram V, Van Kaer L, Hobbs JA, Brutkiewicz RR. Generation of cellular immunity to lymphocytic choriomeningitis virus is independent of CD1d1 expression. Immunology. 2001;104:168–74. doi: 10.1046/j.0019-2805.2001.01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson TR, Hong S, Van Kaer L, Koezuka Y, Graham BS. NK T cells contribute to expansion of CD8(+) T cells and amplification of antiviral immune responses to respiratory syncytial virus. J Virol. 2002;76:4294–03. doi: 10.1128/JVI.76.9.4294-4303.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hobbs JA, Cho S, Roberts TJ, Sriram V, Zhang J, Xu M, Brutkiewicz RR. Selective loss of natural killer T cells by apoptosis following infection with lymphocytic choriomeningitis virus. J Virol. 2001;75:10746–54. doi: 10.1128/JVI.75.22.10746-10754.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grubor-Bauk B, Simmons A, Mayrhofer G, Speck PG. Impaired clearance of herpes simplex virus type 1 from mice lacking CD1d or NKT cells expressing the semivariant V alpha 14-J alpha 281 TCR. J Immunol. 2003;170:1430–4. doi: 10.4049/jimmunol.170.3.1430. [DOI] [PubMed] [Google Scholar]
- 38.Carnaud C, Lee D, Donnars O, Park SH, Beavis A, Koezuka Y, Bendelac A. Cutting edge: cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J Immunol. 1999;163:4647–50. [PubMed] [Google Scholar]
- 39.Eberl G, MacDonald HR. Selective induction of NK cell proliferation and cytotoxicity by activated NKT cells. Eur J Immunol. 2000;30:985–92. doi: 10.1002/(SICI)1521-4141(200004)30:4<985::AID-IMMU985>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 40.Kakimi K, Guidotti LG, Koezuka Y, Chisari FV. Natural killer T cell activation inhibits hepatitis B virus replication in vivo. J Exp Med. 2000;192:921–30. doi: 10.1084/jem.192.7.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gonzalez-Aseguinolaza G, de Oliveira C, Tomaska M, et al. Alpha-galactosylceramide-activated Valpha 14 natural killer T cells mediate protection against murine malaria. Proc Natl Acad Sci USA. 2000;97:8461–6. doi: 10.1073/pnas.97.15.8461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Molano A, Park SH, Chiu YH, Nosseir S, Bendelac A, Tsuji M. Cutting edge. the IgG response to the circumsporozoite protein is MHC class II-dependent and CD1d-independent: exploring the role of GPIs in NKT cell activation and antimalarial responses. J Immunol. 2000;164:5005–9. doi: 10.4049/jimmunol.164.10.5005. [DOI] [PubMed] [Google Scholar]
- 43.Exley MA, Bigley NJ, Cheng O, et al. CD1d-reactive T-cell activation leads to amelioration of disease caused by diabetogenic encephalomyocarditis virus. J Leukoc Biol. 2001;69:713–8. [PubMed] [Google Scholar]
- 44.Giron DJ, Cohen SJ, Lyons SP, Wharton CH, Cerutis DR. Inhibition of virus-induced diabetes mellitus by interferon is influenced by the host strain. Proc Soc Exp Biol Medical. 1983;173:328–31. doi: 10.3181/00379727-173-41651. [DOI] [PubMed] [Google Scholar]
- 45.Huber SA, Babu PG, Craighead JE. Genetic influences on the immunologic pathogenesis of encephalomyocarditis (EMC) virus-induced diabetes mellitus. Diabetes. 1985;34:1186–90. doi: 10.2337/diab.34.11.1186. [DOI] [PubMed] [Google Scholar]
- 46.Gaines KL, Kayes SG, Wilson GL. Factors affecting the infection of the D variant of encephalomyocarditis virus in the B cells of C57BL/6J mice. Diabetologia. 1987;30:419–25. doi: 10.1007/BF00292545. [DOI] [PubMed] [Google Scholar]
- 47.White LL, Smith RA. D variant of encephalomyocarditis virus (EMCV-D) -induced diabetes following natural killer cell depletion in diabetes-resistant male C57BL/6J mice. Viral Immunol. 1990;3:67–76. doi: 10.1089/vim.1990.3.67. [DOI] [PubMed] [Google Scholar]
- 48.Baek HS, Yoon JW. Role of macrophages in the pathogenesis of encephalomyocarditis virus-induced diabetes in mice. J Virol. 1990;64:5708–15. doi: 10.1128/jvi.64.12.5708-5715.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kanda T, Utsugi T, Kawazu S, et al. Induction of virus-induced IDDM in virus resistant mice without lymphocyte maturation. Life Sci. 1998;63:33–40. doi: 10.1016/s0024-3205(98)00233-1. [DOI] [PubMed] [Google Scholar]
- 50.Neal ZC, Splitter GA. Protection against lethal encephalomyocarditis virus infection in the absence of serum-neutralizing antibodies. J Virol. 1998;72:8052–60. doi: 10.1128/jvi.72.10.8052-8060.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ozmen L, Aguet M, Trinchieri G, Garotta G. The in vivo antiviral activity of interleukin-12 is mediated by gamma interferon. J Virol. 1995;69:8147–50. doi: 10.1128/jvi.69.12.8147-8150.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pozzetto B, Gresser I. Role of sex and early interferon production in the susceptibility of mice to encephalomyocarditis virus. J Gen Virol. 1985;66:701–9. doi: 10.1099/0022-1317-66-4-701. [DOI] [PubMed] [Google Scholar]
- 53.McFarland HI, Bigley NJ. Sex-dependent, early cytokine production by NK-like spleen cells following infection with the D variant of encephalomyocarditis virus (EMCV-D) Viral Immunol. 1989;2:205–14. doi: 10.1089/vim.1989.2.205. [DOI] [PubMed] [Google Scholar]
- 54.Curiel RE, Miller MH, Ishikawa R, Thomas DC, Bigley NJ. Does the gender difference in interferon production seen in picornavirus-infected spleen cell cultures from ICR Swiss mice have any in vivo significance? J Interferon Res. 1993;13:387–95. doi: 10.1089/jir.1993.13.387. [DOI] [PubMed] [Google Scholar]
- 55.Bradbury A, Belt KT, Neri TM, Milstein C, Calabi F. Mouse CD1 is distinct from and co-exists with TL in the same thymus. EMBO J. 1988;7:3081–6. doi: 10.1002/j.1460-2075.1988.tb03173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Balk SP, Bleicher PA, Terhorst C. Isolation and expression of cDNA encoding the murine homologues of CD1. J Immunol. 1991;146:768–74. [PubMed] [Google Scholar]
- 57.Mansour SL, Thomas KR, Capecchi MR. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature. 1988;336:348–52. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- 58.Deng C, Wynshaw-Boris A, Zhou F, Kuo A, Leder P. Fibroblast growth factor receptor 3 is a negative regulator of bone growth. Cell. 1996;84:911–21. doi: 10.1016/s0092-8674(00)81069-7. [DOI] [PubMed] [Google Scholar]
- 59.Biron CA. Role of early cytokines, including alpha and beta interferons (IFN- alpha/beta), in innate and adaptive immune responses to viral infections. Semin Immunol. 1998;10:383–90. doi: 10.1006/smim.1998.0138. [DOI] [PubMed] [Google Scholar]
- 60.Kitamura H, Iwakabe K, Yahata T, et al. The natural killer T (NKT) cell ligand alpha-galactosylceramide demonstrates its immunopotentiating effect by inducing interleukin (IL) -12 production by dendritic cells and IL-12 receptor expression on NKT cells. J Exp Med. 1999;189:1121–8. doi: 10.1084/jem.189.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tomura M, Yu WG, Ahn HJ, et al. A novel function of Valpha14+CD4+NKT cells: stimulation of IL-12 production by antigen-presenting cells in the innate immune system. J Immunol. 1999;163:93–101. [PubMed] [Google Scholar]
- 62.Leite-De-Moraes MC, Hameg A, Arnould A, Machavoine F, Koezuka Y, Schneider E, Herbelin A, Dy M. A distinct IL-18-induced pathway to fully activate NKT lymphocytes independently from TCR engagement. J Immunol. 1999;163:5871–6. [PubMed] [Google Scholar]
- 63.Huber SA, Sartini D, Exley M. Vgamma4(+) T cells promote autoimmune CD8(+) cytolytic T-lymphocyte activation in coxsackievirus B3-induced myocarditis in mice: role for CD4(+) Th1 cells. J Virol. 2002;76:10785–90. doi: 10.1128/JVI.76.21.10785-10790.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huber S, Sartini D, Exley M. Role of CD1d in coxsackievirus B3-induced myocarditis. J Immunol. 2003;170:3147–53. doi: 10.4049/jimmunol.170.6.3147. [DOI] [PubMed] [Google Scholar]
- 65.Exley MA, He Q, Cheng O, Wang RJ, Cheney CP, Balk SP, Koziel MJ. Cutting edge. Compartmentalization of Th1-like noninvariant CD1d-reactive T cells in hepatitis C virus-infected liver. J Immunol. 2002;168:1519–23. doi: 10.4049/jimmunol.168.4.1519. [DOI] [PubMed] [Google Scholar]