Abstract
Stress in early life has been associated with insufficient glucocorticoid signaling in adulthood, possibly affecting inflammation processes. Childhood maltreatment has been linked to increased risk of adult disease with potential inflammatory origin. However, the impact of early life stress on adult inflammation is not known in humans. We tested the life-course association between childhood maltreatment and adult inflammation in a birth cohort followed to age 32 years as part of the Dunedin Multidisciplinary Health and Development Study. Regression models were used to estimate the effect of maltreatment on inflammation, adjusting for co-occurring risk factors and potential mediating variables. Maltreated children showed a significant and graded increase in the risk for clinically relevant C-reactive protein levels 20 years later, in adulthood [risk ratio (RR) = 1.80, 95% confidence interval (CI) = 1.26–2.58]. The effect of childhood maltreatment on adult inflammation was independent of the influence of co-occurring early life risks (RR = 1.58, 95% CI = 1.08–2.31), stress in adulthood (RR = 1.64, 95% CI = 1.12–2.39), and adult health and health behavior (RR = 1.76, 95% CI = 1.23–2.51). More than 10% of cases of low-grade inflammation in the population, as indexed by high C-reactive protein, may be attributable to childhood maltreatment. The association between maltreatment and adult inflammation also generalizes to fibrinogen and white blood cell count. Childhood maltreatment is a previously undescribed, independent, and preventable risk factor for inflammation in adulthood. Inflammation may be an important developmental mediator linking adverse experiences in early life to poor adult health.
Keywords: C-reactive protein, development, epidemiology, risk factor, stress
Inflammation is an integral part of the stress response (1–3). In the context of the “fight or flight” reaction, acute psychosocial stress can induce activation of the transcription nuclear factor κB and secretion of proinflammatory cytokines, presumably by adrenergic stimulation (4, 5). These are chief stimulators of acute-phase proteins, such as C-reactive protein, which promote resistance to infection and repair of damaged tissues. Through the production of proinflammatory cytokines, immune activation progressively stimulates the secretion of glucocorticoids, and glucocorticoid signaling, in turn, terminates the inflammatory response once the threat fades (1).
Early life adverse experiences may disrupt the potentially adaptive response to stress. Animal models suggest that maternal care exerts a critical influence on the development of the stress response (6) and may alter the long-term predisposition to inflammation (7). In humans, adults who reported experiences of childhood maltreatment showed a reduced ability of glucocorticoid signaling to control the hypothalamic-pituitary-adrenal axis response to a psychosocial stress test (8). Given the inhibitory influence of glucocorticoids on inflammation, this finding suggests that maltreated children also might show increased levels of inflammation in adulthood.
Also, adults who were abused as children have been shown to be at increased risk of disease with potential inflammatory origin (9, 10). However, the impact of early life stress on inflammation has not been investigated in humans to date. The persistent activation of inflammatory pathways could be one of the mechanisms through which early life adverse experiences alter long-term health outcomes.
Our first aim was to test the hypothesis that maltreated children are characterized by an increased risk of clinically relevant high sensitivity C-reactive protein (hsCRP) levels in adulthood. We chose hsCRP because it is thought to be one of the most reliable indicators of inflammation and recently has been endorsed as an adjunct to traditional risk factor screening for cardiovascular disease by the Centers for Disease Control and Prevention and the American Heart Association (11).
Our second aim was to test whether childhood maltreatment was an independent risk factor for adult inflammation. We tested three alternative explanations for this life-course association. According to the “co-occurring risk hypothesis,” maltreated children may experience other early life risks (12) that could be responsible for inflammation in adulthood. We measured three potential early life risks, low birth weight (13), socioeconomic disadvantage (14), and low intelligence quotient (IQ) (15), and tested whether maltreatment still predicted adult inflammation after controlling for co-occurring risks. According to the “adult stress hypothesis,” maltreated children grow up to be exposed to more stress (12). We measured three indicators of stress that have been linked to inflammation, low status attainment (16), depression (17), and perceived stress (18), and tested whether childhood maltreatment is related to adult inflammation because maltreated children experience more stressful lives when they grow up. According to the “health-behavior hypothesis,” maltreated children may engage in more health-damaging behaviors and show poorer health in adulthood, factors that have been independently associated with inflammation (19, 20). We measured indicators of the metabolic syndrome, smoking, physical activity, and diet, and tested whether the association between childhood maltreatment and adult inflammation is accounted for by these factors. If childhood maltreatment is an independent risk factor, its association with adult inflammation should still be significant after controlling for these confounding effects.
Our third aim was to extend this inquiry to fibrinogen and white blood cells (WBCs), two other common markers of inflammation that also show significant association with cardiovascular disease (21). We tested whether the association between childhood maltreatment and adult inflammation was specific to hsCRP or more generally predicted the clustering of inflammation factors in the same individuals.
Results
Table 1 shows the biomarkers of adult inflammation as well as the risk factors associated with childhood maltreatment. The regression analysis in Table 2 (baseline model) shows that children in the definite maltreatment group were 1.80 [95% confidence interval (CI) = 1.26–2.58] times and children in the probable maltreatment group were 1.18 (95% CI = 0.87–1.60) times more likely to have elevated hsCRP in adulthood compared with nonmaltreated children. Analyses restricted to the sample of participants free from drugs with substantial effect on hsCRP, namely statins and estrogens, yielded overlapping results: Children in the definite maltreatment group were 1.86 (95% CI = 1.23–2.81) times more likely to have elevated hsCRP in adulthood compared with nonmaltreated children. We also sought to ensure that the association between maltreatment and inflammation was not simply a function of individuals having extreme hsCRP values. We excluded all individuals with hsCRP >10 mg/liter (11) and observed that children with a history of maltreatment were still 1.59 (95% CI = 1.02–2.50) times more likely to have elevated hsCRP in adulthood compared with nonmaltreated children.
Table 1.
The association of childhood maltreatment with biomarkers and risk factors
| Risk factor | Level | Maltreatment, % (n)* |
P value† | ||
|---|---|---|---|---|---|
| No |
Probable |
Definite |
|||
| 64 (551) | 27 (232) | 9 (83) | |||
| Adult inflammation: | |||||
| hsCRP (>3 mg/liter), % (n) | 18 (99) | 21.3 (49) | 32.5 (27) | 0.011 | |
| hsCRP, (log) mean (SE) | 0.16 (0.047) | 0.24 (0.074) | 0.51 (0.148) | 0.028 | |
| Fibrinogen, mean (SE) | 2.55 (0.025) | 2.61 (0.036) | 2.72 (0.068) | 0.035 | |
| WBC, mean (SE) | 7.39 (0.071) | 7.93 (0.124) | 8.08 (0.221) | <0.0001 | |
| Co-occurring early life risks: | |||||
| Low birth weight, % (n) | 5.1 (28) | 3.0 (7) | 7.2 (6) | 0.222 | |
| Child SES, % (n) | Low | 14.7 (81) | 25.3 (58) | 37.8 (31) | |
| Medium | 67.8 (373) | 60.3 (138) | 48.8 (40) | ||
| High | 17.5 (96) | 14.4 (33) | 13.4 (11) | <0.0001 | |
| Low child IQ, % (n) | 8.8 (47) | 20.0 (46) | 22.0 (18) | <0.0001 | |
| Adult stress indicators: | |||||
| SES, % (n) | Low | 26.7 (147) | 37.1 (86) | 41.0 (34) | |
| Medium | 35.7 (196) | 32.3 (75) | 32.5 (27) | ||
| High | 37.6 (207) | 30.6 (71) | 26.5 (22) | 0.01 | |
| Major depression, % (n) | 12.7 (70) | 16.8 (39) | 32.5 (27) | <0.0001 | |
| High perceived stress, % (n) | 23.7 (129) | 29.9 (69) | 42.2 (35) | 0.001 | |
| Adult health and health behavior: | |||||
| Cardiovascular risk cluster, % (n) | 14.2 (78) | 22.1 (51) | 18.1 (15) | 0.024 | |
| Smoking, % (n) | Nonsmoker | 63.6 (350) | 53.0 (123) | 36.1 (30) | |
| Up to 10 per day | 17.3 (95) | 19.4 (45) | 18.1 (15) | ||
| 11 to 20 per day | 15.6 (86) | 22.4 (52) | 30.1 (25) | ||
| >20 per day | 3.5 (19) | 5.2 (12) | 15.7 (13) | <0.0001 | |
| Physical activity, % (n) | Light | 26.0 (142) | 23.4 (54) | 21.7 (18) | |
| Moderate | 26.0 (142) | 24.2 (56) | 21.7 (18) | ||
| Hard | 25.1 (137) | 26.0 (60) | 25.3 (21) | ||
| Very hard | 23.0 (126) | 26.4 (61) | 31.3 (26) | 0.712 | |
| Diet (fruit, vegetable intake), % (n) | Very low | 25.8 (142) | 24.2 (56) | 30.1 (25) | |
| Low | 33.8 (186) | 40.3 (93) | 32.5 (27) | ||
| High | 20.6 (113) | 22.1 (51) | 16.9 (14) | ||
| Very high | 19.8 (109) | 13.4 (31) | 20.5 (17) | 0.252 | |
| Others: | |||||
| Male sex, % (n) | 53.5 (295) | 53.9 (125) | 44.6 (37) | 0.295 | |
| Use of antiinflammatory medication, % (n) | 32.8 (179) | 27.7 (64) | 19.8 (16) | 0.035 | |
*Study members analyzed here have the same prevalence of maltreatment as the original birth cohort (no maltreatment, 64%; probable maltreatment, 27%; definite maltreatment, 9%).
†Association with categorical variables has been estimated with the Fisher's exact test, whereas association with continuous variables has been estimated with one-way ANOVA. Some of the cell sizes vary slightly because of missing cases.
Table 2.
The relative risk and 95% CIs from the Cox regression models with robust variance predicting high hsCRP levels (hsCRP > 3 mg/dl)
| Risk factor | Level | Baseline | Model 1 | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|---|---|
| Childhood maltreatment: | No | 1 | 1 | 1 | 1 | 1 |
| Probable | 1.18 (0.87–1.60) | 1.11 (0.81–1.52) | 1.20 (0.89–1.63) | 1.16 (0.86–1.56) | 1.07 (0.78–1.46) | |
| Definite | 1.80 (1.26–2.58) | 1.58 (1.08–2.31) | 1.64 (1.12–2.39) | 1.76 (1.23–2.51) | 1.61 (1.12–2.32) | |
| Co-occurring early life risks: | ||||||
| Low birth weight | 1.61 (1.00–2.57) | 1.34 (0.85–2.12) | — | — | 1.28 (0.80–2.04) | |
| Child SES | Low | 1.96 (1.19–3.25) | 1.77 (1.05–2.97) | — | — | 1.47 (0.88–2.45) |
| Medium | 1.59 (1.00–2.52) | 1.60 (1.00–2.55) | 1.51 (0.95–2.40) | |||
| High | 1 | 1 | 1 | |||
| Low child IQ | 1.44 (1.03–2.01) | 1.27 (0.91–1.78) | — | — | 1.07 (0.73–1.57) | |
| Adult stress indicators: | ||||||
| SES | Low | 1.38 (0.99–1.92) | — | 1.23 (0.87–1.74) | — | 1.16 (0.80–1.70) |
| Medium | 1.19 (0.85–1.67) | 1.14 (0.81–1.60) | 1.08 (0.78–1.51) | |||
| High | 1 | 1 | 1 | |||
| Major depression | 1.45 (1.06–1.99) | — | 1.17 (0.83–1.64) | — | 1.17 (0.83–1.64) | |
| High perceived stress | 1.45 (1.10–1.91) | — | 1.22 (0.90–1.66) | — | 1.17 (0.86–1.58) | |
| Adult health and health behavior: | ||||||
| CV risk cluster | 2.39 (1.84–3.10) | — | — | 2.50 (1.91–3.27) | 2.34 (1.77–3.08) | |
| Smoking | Nonsmoker | 1 | — | — | 1 | 1 |
| Up to 10 per day | 0.88 (0.61–1.28) | 0.93 (0.64–1.35) | 0.92 (0.64–1.34) | |||
| 11 to 20 per day | 0.85 (0.59–1.23) | 0.76 (0.52–1.10) | 0.74 (0.50–1.09) | |||
| >20 per day | 1.18 (0.69–2.03) | 1.14 (0.64–2.04) | 1.06 (0.58–1.93) | |||
| Physical activity | Light | 1.57 (1.05–2.34) | — | — | 1.25 (0.83–1.88) | 1.32 (0.87–1.99) |
| Moderate | 1.47 (0.98–2.21) | 1.16 (0.78–1.73) | 1.27 (0.84–1.92) | |||
| Hard | 1.30 (0.86–1.97) | 1.10 (0.73–1.66) | 1.13 (0.75–1.72) | |||
| Very hard | 1 | 1 | 1 | |||
| Diet (fruit, vegetable) intake | Very low | 1.01 (0.68–1.48) | — | — | 1.09 (0.74–1.61) | 1.03 (0.68–1.55) |
| Low | 0.78 (0.53–1.16) | 0.82 (0.56–1.19) | 0.81 (0.55–1.19) | |||
| High | 1.01 (0.67–1.52) | 0.92 (0.63–1.36) | 0.95 (0.64–1.42) | |||
| Very high | 1 | 1 | 1 | |||
| Others: | ||||||
| Male sex | 0.50 (0.38–0.66) | 0.54 (0.41–0.72) | 0.54 (0.41–0.72) | 0.50 (0.38–0.68) | 0.56 (0.41–0.76) | |
| Use of antiinflammatory medication | 1.33 (1.01–1.75) | 1.34 (1.02–1.76) | 1.37 (1.03–1.81) | 1.35 (1.03–1.77) | 1.37 (1.03–1.82) |
The baseline model shows the bivariate analysis of the association between putative risk factors and high hsCRP. Model 1 indexes the co-occurring risk hypothesis, showing the RR of high hsCRP according to maltreatment experiences adjusted for low birth weight, childhood SES, and low childhood IQ. Model 2 indexes the adult stress hypothesis, showing the RR of high hsCRP according to maltreatment experiences adjusted for adult SES, major depression, and high perceived stress. Model 3 indexes the health-behavior hypothesis, showing the RR of high hsCRP according to maltreatment experiences adjusted for cardiovascular risk cluster, smoking, physical activity, and diet. Model 4 shows the RR of high hsCRP according to maltreatment experiences adjusted for all child and adult risk factors. CI values are shown in parentheses.
Consistent with the co-occurring risk hypothesis, maltreated children were significantly more likely than nonmaltreated children to experience co-occurring early life risks (Table 1). Although early life risks were independently associated with adult inflammation (Table 2, baseline model), even after controlling for these risks the association between maltreatment and elevated adult hsCRP remained significant, risk ratio (RR) = 1.58 (95% CI = 1.08–2.31) (Table 2, model 1).
Consistent with the adult stress hypothesis, maltreated children also were significantly more likely than nonmaltreated children to experience adult stress (Table 1). However, even after controlling for these indicators of stress, the association between childhood maltreatment and elevated adult hsCRP remained significant, RR = 1.64 (95% CI = 1.12–2.39) (Table 2, model 2).
Finally, consistent with the health-behavior hypothesis, maltreated children were more likely than nonmaltreated children to be in poor health and to engage in health-damaging behaviors (Table 1). After controlling for these factors, the association between childhood maltreatment and elevated adult hsCRP still remained significant, RR = 1.76 (95% CI = 1.23–2.51) (Table 2, model 3).
Even after controlling for all co-occurring childhood and adult risk factors simultaneously, the association between childhood maltreatment and elevated adult hsCRP remained significant, RR = 1.61 (95% CI = 1.12–2.32) (Table 2, model 4).
Under the assumptions of causality and independence, we estimated that 11.2% of the cases of high hsCRP in the general population were attributable to childhood maltreatment.
The significant dose–response association between childhood maltreatment and inflammation generalized to continuous measures of (logged) hsCRP, fibrinogen, WBCs, and the composite factor score of inflammation [Table 1, Fig. 1, and supporting information (SI) Tables 3–5].
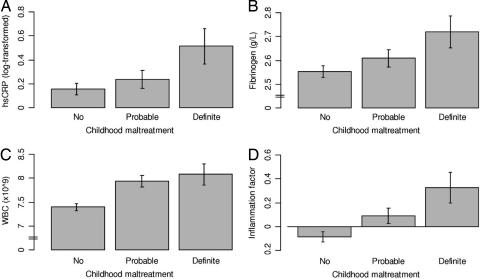
Fig. 1.
The association of childhood maltreatment with biomarkers of inflammation. (A) Mean (and SE) of logged hsCRP according to maltreatment experiences. In an ordinary least-squares regression model adjusted for sex and antiinflammatory drug use, definite maltreatment (b = 0.35, SE = 0.13, t = 2.58; P = 0.01), but not probable maltreatment (b = 0.09, SE = 0.09, t = 1.07; P = 0.29), predicted an increase in logged hsCRP levels. (B) Mean (and SE) of fibrinogen according to maltreatment experiences. Definite maltreatment (b = 0.15, SE = 0.07, t = 2.20; P = 0.03), but not probable maltreatment (b = 0.06, SE = 0.04, t = 1.39; P = 0.17), predicted an increase in fibrinogen levels. (C) Mean (and SE) of WBC count according to maltreatment experiences. Both probable maltreatment (b = 0.55, SE = 0.14, t = 3.95; P < 0.001) and definite maltreatment (b = 0.67, SE = 0.21, t = 3.18; P = 0.002) predicted an increase in WBC count. (D) Mean (and SE) of a factor score for inflammation according to maltreatment experiences. The inflammation factor has a standardized mean of 0 and SD of 1. Both probable maltreatment (b = 0.19, SE = 0.08, t = 2.44; P = 0.02) and definite maltreatment (b = 0.39, SE = 0.12, t = 3.36; P = 0.001) predicted an increase in the factor score for inflammation.
Conclusion
This longitudinal-prospective study links exposure to childhood maltreatment in the first decade of life to specific, clinically significant biomarkers of inflammation in adulthood. In light of the role of inflammation in the pathophysiology of cardiovascular disease, diabetes, and chronic lung disease (22–24), and of the increased risk for such disease in adults maltreated as children (9, 10), we suggest that inflammation may be an important mechanism mediating the adverse effect of early life stress on adult health.
Current evidence suggests the possibility of stress response programming by early life adverse experiences. Experimental research suggests that early life stress can induce a persistent condition of insufficient glucocorticoid signaling (6, 8). In animals, epigenetic programming seems to be responsible for the effects of early life stress on later stress reactivity, by inducing persistent, although reversible, reduction in glucocorticoid receptor gene expression (6). Insufficient glucocorticoid signaling, in turn, might lead to an unrestrained inflammatory state in adults maltreated as children, hampering the extinction of otherwise adaptive responses to stress (1, 2). Similarly, early life stressful experiences might reduce inhibitory cholinergic neurotransmission (25), favoring inflammation persistence (26).
The experimental design needed to directly test causation in humans, namely randomly assigning children to maltreatment, is not ethical. Findings from this longitudinal child-to-adult study therefore provide crucial data and appear to meet several criteria suggestive of a causal association between childhood maltreatment and adult inflammation (27). First, maltreatment preceded the outcome. Second, there was evidence of a dose–response relation between severity of maltreatment and inflammation. Third, the association between maltreatment and inflammation appeared to be independent of a wide range of correlated risk factors, including (i) other early life risk factors for poor adult health, (ii) stress in adulthood, and (iii) adult health factors and health-damaging behaviors. Fourth, the evidence was biologically plausible and consistent with emerging experimental data suggesting that maltreatment may have tangible neurobiological effects, ultimately influencing glucocorticoid signaling (8). Nevertheless, it is possible that residual confounding may account for the long-term association reported here.
If causal, the effect of childhood maltreatment on adult inflammation may have significant public-health implications. First, childhood maltreatment is a novel target for the prevention of inflammation and inflammation-related disease, with the potential to reduce by > 10% the cases of low-grade inflammation in the general population. Second, childhood maltreatment is a preventable and potentially treatable childhood risk factor for poor adult health. Although, for example, improvements in socioeconomic condition and in IQ remain a largely unmet target, effective preventive strategies exist for maltreatment (28). Therapeutic interventions including psychological and pharmacological treatment also might reverse the long-lasting effects of maltreatment (29, 30). Third, childhood maltreatment is a pleiotropic risk factor influencing multiple pathophysiological pathways and outcomes [e.g., psychiatric disorders (31), health behaviors (19), and crime (12)]. The eradication of maltreatment therefore is likely to relieve a public-health burden far bigger than the one related to inflammation alone.
These new findings should be evaluated alongside several limitations. Because we studied a cohort of children born in the early 1970s, we are not yet able to assess clinical endpoints, such as cardiovascular disease, because the study members still are too young. Instead, we focused on intermediate risk factors, such as high hsCRP, that are known to predict future disease in midlife and old age (22–24). Moreover, findings from this New Zealand cohort require replication in other parts of the world. However, given the consistency of our findings regarding other correlates of adult inflammation [e.g., low birth weight (13), low childhood socioeconomic status (SES) (14), and depression (17)] with evidence reported from different population-based studies worldwide, there is reason to believe that our results may be replicated in other settings. Similarly, we do not know whether these findings generalize to all ethnic groups.
Life-course epidemiology adds a temporal perspective to the conventional understanding of health and disease. Findings from the present study, along with evidence emerging from other life-course investigations, suggest that health is not a state but a lifetime achievement. A better understanding of developmental processes and of the mechanisms by which psychosocial experiences “get under the skin” and leave enduring health signatures is needed to unravel complex disease pathophysiology (32, 33).
Methods
Sample.
Participants are members of the Dunedin Multidisciplinary Health and Development Study, a longitudinal investigation of health and behavior in a complete birth cohort. Study members (n = 1,037; 91% of eligible births; 52% male) were born between April 1972 and March 1973 in Dunedin, New Zealand, and participated in the first follow-up assessment at age 3. The cohort represents the full range of SES in the general population of New Zealand's South Island and is primarily white. Assessments have been carried out at ages 3, 5, 7, 9, 11, 13, 15, 18, 21, 26, and 32. Data are collected at the Study Research Unit during a full day of individual data collection.
This investigation is based on participants who completed the age 32 assessment (n = 972; 96% of the 1,015 study members still alive in 2004–2005).
Study protocol was approved by the institutional review boards of the participating universities. Study members gave informed consent before participating.
Measure of Childhood Maltreatment.
As described in ref. 31, evidence of childhood maltreatment during the first decade of life (ages 3–11) was ascertained by using behavioral observations, parental reports, and retrospective reports by study members once they reached adulthood. First, exposure to maternal rejection (reported for 14% of study participants) was assessed at age 3 by observational ratings of mothers' interaction with the study children. Second, exposure to harsh discipline was assessed at ages 7 and 9 according to parental reports of disciplinary behaviors. Parents scoring in the top decile of the samplewide distribution were classified as unusually harsh. Third, exposure to disruptive caregiver changes was assessed through age 11 and defined by two or more changes of the child's primary caregiver (6% of participants). Fourth, exposure to physical abuse (4% of participants) was assessed retrospectively at age 26 on the basis of reports of multiple episodes of severe physical punishment resulting in lasting bruising or injury through age 11. Fifth, exposure to sexual abuse (12% of participants) was assessed retrospectively at age 26 on the basis of reports of unwanted sexual contact before age 11. Given that the effect of stress often appears to be cumulative (2, 9, 19), we derived a cumulative exposure index for each child by counting the number of maltreatment experiences during the first decade of life (31); 64% of children experienced no maltreatment, 27% experienced one indicator of maltreatment (hereafter “probable maltreatment”), and 9% experienced two or more indicators of maltreatment (“definite maltreatment”).
Measures of Adult Inflammation.
Physical examinations and venepunctures (always between 4:15 and 4:45 p.m.) were conducted at age 32: 92% of the participants (n = 892) provided blood samples. Pregnant women (n = 26) were excluded from the reported analyses.
hsCRP (mg/liter) was measured on a Hitachi 917 analyzer (Roche Diagnostics, Mannheim, Germany) by using a particle-enhanced immunoturbidimetric assay. We adopted the Centers for Disease Control and Prevention/American Heart Association definition of high cardiovascular risk (hsCRP > 3 mg/liter) to identify our risk group (11).
Fibrinogen (g/liter) was measured on a Sysmex (Malberg, Germany) CA-1500 by using a fully automated cap piercing coagulation analyzer.
WBC (109/liter) were measured on a Sysmex (Kobe, Japan) XE2100 automated hematology analyzer via flow-cytometry with a semiconductor laser.
Co-Occurring Risk Factors and Potential Mediating Variables.
Low birth weight.
Children's birth weight was obtained from hospital records. Children with weight <2,500 g were classified as having low birth weight.
Low childhood SES.
The SES of study members' families was measured repeatedly from birth through age 15 with a six-point scale assessing parents' occupational status. The scale places each occupation into one of six categories based on educational levels and income associated with that occupation in data from the New Zealand census (34). The variable used in our analyses is the average of the highest SES level of either parent and reflects the socioeconomic conditions experienced by the study members while they grew up.
Low childhood IQ.
The Wechsler Intelligence Scale for Children was administered by trained psychometrists at ages 7, 9, and 11 (35). We averaged scores from the three age periods, standardized the IQ score, and defined children with IQ < 85 (more than one SD below the mean) as having low IQ.
Low SES in adulthood.
At age 32, study members were asked about their current or most recent occupation; homemakers and those not working were pro-rated based on their educational status according to criteria included in the New Zealand Socio-economic Index (36). Information was coded to a six-point scale for occupations in New Zealand.
Major depression.
At age 32, study members were interviewed by using the Diagnostic Interview Schedule (37) with a reporting period of 12 months. Depression was diagnosed by using the Diagnostic and Statistical Manual of Mental Disorders (38).
Perceived stress.
The Perceived Stress Scale (PSS) (39) administered at age 32 is a subjective measure quantifying the degree of unpredictability, uncontrollability, and overload in respondents' lives. Study members in the top quartile of the PSS distribution were considered as having high perceived stress.
Cardiovascular risk cluster.
As described in ref. 40, health risk-factor clustering was assessed by measuring six biomarkers: (i) overweight, (ii) high blood pressure, (iii) high total cholesterol, (iv) low high-density cholesterol, (v) high glycated hemoglobin, and (vi) low VO2 max adjusted for body weight. We assessed multiple risk-factor clustering by summing the number of biomarkers on which the study member was at-risk. Study members were “clustered” if they had at least three risk factors.
Smoking.
We divided study members at age 32 into nonsmokers, light smokers (up to 10 cigarettes per day), moderate smokers (11–20), and heavy smokers (>20).
Physical activity.
At age 32, study members were interviewed about the amount and type of physical activity in the last week and about personal effort involved in carrying out a specific activity. According to guidelines proposed by Ainsworth et al. (41), interviewers rated physical activity as light, moderate, hard, and very hard. The total metabolic equivalent (MET) score for the week was calculated as the weighted sum of the time spent in each activity. We divided the MET score into quartiles for analysis.
Diet.
On the basis of evidence linking Mediterranean-style diet with a reduced risk of inflammation (42), we divided study members according to their daily intake of fruit and vegetables. We divided the sum of fruit and vegetable consumption into quartiles for analysis.
Antiinflammatory drug use.
At age 32, study members were assessed for their use of medications with antiinflammatory effect, including systemic steroids, respiratory steroids, nonsteroidal antiinflammatory drugs, prophylactic aspirin, antigout medications, antirheumatic medications, statins, and estrogens.
Statistical Analysis.
Cox regression models with constant time of follow-up and robust variance (43) were used to estimate the effect of childhood maltreatment on the categorical outcome of high hsCRP at age 32. We present RRs for elevated hsCRP in children classified in the definite and probable maltreatment groups, compared with nonmaltreated children. The regression model was expanded to test alternative explanations for the association between childhood maltreatment and adult inflammation. Results were adjusted for sex and antiinflammatory drug use.
The contribution of childhood maltreatment to the overall burden of inflammation at age 32 years was estimated by calculating the population attributable fraction.
For sensitivity analysis, we estimated the effect of childhood maltreatment on age 32 continuous measures of (logged) hsCRP, fibrinogen, and WBC. Intercorrelations among these three measures ranged from 0.20 to 0.63. A principal-component analysis yielded one factor accounting for 59% of the variance, showing that all three measures index inflammation risk.
Supplementary Material
Acknowledgments
We thank the Dunedin Study members and study founder Dr. Phil Silva. The Dunedin Multidisciplinary Health and Development Research Unit is supported by the New Zealand Health Research Council. This research received support from Medical Research Council Grant G0100527, National Institute of Mental Health Grants MH45070 and MH49414, and the William T. Grant Foundation. A.D. is a Wellcome Trust Research Training Fellow. C.M.P. is a Medical Research Council Research Fellow. A.C. is a Wolfson Merit Award holder.
Abbreviations
- CI
confidence interval
- hsCRP
high sensitivity C-reactive protein
- IQ
intelligence quotient
- RR
risk ratio
- SES
socioeconomic status.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0610362104/DC1.
References
- 1.Chrousos GP. N Engl J Med. 1995;332:1351–1362. doi: 10.1056/NEJM199505183322008. [DOI] [PubMed] [Google Scholar]
- 2.McEwen BS. N Engl J Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 3.Glaser R, Kiecolt-Glaser JK. Nat Rev Immunol. 2005;5:243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- 4.Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D, Ferstl R, von Eynatten M, Wendt T, Rudofsky G, et al. Proc Natl Acad Sci USA. 2003;100:1920–1925. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maes M, Song C, Lin A, De JR, Van GA, Kenis G, Bosmans E, De MI, Benoy I, Neels H, et al. Cytokine. 1998;10:313–318. doi: 10.1006/cyto.1997.0290. [DOI] [PubMed] [Google Scholar]
- 6.Meaney MJ, Szyf M. Trends Neurosci. 2005;28:456–463. doi: 10.1016/j.tins.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Shanks N, Lightman SL. J Clin Invest. 2001;108:1567–1573. doi: 10.1172/JCI14592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, Miller AH, Nemeroff CB. J Am Med Assoc. 2000;284:592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- 9.Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS. Am J Prev Med. 1998;14:245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 10.Dong M, Giles WH, Felitti VJ, Dube SR, Williams JE, Chapman DP, Anda RF. Circulation. 2004;110:1761–1766. doi: 10.1161/01.CIR.0000143074.54995.7F. [DOI] [PubMed] [Google Scholar]
- 11.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, III, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, et al. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 12.Widom CS. Science. 1989;244:160–166. doi: 10.1126/science.2704995. [DOI] [PubMed] [Google Scholar]
- 13.Sattar N, McConnachie A, O'Reilly D, Upton MN, Greer IA, Davey SG, Watt G. Arterioscler Thromb Vasc Biol. 2004;24:583–587. doi: 10.1161/01.ATV.0000118277.41584.63. [DOI] [PubMed] [Google Scholar]
- 14.Taylor SE, Lehman BJ, Kiefe CI, Seeman TE. Biol Psychiatry. 2006;60:819–824. doi: 10.1016/j.biopsych.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 15.Whalley LJ, Deary IJ. BMJ. 2001;322:819. doi: 10.1136/bmj.322.7290.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steptoe A, Owen N, Kunz-Ebrecht S, Mohamed-Ali V. Brain Behav Immun. 2002;16:774–784. doi: 10.1016/s0889-1591(02)00030-2. [DOI] [PubMed] [Google Scholar]
- 17.Ford DE, Erlinger TP. Arch Intern Med. 2004;164:1010–1014. doi: 10.1001/archinte.164.9.1010. [DOI] [PubMed] [Google Scholar]
- 18.Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Proc Natl Acad Sci USA. 2003;100:9090–9095. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anda RF, Croft JB, Felitti VJ, Nordenberg D, Giles WH, Williamson DF, Giovino GA. J Am Med Assoc. 1999;282:1652–1658. doi: 10.1001/jama.282.17.1652. [DOI] [PubMed] [Google Scholar]
- 20.Bazzano LA, He J, Muntner P, Vupputuri S, Whelton PK. Ann Intern Med. 2003;138:891–897. doi: 10.7326/0003-4819-138-11-200306030-00010. [DOI] [PubMed] [Google Scholar]
- 21.Danesh J, Collins R, Appleby P, Peto R. J Am Med Assoc. 1998;279:1477–1482. doi: 10.1001/jama.279.18.1477. [DOI] [PubMed] [Google Scholar]
- 22.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. N Engl J Med. 1997;336:973–979. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 23.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. J Am Med Assoc. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 24.Sin DD, Man SF. Circulation. 2003;107:1514–1519. doi: 10.1161/01.cir.0000056767.69054.b3. [DOI] [PubMed] [Google Scholar]
- 25.Kaufer D, Friedman A, Seidman S, Soreq H. Nature. 1998;393:373–377. doi: 10.1038/30741. [DOI] [PubMed] [Google Scholar]
- 26.Tracey KJ. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 27.Grimes DA, Schulz KF. Lancet. 2002;359:248–252. doi: 10.1016/S0140-6736(02)07451-2. [DOI] [PubMed] [Google Scholar]
- 28.Olds DL, Eckenrode J, Henderson CR, Jr, Kitzman H, Powers J, Cole R, Sidora K, Morris P, Pettitt LM, Luckey D. J Am Med Assoc. 1997;278:637–643. [PubMed] [Google Scholar]
- 29.Nemeroff CB, Heim CM, Thase ME, Klein DN, Rush AJ, Schatzberg AF, Ninan PT, McCullough JP, Jr, Weiss PM, Dunner DL, et al. Proc Natl Acad Sci USA. 2003;100:14293–14296. doi: 10.1073/pnas.2336126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pariante CM, Thomas SA, Lovestone S, Makoff A, Kerwin RW. Psychoneuroendocrinology. 2004;29:423–447. doi: 10.1016/j.psyneuen.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 31.Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, Poulton R. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- 32.Hertzman C. Ann NY Acad Sci. 1999;896:85–95. doi: 10.1111/j.1749-6632.1999.tb08107.x. [DOI] [PubMed] [Google Scholar]
- 33.Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. Proc Natl Acad Sci USA. 2004;101:17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poulton R, Caspi A, Milne BJ, Thomson WM, Taylor A, Sears MR, Moffitt TE. Lancet. 2002;360:1640–1645. doi: 10.1016/S0140-6736(02)11602-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wechsler D. Wechsler Intelligence Scale for Children-Revised. New York: Psychological Corp; 1974. [Google Scholar]
- 36.Davis P, Jenkin G, Coope P. New Zealand Socio-Economic Index 1996. An Update and Revision of the New Zealand Socio-Economic Index of Occupational Status. Wellington, New Zealand: Statistics New Zealand; 2003. [DOI] [PubMed] [Google Scholar]
- 37.Robins LN, Cottler L, Bucholz KK, Compton W. The Diagnostic Interview Schedule for DSM-IV. St. Louis, MO: Washington University; 1995. [Google Scholar]
- 38.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Ed. Washington, DC: Am Psychiatr Assoc; 1994. [Google Scholar]
- 39.Cohen S, Kamarck T, Mermelstein R. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 40.Caspi A, Harrington H, Moffitt TE, Milne BJ, Poulton R. Arch Pediatr Adolesc Med. 2006;160:805–811. doi: 10.1001/archpedi.160.8.805. [DOI] [PubMed] [Google Scholar]
- 41.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O'Brien WL, Bassett DR, Jr, Schmitz KH, Emplaincourt PO, et al. Med Sci Sports Exerc. 2000;32:S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 42.Esposito K, Marfella R, Ciotola M, Di PC, Giugliano F, Giugliano G, D'Armiento M, D'Andrea F, Giugliano D. J Am Med Assoc. 2004;292:1440–1446. doi: 10.1001/jama.292.12.1440. [DOI] [PubMed] [Google Scholar]
- 43.Barros AJ, Hirakata VN. BMC Med Res Methodol. 2003;3:21. doi: 10.1186/1471-2288-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.