Abstract
The objective of this work was to evaluate the influence of exogenous l-arginine on the capillary blood flow of peripheral tissues of normotensive subjects. Rats were anesthetized with sodium pentobarbital, and the blood flow of femoral, dorsal, and ventral skin and gastrocnemius and soleus muscle was measured by laser Doppler flow and microsphere methods to compare the blood flow before and after the l-arginine infusion. l-Arginine lowered the mean blood pressure in a dose-dependent manner, but a statistically significant reduction in mean blood pressure was detected only at a high dose of 500 mg/kg of body weight. The significant blood flow increment was detected after the l-arginine infusion at doses of 50 and 150 mg/kg without causing hypotension. Nicardipine, a calcium channel blocker, also increased the skin blood flow, but the blood flow increment and blood pressure fall were comparable. A significant increment in microperfusion was detected in gastrocnemius, soleus muscle, and ventral skin compared with control group by the microsphere method. No adverse effects were observed during l-arginine and microsphere infusion. The present work indicates that l-arginine infusion increases muscle capillary blood flow in rats that are not performing exercise. Supplementation with l-arginine might provide additional blood flow at rest and during exercise and result in the improvement of muscle performance and exercise capacity.
Keywords: exercise, nitric oxide
Nitric oxide (NO) is a widespread signaling molecule that is involved in mammalian physiological processes including the relaxation of blood vessels (1–3), immune function (4), inhibition of platelet aggregation (5, 6), and neurotransmission (7). The amino acid l-arginine is the natural precursor for the formation of NO (8), and exogenous l-arginine administration causes the relaxation of blood vessels through the action of NO (9, 10). Although NO synthase, the enzyme that produces NO from l-arginine, is theoretically saturated with its natural substrate, exogenous l-arginine administration leads to increased NO production, and this phenomenon is known as the arginine paradox (9, 10). Therefore, many investigators have been interested in the effects of supplementation with l-arginine to produce increased beneficial effects of NO, including prevention of the progression of atherosclerosis (11, 12), prevention of pulmonary artery endothelial dysfunction (13, 14), enhancement of cellular immune response of T lymphocytes (4), and amelioration of hypertension (15, 16). Other studies indicate that oral supplementation of l-arginine also corrects endothelial dysfunction of chronic heart failure (17), and it increases exercise capacity in patients with precapillary pulmonary hypertension by increasing peak oxygen consumption and decreasing pulmonary arterial pressure and pulmonary vascular resistance thorough the action of NO (18).
Because oxygen and carbon dioxide exchange takes place only in the capillary bed, the peripheral blood flow correlates with the rate of oxygen uptake in these tissues (19). Therefore, increased capillary blood flow represents an important function of the l-arginine/NO pathway. Moreover, l-arginine may increase the blood flow not only to the lung but also to the muscle capillary.
Smooth muscle relaxation of blood vessels may induce both blood flow increment and blood pressure fall, but only the blood flow increment is desirable for increasing exercise capacity. Accordingly, to determine whether l-arginine administration can increase blood flow without decreasing the systemic blood pressure, it is necessary to test the effects of various doses of l-arginine. The objective of the present work was to examine the actions of l-arginine infusion on peripheral perfusion and systemic blood pressure in rats.
Results
Influence of l-Arginine Infusion on Mean Blood Pressure (MBP) and Skin Surface Blood Flow: Laser Doppler Study.
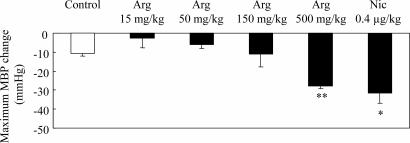
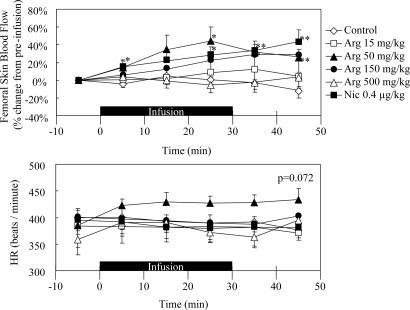
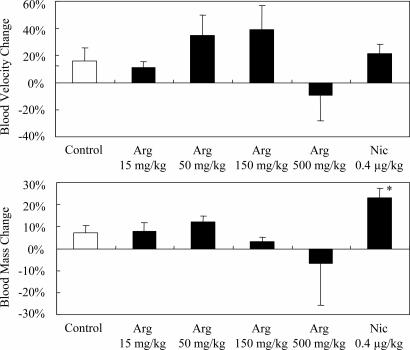
All rats appeared to be healthy, and no adverse effects were observed throughout the study. Typical records of the hemodynamic study are illustrated in Fig. 1, and the effects of l-arginine and nicardipine on the systemic MBP of anesthetized rats are summarized in Fig. 2. Intravenous l-arginine infusion tended to lower the MBP, but a statistically significant response was obtained only at a dose of 500 mg/kg of body weight. The magnitude of the latter hypotensive response was similar to that of nicardipine, which decreased the MBP at a dose of 0.4 μg/kg. Fig. 3 illustrates the effect of sample infusion on the femoral skin surface blood flow. A significant increment in blood flow was detected after l-arginine infusion at doses of 50 and 150 mg/kg. Nicardipine, at a dose of 0.4 μg/kg, also increased the skin blood flow, and the efficacy of nicardipine was similar to that of 50–150 mg of l-arginine per kg. Interestingly, 500 mg of l-arginine per kg failed to increase blood flow, although an increase in blood flow was observed at lower doses. Neither l-arginine nor nicardipine produced significant effects on heart rate (data not shown). l-Arginine infusion tended to increase both blood velocity and blood mass in the 50 mg/kg and 150 mg/kg l-arginine groups, but these differences were not statistically significant (Fig. 4). On the contrary, nicardipine significantly increased blood mass, suggesting that the increment in blood flow produced by nicardipine was mainly the result of the increment in blood mass (Fig. 4).
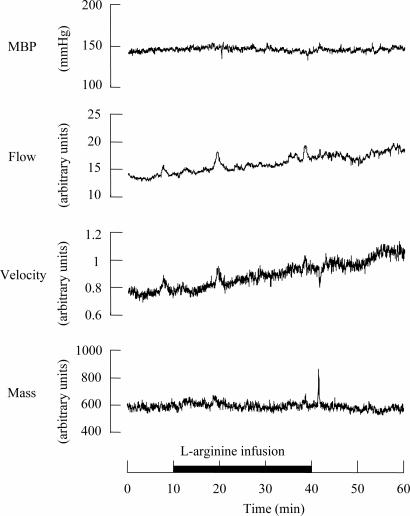
Fig. 1.
Recording of MBP, heart rate and blood flow, blood velocity, and blood mass of femoral skin during l-arginine (150 mg/kg for 30 min) infusion in one rat. Preinfusion values were recorded for >10 min before infusion to confirm stability, and the onset of recording is expressed as time 0. l-Arginine was infused from the 10-min to the 40-min period for 30 min, as indicated by the solid underbar.
Fig. 2.
Responses of MBP during l-arginine infusion at four different doses of 15, 50, 150, and 500 mg/kg, and nicardipine infusion at 0.4 μg/kg. Each column and vertical bar shows the mean ± SEM. ∗, P < 0.05; ∗∗, P < 0.01; significantly different vs. control value determined by factorial analysis of variance followed by Dunnet's test for mean values comparison.
Fig. 3.
Time course changes of femoral skin blood flow by using laser Doppler flow method and heart rate (HR) during l-arginine [Arg 15 (15 mg/kg l-arginine for 30 min), Arg 50 (50 mg/kg l-arginine for 30 min), Arg 150 (150 mg/kg l-arginine for 30 min), Arg 500 (500 mg/kg l-arginine for 30 min)] and nicardipine (Nic; 0.4 μg/kg nicardipine for 30 min) i.v. infusion. (Upper) Each point represents the percent change in MBF from preinfusion at 10-min intervals. (Lower) The individual values of mean heart rate at 10-min intervals are shown. Solid underbars represent sample infusion time. Data are illustrated as means ± SEM. ∗, P < 0.05; ∗∗, P < 0.01; significantly different vs. control value determined by factorial analysis of variance followed by Dunnet's test for mean values comparison.
Fig. 4.
Responses of blood velocity and blood mass in femoral skin during l-arginine infusion at four different doses of 15, 50, 150, and 500 mg/kg, and nicardipine infusion at a dose of 0.4 μg/kg. Each column and vertical bar shows the percent change from preinfusion values, and data are expressed as means ± SEM. ∗, P < 0.05; significantly different vs. control value determined by factorial analysis of variance followed by Dunnet's test for mean values comparison.
Influence of l-Arginine Infusion on Peripheral Blood Flow: Microsphere Study.
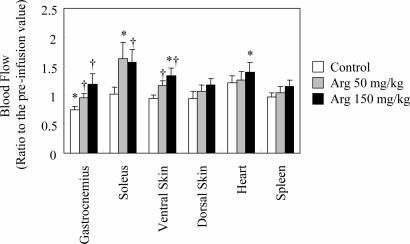
All rats appeared to be healthy, and no adverse effects were observed throughout the study. Because the initial blood flow showed individual variation, blood flow changes were compared by the ratio of postinfusion value to the preinfusion value (Fig. 5). Saline infusion itself caused a significant decrease in gastrocnemius tissue blood flow, but such effects were not observed in other tissues. Intravenous l-arginine infusion tended to increase the microperfusion of all tissues analyzed in this work in a dose-dependent manner, but no decrease in blood flow was observed in internal organs such as duodenum, liver, kidney, and epididymal fat pad (data not shown). l-Arginine infusion, however, elicited significant increases in microperfusion of gastrocnemius, soleus muscle, and ventral skin compared with the control group. No significant changes occurred in spleen. The range of blood flow increment determined by the microsphere method was similar to that measured by the laser Doppler method. In most of the tissues, the peak blood flow response to l-arginine was observed at a dose of 150 mg/kg. Soleus muscle, however, showed a peak blood flow response at a dose of 50 mg of l-arginine per kg.
Fig. 5.
Effect of l-arginine infusion at doses of 50 and 150 mg/kg on regional blood flow. Each column and vertical bar shows the percent change from preinfusion values, and data are expressed as means ± SEM. ∗, Significant difference vs. preinfusion values, P < 0.05; †, significant difference vs. control group, P < 0.05.
Discussion
The objective of the present work was to evaluate the influence of exogenous l-arginine on the blood flow of peripheral tissues and systemic MBP of normotensive rats. Although a great deal of research has been conducted on the effects of exogenous l-arginine on the circulatory system, most of the studies have focused on the effect of l-arginine administration on blood pressure in experimental models of hypertension (20–22) and in patients (16, 23, 24); the improvement of blood flow or exercise capacity in patients with chronic heart failure (17), hypertension (18), and hypercholesterolemia (25); and the prevention of atherosclerosis (11). Few reports exist on the effect of l-arginine on capillary blood flow in normotensive subjects (16, 26–29). The therapeutic effect of l-arginine supplementation in improving exercise capacity in healthy subjects has often been attributed to an increase in plasma growth hormone levels (27–29). However, l-arginine administration does not always increase plasma growth hormone levels (30, 31). The beneficial effects of l-arginine supplementation on exercise capacity are thought to come from increases in blood flow by the NO pathway rather than from growth hormone secretion (32). To our knowledge, the effects of l-arginine on capillary perfusion of muscle and skin of normotensive subjects have not been reported.
Some previous studies have reported that l-arginine failed to show beneficial effects related to NO function such as the improvement of renal function in patients with chronic transplant dysfunction (33), the improvement of exercise capacity in patients with chronic heart failure (34), the amelioration of hypoxia from acute mountain sickness (35), and the attenuation of vasoconstrictor response elicited by application of lower body negative pressure (36), although l-arginine supplementation increased plasma l-arginine levels and NO production, and it was well tolerated without causing any adverse effects. Other studies on healthy subjects indicated that l-arginine supplementation showed no significant effects on forearm blood flow responses to acetylcholine (37) or that infusion of d-arginine, the biologically inactive enantiomer of l-arginine, provided greater vasodilation than l-arginine infusion (38). These negative reports on l-arginine action have led to some confusion regarding the benefit of supplementation of l-arginine in healthy subjects.
In this work, l-arginine prompted an MBP fall and blood flow increase at different doses, whereas nicardipine infusion produced an MBP fall and blood flow increase at the same dose. It is unclear why l-arginine at a high dose of 500 mg/kg decreased MBP without affecting peripheral blood flow. The effects of nicardipine on hemodynamic responses are in agreement with previous reports (39, 40), and similar effects were reported for other calcium channel blockers (41–44). These differences in hemodynamic responses may be attributed to the difference in pharmacological action between calcium channel blockers and l-arginine. Blood viscosity is a parameter that defines the blood velocity (45–47). Therefore, effects on blood viscosity may provide a possible explanation of the difference in pharmacological action between calcium channel blockers and l-arginine. The observation that l-arginine administration can lower the MBP and increase blood flow at different doses is not well understood, and further investigation is needed to clarify the biphasic action of l-arginine on hemodynamics.
Blood flow increases in exercise are caused by vasodilation, muscle pump action, and increased cardiac output, whereas the blood flow increment by l-arginine is caused by vasodilation alone. Muscle pump action, which represents the muscle contraction-induced widening of arteriovenous pressure difference, augments blood flow (48) during locomotion, and it provides a greater increase in blood flow than that caused by the vasodilation alone (48). In this work, the increases in muscle blood flow after l-arginine administration were equal to or less than that caused by exercise, as reported in refs. 50–53. Therefore, the significance of the blood flow increment by l-arginine is that l-arginine administration increased the blood flow in subjects that were at rest.
Accordingly, one question that can be raised is whether the vasodilation at rest caused by l-arginine can improve exercise capacity. l-Arginine has already been reported to be useful in reducing exercise-induced increases in plasma lactate and ammonia (54). Improved muscle blood flow after l-arginine administration can explain the reduction in muscle and plasma lactate and ammonia levels. Flaim et al. (51) reported that exercise increases blood flow in muscle and brain, whereas splanchnic blood flow decreases, which may involve the shunting of blood away from splanchnic regions and into the muscle area. In contrast, l-arginine itself may not cause shunting of blood from splanchnic regions, and splanchnic blood flow may be maintained. Blood flow increment at rest might be beneficial to avoid oxygen deficit and/or to cancel an oxygen debt during and after the exercise.
In this work, gastrocnemius and soleus muscle, which represent fast-twitch and slow-twitch muscles of the leg, respectively, were studied to evaluate the impact of l-arginine on both types of muscle. Treadmill and aquatic exercise were shown to increase the blood flow and oxygen transport in both types of muscle (51, 52). Although l-arginine infusion increased blood flow in both types of muscle, the response to l-arginine infusion at a dose of 50 mg/kg was greater in soleus muscle than in gastrocnemius, which strongly suggests that slow-twitch muscle provides the greatest response to NO-derived vasodilation compared with fast-twitch muscle and other organs. Slow-twitch muscle is important in endurance exercise, and performance of endurance exercise depends on the oxygen supply to the muscle (55). Other studies describe that endurance training itself increases oxygen transport by increasing diffusing capacity and blood flow (56), and exercise increases insulin sensitivity by increasing blood flow and glucose uptake in muscle (57). l-Arginine infusion also increases glucose clearance in muscle by enhancing NO production rather than by increasing insulin secretion from the pancreas (58). Therefore, increases in muscle blood flow might mimic the impact of endurance training on muscle metabolic capacity to some degree, and l-arginine supplementation might also elicit these effects by increasing blood flow in muscle. These observations suggest that l-arginine supplementation might provide beneficial effects in sports nutrition.
The present work indicates that l-arginine infusion increases muscle capillary blood flow in normotensive subjects, at least in rats, and that a dietary supplement can boost muscle blood flow in animals that are not performing exercise. These observations indicate that l-arginine supplementation, in addition to reducing the severity and progression of atherosclerosis (11, 12), might also provide beneficial effects in healthy subjects by improving skeletal muscle blood flow. The effect of l-arginine on muscle blood flow is likely attributed to an increase in vascular endothelial NO production, as is the case for the antiatherosclerotic action of l-arginine (11, 59). Dietary supplementation with l-arginine may, therefore, provide therapeutic benefit in both healthy individuals and in subjects with cardiovascular disease.
Materials and Methods
All procedures performed on the animals were performed according to the Guidelines for Experiments of the Pharmaceutical Research Laboratories, Ajinomoto Co., Inc. (Kawasaki, Japan).
Subjects.
Sixty-six male Sprague–Dawley rats weighing 310–490 g were obtained from Charles River Japan (Yokohama, Japan) and used in this work. Animals were housed in a controlled 12-h light/12-h dark photoperiod with food and water available ad libitum.
Recording of MBP and Blood Flow by Laser Doppler Method.
Rats were anesthetized with sodium pentobarbital (50 mg/kg, i.p.), and anesthesia was maintained by constant infusion of sodium pentobarbital (20 mg/kg per h). The right common carotid artery was cannulated for the measurement of MBP with a pressure transducer (TP-400; Nihon Koden, Tokyo, Japan), and the right jugular vein was also cannulated for drug administration. A laser Doppler blood flow meter (FLO-N1; Omegawave, Tokyo, Japan) probe was positioned in the left femur surface to monitor skin blood velocity, blood mass, and blood flow. MBP and blood flow were recorded with the aid of the MacLab system (ADInstruments Japan, Nagoya, Japan). The body temperature was maintained at 37–38°C with the aid of a heating pad.
After the stabilization period, test agents were infused i.v. through the cannula for 30 min: Arg 15, 15 mg/kg l-arginine for 30 min; Arg 50, 50 mg/kg l-arginine for 30 min; Arg 150, 150 mg/kg l-arginine for 30 min; Arg 500, 500 mg/kg l-arginine for 30 min; Nic, 0.4 μg/kg nicardipine for 30 min; control, saline. These agents were infused continuously with a motor-driven syringe pump (CFV-3200; Nihon Koden). MBP, heart rate, and blood flow of the left femur skin were observed and recorded throughout the study.
Blood Flow Analysis by Microsphere Method.
Microperfusion analysis using microspheres was performed as described in refs. 49, 60, and 61 with minor modification. Thirty-three male Sprague–Dawley rats were sedated with a sodium pentobarbital bolus (50 mg/kg, i.p.) followed by constant infusion of sodium pentobarbital (20 mg/kg per h). A polyethylene tube was implanted in the left ventricle of the heart through the right common carotid artery to inject microspheres (15-μm diameter; Primetech, Tokyo, Japan), and the right femoral artery was cannulated to collect reference blood. Both catheters were filled with heparinized saline. The right jugular vein was also cannulated to infuse test agents.
After the stabilization period, the first microspheres were injected. Microspheres were sonicated and vigorously agitated with a vortex mixer before the injection; then 300,000 microspheres were injected and flushed with 400 μl of saline into the left ventricle of the heart over a 60-s period. Starting 10 s before injection of the microspheres, the reference blood was withdrawn from the femoral arterial cannula at a rate of 300 μl/min for 90 s with a syringe pump (CFV-3200). Microspheres were distributed and trapped in the microcirculation of different organs in proportion to regional blood flow. l-Arginine solution or saline was infused into the jugular vein 10 min after the first microsphere injection for 30 min: Arg 50, 50 mg/kg l-arginine for 30 min; Arg 150, 150 mg/kg l-arginine for 30 min; control, saline; followed by the second microsphere injection. The time of the second microsphere injection was determined after the time of peak blood flow. The rats were killed by acute sodium pentobarbital injection, and gastrocnemius, soleus and heart muscle, dorsal and ventral skin, and spleen were excised. Organs and blood samples were digested in 4 mol/liter potassium hydroxide at 70°C for 4 h, and they were filtered through a polyester filter to trap the microspheres. After the filtration, polyester filters were transferred into a 5-ml polyethylene sample tube and dried at 50°C for 1 h. Three hundred microliters of acidified cellosolve acetate was then added to dissolve the microspheres, and tubes were centrifuged at 1,500 × g for 10 min to separate undissolved materials. Two hundred microliters of the supernatant was taken from the tube and put on 96-well glass plates (FB-96; Nippon Sheet Glass, Tokyo, Japan). Photometric absorption of each solution was measured with the aid of a microplate reader (Benchmark Plus; Bio-Rad Laboratories Japan, Tokyo, Japan). The number of microspheres was calculated by using the specific absorbance value of the dyes used in each experiment. Organ blood flow was calculated according to the formula: organ flow = microsphere number in organ × reference blood flow per minute divided by the microsphere number in reference blood.
Data Analysis.
Results were expressed as means ± SEM, and they represent unpaired data. The statistical significances within a parameter were evaluated by factorial analysis of variance followed by Dunnet's test for mean values comparison. A P value <0.05 was considered statistically significant.
Acknowledgments
We thank Shinobu Tanimoto and Kou Tokumitsu for technical assistance.
Abbreviations
- MBP
mean blood pressure
- NO
nitric oxide.
Footnotes
The authors declare no conflict of interest.
References
- 1.Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Proc Natl Acad Sci USA. 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palmer RM, Rees DD, Ashton DS, Moncada S. Biochem Biophys Res Commun. 1988;153:1251–1256. doi: 10.1016/s0006-291x(88)81362-7. [DOI] [PubMed] [Google Scholar]
- 3.Pedrinelli R, Ebel M, Catapano G, Dell'Omo G, Ducci M, Del Chicca M, Clerico A. Eur J Clin Pharmacol. 1995;48:195–201. doi: 10.1007/BF00198298. [DOI] [PubMed] [Google Scholar]
- 4.Ochoa JB, Strange J, Kearney P, Gellin G, Endean E, Fitzpatrick E. J Parenter Enteral Nutr. 2001;25:23–29. doi: 10.1177/014860710102500123. [DOI] [PubMed] [Google Scholar]
- 5.Mellion BT, Ignarro LJ, Myers CB, Ohlstein EH, Ballot BA, Hyman AL, Kadowitz PJ. Mol Pharmacol. 1983;23:653–664. [PubMed] [Google Scholar]
- 6.Furlong B, Henderson AH, Lewis MJ, Smith JA. Br J Pharmacol. 1987;90:687–692. doi: 10.1111/j.1476-5381.1987.tb11221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rajfer J, Aronson WJ, Bush PA, Dorey FJ, Ignarro LJ. N Engl J Med. 1992;326:90–94. doi: 10.1056/NEJM199201093260203. [DOI] [PubMed] [Google Scholar]
- 8.Palmer RM, Ashton DS, Moncada S. Nature. 1988;333:664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- 9.Boger RH, Bode-Boger SM. Annu Rev Pharmacol Toxicol. 2001;41:79–99. doi: 10.1146/annurev.pharmtox.41.1.79. [DOI] [PubMed] [Google Scholar]
- 10.Gornik HL, Creager MA. J Nutr. 2004;134:2880S–2887S. doi: 10.1093/jn/134.10.2880S. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi T, Juliet PA, Matsui-Hirai H, Miyazaki A, Fukatsu A, Funami J, Iguchi A, Ignarro LJ. Proc Natl Acad Sci USA. 2005;102:13681–13686. doi: 10.1073/pnas.0506595102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marchesi S, Lupattelli G, Siepi D, Roscini AR, Vaudo G, Sinzinger H, Mannarino E. J Clin Pharm Ther. 2001;26:343–349. doi: 10.1046/j.1365-2710.2001.00362.x. [DOI] [PubMed] [Google Scholar]
- 13.Qi J, Du J, Wang L, Zhao B, Tang C. Chin Med J (Engl) 2001;114:933–936. [PubMed] [Google Scholar]
- 14.Hutchison SJ, Sievers RE, Zhu BQ, Sun YP, Stewart DJ, Parmley WW, Chatterjee K. Chest. 2001;120:2004–2012. doi: 10.1378/chest.120.6.2004. [DOI] [PubMed] [Google Scholar]
- 15.Kelly BS, Alexander JW, Dreyer D, Greenberg NA, Erickson A, Whiting JF, Ogle CK, Babcock GF, First MR. J Parenter Enteral Nutr. 2001;25:194–202. doi: 10.1177/0148607101025004194. [DOI] [PubMed] [Google Scholar]
- 16.Siani A, Pagano E, Iacone R, Iacoviello L, Scopacasa F, Strazzullo P. Am J Hypertens. 2000;13:547–551. doi: 10.1016/s0895-7061(99)00233-2. [DOI] [PubMed] [Google Scholar]
- 17.Hambrecht R, Hilbrich L, Erbs S, Gielen S, Fiehn E, Schoene N, Schuler G. J Am Coll Cardiol. 2000;35:706–713. doi: 10.1016/s0735-1097(99)00602-6. [DOI] [PubMed] [Google Scholar]
- 18.Nagaya N, Uematsu M, Oya H, Sato N, Sakamaki F, Kyotani S, Ueno K, Nakanishi N, Yamagishi M, Miyatake K. Am J Respir Crit Care Med. 2001;163:887–891. doi: 10.1164/ajrccm.163.4.2007116. [DOI] [PubMed] [Google Scholar]
- 19.Stainsby WN, Otis AB. Am J Physiol. 1964;206:858–866. doi: 10.1152/ajplegacy.1964.206.4.858. [DOI] [PubMed] [Google Scholar]
- 20.Kelly JJ, Williamson P, Martin A, Whitworth JA. J Hypertens. 2001;19:263–268. doi: 10.1097/00004872-200102000-00013. [DOI] [PubMed] [Google Scholar]
- 21.de la Riva IJ, Roson MI, Vega GW, Speziale N, Albornoz LE, Palumbo EL, Ferrero AJ, Damiano PF. Arch Physiol Biochem. 2000;108:415–421. doi: 10.1076/apab.108.5.415.4298. [DOI] [PubMed] [Google Scholar]
- 22.Zhou MS, Kosaka H, Tian RX, Abe Y, Chen QH, Yoneyama H, Yamamoto A, Zhang L. J Hypertens. 2001;19:421–429. doi: 10.1097/00004872-200103000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Surdacki A, Zmudka K, Bieron K, Kostka-Trabka E, Dubiel JS, Gryglewski RJ. Wien Klin Wochenschr. 1994;106:521–526. [PubMed] [Google Scholar]
- 24.Wolf SC, Erley CM, Kenner S, Berger ED, Risler T. Clin Nephrol. 1995;43:S42–S46. [PubMed] [Google Scholar]
- 25.Duan J, Murohara T, Ikeda H, Katoh A, Shintani S, Sasaki K, Kawata H, Yamamoto N, Imaizumi T. Circulation. 2000;102:370–376. doi: 10.1161/01.cir.102.suppl_3.iii-370. [DOI] [PubMed] [Google Scholar]
- 26.Morikawa E, Moskowitz MA, Huang Z, Yoshida T, Irikura K, Dalkara T. Stroke. 1994;25:429–435. doi: 10.1161/01.str.25.2.429. [DOI] [PubMed] [Google Scholar]
- 27.Eto B, Le Moel G, Porquet D, Peres G. Arch Physiol Biochem. 1995;103:160–164. doi: 10.3109/13813459508996129. [DOI] [PubMed] [Google Scholar]
- 28.Wideman L, Weltman JY, Patrie JT, Bowers CY, Shah N, Story S, Veldhuis JD, Weltman A. Am J Physiol. 2000;279:R1467–R1477. doi: 10.1152/ajpregu.2000.279.4.R1467. [DOI] [PubMed] [Google Scholar]
- 29.Fry AC, Kraemer WJ, Stone MH, Warren BJ, Kearney JT, Maresh CM, Weseman CA, Fleck SJ. Int J Sport Nutr. 1993;3:306–322. doi: 10.1123/ijsn.3.3.306. [DOI] [PubMed] [Google Scholar]
- 30.Suminski RR, Robertson RJ, Goss FL, Arslanian S, Kang J, DaSilva S, Utter AC, Metz KF. Int J Sport Nutr. 1997;7:48–60. doi: 10.1123/ijsn.7.1.48. [DOI] [PubMed] [Google Scholar]
- 31.Marcell TJ, Taaffe DR, Hawkins SA, Tarpenning KM, Pyka G, Kohlmeier L, Wiswell RA, Marcus R. J Gerontol A Biol Sci Med Sci. 1999;54:M395–M399. doi: 10.1093/gerona/54.8.m395. [DOI] [PubMed] [Google Scholar]
- 32.Paddon-Jones D, Borsheim E, Wolfe RR. J Nutr. 2004;134:2888S–2894S. doi: 10.1093/jn/134.10.2888s. [DOI] [PubMed] [Google Scholar]
- 33.Zhang XZ, Ardissino G, Ghio L, Tirelli AS, Dacco V, Colombo D, Pace E, Testa S, Claris-Appiani A. Clin Nephrol. 2001;55:453–459. [PubMed] [Google Scholar]
- 34.Kanaya Y, Nakamura M, Kobayashi N, Hiramori K. Heart. 1999;81:512–517. doi: 10.1136/hrt.81.5.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mansoor JK, Morrissey BM, Walby WF, Yoneda KY, Juarez M, Kajekar R, Severinghaus JW, Eldridge MW, Schelegle ES. High Alt Med Biol. 2005;6:289–300. doi: 10.1089/ham.2005.6.289. [DOI] [PubMed] [Google Scholar]
- 36.Hind JM, Doodson AC. Clin Auton Res. 1994;4:293–297. doi: 10.1007/BF01821527. [DOI] [PubMed] [Google Scholar]
- 37.Chin-Dusting JP, Alexander CT, Arnold PJ, Hodgson WC, Lux AS, Jennings GL. J Cardiovasc Pharmacol. 1996;28:158–166. doi: 10.1097/00005344-199607000-00023. [DOI] [PubMed] [Google Scholar]
- 38.Rhodes P, Barr CS, Struthers AD. Eur J Clin Invest. 1996;26:325–331. doi: 10.1046/j.1365-2362.1996.144277.x. [DOI] [PubMed] [Google Scholar]
- 39.Lambert CR, Pepine CJ. Am J Cardiol. 1989;64:8H–15H. doi: 10.1016/0002-9149(89)90974-0. [DOI] [PubMed] [Google Scholar]
- 40.Saitoh C, Momose K, Tanaka H, Sato S, Taguchi K, Saitoh M, Maehara J, Asano M, Usuda S. Arzneimittelforschung. 1998;48:125–132. [PubMed] [Google Scholar]
- 41.Guha A, Tator CH, Piper I. J Neurosurg. 1985;63:250–259. doi: 10.3171/jns.1985.63.2.0250. [DOI] [PubMed] [Google Scholar]
- 42.Magorien RD, Leier CV, Kolibash AJ, Barbush TJ, Unverferth DV. Circulation. 1984;70:884–890. doi: 10.1161/01.cir.70.5.884. [DOI] [PubMed] [Google Scholar]
- 43.Knight DR, Kirby DA, Vatner SF. Hypertension. 1985;7:380–385. [PubMed] [Google Scholar]
- 44.Takahara A, Konda T, Enomoto A, Kondo N. Biol Pharm Bull. 2004;27:1388–1391. doi: 10.1248/bpb.27.1388. [DOI] [PubMed] [Google Scholar]
- 45.Grotta J, Ackerman R, Correia J, Fallick G, Chang J. Stroke. 1982;13:296–301. doi: 10.1161/01.str.13.3.296. [DOI] [PubMed] [Google Scholar]
- 46.Rim SJ, Leong-Poi H, Lindner JR, Wei K, Fisher NG, Kaul S. Circulation. 2001;104:2704–2709. doi: 10.1161/hc4701.099580. [DOI] [PubMed] [Google Scholar]
- 47.House SD, Johnson PC. Am J Physiol. 1986;250:H828–H837. doi: 10.1152/ajpheart.1986.250.5.H828. [DOI] [PubMed] [Google Scholar]
- 48.Sheriff DD, Rowell LB, Scher AM. Am J Physiol. 1993;265:H1227–H1234. doi: 10.1152/ajpheart.1993.265.4.H1227. [DOI] [PubMed] [Google Scholar]
- 49.Kobayashi N, Kobayashi K, Kouno K, Horinaka S, Yagi S. Am J Physiol. 1994;266:H1910–H1917. doi: 10.1152/ajpheart.1994.266.5.H1910. [DOI] [PubMed] [Google Scholar]
- 50.Peterson DF, Armstrong RB, Laughlin MH. J Appl Physiol. 1988;65:434–440. doi: 10.1152/jappl.1988.65.1.434. [DOI] [PubMed] [Google Scholar]
- 51.Flaim SF, Minteer WJ, Clark DP, Zelis R. J Appl Physiol. 1979;46:302–308. doi: 10.1152/jappl.1979.46.2.302. [DOI] [PubMed] [Google Scholar]
- 52.Musch TI, Terrell JA. Am J Physiol. 1992;262:H411–H419. doi: 10.1152/ajpheart.1992.262.2.H411. [DOI] [PubMed] [Google Scholar]
- 53.Rowell LB, Saltin B, Kiens B, Christensen NJ. Am J Physiol. 1986;251:H1038–H1044. doi: 10.1152/ajpheart.1986.251.5.H1038. [DOI] [PubMed] [Google Scholar]
- 54.Schaefer A, Piquard F, Geny B, Doutreleau S, Lampert E, Mettauer B, Lonsdorfer J. Int J Sports Med. 2002;23:403–407. doi: 10.1055/s-2002-33743. [DOI] [PubMed] [Google Scholar]
- 55.Paterson ND, Kowalchuk JM, Paterson DH. J Appl Physiol. 2005;99:683–690. doi: 10.1152/japplphysiol.00707.2004. [DOI] [PubMed] [Google Scholar]
- 56.Roca J, Agusti AG, Alonso A, Poole DC, Viegas C, Barbera JA, Rodriguez-Roisin R, Ferrer A, Wagner PD. J Appl Physiol. 1992;73:1067–1076. doi: 10.1152/jappl.1992.73.3.1067. [DOI] [PubMed] [Google Scholar]
- 57.Hardin DS, Azzarelli B, Edwards J, Wigglesworth J, Maianu L, Brechtel G, Johnson A, Baron A, Garvey WT. J Clin Endocrinol Metab. 1995;80:2437–2446. doi: 10.1210/jcem.80.8.7629239. [DOI] [PubMed] [Google Scholar]
- 58.McConell GK, Huynh NN, Lee-Young RS, Canny BJ, Wadley GD. Am J Physiol. 2005;290:E60–E66. doi: 10.1152/ajpendo.00263.2005. [DOI] [PubMed] [Google Scholar]
- 59.Dhawan V, Handu SS, Nain CK, Ganguly NK. Mol Cell Biochem. 2005;269:1–11. doi: 10.1007/s11010-005-1810-4. [DOI] [PubMed] [Google Scholar]
- 60.Nishiyama K, Nishiyama A, Frohlich ED. Am J Physiol. 1976;230:691–698. doi: 10.1152/ajplegacy.1976.230.3.691. [DOI] [PubMed] [Google Scholar]
- 61.Kobayashi N, Kobayashi K, Kono K, Akabane T, Kaneko H, Takada M, Tsuchiya N, Yagi S. Jpn Heart J. 1994;35:467–475. doi: 10.1536/ihj.35.467. [DOI] [PubMed] [Google Scholar]