Introduction
From the numerous surface markers of a B lymphocyte, the B-cell antigen receptor (BCR) complex is probably the most powerful marker influencing the developmental processes of the cell. The BCR consists of the membrane-bound immunoglobulin (mIg) but, depending on the state of differentiation, may be associated with a couple of other transmembrane proteins, most notably Igα (CD79a) and Igβ (CD79b).1 The N-terminal end of the mIg harbours the antigen-binding site, characterized by an incredibly high potential for diversification and built up by the variable regions of heavy and light chains. Followed by three or four constant domains, depending on the selected immunoglobulin-isotype, the mIg finally expresses two further domains: a transmembrane domain and a cytoplasmic tail, both of which vary in their isotype-specific amino acid composition.2 So far, the sheath proteins Igα and Igβ are known as the signal transduction component of the BCR complex, connecting the antigen receptor to the tyrosine phosphorylation pathways in the cell. All isotypes of mIg can form a complex with Igα and Igβ,3 indicating an involvement of all isotypes in the signal transduction pathway.
Venkitaraman et al.3 showed that for mIgG and mIgD the Igα/Igβ sheath is not required for surface expression. However, Igα/Igβ is the minimum requirement for signal transduction4 and, in the case of IgM, is responsible for the release from intracellular retention sites.5 Igα is expressed by the mb-1 gene and is a 32 000 MW glycoprotein. Igβ (B29 gene) can be expressed in two different isoforms of 37 000 and 39 000 MW, respectively. Interestingly, Igα can be differentially glycosylated. Pogue and Goodnow6 suggested that the extracellular spacer domain of mIgD is necessary and sufficient to confer the mIgD-specific glycosylation pattern of the mb-1 gene.6 However, it remains to be elucidated if and how alternative glycosylation of mb-1 may affect signalling competence or internalization.
The transmembrane domain of mIgs
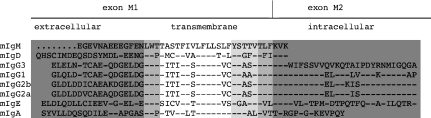
Based on original studies, the transmembrane domain of mIgs was identified as a stretch of 25 uncharged amino acids between charged residues of the putative extracellular and intracellular regions.7 This implies a very short cytoplasmic tail of only three amino acids lysine (K), valine (V), lysine (K) for the μ and δ heavy chains. However, according to the neural network protein prediction method (PHDtopology)8 the transmembrane domain is assumed to be six amino acids shorter, resulting in cytoplasmic tails six amino acids in length for IgM and IgD. A third algorithm,9 even suggests a μ-cytoplasmic tail of 11 amino acid residues. Undoubtedly, this would have an effect on the strength of the immunoglobulin tails to interact with signal-transducing components, as supposed by Cambier and colleagues.2
Most of the functional data concerning the transmembrane domain are deduced from point mutation studies. It could be shown that two polar motifs, the –YSTTVT- and the –TTAST- patches within the μ transmembrane domain are of crucial importance for correct functioning of the BCR. Amino acid residues of these regions contain structural information sufficient for association of μ chains with the Igα/Igβ sheath.10 This assembly may be additionally affected by the glycosylation pattern of the extracellular domain of the BCR.11 Pleiman et al.12 reported, that distinct point mutations within the –YSTTVT- motif totally inhibited antigen-induced signal transduction, though the interaction with Igα was not impaired. However, the use of polyclonal anti-μ serum restored signal transduction probably as a result of more efficient cross-linking of the BCR. Pleiman et al.12 further hypothesized that the presence of Igα/Igβ, although absolutely required, is not sufficient for signal induction, suggesting the existence of a kinase prebound to the μ heavy chain. This assumption supports the finding of Williams et al.13 who identified a serine/threonine kinase associated with the IgM-BCR independently of the Igα/Igβ sheath. Mitchell et al.14 mutated the tyrosine residue within the –YSTTVT- to phenylalanine and showed that the signalling competence was not influenced, but efficient processing and presentation of antigen was affected. The mutation did not affect the association with the Igα/Igβ sheath, pointing to a role of the mIg in intracellular trafficking to class II-rich processing vesicles. Whether these two polar motifs also influence the selective binding of the BCR-associated proteins (BAPs) remains to be investigated. Five BAPs have been identified so far, three of which exclusively bind mIgM (BAP32, BAP37 and BAP41)15 and two interact with mIgD (BAP29 and BAP31).16
The –TTAST- and –YSTTVT- patches have also been suggested to be critical for endoplasmic reticulum retention of the BCR. In addition to the transmembrane domain, the exoplasmic constant region was shown also to play a role in endoplasmic reticulum retention: deletion of the first constant exon CH1 of the μ heavy chain allowed surface expression in the absence of mb-1.17 It is likely that immunoglobulin chains are retained in the endoplasmic reticulum by chaperons such as calnexin,18 BiP and GRP94, preventing surface expression until their binding domains are masked by the Igα/Igβ dimer.19 This mechanism may provide correct assembly of the BCR and degradation of incorrectly folded or incomplete complexes.20
To date, most studies were performed using the μ heavy chain. However, neither the –YSTTVT- nor the –TTAST- patch is conserved among the different isotypes, suggesting further motifs to be responsible for Igα association. Furthermore, there is a remarkable clonal variation among the cell lines transfected with immunoglobulin heavy chain constructs concerning expression and surface transport, complicating a precise interpretation of the cellular and molecular events leading to correct assembly of functional BCRs.3,21
The cytoplasmic tails of mIgs
All immunoglobulin cytoplasmic tails differ in size and amino acid composition. However, with the exception of IgA, the first three amino acids (KVK; Kyte algorithm) are conserved among the different isotypes (Fig. 1). Probably, the putative three amino acid residues KVK of the mIgM and mIgD would be far too short to interact with any signal transducers, whereas a tail of six amino acids (as predicted by PHDtopology) or 11 amino acids (Kyte and Doolittle7) could be sufficient for active protein–protein interactions. Apparently, the charge of the tail rather than the precise amino acid composition of the KVK tail (at least in human μ chains) is responsible for proper signalling and internalization. An exchange of KVK to RIR in transfected cell lines impairs neither calcium mobilization nor antigen internalization. On the other hand, a negatively charged mIgM tail prevents the surface expression of the receptor, probably by interfering with the negatively charged phospholipids within the membrane.22 However, deletion of the KVK tail of the μ chain results in the generation of a phosphatidyl-inositol-linked membrane protein, lacking any competence for signalling and antigen presentation in transfected cell lines.23 The dramatic effects of removing or exchanging the cytoplasmic tail could indicate that the tail is strongly involved in the interaction with immunoglobulin-associated proteins. Alternatively, the deletion of the cytoplasmic domain could induce an inactivating allosteric conformational change in the structure of the mIgM molecule because of the covalent attachment to the membrane lipids.
Figure 1.
Amino acid alignment of the C-terminal domains of mouse mIg-isotypes. Light shading, domains as predicted by the Kyte and Doolittle algorithm; medium shading, domains as predicted by PHDtopology; dark shading, domains as predicted by the Klein algorithm. In the case of mIgA, the transmembrane and cytoplasmic domains are encoded by just one exon. Concerning the extracellular domain, light and dark shade are coherent.
In contrast to mIgM, the tails of the other isotypes (γ, ε and α) are strongly suggested to interact with additional signalling units. Truncation of the murine ε and γ1 tails from 28 amino acids to the conserved KVK sequence in knock-out mice result in severely reduced quantity and affinity of the respective secreted antibody in both primary and secondary responses, though class switch was not affected.24,25 In a recent work Luger et al.26 additionally described that somatic diversity of the immunoglobulin repertoire is influenced by the cytoplasmic tails of mIgs. Furthermore, transfectants of γ1 tail-truncated constructs25 exhibit a dramatic reduction in surface expression. Weiser et al.27 showed that mIgG2a, without the Igα/Igβ sheath, is efficiently internalized after antigen binding, demonstrating the capacity of the tail to interact with the internalization machinery independently from sheath proteins. According to Patel and Neuberger,28 other isotypes require the Igα/Igβ sheath for antigen internalization, though the process of internalization may be driven independently on the tyrosine residues within the sheath. For correct intracellular targeting of the BCR however, Igα/Igβ may not be sufficient.14
Martin and Goodnow29 could show that an exchange of the transmembrane and cytoplasmic domain of mIgM for mIgG1 strongly enhances plasma cell formation by increasing clonal expansion and decreasing cell loss during the germinal centre reaction. They suggested that the longer tail is responsible for the enhanced secondary immune response.
Summarizing, one can speculate whether the reduced serum response in tail-truncated mice is simply the result of reduced surface expression, or if the tail serves as the actual docking site for additional signalling components or adaptor proteins, thus affecting BCR-mediated signalling or antigen processing and presentation.30 One could argue that only the transmembrane regions are important for surface expression, as previously discussed, but regarding the slight variations in the transmembrane domain of the ε and γ subtypes, the longer tails could be supposed to compensate for a diminished potency of the transmembrane domain for Igα association.
Receptor stimulation
One of the first events after receptor engagement is the subsequent phosphorylation of the ITAMs (immunoreceptor tyrosine-based activation motif with the sequence –YxxLx7YxxI/L-, which are present as single copy in the cytoplasmic domains of Igα and Igβ (Fig. 2). This phosphorylation is mediated by members of the Src-family kinases Lyn, Blk, Fyn and/or Lck. The phosphorylated ITAMs provide a binding site for the SH2-containing kinase Syk, which in turn is subsequently activated by Src-family kinases, resulting in an enhanced binding capacity towards the phosphorylated ITAMs (src-family kinases also bear SH2 domains). This activation cascade leads to the recruitment of additional effector and scaffold molecules, most notably BLNK (SLP65 or BASH), HS1 and SHC which are further substrates for the protein tyrosine kinases (PTKs) leading to the formation of a putative stable signalling complex, the signalosome. The immediate-early signalling pathways activated upon receptor engagement are well characterized and include most notably microtubule-associated protein (MAP) kinases, phospholipase C-γ (PLC-γ) (accompanied by calcium release) and the phosphatidyl inositol-3 kinase. All these pathways are extensively reviewed elsewhere.31–34 Conversely, little is known about how the initial phosphorylation of the ITAMs within the Igα/Igβ sheath is mediated. In principle, two models (Fig. 3) for the initiation of BCR signalling are discussed: the allosteric activation of cytoplasmic protein–tyrosine kinases upon antigen binding, and the activation of a cytoplasmic tyrosine kinase upon clustering of two or more BCRs on the membrane. A further matter of discussion deals with the initial presence of the kinases leading to ITAM phosphorylation. The kinases could either be (1) preassociated or otherwise, the antigen-induced clustering of BCRs could result in their (2) translocation into specialized signalling domains. Thus, for the allosteric as well as the clustering model two further models can be brought forward: the preassociation model and the signalling domain model (lipid raft model).
Figure 2.
Model of the BCR complex. The mIg backbone, comprising two immunoglobulin heavy chains, two immunoglobulin light chains, the transmembrane region and the cytoplasmic domain, is associated with the two coat proteins Igα and Igβ. Both sheath proteins, inside their intracellular tail harbour an ITAM motif. On antigen contact, the tyrosines of the ITAMs are phosphorylated by members of the Src-family kinsases.
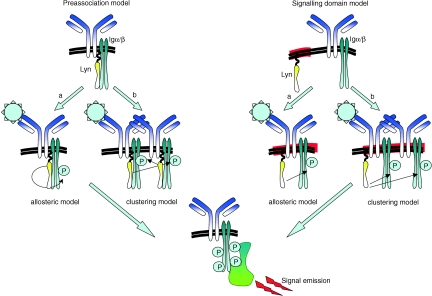
Figure 3.
Model for the initial phosphorylation of the ITAMs within the Igα/Igβ sheath. In principle, two models for the initiation of BCR signalling are discussed: the allosteric activation of cytoplasmic protein–tyrosine kinases upon antigen binding (a), and the activation of cytoplasmic protein–tyrosine kinases upon clustering of two or more BCRs on the membrane (b). However, the kinases leading to the initial phosphorylation event could be preassociated to the cytoplasmic tails of Igα/Igβ (Pre-association model), or the BCRs, as a result of the clustering, are translocated to specialized membrane locations (signalling domain or lipid raft model).
The allosteric activation model (Fig. 3)
As first suggested by Cambier et al.,2 antigen binding could induce a conformational change in the structure of the cytoplasmic portion of the mIg, thereby activating a prebound protein tyrosine kinase that triggers the cytoplasmic signalling cascade. Alternatively, Reth35 proposes that the conformational change of the mIg upon antigen binding may lead to the production of hydrogen peroxide, which activates the receptor proximal kinases by selective inhibition of phosphatases. A third possibility is that the conformational change induces the translocation of the BCR into distinct signalling domains (rafts) within the membrane.
The clustering model (Fig. 3)
In general, this model proposes that the local proximity of two or more BCRs induces the activation of cytoplasmic kinases. Approximately 5 × 104−25 × 104 BCRs constantly cover the surface of a B cell.22 According to this model, a signal is either triggered by cross-linking of BCRs through multivalent antigen or as a result of stochastic co-location of multiple receptors by passive diffusion on the cell surface. The latter mode of signal activation proposes that once a receptor becomes stabilized through interaction with antigen, the probability of the prolonged co-location of a second receptor is slightly enhanced. In other words, the antigen alters the magnitude of membrane diffusion of the receptors depending on its Km value. The higher the affinity of the BCR for the antigen, the more probable the prolonged co-location of one or more receptors on the cell surface and the higher the net signal strength. This model of stochastic signalling was initially proposed for the T-cell receptor36 but it seems plausible that BCR signal induction may act in a similar fashion because higher molecular complexes of clustered BCRs can be isolated from B-cell surfaces even in the absence of antigen.37
The preassociation model (Fig. 3)
The simplest conception is that Lyn is preassociated with the BCR so that surface approximation of multiple receptors allows Lyn to phosphorylate the ITAMs of the adjacent BCR complex. Otherwise, the conformational change upon receptor engagement could allow Lyn to act on the ITAMs. According to this model, Lyn binds to Igα/Igβ without receptor engagement, however, it remains speculative whether the binding of the PTK to Igα is mediated by the SH-2 domain as a result of background phosphorylation of the ITAMs or if the mode of interaction is independent from phosphotyrosines.33 Alternatively, the src-family PTKs could be preassociated with the mIg itself, without requiring the presence of the Igα/Igβ sheath, as predicted by two groups.12,38 However, there is also evidence for a transducer complex prebound to the inactive BCR as postulated by Reth and Wienands.33 This transducer complex may involve the BAPs and is assumed to bind to unphosphorylated ITAMs of the Igα/Igβ sheath, thereby preventing the uncontrolled phosphorylation of the ITAMs. BCR engagement could result in the release of the transducer complex from the BCR and its subsequent activation, whereupon the ITAM-tyrosines become accessible for phosphorylation by the src-family kinases resulting in the initiation of several signalling pathways.
The signalling domain model (Fig. 3)
Recently it was found that Lyn is constitutively present in glycolipid-enriched membrane microdomains, termed GEMs or lipid rafts, via its doubly acetylated N terminus, whereas the monomeric BCR seems to be excluded from rafts. On cross-linking by antigen, the BCR immediately translocates into rafts.39 This indicates that the equilibrium distribution of the BCR within lipid rafts would regulate the phosphorylation of its ITAMs by raft-associated Lyn.
Another important finding is that Lyn itself is not constitutively active but tightly regulated. In fact, two tyrosine residues (Tyr416 and Tyr527) within the catalytic domain contribute to the activity of Lyn. Autophosphorylation of Tyr416 is stimulatory, whereas phosphorylation of Tyr527 provokes the intramolecular association with the SH2 domain, thereby inhibiting kinase activity. Phosphorylation of Tyr527 is negatively controlled by CSK tyrosine kinase (C-terminal Src kinase or p50csk) and positively influenced by the phosphatase CD45.40
CD45 is a transmembrane protein tyrosine phosphatase (PTPase) that is highly expressed in all haematopoietic lineages.41 However, each cell type produces a distinct CD45 isoform, ranging in molecular weight from 180 000 to 235 000. The various isoforms all share the same intracellular PTPase domain, but differ in their extracellular domain concerning length and pattern of glycosylation. The isoform characteristic for the B-cell lineage is CD45R (also called B220, because of its molecular weight of 220 000).42 It is suggested that CD45 can act on a broad spectrum of different substrates, thereby modulating the activity of multiple receptor proximal elements in either a positive or negative fashion. Most findings led to the prevailing view that CD45 enhances BCR signalling by dephosphorylation of tyrosine 527 of the lyn kinase;42 conversely, Yanagi et al. found that CD45-deficient DT40 B cells exhibit hyperphosphorylation of Lyn at both the positive and negative regulatory tyrosine residues.43 In addition, CD45-negative variants of WEHI-231 cells show increased tyrosine phosphorylation of cellular proteins at the resting state accompanied by prolonged calcium release upon BCR challenge.44 These apparent discrepancies suggest CD45 to have both positive and negative roles in BCR signalling and have been explained by selective exclusion of CD45 from or inclusion in sites of receptor clustering.41 The sorting of signalling modules is most likely achieved by lipid rafts.42 Moreover, it could be shown that the enzymatic activity of CD45 was increased upon its phosphorylation by CSK, leading to the direct interaction of the src-family kinase lck with CD45.45 Thus, a tight pattern of reciprocal interference of CSK and CD45 allow or prohibit transmission signals from the BCR.
The kinase CSK negatively regulates BCR-mediated signalling by phosphorylation of the tyrosine 527 within the catalytic domain of Lyn.40 The myristylated N terminus of Lyn enables constitutive anchoring to the plasma membrane, whereas CSK lacks a long, saturated acyl chain and therefore is located in the cytoplasm. However, recently a transmembrane adaptor molecule termed PAG (phosphoprotein associated with glycosphingolipid-enriched microdomains, also called CBP) was identified that is present within lipid rafts. The phosphorylated PAGs provide a binding site for CSK via its SH2 domain.46 It is thought that in resting B cells PAG is constitutively phosphorylated, implying the inhibition of Lyn activity by the active, membrane-proximal CSK. Upon BCR engagement, PAG becomes dephosphorylated, leading to the release of CSK from the PAG and the activation of Lyn.40,47 The PTPase that mediates dephosphorylation of the PAGs remains to be identified, but CD45 could be a possible candidate.42
A simplified scenario of signal initiation according to the lipid raft model could be summarized as follows: in the resting state, Lyn is kept inactive within membrane rafts by PAG-associated CSK, whereas monomeric BCRs and CD45 are excluded from rafts. Upon receptor engagement, the clustered BCRs gain access to rafts – probably independent from PTK activity – thereby modulating the molecule composition of the rafts, which allows entrance of the PTPase CD45. The subsequent dephosphorylation of PAGs leads to the release of CSK and the activation of Lyn, which in turn can mediate phosphorylation of the ITAMs. Prolonged co-location of CD45 and Lyn could finally terminate the signal by dephosphorylation of tyrosine 416 within Lyn. Together, all the different models discussed above could work alone or in combination to elicit a signal from the engaged BCR.
Activation of different signalling pathways by the bcr
Strikingly, all the distinct cytoplasmic signals wired from the BCR upon engagement can differ not just in quantity, but more surprisingly even in quality.48 Thus the BCR is able selectively to turn on a specific signalling pathway, by keeping another quiescent. The putative mechanisms, contributing to the switchboard functioning of the BCR are briefly discussed.
Different receptor isotypes may evoke different signals
Mature B cells are normally double positive for mIgM and mIgD. However, the signals initiated from either isotype differ concerning kinetics and intensity. Kim and Reth49 propose that IgM but not mIgD is under control of a negative feedback loop, thereby promoting enhanced apoptosis. These data are consistent with the findings that engagement of IgM on immature cells in vivo and in μ-transfectants in vitro result in their apoptotic deletion, whereas engagement of mIgD does not have this effect.50–52 In addition, the glycosyl-phosphatidylinositol (GPI)-linked isoform of mIgD53 selectively activates cAMP-, but not Ca2+-dependent signalling pathways, thereby preventing apoptotic cell death and contributing to B-cell activation.54 Peckham et al. gained the reciprocal results, assessing that apoptosis induction is far more susceptible to δ-mediated signalling than signals initiated by the μ isotype.55 However, in vivo, the BCR of either isotype seems to be able to compensate for the loss of the other since mice deficient for mIgM or mIgD show only weak phenotypes.56,57 In these mice the IgM-BCR probably mimics the IgD-BCR and vice versa. Furthermore, in immunoglobulin-transgenic mice carrying either hen-egg-lysozyme (HEL) -specific mIgM or mIgD, the response to HEL was comparable to that of the double transgenics in both tolerance induction and activation.58
Concerning other isotypes, it has been clearly shown24,25 that the cytoplasmic tails of IgG1 and IgE influence the quantity and quality of the immune response. In mice with mutations in the cytoplasmic tails of IgG1 and IgE, aimed to mimic the cytoplasmic tail of IgM, a dramatic decrease of the serum level of the respective isotype was found.
These differences might be the result of signalling proteins interacting with distinct BCR-isotypes as a couple of proteins have been identified that selectively interact with mIgM (BAP32, BAP37 and BAP41),15 mIgD (BAP29 and BAP31),16 mIgG2a59 and human mIgE.60
Indeed, in our laboratory we recently identified a potential candidate, the haematopoietic progenitor kinase 1 (HPK1) as interaction partner for the cytoplasmic tails of mIgE and mIgGs.61
Furthermore as already mentioned, the glycosylation pattern of Igα may be isotype specific, thereby recruiting distinct signalling molecules to the membrane. Also the relative abundances of the respective isotypes on the cell membrane could give rise to alternative signal emission, taking into account that the ratio of μ and δ expression on double-positive B cells is strongly dependent on whether the cell is transitional, mature, anergic, or of the B1 phenotype. Together, the existing data raise the possibility that isotype-dependent signal transduction pathways exist in parallel with the well-characterized signal transduction pathways via the Igα/Igβ sheath proteins.
The developmental status of the B cell may turn on the signals according to the pre-existing set of signalling modules present
In this model, the developmental pattern of gene expression and chromatin structure would allow a signal to regulate different sets of genes. Indeed, there are slight differences in calcium release, tyrosine phosphorylation, raft association and induction of transcription factors in splenic B cells from adult compared to neonatal mice,62 as well as in mouse cell lines representing different stages of development.63 Seyfert et al.64 published developmentally regulated methylation of egr-1 in immature B cells and in a review by Baldwin,65 different subunits of NFκB in mature and immature B cells have been postulated. Recently, it was supposed also that developmental differences in lipid raft composition could alter the functional outcome of BCR signalling and selectively recruit a specific set of signal transducers to the membrane;39 this assumption could explain the finding that BCR signalling is induced outside lipid rafts in immature and anergic B cells.66,67
Signalling pathways, that have a certain threshold for activation are induced depending on the signal strength; thus, signal quantity could affect signal quality
A quantitative model would imply the existence of genes that respond to different signal amplitudes. Since signal strength is thought to be a direct result of the amount of receptor clustering, the avidity of the antigen would allow a distinct alteration of signal emission. Indeed, the notion that signal strength can influence the cellular response is consistent with several experimental findings where antigens or autoantigens of different avidity and affinity strongly affect B-cell maturation in the bone marrow as well as B-cell activation in the periphery. The most intriguing examples for this model are found in experiments on tolerance induction. Goodnow et al.68 showed the functional silencing of self-reactive B cells when the affinity of the BCR for the autoantigen exceeds a certain threshold. In transgenic mice bearing a HEL-specific mIg and expressing soluble HEL ubiquitously, the B cells producing the transgenic HEL-specific BCR become anergic when the concentration of soluble HEL corresponds to a certain minimum receptor occupancy. The characteristics of anergic cells are their unresponsiveness to the respective antigen and their down-regulation of mIgM. In contrast to soluble antigen, membrane-bound or multimeric soluble HEL induce clonal deletion of HEL-specific B cells in the bone marrow.69 Thus, tolerance induction of B cells seems to be regulated in a signal quantitative manner. Interestingly, mature peripheral HEL-specific B cells become anergic either when HEL production is induced in adult mice, or when they are transferred to HEL-transgenic mice, accompanied by characteristic mIgM down-regulation.68 On the other hand, anergy is reversed when the B cells are ‘parked’ in non-HEL-transgenic littermates for several months, although the low expression of mIgM was not converted to the normal phenotype.70 This dissociation between recovery from unresponsiveness and lack of mIgM up-regulation points rather to a mere ‘signal quantity’ model of tolerance induction than to the assumption that mIgM and mIgD transmit signals of different quality, as discussed above. Nonetheless, BCR signalling below the critical threshold for tolerance induction is necessary for the survival of B cells at any stage of development. The first line of evidence for this came from Syk-deficient mice. Syk–/– B cells show severely impaired differentiation at the immature stage, apparently because of the loss of BCR signalling.71 Lam et al.72 published that the inducible deletion of a LoxP-flanked transgenic heavy chain in mature B cells causes enhanced apoptosis, which implies that a low level of signalling through the BCR is required for the survival of both mature and immature B cells. This signal can either be the result of the stochastic (ligand-independent) signalling of the BCR as proposed by Pracht et al.,73 or the BCR needs a low affinity for self structures. Investigating transitional B cells, the latter assumption is becoming more likely. The transition of B cells from the immature to the mature stage is accompanied by a dramatic cell loss of about 70–90%. Investigating the nature of this cell loss revealed a non-stochastic positive selection process of B cells bearing a distinct set of heavy–light chain pairs. If the survival of transitional B cells depended on a tonic signal from the BCR, the cell loss would be stochastic rather than selective, thus pointing to a low-affinity interaction of the BCR with self tissue (or with a yet unidentified ligand) as a prerequisite for cell survival.74 Interestingly, Charles Janeway75 suggests that secretory immunoglobulin regulates the selection of the BCR according to the idiotypic network theory predicted by Niels Jerne. He found that B cells unable to secrete their antibodies die by apoptosis as do mIg-deficient B cells. However, Janeway's group could not restore positive selection by re-supplying the secreted immunoglobulin.
Whether BCR signal strength regulates, with regard to maintenance and tolerance induction, memory B cells in the same way as mature B cells, is still a matter of controversy. Undoubtedly, they do depend on a correctly assembled BCR but not on specific antigen for survival.76 However, it is not yet clear whether persisting antigen in the absence of danger can elicit the silencing of memory B cells in a similar fashion as shown for mature B cells.
Together, there is strong experimental evidence that B cells constantly need a low amount of signalling from the BCR to survive, thereby resembling T cells that also depend on low affinity interaction of their T-cell receptors with self peptides presented by major histocompatibility complex molecules expressed on the surface of peripheral tissue.75 Exceeding this threshold without T-cell help results in clonal silencing, indicating that signal strength has a huge impact on the cellular response.
BCR-induced signals could be modulated through the complex interplay with the signals from other receptors (i.e. co-receptors, Toll-like receptors, integrins, cytokine receptors, receptors for T-cell interaction)
Clearly cytokines and T-cell help pivotally shape the B-cell response to antigen. Nonetheless, their interconnection with the immediate-early wave of signalling triggered by the BCR is hard to assess because of technical limitations. One can speculate whether the various receptor species on a cell wire separate signals to the nucleus or whether they form a network of reciprocal influence.
CD45 was recently shown to regulate cytokine-mediated signalling as a Janus kinase (JAK) phosphatase.42 Taking into account that CD45 is involved in BCR signalling, this finding could point to an influence of the cytokine pattern on BCR activation, possibly by affecting the threshold settings for BCR signalling.
Integrins might also be a candidate for modulating BCR signalling. Integrins, such as very late antigen 4 (VLA-4) and leucocyte function-associated molecule 1 (LFA1), are the major family of cell surface receptors that mediate attachment to the extracellular matrix and cell–cell adhesive interactions. Integrin-mediated adhesion and signalling both play a critical role in the differentiation of germinal centre B cells77 and in the formation of immunological synapses (reviewed in ref. 78). Considering that integrins share similar upstream signalling proteins with the BCR, e.g. CSK, SYK and CRK,79 it seems plausible that integrins could concentrate on the site of antigen acquisition by the BCR, contributing to the synapse formation of GC B cells with follicular dendritic cells,80 thereby directly affecting BCR signalling in a positive fashion.
Signalling through the Toll-like receptors (TLRs) also strongly determines the B-cell fate. TLRs comprise a group of evolutionarily highly conserved transmembrane proteins, first discovered in the fruit-fly, where they mediate the release of anti-fungal peptides. The mammalian homologues were found to recognize distinct patterns of microbial origin (e.g. lipopolysaccharide, dsRNA, peptidoglycan) and structures released from stressed self-tissue (e.g. heat-shock proteins), which enables the immune system rapidly to trigger a host defence response (reviewed in refs 81,82). TLRs are expressed on antigen-presenting cells, including B cells. There, the simultaneous recognition of antigen by the BCR and engagement of the TLR causes a synergistic signal that leads to enhanced B-cell activation and plasma cell formation, circumventing T-cell help.83,84
In contrast to cytokine receptors, integrins and TLRs (all of which only possibly affect BCR-mediated signalling directly), there is a certain group of receptors with a well-established potential to interact physically with the BCR within the B-cell membrane to modulate directly the signal strength and probably signal quality upon antigen binding. This group comprises the co-receptors (CD19, CD21, CD22, CD23, PIR-A, PIR-B and FcγRIIb).
In general, co-ligation of the co-receptors with the BCR results in subsequent tyrosine phosphorylation of their cytoplasmic tails by BCR-associated PTKs. This leads to the initiation of a signal that is either stimulatory or inhibitory, depending on whether the tail of the co-receptor bears an ITAM or an ITIM (immunoreceptor tyrosine-based inhibitory motif).40 ITAMs provide a binding site for stimulatory tyrosine kinases, whereas ITIMs recruit phosphatases, such as SHP (SH-2 domain containing protein tyrosine phosphatase) and SHIP (SH-2 domain containing inositol 5-phosphatase) to the BCR complex, thereby opposing the activation that is mediated by the BCR.40
Two murine complement receptors have been described, CR1 (CD35) and CR2 (CD21), which are derived from the same gene by alternative splicing. On B cells only CR2 is expressed, forming a stable receptor complex within the membrane together with CD19, TAPA-1 (CD81) and LEU13. Cross-linking of CD21 to the mIg by antigen complexed with complement drastically lowers the threshold for BCR-mediated signalling and facilitates B-cell activation.85 A second ligand for CD21 is CD23, a lectin-like membrane protein on the surface of B cells and follicular dendritic cells.86
An important function on BCR-mediated signalling is also performed by the receptor for sialic acid (CD22). CD22 recognizes sialic-acid-bearing ligands, which are present ubiquitously on self tissue. Co-ligation of CD22 to the mIg represses BCR-induced responses, as in CD22 knockout mice, a severe hyper-responsiveness to BCR stimulation could be observed.87 Moreover, the constitutive binding to ligands in cis apparently supports the inhibitory function of CD22 on BCR signalling.88 This mechanism might contribute to the prevention of humoral autoimmunity.32 However, CD22 was shown to dampen selectively the signalling through IgM and IgD BCRs but not that through IgG BCRs because the longer tail of IgG prevents phosphorylation of CD22.89 However, this is in contradiction to older studies, where cross-linking of endogenous IgG on A20 cells indeed led to CD22 phosphorylation.13
The paired immunoglobulin like receptors (PIR) A and B are probably the less well-characterized co-receptors. They are thought to share the same ligand, though this ligand remains to be identified. PIRA has a positive effect on BCR signalling, while PIRB has a negative effect.40
Receptors for secreted antibodies (Fc receptors) are widely distributed among immune cells. They are found on macrophages, monocytes, neutrophils, mast cells, dendritic cells and lymphoid cells. Several receptors for IgG (FcγRI, FcγRIIA, FcγRIIB, FcγRIII) and IgE (FcεRI, FcεRII, FcγRIIB, FcγRIII) have been identified, and most of them stimulate the immune response by enhancing antigen uptake for subsequent presentation to T cells.85,90 However, only two Fc receptors are normally found on B cells, the FcγRIIB (CD32) and FcεRII (CD23). In contrast to the other FcγRs, FcγRIIB contains an ITIM within its cytoplasmic tail, thus contributing to the dampening of BCR-induced signals. Most probably, FcγRIIB negatively modulates BCR signalling not to prevent production of antibodies completely, but to prevent them from reaching abnormal levels.85 CD23 is mainly restricted to B cells and follicular dendritic cells, but can also be found on a small portion of T cells.91 Proteolytic cleavage by an endogenous metalloprotease results in the release of soluble CD23,92 which is still able to bind its natural ligands CD21 and IgE.91 Initially, the role of CD23 during an immune response has been a matter of controversy, since IgE production in CD23 knockout mice was shown to be either enhanced93 or unaffected.94 However, further studies have led to the prevailing view of CD23 positively modulating the humoral response by either enhancing BCR signalling and/or increasing antigen uptake for processing and presentation.85
Conclusion
The ultimate fate of a B lymphocyte is the production of antibodies. However, antibody production is regulated in multiple complex ways, reflected by the fact that B cells must be able to respond to an almost unlimited number of antigens. The link between the ‘outer’ and the ‘inner’ environment is represented by the B-cell antigen receptor and its ability to transmit signals that accompany the B cell along its differentiation pathway. The total antigen receptor is a multimeric protein complex consisting of the membrane form of the selected immunoglobulin isotype and at least two further coat proteins Igα and Igβ. So far, the membrane immunoglobulin has been viewed as the backbone of the receptor complex, while the cytoplasmic tails of the coat proteins were identified as the signal transducing component, connecting the antigen receptor to the tyrosine phosphorylation pathway in the cell. However, expression of additional co-receptors and signalling effectors is important for the regulation and transmission of exact signals, leading to a non-autoreactive, plasma cell or memory cell compartment. It is not yet clear whether the cytoplasmic tails of the immunoglobulin molecules or the cytoplasmic tails of the coat proteins or both tails together are necessary for transducing these signals. It has become clear that BCR-mediated signalling at different maturational stages has distinct biological consequences. However, there are very few data defining the biochemical linkage of Igα/Igβ/mIg-mediated signals to these unique biological responses. Therefore, a pressing challenge for future research lies in unravelling the differences in these pathways and identifying operative effectors.
Acknowledgments
Experimental work and publication charges were supported by the Austrian Science Foundation (S8809-MED), the Austrian National Bank (OENB grant: 9546) and the Swiss National Science Foundation (grant 3100·63381).
References
- 1.Reth M. Antigen receptors on B lymphocytes. Annu Rev Immunol. 1992;10:97. doi: 10.1146/annurev.iy.10.040192.000525. [DOI] [PubMed] [Google Scholar]
- 2.Cambier JC, Pleiman CM, Clark MR. Signal transduction by the B cell antigen receptor and its coreceptors. Annu Rev Immunol. 1994;12:457. doi: 10.1146/annurev.iy.12.040194.002325. [DOI] [PubMed] [Google Scholar]
- 3.Venkitaraman AR, Williams GT, Dariavach P, Neuberger MS. The B-cell antigen receptor of the five immunoglobulin classes. Nature. 1991;352:777. doi: 10.1038/352777a0. [DOI] [PubMed] [Google Scholar]
- 4.Sanchez M, Misulovin Z, Burkhardt AL, Mahajan S, Costa T, Franke R, Bolen JB, Nussenzweig M. Signal transduction by immunoglobulin is mediated through Ig alpha and Ig beta. J Exp Med. 1993;178:1049. doi: 10.1084/jem.178.3.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grupp SA, Mitchell RN, Schreiber KL, McKean DJ, Abbas AK. Molecular mechanisms that control expression of the B lymphocyte antigen receptor complex. J Exp Med. 1995;181:161. doi: 10.1084/jem.181.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pogue SL, Goodnow CC. Ig heavy chain extracellular spacer confers unique glycosylation of the Mb-1 component of the B cell antigen receptor complex. J Immunol. 1994;152:3925. [PubMed] [Google Scholar]
- 7.Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 8.Zidovetzki R, Rost B, Pecht I. Role of transmembrane domains in the functions of B- and T-cell receptors. Immunol Lett. 1998;64:97. doi: 10.1016/s0165-2478(98)00100-x. [DOI] [PubMed] [Google Scholar]
- 9.Klein P, Kanehisa M, DeLisi C. The detection and classification of membrane-spanning proteins. Biochim Biophys Acta. 1985;815:468. doi: 10.1016/0005-2736(85)90375-x. [DOI] [PubMed] [Google Scholar]
- 10.Stevens TL, Blum JH, Foy SP, Matsuuchi L, DeFranco AL. A mutation of the mu transmembrane that disrupts endoplasmic reticulum retention. Effects on association with accessory proteins and signal transduction. J Immunol. 1994;152:4397. [PubMed] [Google Scholar]
- 11.Li Q, Santini R, Rosenspire AR. Glycosylated extracellular domains of membrane immunoglobulin M contribute to its association with mb-1/B29 gene products and the B cell receptor complex. Immunol Invest. 1998;27:57. doi: 10.3109/08820139809070890. [DOI] [PubMed] [Google Scholar]
- 12.Pleiman CM, Chien NC, Cambier JC. Point mutations define a mIgM transmembrane region motif that determines intersubunit signal transduction in the antigen receptor. J Immunol. 1994;152:2837. [PubMed] [Google Scholar]
- 13.Williams GT, Peaker CJ, Patel KJ, Neuberger MS. The alpha/beta sheath and its cytoplasmic tyrosines are required for signaling by the B-cell antigen receptor but not for capping or for serine/threonine-kinase recruitment. Proc Natl Acad Sci USA. 1994;91:474. doi: 10.1073/pnas.91.2.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitchell RN, Barnes KA, Grupp SA, Sanchez M, Misulovin Z, Nussenzweig MC, Abbas AK. Intracellular targeting of antigens internalized by membrane immunoglobulin in B lymphocytes. J Exp Med. 1995;181:1705. doi: 10.1084/jem.181.5.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terashima M, Kim KM, Adachi T, Nielsen PJ, Reth M, Kohler G, Lamers MC. The IgM antigen receptor of B lymphocytes is associated with prohibitin and a prohibitin-related protein. EMBO J. 1994;13:3782. doi: 10.1002/j.1460-2075.1994.tb06689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim KM, Adachi T, Nielsen PJ, Terashima M, Lamers MC, Kohler G, Reth M. Two new proteins preferentially associated with membrane immunoglobulin D. EMBO J. 1994;13:3793. doi: 10.1002/j.1460-2075.1994.tb06690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cherayil BJ, MacDonald K, Waneck GL, Pillai S. Surface transport and internalization of the membrane IgM H chain in the absence of the Mb-1 and B29 proteins. J Immunol. 1993;151:11. [PubMed] [Google Scholar]
- 18.Wu Y, Pun C, Hozumi N. Roles of calnexin and Ig-alpha beta interactions with membrane Igs in the surface expression of the B cell antigen receptor of the IgM and IgD classes. J Immunol. 1997;158:2762. [PubMed] [Google Scholar]
- 19.Foy SP, Matsuuchi L. Association of B lymphocyte antigen receptor polypeptides with multiple chaperone proteins. Immunol Lett. 2001;78:149. doi: 10.1016/s0165-2478(01)00256-5. [DOI] [PubMed] [Google Scholar]
- 20.Hendershot LM. Immunoglobulin heavy chain and binding protein complexes are dissociated in vivo by light chain addition. J Cell Biol. 1990;111:829. doi: 10.1083/jcb.111.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wienands J, Hombach J, Radbruch A, Riesterer C, Reth M. Molecular components of the B cell antigen receptor complex of class IgD differ partly from those of IgM. EMBO J. 1990;9:449. doi: 10.1002/j.1460-2075.1990.tb08130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaw AC, Mitchell RN, Weaver YK, Campos-Torres J, Abbas AK, Leder P. Mutations of immunoglobulin transmembrane and cytoplasmic domains: effects on intracellular signaling and antigen presentation. Cell. 1990;63:381. doi: 10.1016/0092-8674(90)90171-a. [DOI] [PubMed] [Google Scholar]
- 23.Mitchell RN, Shaw AC, Weaver YK, Leder P, Abbas AK. Cytoplasmic tail deletion converts membrane immunoglobulin to a phosphatidylinositol-linked form lacking signaling and efficient antigen internalization functions. J Biol Chem. 1991;266:8856. [PubMed] [Google Scholar]
- 24.Achatz G, Nitschke L, Lamers MC. Effect of transmembrane and cytoplasmic domains of IgE on the IgE response. Science. 1997;276:409. doi: 10.1126/science.276.5311.409. [DOI] [PubMed] [Google Scholar]
- 25.Kaisho T, Schwenk F, Rajewsky K. The roles of gamma 1 heavy chain membrane expression and cytoplasmic tail in IgG1 responses. Science. 1997;276:412. doi: 10.1126/science.276.5311.412. [DOI] [PubMed] [Google Scholar]
- 26.Luger E, Lamers M, Achatz-Straussberger G, Geisberger R, Infuhr D, Breitenbach M, Crameri R, Achatz G. Somatic diversity of the immunoglobulin repertoire is controlled in an isotype-specific manner. Eur J Immunol. 2001;31:2319. doi: 10.1002/1521-4141(200108)31:8<2319::aid-immu2319>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 27.Weiser P, Riesterer C, Reth M. The internalization of the IgG2a antigen receptor does not require the association with Ig-alpha and Ig-beta but the activation of protein tyrosine kinases does. Eur J Immunol. 1994;24:665. doi: 10.1002/eji.1830240327. [DOI] [PubMed] [Google Scholar]
- 28.Patel KJ, Neuberger MS. Antigen presentation by the B cell antigen receptor is driven by the alpha/beta sheath and occurs independently of its cytoplasmic tyrosines. Cell. 1993;74:939. doi: 10.1016/0092-8674(93)90473-4. [DOI] [PubMed] [Google Scholar]
- 29.Martin F, Kearney JF. Marginal-zone B cells. Nat Rev Immunol. 2002;2:323. doi: 10.1038/nri799. [DOI] [PubMed] [Google Scholar]
- 30.Tarlinton D. Antigen presentation by memory B cells: the sting is in the tail. Science. 1997;276:374. doi: 10.1126/science.276.5311.374. [DOI] [PubMed] [Google Scholar]
- 31.Wienands J, Larbolette O, Reth M. Evidence for a preformed transducer complex organized by the B cell antigen receptor. Proc Natl Acad Sci USA. 1996;93:7865. doi: 10.1073/pnas.93.15.7865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kurosaki T. Genetic analysis of B cell antigen receptor signaling. Annu Rev Immunol. 1999;17:555. doi: 10.1146/annurev.immunol.17.1.555. [DOI] [PubMed] [Google Scholar]
- 33.Reth M, Wienands J. Initiation and processing of signals from the B cell antigen receptor. Annu Rev Immunol. 1997;15:453. doi: 10.1146/annurev.immunol.15.1.453. [DOI] [PubMed] [Google Scholar]
- 34.DeFranco AL. The complexity of signaling pathways activated by the BCR. Curr Opin Immunol. 1997;9:296. doi: 10.1016/s0952-7915(97)80074-x. [DOI] [PubMed] [Google Scholar]
- 35.Reth M. Hydrogen peroxide as second messenger in lymphocyte activation. Nat Immunol. 2002;3:1129. doi: 10.1038/ni1202-1129. [DOI] [PubMed] [Google Scholar]
- 36.Germain RN. The art of the probable: system control in the adaptive immune system. Science. 2001;293:240. doi: 10.1126/science.1062946. [DOI] [PubMed] [Google Scholar]
- 37.Schamel WW, Reth M. Monomeric and oligomeric complexes of the B cell antigen receptor. Immunity. 2000;13:5. doi: 10.1016/s1074-7613(00)00003-0. [DOI] [PubMed] [Google Scholar]
- 38.Hutchcroft JE, Harrison ML, Geahlen RL. Association of the 72-kDa protein-tyrosine kinase PTK72 with the B cell antigen receptor. J Biol Chem. 1992;267:8613. [PubMed] [Google Scholar]
- 39.Pierce SK. Lipid rafts and B-cell activation. Nat Rev Immunol. 2002;2:96. doi: 10.1038/nri726. [DOI] [PubMed] [Google Scholar]
- 40.Kurosaki T. Regulation of B-cell signal transduction by adaptor proteins. Nat Rev Immunol. 2002;2:354. doi: 10.1038/nri801. [DOI] [PubMed] [Google Scholar]
- 41.Thomas ML, Brown EJ. Positive and negative regulation of Src-family membrane kinases by CD45. Immunol Today. 1999;20:406. doi: 10.1016/s0167-5699(99)01506-6. [DOI] [PubMed] [Google Scholar]
- 42.Penninger JM, Irie-Sasaki J, Sasaki T, Oliveira-dos-Santos AJ. CD45: new jobs for an old acquaintance. Nat Immunol. 2001;2:389. doi: 10.1038/87687. [DOI] [PubMed] [Google Scholar]
- 43.Yanagi S, Sugawara H, Kurosaki M, Sabe H, Yamamura H, Kurosaki T. CD45 modulates phosphorylation of both autophosphorylation and negative regulatory tyrosines of Lyn in B cells. J Biol Chem. 1996;271:30487. doi: 10.1074/jbc.271.48.30487. [DOI] [PubMed] [Google Scholar]
- 44.Ogimoto M, Katagiri T, Mashima K, Hasegawa K, Mizuno K, Yakura H. Negative regulation of apoptotic death in immature B cells by CD45. Int Immunol. 1994;6:647. doi: 10.1093/intimm/6.4.647. [DOI] [PubMed] [Google Scholar]
- 45.Autero M, Saharinen J, Pessa-Morikawa T, et al. Tyrosine phosphorylation of CD45 phosphotyrosine phosphatase by p50csk kinase creates a binding site for p56lck tyrosine kinase and activates the phosphatase. Mol Cell Biol. 1994;14:1308. doi: 10.1128/mcb.14.2.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kawabuchi M, Satomi Y, Takao T, Shimonishi Y, Nada S, Nagai K, Tarakhovsky A, Okada M. Transmembrane phosphoprotein Cbp regulates the activities of Src-family tyrosine kinases. Nature. 2000;404:999. doi: 10.1038/35010121. [DOI] [PubMed] [Google Scholar]
- 47.Brdicka T, Pavlistova D, Leo A, et al. Phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG), a novel ubiquitously expressed transmembrane adaptor protein, binds the protein tyrosine kinase csk and is involved in regulation of T cell activation. J Exp Med. 2000;191:1591. doi: 10.1084/jem.191.9.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Healy JI, Goodnow CC. Positive versus negative signaling by lymphocyte antigen receptors. Annu Rev Immunol. 1998;16:645. doi: 10.1146/annurev.immunol.16.1.645. [DOI] [PubMed] [Google Scholar]
- 49.Kim KM, Reth M. Function of B-cell antigen receptor of different classes. Immunol Lett. 1995;44:81. doi: 10.1016/0165-2478(94)00219-h. [DOI] [PubMed] [Google Scholar]
- 50.Ales-Martinez JE, Warner GL, Scott DW. Immunoglobulins D and M mediate signals that are qualitatively different in B cells with an immature phenotype. Proc Natl Acad Sci USA. 1988;85:6919. doi: 10.1073/pnas.85.18.6919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carsetti R, Kohler G, Lamers MC. A role for immunoglobulin D. interference with tolerance induction. Eur J Immunol. 1993;23:168. doi: 10.1002/eji.1830230127. [DOI] [PubMed] [Google Scholar]
- 52.Tisch R, Roifman CM, Hozumi N. Functional differences between immunoglobulins M and D expressed on the surface of an immature B-cell line. Proc Natl Acad Sci USA. 1988;85:6914. doi: 10.1073/pnas.85.18.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wienands J, Reth M. Glycosyl-phosphatidylinositol linkage as a mechanism for cell-surface expression of immunoglobulin D. Nature. 1992;356:246. doi: 10.1038/356246a0. [DOI] [PubMed] [Google Scholar]
- 54.Chaturvedi A, Siddiqui Z, Bayiroglu F, Rao KV. A GPI-linked isoform of the IgD receptor regulates resting B cell activation. Nat Immunol. 2002;3:951. doi: 10.1038/ni839. [DOI] [PubMed] [Google Scholar]
- 55.Peckham D, Andersen-Nissen E, Finkelman FD, Stunz LL, Ashman RF. Difference in apoptosis induction between surface IgD and IgM. Int Immunol. 2001;13:285. doi: 10.1093/intimm/13.3.285. [DOI] [PubMed] [Google Scholar]
- 56.Nitschke L, Kosco MH, Kohler G, Lamers MC. Immunoglobulin d-deficient mice can mount normal immune responses to thymus-independent and -dependent antigens. Proc Natl Acad Sci USA. 1993;90:1887. doi: 10.1073/pnas.90.5.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lutz C, Ledermann B, Kosco-Vilbois MH, Ochsenbein AF, Zinkernagel RM, Kohler G, Brombacher F. IgD can largely substitute for loss of IgM function in B cells. Nature. 1998;393:797. doi: 10.1038/31716. [DOI] [PubMed] [Google Scholar]
- 58.Brink R, Goodnow CC, Crosbie J, Adams E, Eris J, Mason DY, Hartley SB, Basten A. Immunoglobulin M and D antigen receptors are both capable of mediating B lymphocyte activation, deletion, or anergy after interaction with specific antigen. J Exp Med. 1992;176:991. doi: 10.1084/jem.176.4.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weiser P, Muller R, Braun U, Reth M. Endosomal targeting by the cytoplasmic tail of membrane immunoglobulin. Science. 1997;276:407. doi: 10.1126/science.276.5311.407. [DOI] [PubMed] [Google Scholar]
- 60.Batista FD, Anand S, Presani G, Efremov DG, Burrone OR. The two membrane isoforms of human IgE assemble into functionally distinct B cell antigen receptors. J Exp Med. 1996;184:2197. doi: 10.1084/jem.184.6.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Geisberger R, Prlic M, Achatz-Straussberger G, et al. Phage display based cloning of proteins interacting with the cytoplasmic tail of membrane immunoglobulins. Clin Dev Immunol. 2003;9:127. doi: 10.1080/1044667031000137584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yellen AJ, Glenn W, Sukhatme VP, Cao XM, Monroe JG. Signaling through surface IgM in tolerance-susceptible immature murine B lymphocytes. Developmentally regulated differences in transmembrane signaling in splenic B cells from adult and neonatal mice. J Immunol. 1991;146:1446. [PubMed] [Google Scholar]
- 63.Kovesdi D, Koncz G, Ivanyi-Nagy R, et al. Developmental differences in B cell receptor-induced signal transduction. Cell Signal. 2002;14:563. doi: 10.1016/s0898-6568(01)00274-1. [DOI] [PubMed] [Google Scholar]
- 64.Seyfert VL, McMahon SB, Glenn WD, Yellen AJ, Sukhatme VP, Cao XM, Monroe JG. Methylation of an immediate-early inducible gene as a mechanism for B cell tolerance induction. Science. 1990;250:797. doi: 10.1126/science.2237429. [DOI] [PubMed] [Google Scholar]
- 65.Baldwin AS., Jr The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 66.Weintraub BC, Jun JE, Bishop AC, Shokat KM, Thomas ML, Goodnow CC. Entry of B cell receptor into signaling domains is inhibited in tolerant B cells. J Exp Med. 2000;191:1443. doi: 10.1084/jem.191.8.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sproul TW, Malapati S, Kim J, Pierce SK. Cutting edge: B cell antigen receptor signaling occurs outside lipid rafts in immature B cells. J Immunol. 2000;165:6020. doi: 10.4049/jimmunol.165.11.6020. [DOI] [PubMed] [Google Scholar]
- 68.Goodnow CC, Crosbie J, Jorgensen H, Brink RA, Basten A. Induction of self-tolerance in mature peripheral B lymphocytes. Nature. 1989;342:385. doi: 10.1038/342385a0. [DOI] [PubMed] [Google Scholar]
- 69.Hartley SB, Crosbie J, Brink R, Kantor AB, Basten A, Goodnow CC. Elimination from peripheral lymphoid tissues of self-reactive B lymphocytes recognizing membrane-bound antigens. Nature. 1991;353:765. doi: 10.1038/353765a0. [DOI] [PubMed] [Google Scholar]
- 70.Goodnow CC, Brink R, Adams E. Breakdown of self-tolerance in anergic B lymphocytes. Nature. 1991;352:532. doi: 10.1038/352532a0. [DOI] [PubMed] [Google Scholar]
- 71.Cheng AM, Rowley B, Pao W, Hayday A, Bolen JB, Pawson T. Syk tyrosine kinase required for mouse viability and B-cell development. Nature. 1995;378:303. doi: 10.1038/378303a0. [DOI] [PubMed] [Google Scholar]
- 72.Lam KP, Kuhn R, Rajewsky K. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell. 1997;90:1073. doi: 10.1016/s0092-8674(00)80373-6. [DOI] [PubMed] [Google Scholar]
- 73.Pracht C, Gimborn K, Reth M, Huber M. BCR mutants deficient in ligand-independent and more sensitive for ligand-dependent signaling. Eur J Immunol. 2002;32:1614. doi: 10.1002/1521-4141(200206)32:6<1614::AID-IMMU1614>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 74.Levine MH, Haberman AM, Sant'Angelo DB, Hannum LG, Cancro MP, Janeway CA, Jr, Shlomchik MJ. A B-cell receptor-specific selection step governs immature to mature B cell differentiation. Proc Natl Acad Sci USA. 2000;97:2743. doi: 10.1073/pnas.050552997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Janeway CA., Jr How the immune system works to protect the host from infection: a personal view. Proc Natl Acad Sci USA. 2001;98:7461. doi: 10.1073/pnas.131202998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maruyama M, Lam KP, Rajewsky K. Memory B-cell persistence is independent of persisting immunizing antigen. Nature. 2000;407:636. doi: 10.1038/35036600. [DOI] [PubMed] [Google Scholar]
- 77.Koopman G, Keehnen RM, Lindhout E, Newman W, Shimizu Y, van Seventer GA, de Groot C, Pals ST. Adhesion through the LFA-1 (CD11a/CD18) -ICAM-1 (CD54) and the VLA-4 (CD49d) -VCAM-1 (CD106) pathways prevents apoptosis of germinal center B cells. J Immunol. 1994;152:3760. [PubMed] [Google Scholar]
- 78.Davis DM. Assembly of the immunological synapse for T cells and NK cells. Trends Immunol. 2002;23:356. doi: 10.1016/s1471-4906(02)02243-3. [DOI] [PubMed] [Google Scholar]
- 79.Clark EA, Brugge JS. Integrins and signal transduction pathways: the road taken. Science. 1995;268:233. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- 80.Batista FD, Iber D, Neuberger MS. B cells acquire antigen from target cells after synapse formation. Nature. 2001;411:489. doi: 10.1038/35078099. [DOI] [PubMed] [Google Scholar]
- 81.Imler JL, Hoffmann JA. Toll receptors in innate immunity. Trends Cell Biol. 2001;11:304. doi: 10.1016/s0962-8924(01)02004-9. [DOI] [PubMed] [Google Scholar]
- 82.Takeda K, Akira S. Roles of Toll-like receptors in innate immune responses. Genes Cells. 2001;6:733. doi: 10.1046/j.1365-2443.2001.00458.x. [DOI] [PubMed] [Google Scholar]
- 83.Vinuesa CG, Goodnow CC. Immunology: DNA drives autoimmunity. Nature. 2002;416:595. doi: 10.1038/416595a. [DOI] [PubMed] [Google Scholar]
- 84.Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 85.Heyman B. Regulation of antibody responses via antibodies, complement, and Fc receptors. Annu Rev Immunol. 2000;18:709. doi: 10.1146/annurev.immunol.18.1.709. [DOI] [PubMed] [Google Scholar]
- 86.Fearon DT, Carter RH. The CD19/CR2/TAPA-1 complex of B lymphocytes: linking natural to acquired immunity. Annu Rev Immunol. 1995;13:127. doi: 10.1146/annurev.iy.13.040195.001015. [DOI] [PubMed] [Google Scholar]
- 87.Nitschke L, Carsetti R, Ocker B, Kohler G, Lamers MC. CD22 is a negative regulator of B-cell receptor signalling. Curr Biol. 1997;7:133. doi: 10.1016/s0960-9822(06)00057-1. [DOI] [PubMed] [Google Scholar]
- 88.Kelm S, Gerlach J, Brossmer R, Danzer CP, Nitschke L. The ligand-binding domain of CD22 is needed for inhibition of the B cell receptor signal, as demonstrated by a novel human CD22-specific inhibitor compound. J Exp Med. 2002;195:1207. doi: 10.1084/jem.20011783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wakabayashi C, Adachi T, Wienands J, Tsubata T. A distinct signaling pathway used by the IgG-containing B cell antigen receptor. Science. 2002;298:2392. doi: 10.1126/science.1076963. [DOI] [PubMed] [Google Scholar]
- 90.Gerber JS, Mosser DM. Stimulatory and inhibitory signals originating from the macrophage Fcgamma receptors. Microbes Infect. 2001;3:131. doi: 10.1016/s1286-4579(00)01360-5. [DOI] [PubMed] [Google Scholar]
- 91.Payet-Jamroz M, Helm SL, Wu J, et al. Suppression of IgE responses in CD23-transgenic animals is due to expression of CD23 on nonlymphoid cells. J Immunol. 2001;166:4863. doi: 10.4049/jimmunol.166.8.4863. [DOI] [PubMed] [Google Scholar]
- 92.Karagiannis SN, Warrack JK, Jennings KH, Murdock PR, Christie G, Moulder K, Sutton BJ, Gould HJ. Endocytosis and recycling of the complex between CD23 and HLA-DR in human B cells. Immunology. 2001;103:319. doi: 10.1046/j.1365-2567.2001.01238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yu P, Kosco-Vilbois M, Richards M, Kohler G, Lamers MC. Negative feedback regulation of IgE synthesis by murine CD23. Nature. 1994;369:753. doi: 10.1038/369753a0. [DOI] [PubMed] [Google Scholar]
- 94.Fujiwara H, Kikutani H, Suematsu S, et al. The absence of IgE antibody-mediated augmentation of immune responses in CD23-deficient mice. Proc Natl Acad Sci USA. 1994;91:6835. doi: 10.1073/pnas.91.15.6835. [DOI] [PMC free article] [PubMed] [Google Scholar]