Abstract
Two members of the ADAM (a disintegrin and metalloprotease)-family, MADDAM and decysin, were described as dendritic cell (DC) maturation markers. We are interested in monocyte differentiation and investigated in particular the expression pattern of both genes during the differentiation of human monocytes into DC and macrophages (MAC). Both genes are weakly expressed in freshly isolated monocytes. In immature DC decysin mRNA was absent, even after induction of the terminal differentiation of DC by CD40L or tumour necrosis factor-α (TNF-α). Only in DC maturated by lipopolysaccharide (LPS) strong signals of decysin mRNA were detected. However, MADDAM mRNA was expressed in immature DC and the expression was markedly increased after induction of the terminal DC differentiation by various stimuli. In contrast, MAC showed a high constitutive decysin mRNA expression, but expressed no MADDAM mRNA. On protein level similar results of MADDAM expression were obtained. Stimulation of MAC by LPS did not induce MADDAM mRNA expression, while decysin mRNA expression was strongly increased. Further investigations revealed that the well-known inducer of MAC differentiation, 1α,25-dihydroxyvitamin D3 up-regulated decysin mRNA expression during the differentiation of primary monocytes and myelomonocytic THP-1 cells into MAC. In vivo decysin mRNA expression was only detected in human colon, but not in other tissues we examined. Accordingly, isolated intestinal MAC expressed decysin mRNA. In conclusion, decysin and MADDAM mRNA expression were regulated in an opposite way during monocyte differentiation: MADDAM mRNA and protein was mainly detected in DC, whereas decysin mRNA expression was mainly found in MAC. Therefore only MADDAM, but not decysin is a suitable marker for human monocyte-derived DC.
Introduction
Macrophages (MAC) and some subpopulations of dendritic cells (DC) resemble each other in several functions and surface antigen expression as they originate from the same myeloid progenitor cells.1In vitro both cell types can be generated from human blood monocytes under different culture conditions. The differentiation of MAC is induced by culturing of monocytes with human serum2 while DC are generated from monocytes in the presence of fetal calf serum (FCS), interleukin-4 (IL-4) and granulocyte–macrophage colony-stimulating factor (GM-CSF).3–5 The signals that determine whether the monocytic progenitor cells differentiate in vivo into MAC or DC are still being discussed. Randolph and colleagues showed that phagocytosis is an important key event for DC differentiation, but not for MAC differentation.6 Another factor that may play a role in this decision is 1α,25-dihydroxyvitamin D3. In vitro, 1α,25-dihydroxyvitamin D3 initiates the differentiation of monocytes into MAC even under serum-free conditions;7,8 but suppresses DC differentiation.9–12 Several genes are known to be regulated by 1α,25-dihydroxyvitamin D3 during monocyte differentiation. One of these genes is MADDAM (metalloprotease and disintegrin dendritic cell antigen marker) also called ADAM19.13 Because of its expression which is absent in MAC, but strongly present in DC, MADDAM is a useful molecular marker for the distinction between myeloid DC and MAC.13
MADDAM belongs to the ADAM-family that represents a fast-growing family of mainly transmembrane glycoproteins with a high similarity to the ‘snake venom metalloproteases and disintegrins’.14–16 So far more than 30 members of the ADAM-family are identified in different species. These proteins comprise several protein modules, the prodomain, the metalloprotease and disintegrin domain, the cysteine-rich region, the EGF-repeat domain, the transmembrane region and the cytosolic tail. Because of their special structure they are engaged in different functions17 such as shedding of extracellular proteins like tumour necrosis factor-α (TNF-α), cell-cell fusion and adhesion and/or intracellular signaling.15,18–23
Beside MADDAM, another member of the ADAM-family, decysin, is also described as a gene expressed in monocytes and mature DC.24 The aim of our study was to compare the expression pattern of decysin and MADDAM during monocyte differentiation and to investigate the regulation of decysin expression by 1α,25-dihydroxyvitamin D3, an inducer of MAC differentiation and suppressor of DC maturation.
Materials and methods
Chemicals
All chemical reagents used were purchased from Roche (Mannheim, Germany) unless otherwise noted. CD40L-transfected murine fibroblasts were kindly provided by Joachim L. Schultze.25 1α,25-dihydroxyvitamin D3 was a gift from Roche (Basel, Switzerland) and LPS extracted from Salmonella abortus equi was kindly provided by Chris Galanos (Freiburg, Germany).
Cell separation and culture
Blood mononuclear cells of healthy donors were isolated by leukapheresis and gradient centrifugation over Ficoll/Paque (Pharmacia, Freiburg, Germany). Subsequently a countercurrent centrifugation in a J6M-E centrifuge (Beckmann, München, Germany) was performed as described in order to obtain purified monocytes.26 The purity (>90%) of the monocytes was determined by morphology and expression of CD14 antigen. After isolation monocytes were cultured at a density of 106 cells/ml with RPMI (Biochrom, Berlin, Germany) supplemented with 50 mm mercaptoethanol, antibiotics (0·5 U/ml penicillin and 0·5 mg/ml streptomycin), 1 mm sodium pyruvate, 1× minimal essential medium (MEM) non-essential amino acids, 1× MEM vitamins and 0·22 mg/ml l-glutamine (Gibco BRL, Eggenstein, Germany).
Differentiation of monocytes into macrophages was achieved by cultivation of monocytes for up to 7 days in Petri dishes containing medium and 2% human AB-serum (Sigma, Deisenhofen, Germany) in the absence or presence of 10−7 m 1α,25-dihydroxyvitamin D3. In order to obtain DC, monocytes were seeded in culture flasks for up to 7 days with medium containing 10% FCS (Bio Whittaker, Taufkirchen, Germany), 35 ng/ml human recombinant GM-CSF (Sandoz-Essex, Munich, Germany) and 500 U/ml human recombinant IL-4 (Promocell, Heidelberg, Germany). For induction of terminal DC differentiation 10 ng/ml TNF-α (Promocell), 10 ng/ml lipopolysaccharide (LPS) or paraformaldehyde-fixed CD40L-transfected murine fibroblasts (ratio DC/fibroblasts = 1 : 1) were added at day 5 and the cells were cultured for 2 days.27–29
Cells of the myelomonocytic cell lines THP-1 and HL-60 were cultured in RPMI supplemented with the additives used for monocytes and 10% FCS. The differentiation of these cells into MAC-like cells was triggered by 10−7 m 1α,25-dihydroxyvitamin D3 or 10−8 m phorbol myristate acetate (PMA) for up to 2 days.30–33
Intestinal MAC were isolated from biopsy specimens of inflamed and normal mucosa by collagenase digestion and purified by magnetic cell sorting (Miltenyi Biotech, Bergisch Gladbach, Germany) with anti-CD33 magnetic beads as previously described.34,35 Final purity of >95% MAC was confirmed by fluorescence-activated cell sorting (FACS) analysis using phycoerythrin (PE)-conjugated goat anti-mouse immunoglobulin G (IgG; Caltag, Hamburg, Germany). The study was approved by the University of Regensburg Ethics Committee.
RNA preparation
Total RNA was extracted from primary cells and cell lines by the method of Chomczynski and Sacchi36 and mRNA of intestinal MAC was isolated by polyT magnetic beads (Dynal, Oslo, Norway) from CD33+ cells according to the manufacturer's protocol.
Flow cytometry
Cells were harvested, washed twice with cold phosphate-buffered saline (PBS) containing 0·1% sodium azide and 0·6 mg/ml human immunoglobulin and incubated for 30 min at 4° with specific monoclonal antibodies conjugated with fluorescein isothiocyanate (FITC) or PE. The following antibodies were used: CD1a-PE (Coulter, Krefeld, Germany), CD14-FITC (Coulter) and CD83-PE (Immunotech, Marseille, France). Control cells were stained with the respective isotypes. After two washes, cells were fixed with 1% paraformaldehyde in PBS and analysis was performed using a FACScan (Becton Dickinson, Mountain View, CA).
Northern blot analysis
Total RNA (10 µg/lane) was electrophoretically separated on an 1% agarose formaldehyde gel, transferred to nylon membranes (Magna NT, MSI, Westborough, MA) and UV cross-linked. The cDNA fragment of decysin complementary to the nucleic acids +622 bp to +1127 bp24 was 32P-labelled with gene-specific primers and Klenow enzyme (Roche). Hybridization was performed overnight at 65° in church buffer (0·5 m sodium phosphate buffer pH 7·2, 7% sodium dodecyl sulphate (SDS), 1 mm ethylenediaminetetraacetic acid (EDTA) and 150 µg/ml tRNA). For RNA loading control membranes were rehybridized with an 18S rRNA-oligonucleotide (5′-ACG GTA TCT GAT CGT CTT CGA ACC-3′) labelled by T4 Kinase (5′-End Labelling Kit, Amersham, Amersham, UK).
Western blot analysis
After lysis of the cells (60 mm Tris/HCl with pH 6·8, 2% SDS, 5 mm EDTA, 10 mm dithiothreitol, 1 mm phenylmethylsulphonyl fluoride) the samples were boiled for 10 min at 95°, separated on a 10% SDS–polyacrylamide gel electrophoresis (PAGE) gel and transferred to Hybond-ECL membranes (Amersham). Membranes were incubated overnight by 4° in blocking puffer (PBS with 5% bovine serum albumin) before incubation for 1 hr in blocking buffer containing the polyclonal antibody RP1 against the propeptide domain of human ADAM19 (Triple Point Biologics, Portland, OR) in a concentration of 1 µg/ml and 0·05% Tween-20. After three washing steps with blocking buffer containing 0·05% Tween-20, membranes were incubated for 1 hr in blocking buffer with horseradish peroxidase-conjugated goat anti-rabbit immunoglobulins (1 : 2500 diluted; DAKO, Hamburg, Germany) and 0·05% Tween-20. After three washes in PBS with 0·05% Tween-20, signals were developed with ECL Western blotting reagents (Amersham) according to the manufacturer's instructions and detected by exposition to Kodak film.
Reverse transcriptase–polymerase chain reaction (RT–PCR)
cDNA of intestinal MAC was obtained by using the Reverse Transcription System of Promega (Mannheim, Germany) following the manufacturer's instructions. For amplification of the cDNAs the following primer pairs were used:
Decysin: 5′-TGG TGT GAA GAG CAC TGA CGG GAA AC-3′ (sense); 5′-GGC CCA GCT CAT GTG ACA TCA CTC C-3′ (antisense); β-actin : 5′-TGA CGG GGT CAC CCA CAC TGT GCC CAT CTA-3′ (sense); 5′-CTA GAA GCA TTT GCG GTG GAC GAT GGA GGG-3 (antisense).
The amplification of cDNA was performed with 1 µm sense and antisense primer, 25 µm of each deoxynucleotide triphosphate and 5 U Taq DNA polymerase (Roche) in a total volume of 50 µl. The following PCR conditions were used: 94°, 30 s, 65° (Decysin PCR) or 68° (β-actin PCR), 30 s and 72°, 2 min for up to 40 cycles (Decysin PCR) or 35 cycles (β-actin PCR). The high number of cycles were used because of the low amount of available cDNA of isolated intestinal MAC. After amplification 8 µl of the PCR products were analysed in an 1·5% agarose gel containing 0·5 µg/ml ethidium bromide.
Results
Decysin and MADDAM expression in monocytes, MAC and DC
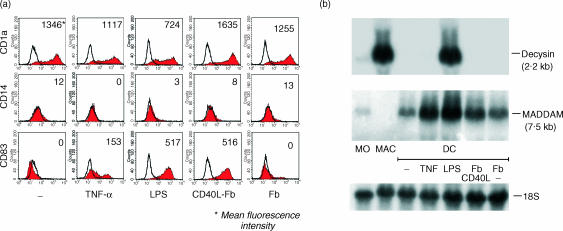
Decysin and MADDAM are described as specific markers of activated/mature DC and we compared the expression pattern of both genes in freshly isolated monocytes, monocyte-derived MAC and monocyte-derived immature and mature DC. The terminal maturation of DC was achieved by stimulation of the cells with TNF-α, LPS or CD40L. In order to control the DC maturation stage the cells were characterized by flow cytometry and stained with antibodies against the CD1a antigen (marker for DC), the CD14 antigen (marker for monocytes/MAC) or against the CD83 antigen (marker for mature DC) (Fig. 1a). As expected, immature and mature DC expressed no CD14 antigen and immature DC were negative for CD83 staining. DC terminal differentiated by TNF-α, LPS or CD40L stimulation showed a strong expression of CD83 antigen (Fig. 1a).
Figure 1.
Comparison of decysin and MADDAM expression in monocytes, MAC and DC. Monocytes were cultured along the MAC differentiation pathway or along the DC differentiation pathway. The terminal differentiation of the immature DC (−) was induced by stimulation with TNF-α, LPS or CD40L-transfected fibroblasts (Fb CD40L). As control nontransfected fibroblasts (Fb) were used. (a) The phenotype and maturation stage of the DC was monitored by flow cytometry. The DC were stained for CD1a, CD14 and CD83. Filled histograms represent staining with the specific antibodies and open histograms represent isotype controls. In addition, the mean fluorescence intensity is shown. (b) Northern blot analysis with P32-labelled cDNA of MADDAM and decysin were performed with RNA of monocytes, MAC and immature and mature DC. As RNA loading control membranes were rehybridized with an 18S rRNA-oligonucleotide.
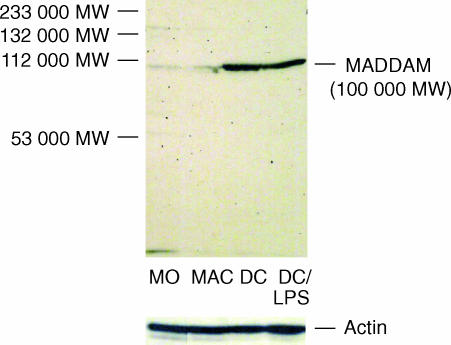
In freshly isolated monocytes decysin and MADDAM were weakly expressed. However, monocyte-derived MAC expressed high levels of decysin mRNA, while the expression of MADDAM mRNA was completely absent in MAC (Fig. 1b). Immature DC generated from monocytes cultured for 7 days with IL-4 and GM-CSF showed no decysin expression, but expressed MADDAM mRNA. Terminal differentiation of these DC by TNF-α, LPS or CD40L led to a strong increase of MADDAM mRNA expression. In contrast, only in DC maturated by LPS stimulation a strong induction of decysin expression was found, while triggering of the DC maturation by TNF-α or CD40L stimulation did not induce decysin expression. (Fig. 1b). On protein level a similar pattern of MADDAM expression was observed during monocyte differentiation. While monocytes and MAC showed a very weak expression of MADDAM protein, a strong signal was detected in DC. However, there was no difference between immature and mature DC (Fig. 2).
Figure 2.
MADDAM expression on protein level. Western blot analysis with a polyclonal antibody against the propeptide domain of the human ADAM19/MADDAM was performed with cell lysates of monocytes, MAC and immature and mature DC. As control Western blot analysis with a polyclonal antibody against actin was carried out.
Influence of LPS on decysin expression in MAC
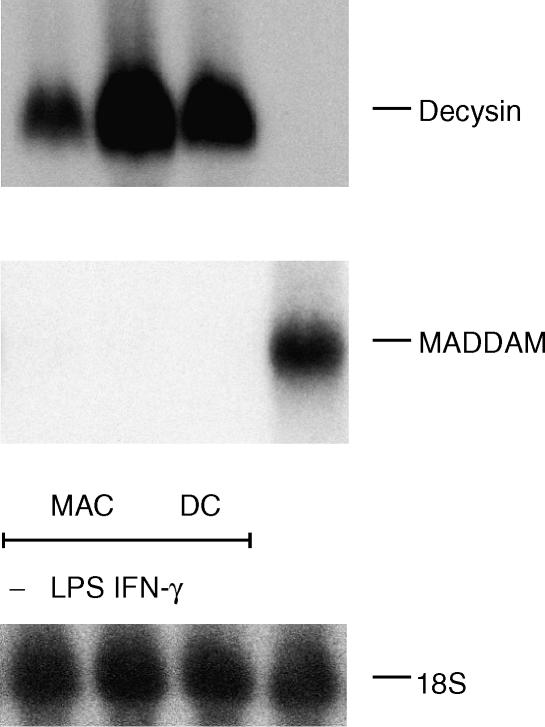
In contrast to LPS, TNF-α and CD40L had no effect on decysin mRNA expression. This indicated that the increase of decysin mRNA expression by LPS is based on an activation event and independent of the terminal DC differentiation. Therefore, we considered whether LPS is also involved in the regulation of decysin expression in MAC. We stimulated MAC overnight with 100 ng/ml LPS or for comparison with 200 U/ml interferon-γ (IFN-γ), another strong stimulus for MAC activation. LPS-activation of MAC resulted in a strong increase of decysin expression, while the decysin expression of INF-γ activated MAC was only weakly affected (Fig. 3). In contrast to decysin, the expression of MADDAM was not altered by LPS or INF-γ in MAC, as its expression was absent in non-stimulated and stimulated MAC (Fig. 3).
Figure 3.
Decysin and MADDAM expression in activated MAC. Northern blot analysis was carried out with a decysin- and MADDAM-specific radioactive probe in order to investigate decysin and MADDAM expression in nonactivated MAC (−) or MAC activated overnight with LPS or IFN-γ. As RNA loading control membranes were rehybridized with an 18S rRNA-oligonucleotide.
Regulation of decysin expression during differentiation of monocytes into MAC
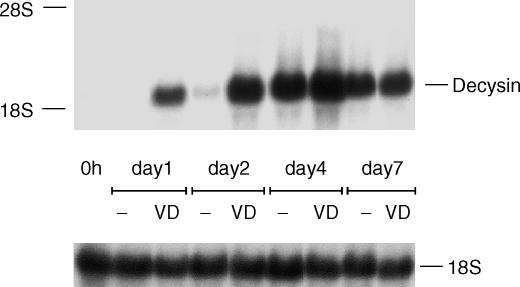
In order to investigate in particular the time course and regulation of decysin expression especially by 1α,25-dihydroxyvitamin D3 during the differentiation of monocytes into MAC we cultured monocytes for up to 7 days with human AB serum in the absence or presence of 1α,25-dihydroxyvitamin D3. After a period of 48 hr a weak induction of decysin mRNA expression was found in monocytes cultured with human serum alone that was further increased during the following 3 days. At day 7 of culture a slight decrease of decysin expression was found. When we cultured the monocytes in combination of human serum and 1α,25-dihydroxyvitamin D3 the induction of decysin expression occurred earlier (after 24 hr) compared to serum alone, but in the later progress of differentiation no difference between both culture conditions was found (Fig. 4).
Figure 4.
Decysin expression during the differentiation of monocytes into MAC. Monocytes were cultured for the indicated time with human serum in the absence (−) or presence of 1α,25-dihydroxyvitamin D3 (VD). Northern blot analysis was performed with a P32-labelled cDNA of decysin and as RNA loading control membranes were rehybridized with an 18S rRNA-oligonucleotide.
Regulation of decysin expression in THP-1 cells
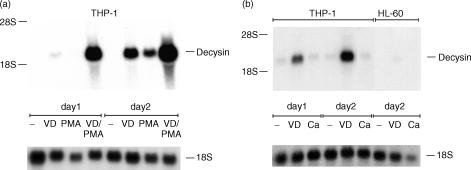
For further verification of decysin expression in MAC we used an alternative to generate MAC-like cells in vitro. We cultured the human myelomonocytic cell lines THP-1 and HL-60 in the presence of PMA or 1α,25-hydroxyvitamin D3. THP-1 cells showed only a weak constitutive expression of decysin mRNA, but cultivation of the cells with 1α,25-dihydroxyvitamin D3 revealed a strong decysin mRNA expression after 48 hr (Fig. 5a). Compared to 1α,25-dihydroxyvitamin D3 PMA showed a weaker influence on decysin expression. However, the combination of both stimuli strongly increased decysin expression in THP-1 cells than 1α,25-dihydroxyvitamin D3 alone (Fig. 5a). In contrast to THP-1 cells, HL-60 cells do not express decysin mRNA after 2 days of culture with 1α,25-dihydroxyvitamin D3 (Fig. 5b).
Figure 5.
Decysin expression in human myelomonocytic cell lines. Decysin expression was examined during the differentiation of THP-1 cells and HL-60 cells into MAC-like or DC-like cells by Northern blot analysis with a P32-labelled cDNA of decysin. As RNA loading control membranes were rehybridized with an 18S rRNA-oligonucleotide. (a) The differentiation of THP-1 cells into MAC was induced by stimulation of the cells for up to 2 days with 1α,25-dihydroxyvitamin D3 (VD), PMA or the combination of both stimuli. (b) THP-1 and HL-60 cells were cultured along the DC differentiation pathway by adding of the calcium ionophore A23178 (Ca) for up to 2 days to the culture. For comparison the cells were in parallel cultured with 1α,25-dihydroxyvitamin D3 (VD).
As described by Koski and colleagues THP-1 and HL-60 cells can also differentiate into DC-like cells after stimulation with the calcium ionophore A23178 for up to 2 days.37,38 Similar to the results obtained by Northern blot analysis with monocyte-derived DC, we found no expression of decysin mRNA in THP-1 or HL-60 cells differentiating along the DC pathway (Fig. 5b).
Distribution of decysin expression in different human tissues
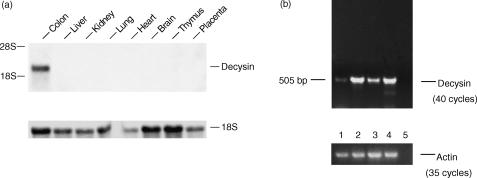
Decysin expression in different tissues of the gut, such as the small intestine and appendix, has already been shown.24 As MAC are distributed in many different tissues like lung and kidney, we investigated the expression of decysin in various tissues. In liver, kidney, lung, heart, brain, thymus and placenta decysin expression was absent, only in the colon a strong decysin mRNA expression was found (Fig. 6a).
Figure 6.
Expression of decysin mRNA in human tissue. (a) Northern blot analysis was performed with a P32 -labelled cDNA of decysin and RNA of different human tissues. As RNA loading control membranes were rehybridized with an 18S rRNA-oligonucleotide. (b) RT–PCR of human intestinal MAC was carried out. The MAC were isolated from biopsies of patients with ulcerative colitis (lanes 1 and 3) and Morbus Crohn's disease (lane 4) or from biopsies of non-inflamed colon (lane 2). The control without DNA is shown in lane 5. For control of equal cDNA amounts used for PCR actin cDNA was amplified.
In order to investigate whether MAC present in the gut expressed decysin, MAC were isolated by magnetic cell sorting from intestinal biopsies of patients with ulcerative colitis (lanes 1 and 3) and Morbus Crohn's disease (lane 4) or from biopsies of non-inflamed colon (lane 2). The expression of decysin mRNA was examined by RT–PCR which revealed decysin mRNA expression in intestinal MAC isolated from inflamed (lanes 1, 3 and 4) and non-inflamed biopsies as well (lane 2; Fig. 6b).
Discussion
Members of the ADAM-family such as ADAM9, ADAM10, ADAM15 and ADAM17 are ubiquitously expressed in humans,39–42 while the expression of decysin and MADDAM seems more restricted.13,24 A common feature of decysin and MADDAM is their description as markers for mature DC derived from either monocytes or CD34-positive cells,13,24 indicating a role of these members of the ADAM-family for the DC differentiation, but not MAC differentiation. However, our results revealed a strong expression of decysin in MAC, but no expression in immature DC. In addition, we and Vandenabeele et al.43 detected no decysin expression in monocyte-derived CD40L-activated DC, although decysin was originally characterized as a gene expressed in these cells.24 Accordingly, monocyte-derived MAC, cells of the monoblastic cell line THP-1 cultured along the MAC differentiation pathway expressed high levels of decysin mRNA as well, but in the myeloblastic HL-60 cells44 that show a less differentiated monocytic phenotype than THP-1 cells45 no decysin expression was observed after induction of MAC differentiation. In contrast to decysin, MADDAM mRNA and protein expression was as previously described found in immature and mature DC, but not in MAC.13 Therefore, decysin and MADDAM are inversely regulated during the differentiation of monocytes into DC and MAC. Based on its high expression in MAC decysin is not an appropriate marker for human monocyte-derived DC, which was already shown for the murine system.46
Beside CD40L, TNF-α and LPS are well-known inducers of terminal differentiation of DC resulting in mature DC, which differ in their morphology and function.47 However, all of them show an up-regulation of the DC maturation markers CD835 and MADDAM, useful tools for the distinction between DC and MAC. Like CD40L, TNF-α had no effect regarding the decysin mRNA expression in DC. However, triggering of DC maturation by LPS led to a strong induction of decysin expression. Also in MAC, LPS stimulation strongly increased decysin mRNA expression, while no MADDAM expression was found in MAC even after LPS stimulation. Therefore, the LPS-dependent induction of decysin expression is most likely an LPS activation event and not dependent on terminal DC differentiation indicating that LPS is an important regulator of decysin expression. In line with these findings a strong expression of decysin was found in different tissues of the bowel24 the only organ where LPS is found at high concentrations in vivo. As we found decysin expression in MAC isolated from intestine, MAC are most likely the source of decysin expression in this tissue. In other MAC-containing tissues like lung or kidney no decysin expression was found. Beside LPS, the key enzyme for the conversion of 25-hydroxyvitamin D3 into the 1α,25-dihydroxyvitamin D3 the 25-hydroxyvitamin D3–1α-hydroxylase is also present in the epithelial cells of the bowel.48 Therefore two important regulators of decysin expression, LPS and 1α,25-dihydroxyvitamin D3, are available in the bowel, which may explain the strong decysin expression especially in this tissue. As MAC are also able to produce 1α,25-dihydroxyvitamin D3 in high concentrations after LPS stimulation,49,50 they could regulate decysin expression in an autocrine and paracrine way. Also in DC stimulated by LPS conversion of 25-hydroxyvitamin D3 into 1α,25-dihydroxyvitamin D3 was observed (own unpublished data) indicating a correlation between 1α,25-dihydroxyvitamin D3 production and decysin expression. A regulatory effect of 1α,25-dihydroxyvitamin D3 was already described for other members of the ADAM-family, the ADAM12 and ADAM19/MADDAM13,51,52 that suggests a general role of this secosteroid in the regulation of members of the ADAM-family.
Decysin has no transmembrane region and was recently characterized as a secreted metalloprotease.46 The function of decysin remains still unclear. Decysin produced by MAC may be involved in the shedding of membrane- or matrix-bound proteins in the inflammatory microenvironment comparable to ADAM9, ADAM10 and ADAM17/TACE, which cleaves fibronectin, gelatin, TNF-α or macrophage colony-stimulating factor-receptor.22,40,53,54
In conclusion, decysin is a maturation-associated gene in MAC and not a suitable marker for the distinction of monocyte-derived DC and MAC. The regulation of decysin by LPS and 1α,25-dihydroxyvitamin D3 suggests a role of decysin in inflammation.
Acknowledgments
This work was supported by grants of the Deutsche Forschungsgesellschaft (Kr1418/4–1) and the University of Regensburg (ReForM A).
Abbreviations
- ADAM-family
a disintegrin and metalloprotease-family
- DC
dendritic cells
- FCS
fetal calf serum
- FITC
fluorescein isothiocyanate
- GM-CSF
granulocyte–macrophage colony-stimulating factor
- IFN-γ
interferon-γ
- IL-4
interleukin-4
- LPS
lipopolysaccharide
- MAC
macrophages
- MADDAM
metalloprotease and disintegrin dendritic cell antigen marker
- PE
phycoerythrin
- PMA
phorbol myristate acetate
- TNF-α
tumour necrosis factor-α
References
- 1.Peters JH, Gieseler R, Thiele B, Steinbach F. Dendritic cells: from ontogenetic orphans to myelomonocytic descendants. Immunol Today. 1996;17:273–8. doi: 10.1016/0167-5699(96)80544-5. [DOI] [PubMed] [Google Scholar]
- 2.Andreesen R, Picht J, Lohr GW. Primary cultures of human blood-born macrophages grown on hydrophobic teflon membranes. J Immunol Methods. 1983;56:295–304. doi: 10.1016/s0022-1759(83)80019-2. [DOI] [PubMed] [Google Scholar]
- 3.Peters JH, Ruhl S, Friedrichs D. Veiled accessory cells deduced from monocytes. Immunobiology. 1987;176:154–66. doi: 10.1016/s0171-2985(87)80107-9. [DOI] [PubMed] [Google Scholar]
- 4.Pickl WF, Majdic O, Kohl P, Stockl J, Riedl E, Scheinecker C, Bello Fernandez C, Knapp W. Molecular and functional characteristics of dendritic cells generated from highly purified CD14+ peripheral blood monocytes. J Immunol. 1996;157:3850–9. [PubMed] [Google Scholar]
- 5.Zhou LJ, Tedder TF. CD14+ blood monocytes can differentiate into functionally mature CD83+ dendritic cells. Proc Natl Acad Sci USA. 1996;93:2588–92. doi: 10.1073/pnas.93.6.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Randolph GJ, Beaulieu S, Lebecque S, Steinman RM, Muller WA. Differentiation of monocytes into dendritic cells in a model of transendothelial trafficking. Science. 1998;282:480–3. doi: 10.1126/science.282.5388.480. [DOI] [PubMed] [Google Scholar]
- 7.Kreutz M, Andreesen R. Induction of human monocyte to macrophage maturation in vitro by 1,25-dihydroxyvitamin D3. Blood. 1990;76:2457–61. [PubMed] [Google Scholar]
- 8.Choudhuri U, Adams JA, Byrom N, McCarthy DM, Barrett J. 1,25-Dihydroxyvitamin D3 induces normal mononuclear blood cells to differentiate in the direction of monocyte-macrophages. Haematologia Budap. 1990;23:9–19. [PubMed] [Google Scholar]
- 9.Berer A, Stockl J, Majdic O, Wagner T, Kollars M, Lechner K, Geissler K, Oehler L. 1,25-Dihydroxyvitamin D (3) inhibits dendritic cell differentiation and maturation in vitro. Exp Hematol. 2000;28:575–83. doi: 10.1016/s0301-472x(00)00143-0. [DOI] [PubMed] [Google Scholar]
- 10.Griffin MD, Lutz W, Phan VA, Bachman LA, McKean DJ, Kumar R. Dendritic cell modulation by 1alpha,25 dihydroxyvitamin D3 and its analogs. A vitamin D receptor-dependent pathway that promotes a persistent state of immaturity in vitro and in vivo. Proc Natl Acad Sci USA. 2001;98:6800–5. doi: 10.1073/pnas.121172198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Penna G, Adorini L. 1 Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol. 2000;164:2405–11. doi: 10.4049/jimmunol.164.5.2405. [DOI] [PubMed] [Google Scholar]
- 12.Piemonti L, Monti P, Sironi M, Fraticelli P, Leone BE, Dal Cin E, Di Allavena PCV. Vitamin D3 affects differentiation, maturation, and function of human monocyte-derived dendritic cells. J Immunol. 2000;164:4443–51. doi: 10.4049/jimmunol.164.9.4443. [DOI] [PubMed] [Google Scholar]
- 13.Fritsche J, Moser M, Faust S, Peuker A, Buttner R, Andreesen R, Kreutz M. Molecular cloning and characterization of a human metalloprotease disintegrin – a novel marker for dendritic cell differentiation. Blood. 2000;96:732–9. [PubMed] [Google Scholar]
- 14.Blobel CP. Metalloprotease-disintegrins: links to cell adhesion and cleavage of TNF alpha and Notch. Cell. 1997;90:589–92. doi: 10.1016/s0092-8674(00)80519-x. [DOI] [PubMed] [Google Scholar]
- 15.Wolfsberg TG, Straight PD, Gerena RL, Huovila AP, Primakoff P, Myles DG, White JM. ADAM, a widely distributed and developmentally regulated gene family encoding membrane proteins with a disintegrin and metalloprotease domain. Dev Biol. 1995;169:378–83. doi: 10.1006/dbio.1995.1152. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto S, Higuchi Y, Yoshiyama K, Shimizu E, Kataoka M, Hijiya N, Matsuura K. ADAM family proteins in the immune system. Immunol Today. 1999;20:278–84. doi: 10.1016/s0167-5699(99)01464-4. [DOI] [PubMed] [Google Scholar]
- 17.Primakoff P, Myles DG. The ADAM gene family: surface proteins with adhesion and protease activity. Trends Genet. 2000;16:83–7. doi: 10.1016/s0168-9525(99)01926-5. [DOI] [PubMed] [Google Scholar]
- 18.Black RA, Rauch CT, Kozlosky CJ, et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385:729–33. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- 19.Blobel CP, Wolfsberg TG, Turck CW, Myles DG, Primakoff P, White JM. A potential fusion peptide and an integrin ligand domain in a protein active in sperm–egg fusion. Nature. 1992;356:248–52. doi: 10.1038/356248a0. [DOI] [PubMed] [Google Scholar]
- 20.Kang Q, Cao Y, Zolkiewska A. Metalloprotease-disintegrin ADAM 12 binds to the SH3 domain of Src and activates Src tyrosine kinase in C2C12 cells. Biochem J. 2000;352 Part 3:883–92. [PMC free article] [PubMed] [Google Scholar]
- 21.Lum L, Wong BR, Josien R, et al. Evidence for a role of a tumor necrosis factor-alpha (TNF-alpha)-converting enzyme-like protease in shedding of TRANCE, a TNF family member involved in osteoclastogenesis and dendritic cell survival. J Biol Chem. 1999;274:13613–8. doi: 10.1074/jbc.274.19.13613. [DOI] [PubMed] [Google Scholar]
- 22.Moss ML, Jin SL, Milla ME, et al. Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-alpha. Nature. 1997;385:733–6. doi: 10.1038/385733a0. [DOI] [PubMed] [Google Scholar]
- 23.Yagami Hiromasa T, Sato T, Kurisaki T, Kamijo K, Nabeshima Y, Fujisawa Sehara A. A metalloprotease-disintegrin participating in myoblast fusion. Nature. 1995;377:652–6. doi: 10.1038/377652a0. [DOI] [PubMed] [Google Scholar]
- 24.Mueller CG, Rissoan MC, Salinas B, et al. Polymerase chain reaction selects a novel disintegrin proteinase from CD40-activated germinal center dendritic cells. J Exp Med. 1997;186:655–63. doi: 10.1084/jem.186.5.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schultze JL, Cardoso AA, Freeman GJ, Seamon MJ, Daley J, Pinkus GS, Gribben JG, Nadler LM. Follicular lymphomas can be induced to present alloantigen efficiently: a conceptual model to improve their tumor immunogenicity. Proc Natl Acad Sci USA. 1995;92:8200–4. doi: 10.1073/pnas.92.18.8200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andreesen R, Brugger W, Scheibenbogen C, Kreutz M, Leser HG, Rehm A, Lohr GW. Surface phenotype analysis of human monocyte to macrophage maturation. J Leukoc Biol. 1990;47:490–7. doi: 10.1002/jlb.47.6.490. [DOI] [PubMed] [Google Scholar]
- 27.Buelens C, Verhasselt V, De Groote D, Thielemans K, Goldman M, Willems F. Human dendritic cell responses to lipopolysaccharide and CD40 ligation are differentially regulated by interleukin-10. Eur J Immunol. 1997;27:1848–52. doi: 10.1002/eji.1830270805. [DOI] [PubMed] [Google Scholar]
- 28.Rescigno M, Winzler C, Delia D, Mutini C, Lutz M, Ricciardi Castagnoli P. Dendritic cell maturation is required for initiation of the immune response. J Leukoc Biol. 1997;61:415–21. [PubMed] [Google Scholar]
- 29.Thurnher M, Radmayr C, Ramoner R, Ebner S, Bock G, Klocker H, Romani N, Bartsch G. Human renal-cell carcinoma tissue contains dendritic cells. Int J Cancer. 1996;68:1–7. doi: 10.1002/(SICI)1097-0215(19960927)68:1<1::AID-IJC1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 30.Auwerx JH, Deeb S, Brunzell JD, Peng R, Chait A. Transcriptional activation of the lipoprotein lipase and apolipoprotein E genes accompanies differentiation in some human macrophage-like cell lines. Biochemistry. 1988;27:2651–5. doi: 10.1021/bi00408a003. [DOI] [PubMed] [Google Scholar]
- 31.Bar-Shavit Z, Teitelbaum SL, Reitsma P, Hall A, Pegg LE, Trial J, Kahn AJ. Induction of monocytic differentiation and bone resorption by 1,25-dihydroxyvitamin D3. Proc Natl Acad Sci USA. 1983;80:5907–11. doi: 10.1073/pnas.80.19.5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cassileth PA, Suholet D, Cooper RA. Early changes in phosphatidylcholine metabolism in human acute promyelocytic leukemia cells stimulated to differentiate by phorbol ester. Blood. 1981;58:237–43. [PubMed] [Google Scholar]
- 33.Schwende H, Fitzke E, Ambs P, Dieter P. Differences in the state of differentiation of THP-1 cells induced by phorbol ester and 1,25-dihydroxyvitamin D3. J Leukoc Biol. 1996;59:555–61. [PubMed] [Google Scholar]
- 34.Hausmann M, Spottl T, Andus T, Rothe G, Falk W, Scholmerich J, Herfarth H, Rogler G. Subtractive screening reveals up-regulation of NADPH oxidase expression in Crohn's disease intestinal macrophages. Clin Exp Immunol. 2001;125:48–55. doi: 10.1046/j.1365-2249.2001.01567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rogler G, Hausmann M, Vogl D, et al. Isolation and phenotypic characterization of colonic macrophages. Clin Exp Immunol. 1998;112:205–15. doi: 10.1046/j.1365-2249.1998.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 37.Koski GK, Schwartz GN, Weng DE, Czerniecki BJ, Carter C, Gress RE, Cohen PA. Calcium mobilization in human myeloid cells results in acquisition of individual dendritic cell-like characteristics through discrete signaling pathways. J Immunol. 1999;163:82–92. [PubMed] [Google Scholar]
- 38.Koski GK, Schwartz GN, Weng DE, Gress RE, Engels FH, Tsokos M, Czerniecki BJ, Cohen PA. Calcium ionophore-treated myeloid cells acquire many dendritic cell characteristics independent of prior differentiation state, transformation status, or sensitivity to biologic agents. Blood. 1999;94:1359–71. [PubMed] [Google Scholar]
- 39.Howard L, Lu X, Mitchell S, Griffiths S, Glynn P. Molecular cloning of MADM, a catalytically active mammalian disintegrin-metalloprotease expressed in various cell types. Biochem J. 1996;317(1):45–50. doi: 10.1042/bj3170045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosendahl MS, Ko SC, Long DL, et al. Identification and characterization of a pro-tumor necrosis factor-alpha-processing enzyme from the ADAM family of zinc metalloproteases. J Biol Chem. 1997;272:24588–93. doi: 10.1074/jbc.272.39.24588. [DOI] [PubMed] [Google Scholar]
- 41.Wu E, Croucher PI, McKie N. Expression of members of the novel membrane linked metalloproteinase family ADAM in cells derived from a range of haematological malignancies. Biochem Biophys Res Commun. 1997;235:437–42. doi: 10.1006/bbrc.1997.6714. [DOI] [PubMed] [Google Scholar]
- 42.Patel IR, Attur MG, Patel RN, Stuchin SA, Abagyan RA, Abramson SB, Amin AR. TNF-alpha convertase enzyme from human arthritis-affected cartilage: isolation of cDNA by differential display, expression of the active enzyme, and regulation of TNF-alpha. J Immunol. 1998;160:4570–9. [PubMed] [Google Scholar]
- 43.Vandenabeele S, Hochrein H, Mavaddat N, Winkel K, Shortman K. Human thymus contains 2 distinct dendritic cell populations. Blood. 2001;97:1733–41. doi: 10.1182/blood.v97.6.1733. [DOI] [PubMed] [Google Scholar]
- 44.Dalton WT, Jr, Ahearn MJ, McCredie KB, Freireich EJ, Stass SA, Trujillo JM. HL-60 cell line was derived from a patient with FAB-M2 and not FAB-M3. Blood. 1988;71:242–7. [PubMed] [Google Scholar]
- 45.Tsuchiya S, Yamabe M, Yamaguchi Y, Kobayashi Y, Konno T, Tada K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1) Int J Cancer. 1980;26:171–6. doi: 10.1002/ijc.2910260208. [DOI] [PubMed] [Google Scholar]
- 46.Mueller CG, Cremer I, Paulet PE, Niida S, Maeda N, Lebeque S, Fridman WH, Sautes-Fridman C. Mannose receptor ligand-positive cells express the metalloprotease decysin in the B cell follicle. J Immunol. 2001;167:5052–60. doi: 10.4049/jimmunol.167.9.5052. [DOI] [PubMed] [Google Scholar]
- 47.Gatti E, Velleca MA, Biedermann BC, et al. Large-scale culture and selective maturation of human Langerhans cells from granulocyte colony-stimulating factor-mobilized CD34+ progenitors. J Immunol. 2000;164:3600–7. doi: 10.4049/jimmunol.164.7.3600. [DOI] [PubMed] [Google Scholar]
- 48.Zehnder D, Bland R, Williams MC, McNinch RW, Howie AJ, Stewart PM, Hewison M. Extrarenal expression of 25-hydroxyvitamin d (3)-1 alpha-hydroxylase. J Clin Endocrinol Metab. 2001;86:888–94. doi: 10.1210/jcem.86.2.7220. [DOI] [PubMed] [Google Scholar]
- 49.Kreutz M, Andreesen R, Krause SW, Szabo A, Ritz E, Reichel H. 1,25-dihydroxyvitamin D3 production and vitamin D3 receptor expression are developmentally regulated during differentiation of human monocytes into macrophages. Blood. 1993;82:1300–7. [PubMed] [Google Scholar]
- 50.Reichel H, Koeffler HP, Bishop JE, Norman AW. 25-Hydroxyvitamin D3 metabolism by lipopolysaccharide-stimulated normal human macrophages. J Clin Endocrinol Metab. 1987;64:1–9. doi: 10.1210/jcem-64-1-1. [DOI] [PubMed] [Google Scholar]
- 51.Abe E, Mocharla H, Yamate T, Taguchi Y, Manolagas SC. Meltrin-alpha, a fusion protein involved in multinucleated giant cell and osteoclast formation. Calcif Tissue Int. 1999;64:508–15. doi: 10.1007/s002239900641. [DOI] [PubMed] [Google Scholar]
- 52.Inoue D, Reid M, Lum L, Kratzschmar J, Weskamp G, Myung YM, Baron R, Blobel CP. Cloning and initial characterization of mouse meltrin beta and analysis of the expression of four metalloprotease-disintegrins in bone cells. J Biol Chem. 1998;273:4180–7. doi: 10.1074/jbc.273.7.4180. [DOI] [PubMed] [Google Scholar]
- 53.Schwettmann L, Tschesche H. Cloning and expression in Pichia pastoris of metalloprotease domain of ADAM 9 catalytically active against fibronectin. Protein Expr Purif. 2001;21:65–70. doi: 10.1006/prep.2000.1374. [DOI] [PubMed] [Google Scholar]
- 54.Rovida E, Paccagnini A, Del Rosso M, Peschon J, Dello SP. TNF-alpha-converting enzyme cleaves the macrophage colony-stimulating factor receptor in macrophages undergoing activation. J Immunol. 2001;166:1583–9. doi: 10.4049/jimmunol.166.3.1583. [DOI] [PubMed] [Google Scholar]