Abstract
Dynamic microarrays hold great promise for advancing research in proteomics, diagnostics and drug discovery. However, this potential has yet to be fully realized due to the lack of reliable multifunctional platforms to transport and immobilize particles, infuse reagents, observe the reaction, and retrieve selected particles. We achieved all these functions in a single integrated device through the combination of hydrodynamic and optical approaches. Hydrodynamic forces allow simultaneous transportation and immobilization of large number of particles, whereas optical-based microbubble technique for bead retrieval gives dexterity in handling individual particles without complicated circuitry. Based on the criterion derived in this paper, the device was designed, and fabricated using standard photolithography and soft lithography methods. We examined the dynamics of bubble formation and dissipation in the device, and parametric studies revealed that higher power settings at short intervals were more efficient than low power settings at longer intervals for bead retrieval. We also demonstrated the capabilities of our device and its potential as a tool for screening methods such as the “one-bead-one-compound” (OBOC) combinatorial library method. Although both approaches, hydrodynamic confinement and optical-based microbubbles, are presented in one device, they can also be separately used for other applications in microchip devices.
Keywords: high throughput screening, lab-on-a-chip, MEMS, bead-based assay, microbubble
Microarray applications are extensive, and have been successfully used in basic scientific studies (1–4), drug-discovery (5, 6), and diagnostic purposes (7). Microarrays can be broadly classified into two categories: static and dynamic. In static microarrays, biomolecules and chemicals are immobilized as microspots on a static solid support, and can be fabricated using a variety of technologies, including printing with high-speed arrayer onto glass slides (7), photolithography using premade masks (8), or photolithography using micromirror devices (9). In the case of dynamic microarrays, instead of stationary solid supports, bio-molecules and chemicals are immobilized onto mobile substrates, usually microbeads (10, 11). Besides bead-based microarrays, dynamic microarrays also include cell-based arrays (12, 13). Compared with their static counterparts, dynamic microarrays have several advantages: (i) ability to mix-and-match the beads (or cells) to cater for the type of screening to be performed, and introduce them into the microarray on demand offer great versatility; (ii) beads (or cells) can be replaced, resulting in a reusable format that greatly reduces the cost of operation; (iii) reaction on beads tends to be faster compared with conventional planar surfaces, as microbeads have increased surface area, thus higher binding capacity. To fully realize the potential of dynamic microarrays, it will be necessary to build a platform that allows us to transport particles, to immobilize them for convenient signal detection, to deliver reagents to them while under continual observation, and to retrieve selected particles. However, despite the progress in particle handling techniques, there is still no reliable, inexpensive and robust device that can perform these functions.
Here, we propose a microchannel design that accomplishes all these functions (transportation, immobilization, infusion of reagents, observation, and retrieval) in a single integrated device (Fig. 1), and apply it to dynamic microarray applications. The principle behind this design is simple: Fluidic resistance along the straight channel is smaller, and beads in the flow will be carried into the trap; but once the traps are filled, the flow will be redirected to the loop channels. A few groups (14–16) have also reported using hydrodynamic forces to immobilize beads/cells, but none managed to handle and release selected particles from their devices. Moreover, our design criterion allows one to design the device without any trial and error. We can achieve one-bead-to-one-trap in the array and the characteristic of the flow allows us to retrieve a trapped particle from the array by displacing it back into the main flow. This retrieval of selected beads from the array is achieved by microbubbles. The microbubble technique is ideal for our application compared with electrokinetic (17–19), optical (20), and other techniques (21) because the force exerted by an expanding bubble is enough to counter the flow. However, conventional microbubble-based devices (22–24) suffer the drawback of complicated circuits and connections. In this device, the microbubbles are formed by using a new optical technique that does away with the need of complicated on-chip integrated circuits and connection to external control electronics, greatly simplifying fabrication and operation. Consequently, our trap-and-release device can be easily implemented with conventional and soft lithography methods. The combination of hydrodynamic and optical approaches allows us to eliminate the shortcomings of each method; hydrodynamic approach allows simultaneous manipulation of large number of particles, whereas optical-based technique for retrieval gives dexterity in accessing individual particles.
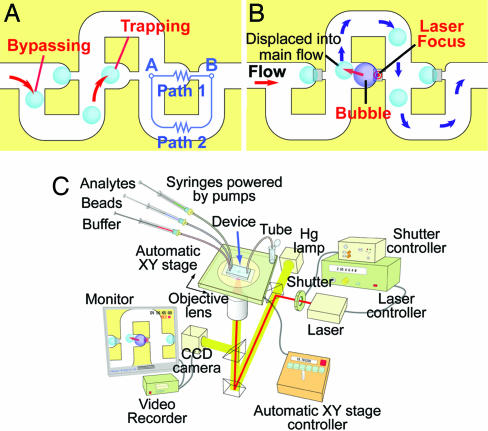
Fig. 1.
Trap-and-release mechanism and experimental setup. (A) Schematic diagram of the μ-Fluidic trap. When the trap is empty, flow resistance along the straight channel is lower than that of the loop channel, and the main stream flows along the straight channel. A bead in the flow is carried by the main stream into the trap if it is empty (trapping mode). Beads will be carried along the loop channel if the trap is filled, bypassing the occupied trap (bypassing mode). This design allows for one-bead-to-one-trap. (B) Release mechanism using microbubble. IR laser is focused onto the aluminum pattern, causing localized heating and bubble formation. The formed bubble displaces the trapped particle from the trap into the main flow. The particle is then carried by the main flow out of the device. (C) Experimental setup.
As a demonstration, we illustrated how this device might be used as a tool for screening methods such as the “one-bead-one-compound” (OBOC) combinatorial library method (2, 4, 10): beads were introduced and arrayed in the device, analyte was perfused over the beads, and selected positive beads were retrieved from the device. The device and methodology presented here lay the groundwork for highly parallel economical bead-based chemical microarrays, and with modifications, it might even be applicable for cells. We also believe that the techniques, hydrodynamic confinement and optical-based microbubbles, can be separately used for other applications in microchip devices.
Concept and Design Criterion
A Simple Hydrodynamic Trap (μ-Fluidic Trap).
Fig. 1A shows the schematic of the μ-Fluidic trap. It composes of square-wave shaped loop channels superimposed onto a straight channel, with narrowed regions along the straight channel functioning as traps. The channels are designed such that when a trap is empty, the straight channel has a lower flow resistance than that of the loop channel. As a result, we have bulk of the fluid flowing along the straight channel. A particle in the flow will be carried by this main stream into the trap (trapping mode). This particle acts as a plug, increasing the flow resistance drastically along the straight channel, and redirecting the main flow to the loop channel. Subsequent particles will then be carried along the loop channel, bypassing the filled trap (bypassing mode). Based on a simple model, the design criterion for this trap will be derived.
Pressure drop in a microchannel.
Using the Darcy–Weisbach equation to determine the pressure drop or pressure difference in a microchannel and solving the continuity and momentum equations for the Hagen–Poiseuille flow problem, we obtain the pressure difference Δp = ƒLρV2/2D, where ƒ is the Darcy friction factor, L is the length of the channel, ρ is the fluid density, V is the average velocity of the fluid, and D is the hydraulic diameter, respectively. D can be further expressed as 4A/P for a rectangular channel, and V as Q/A, where A and P are the cross-sectional area and perimeter of the channel, and Q is the volumetric flow rate. The Darcy friction factor, ƒ, is related to aspect ratio, α, and Reynolds number, Re = ρVD/μ, where μ is the fluid viscosity. The aspect ratio is defined as either height/width or width/height such that 0≤ α ≤1. The product of the Darcy friction factor and Reynolds number is a constant that depends on the aspect ratio, i.e., ƒ × Re = C(α), where C(α) denotes a constant that is a function of α [refer to supporting information (SI) Table 2 for solutions of C(α) (25)]. After simplifications, we obtain the expression
Design criterion for μ-Fluidic trap.
In Fig. 1A, we have the simplified circuit diagram of the trap. Fluid can flow from junction A to B via path 1 or 2. Ignoring minor losses due to bends, widening/narrowing, etc., Eq. 1 is applied separately for paths 1 and 2, and because the pressure drop is the same for both paths, we equate both expressions to yield
where subscripts 1 and 2 denote paths 1 and 2, respectively. For path 1, the length, L1, is assumed to be that of the narrow channel to simplify analysis. This is valid because most of the pressure drop occurs along the narrow channel. For the trap to work, the volumetric flow rate along path 1 must be greater than that of path 2, i.e., Q1 > Q2. Using the relationships A = W × H and P = 2(W + H), where H is the height of the channels, we arrive at
Note that this final expression does not contain any fluid velocity term, implying that a properly designed trap will work for all velocities in the laminar flow regime.
Optical-Based Microbubble Retrieval System.
In our μ-Fluidic trap device, once all of the traps are occupied, the main flow will be redirected to the loop channels. Subsequent particles, not being able to enter occupied traps, will follow the main flow out of the device. Taking advantage of this characteristic, we can retrieve a trapped particle from the array by displacing it back into the main flow using microbubbles. Here, we propose a simple optical-based method to create microbubbles without any need for circuits and connections. Fig. 1B shows the schematic of our method. Aluminum patterns, functioning as heaters, are located near the narrowed region of the μ-Fluidic traps. When we focus an IR laser onto the aluminum pattern, localized heating results in bubble formation and the expanding bubble displaces the immobilized particle from the μ-Fluidic trap into the main flow. The displaced particle is then carried by the flow out of the device where it can be collected. Size of the bubble can be controlled by varying the laser power and duration of the applied laser.
The schematic of the whole system is depicted in Fig. 1C. The device is mounted onto an inverted microscope with an automatic XY stage, which is controlled with a manipulator joystick. Other controllers regulate the intensity and duration of the laser, which is focused through the objective lens. The infusion system (pumps), laser system, and manipulation system can all be controlled by computer, allowing total automation of the system in the future.
Results and Discussion
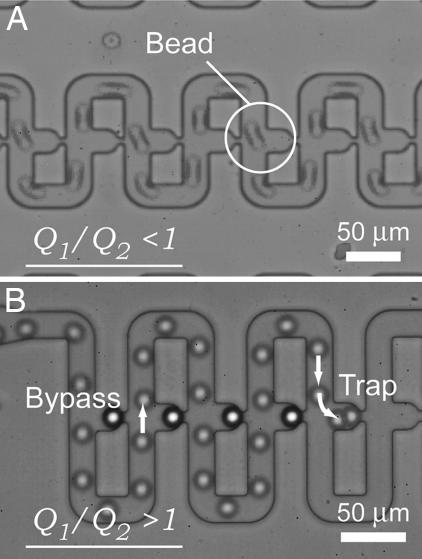
We connected the μ-Fluidic traps in series to create an array for high density immobilization of beads. Two devices, one that met and one that did not meet the design criterion (Eq. 3), were fabricated and tested. Table 1 lists the dimensions of both designs. By superimposing time-lapsed images taken with a high-speed camera, we retraced the paths taken by beads in the flow for both devices. In device A, Q1/Q2 had a value smaller than 1, indicating that the bulk of the flow was along the loop channel. As expected, none of the beads in Device A was immobilized in the μ-Fluidic traps; all of the beads followed the main flow, zigzagging through the device without entering the traps (Fig. 2A, see SI Movie 1). On the other hand, Q1/Q2 equaled 3.95 for device B. With ≈80% of the flow along the straight channel, all of the beads were sequentially carried into the traps and immobilized (Fig. 2B, see SI Movie 2). Arrows in Fig. 2B show the bypassing and trapping mode, respectively. One hundred microbeads can be arrayed in <20 s.
Table 1.
Geometric dimensions of μ–Fluidic traps
|
W1, μm |
L1, μm |
W2, μm |
L2, μm |
H, μm |
Q1/Q2 |
|
|---|---|---|---|---|---|---|
| Device A | 6 | 5.5 | 25 | 163 | 28 | 0.76 |
| Device B | 7.5 | 4.5 | 20 | 172.5 | 18 | 3.95 |
Fig. 2.
Superimposed time-lapsed high speed camera images showing the path taken by beads in devices A (A) and B (B). Arrows show the bypassing and trapping modes.
Our proposed design for hydrodynamic confinement is based on the principle of fluidic resistance. This passive trapping mechanism is robust and insensitive to fluctuations caused by occasional gas bubbles introduced during the switching of solutions. It is also extremely efficient, thus highly suitable for handling small samples. Compared with previously reported hydrodynamic traps (14–16), fabrication is extremely simple, and the design criterion allows one to design the device without any trial and error. When designing the trap, height of the microchannel, H, is an important parameter to consider. It should be set to a value larger than that of the target particle's diameter (φp). However, if H is too large, the excess leakage after a particle occupies a trap might result in multiple particles in a single trap. Based on our experience, H should be set to φp < H < 1.4 φp for the μ-Fluidic trap to achieve one-bead-to-one-trap. Our device is designed to trap particles of a specific size, and we have worked successfully with beads having a coefficient of variation of ≈4%. Using a mixture of beads that exhibits high polydispersity might result in suboptimal performance due to the trapping of multiple beads per site. With the same design criterion, we have also fabricated a high-density (1 × 104) device for immobilization of beads (see SI Text and SI Fig. 5).
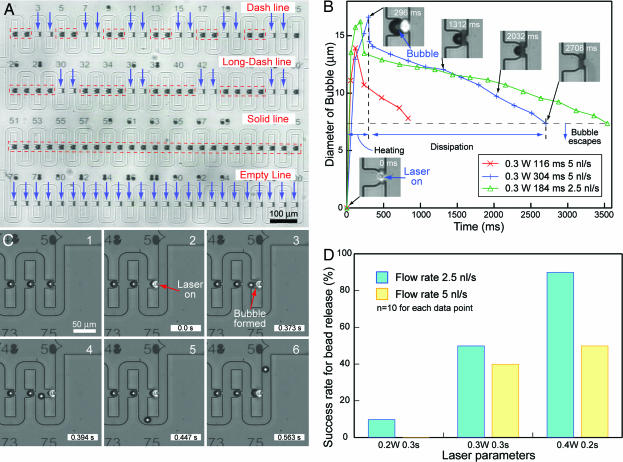
The actual trap-and-release device used in our experiments is shown in Fig. 3A. This device is designed to immobilize 100 beads, and has traps that are numbered for individual addressability. To demonstrate the individual addressability of the bead microarray, and the ease of operation of our trap-and-release device, beads were arrayed and subsequently selected beads were released to form patterned lines. This entire procedure was accomplished within a few minutes. One characteristic of this device revealed by the demonstration is that it is necessary to release the beads in a predetermined order, from the upstream to the downstream of the flow, during retrieval of multiple beads from the array. In this case, beads were released sequentially from left to right. Failure to follow this order would result in released beads re-entering vacant traps farther downstream. Fig. 3B shows the formation and dissipation of optical-based microbubbles during a typical run. During the application of the laser, the size of bubbles increased, and once the laser was switched off, these bubbles started to dissipate, shrinking in size. Removal of heat by the flow, loss of heat through the thin cover glass, and the use of porous polymer matrix [poly(dimethylsiloxane)] probably contributed to the dissipation of the bubbles. After a bubble has shrunk enough to pass through the narrow neck of the trap, it will usually be washed out by the flow. Bubbles that do not get washed out will disappear after a few seconds. Also, as expected, higher flow rates lead to faster dissipation of the bubbles. Conventional microbubble-based devices (22) are beset with problems; besides complicated fabrication process, wiring connections and control systems, residual bubbles can also cause obstructions if they do not dissipate after the heat source is switched off. Difficulty in dissipation of these bubbles is attributed to the formation of stable gas bubbles instead of vapor bubbles, due to the effervescence of dissolved gases from the solution. In our device, bubbles formed in the traps and did not impede the operation of the device in any way. These bubbles readily dissipated and were removed during operation.
Fig. 3.
Bead retrieval experimental results. (A) Beads were selectively retrieved to form patterned lines to demonstrate the ease of operation of our trap-and-release device. In the first row, after every two beads, two consecutive beads were released to form a “dash line.” In the second row, after every four beads, two consecutive beads were released to form a “long-dash line.” Arrow indicates position of released beads. No beads were released from the third row, forming a “solid line” with the beads. All the beads were released from the fourth row to form an “empty line.” Flow direction of buffer is from left to right. Beads have to be released from the upstream to the downstream of the flow. In this case, the beads were released sequentially from left to right. The entire procedure was accomplished within a few minutes using an automatic X–Y stage. (B) Graph showing dynamics of optical-based bubble during formation and dissipation. (C) Sequence of high speed camera images showing the retrieval mechanism using bubble. Photo showing trapped beads before application of laser (1), application of IR laser (2), bubble formation after 0.373 s (3), bubble displacing the bead out of the cavity (4), bead being carried along the loop channel (5), and bead moving toward the outlet (6). Bubble cooled down, shrank, and disappeared ≈3 s after the laser was switched off, and another bead could enter the trap again. (D) Graph showing the success rate of bead release at different laser settings.
High-speed camera images captured the instant at which a trapped bead was displaced by an optical-based microbubble (Fig. 3C, see SI Movie 3). IR laser set to a power of 0.3 W was focused on the aluminum pattern (t = 0.0 s), and after 373 ms, bubble formation started. The expanding bubble displaced the previously immobilized bead into the main channel, where it was carried out of the array. This retrieval procedure typically took <0.6 s to complete. After the laser was switched off, the bubble cooled down, shrank and disappeared in ≈3 s. If the need arises, this emptied trap can be used to immobilize another particle. Studies on the effect of different laser settings on bead release probability at different flow rates revealed that higher power settings at short intervals were more efficient than low power settings at longer intervals (Fig. 3D). Higher release probability is attributed to the ease of bubble formation because high power settings translate to higher heat generation and at short intervals, heat loss to forced convection is limited. Release efficiency can be further improved by reducing the flow rate. Also, in an automated system, it will be possible to implement multiple retrieval attempts to increase the success probability significantly.
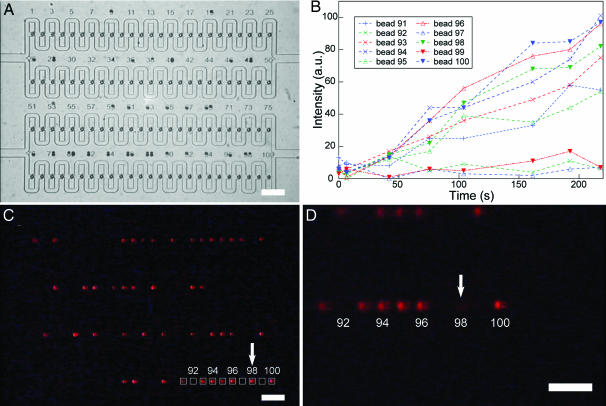
Here, we will also demonstrate the potential of this device as a tool for screening methods such as the OBOC combinatorial library method. In this demonstration, two kinds of beads, biotinylated and nonbiotinylated beads, were mixed and arrayed (Fig. 4A). These beads represented beads coated with different chemical entities in the actual OBOC combinatorial library method. When a solution of analyte, in this case, a solution of streptavidin conjugated with Alexa Fluor 546, was perfused over the beads (10 nl/s for 3 min), streptavidin started to bind to biotinylated beads, which could be seen as an increase in fluorescent response of these beads (Fig. 4B). These fluorescent beads would correspond to positive beads in the actual OBOC combinatorial library method. After 3 min of infusion, the streptavidin flow was stopped and washing buffer was perfused at 10 nl/s. We then selected a particular fluorescent (positive) bead and retrieved it from the array. Fig. 4C shows the image of fluorescent beads in the array taken 218 s after the infusion of streptavidin solution, and Fig. 4D shows the image taken after a selected fluorescent bead was retrieved from the array. This bead could then be collected at the device's outlet and sent for subsequent structural analysis to identity the chemical compound on it. Existing OBOC assays in Petri dishes and methods using COPAS (4), an automated fluorescent activated bead-sorter, to screen OBOC combinatorial libraries are capable of handling large number of beads within a relatively short time. Compared with these methods, our microfluidic bead array method offers a promising alternative because it only requires minute amounts of reagents, and allows continual observation of the reaction throughout screening. Moreover, microfluidics allows the possibility to generate a wide spectrum of concentration (26) from small volumes of reagents for screening purposes, which is challenging for other methods. Even for applications that do not require post analyses such as chemical detection and clinical diagnostic purposes whereby identities of the probes are known in advance, our purposed device has its advantages. After the test, all of the beads can be removed and replaced for another round of screening. Such a flexible and reusable format will greatly reduce the cost of bead-based assays.
Fig. 4.
Demonstration of the use of trap-and-release device in chemical microarray applications. (A) Biotinylated and nonbiotinylated beads were randomly arrayed, and a solution of streptavidin conjugated with Alexa Fluor 546 was perfused over the beads. (B) Graph showing the increase in fluorescent response of 10 of the beads (bead 91–100) in the device. White boxes in C show the observation windows (15 × 15 pixels) for these beads. The marked increase in fluorescence of the biotinylated beads can be easily distinguished from the nonbiotinylated beads (beads 92, 97, and 99). (C) Fluorescent image of the micro-bead array taken at 218 s after the infusion of streptavidin solution. Arrow indicates the fluorescent bead to be retrieved. (D) Close-up fluorescent image of the device. Arrow indicates the position of the retrieved fluorescent bead (bead 98). (Scale bar, 100 μm.)
Discussion will not be complete without considering the possible denaturing of biomolecules caused by heating during the retrieval process. After the laser is switched off, the small amount of heat stored in the aluminum heater is readily removed by an inflow of cooler water upstream (average velocity ≈7–14 mm/s). This dissipation occurs over several hundred milliseconds, which is the time scale for all of the water in the array to be replaced during operation. Although the temperature of the aluminum heater is >132°C (22) during bubble formation, it is of no concern in cases where the microbubbles are merely used to reset the device for reuse. As for screening methods such as the OBOC combinatorial library method, where biomolecules can be cleaved off the beads and analyzed, the condition of these biomolecules should have minimal impact on mass spectrometry analysis. Moreover, only a small part of the bead will be actually in contact with the bubble, and for a very short span of time (<300 ms) before it is brought away by the flow. Nevertheless, there are several ways to reduce the heat generated. The simplest way is to introduce cavities on the aluminum patterns to provide for controlled heterogeneous nucleation sites for bubble formation (22). In addition, we can reduce the saturation temperature of water in the device by switching from an infusion-based to a withdrawal-based (suction) system to drive fluids, resulting in the lowering of temperature during bubble formation. Both methods will reduce the required laser power and applied time interval for bubble formation. Another approach would be to use the expanding bubble to power a jet stream to eject the bead from the trap instead of using the bubble directly (22). This approach creates a buffer zone between the bubble and the bead, insulating the bead from the heat produced.
Conclusions
Our main motivation was to develop robust and efficient methods to manipulate particles and apply them to dynamic microarray applications. Our device consists of essentially two key strategies: (i) hydrodynamic confinement based on the principle of fluidic resistance, and (ii) optical-based microbubbles. The optical approach does away with complicated on-chip integration of circuits, greatly simplifying fabrication, packaging, and control. The device is highly amenable to automatic processing, and can be easily scaled up (see SI Text) to cater for fast, high-throughput, and highly parallel screening. We envision such a reliable, flexible and reusable dynamic microarray platform that allows transportation, immobilization, infusion of reagents, observation, and retrieval in a single integrated device will be sought after by many in various fields. This tool if coupled with methods such as OBOC combinatorial bead-library has the potential to hasten research and discovery in the fields of proteomics, diagnostics and drug discovery. With modifications (e.g., reduction in flow rate) to reduce mechanical stresses on cells during immobilization, and use of biocompatible materials (e.g., gold or platinum) to replace the aluminum patches, we believe that it will be possible to use this device as a platform for cell-based arrays (12, 13) to facilitate studies of pathological and physiological phenomena in cells, which will have enormous potential for cell-based diagnostic applications, drug testing and toxicology studies. Although we presented both components in one device, they are by no means limited to be used jointly, nor are they limited to microarray applications. For example, in the case of disposable diagnostic chips, where retrieval of beads is not necessary, an array made up of μ-Fluidic traps alone will be sufficient for observation purposes. As for the optical-based microbubbles technique developed here, we believe it has many other potential uses beyond microarrays applications; it can be used as actuators, valves, and pumps to replace conventional bubbles formed by resistive heating (22, 23) or electrochemical means (24).
Materials and Methods
Device Fabrication.
Aluminum heaters (≈100 nm thick, 7.5 μm × 15 μm) were patterned on cover glass (30 mm × 40 mm, thickness = 0.12–0.17 mm; Matsunami, Osaka, Japan) using standard lithography techniques, and the microchannels were fabricated with poly(dimethylsiloxane) (PDMS) (Sylgard 184; Dow Corning, Ithaca, NY) using soft lithography techniques. The aluminum patterns and PDMS slab were subsequently exposed to O2 plasma, aligned under microscope, and sealed irreversibly. We then baked the device on a hotplate for 1 h at 76°C to strengthen the bonding. See SI Text for further details.
Reagent Preparation.
Twenty microliters of polystyrene beads (φ = 15 μm, coefficient of variation = 2%; Fluka, Steinheim, Germany) were suspended in 1 ml of ultrapure water (specific resistance of 18 MΩ·cm) with 4 μl of Tween 20 (Kanto Chemical, Tokyo, Japan) added as surfactant. This suspension was used for all of the experiments apart from the biotin-streptavidin demonstration. For this experiment, the suspension was prepared by adding 60 μl of biotinylated beads (φ = 15.3 μm, coefficient of variation < 4%, Sphero Biotin-polystyrene particles crosslinked, Spherotech, Libertyville, IL) and 5 μl of polystyrene beads (Fluka) to 500 μl of PBS solution with 1 μl of Tween 20. The streptavidin solution was prepared by dissolving Streptavidin Alexa Fluor 546 conjugate (Invitrogen, Carlsbad, CA) in PBS to obtain a concentration of 0.05 mg/ml PBS.
Device Operation.
Micro manipulation system (Sigma Koki, Saitama, Japan) was used to create microbubbles in our experiments. This system comprised an IR laser (1,064 nm), a laser controller for adjusting laser intensity, an automatic XY stage manipulator, and a shutter controller for controlling the duration of laser exposure. Syringe pumps (Micro 4; World Precision Instruments, Sarasota, FL) were used to control the flow rates, and reagents were infused into the device using 250- or 500-μl Hamilton Gastight syringes (1700 series, TLL). Video images were taken with Hamamatsu CCD camera, and high-speed images were captured with Photron high speed camera mounted onto an inverted microscope (Eclipse, TE300; Nikon, Tokyo).
Supplementary Material
Acknowledgments
We thank Dr. Yuichi Hiratsuka and Mr. Naoya Fukushima for helpful discussions. W.-H.T. was supported by a Ministry of Education, Culture, Sports, Science, and Technology (MEXT), Government of Japan scholarship. This work was supported in part by Grants-in-Aid for Scientific Research on Priority Areas (innovative nanoscience) from MEXT, Japan.
Abbreviation
- OBOC
one-bead-one-compound.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission. R.A.M. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0606625104/DC1.
References
- 1.Winssinger N, Ficarro S, Schultz PG, Harris JL. Proc Natl Acad Sci USA. 2002;99:11139–11144. doi: 10.1073/pnas.172286899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lam KS, Liu R, Miyamoto S, Lehman AL, Tuscano JM. Acc Chem Res. 2003;36:370–377. doi: 10.1021/ar0201299. [DOI] [PubMed] [Google Scholar]
- 3.Gunderson KL, Kruglyak S, Graige MS, Garcia F, Kermani BG, Zhao C, Che D, Dickinson T, Wickham E, Bierle J, Doucet D, et al. Genome Res. 2004;14:870–877. doi: 10.1101/gr.2255804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hwang SH, Lehman A, Cong X, Olmstead MM, Lam KS, Lebrilla CB, Kurth MJ. Org Lett. 2004;6:3829–3832. doi: 10.1021/ol048408e. [DOI] [PubMed] [Google Scholar]
- 5.Schlessinger J. Nat Biotechnol. 2002;20:232–233. doi: 10.1038/nbt0302-232. [DOI] [PubMed] [Google Scholar]
- 6.Salmon SE, Liu-Stevens RH, Zhao Y, Lebl M, Krchñák V, Wertman K, Sepetov N, Lam KS. Mol Divers. 1996;2:57–63. doi: 10.1007/BF01718701. [DOI] [PubMed] [Google Scholar]
- 7.Robinson WH, DiGennaro C, Hueber W, Haab BB, Kamachi M, Dean EJ, Fournel S, Fong D, Genovese MC, Neuman de Vegvar HE, et al. Nat Med. 2002;8:295–301. doi: 10.1038/nm0302-295. [DOI] [PubMed] [Google Scholar]
- 8.Fodor SP, Read JL, Pirrung MC, Stryer L, Lu AT, Solas D. Science. 1991;251:767–773. doi: 10.1126/science.1990438. [DOI] [PubMed] [Google Scholar]
- 9.Singh-Gasson S, Green RD, Yue Y, Nelson C, Blattner F, Sussman MR, Cerrina F. Nat Biotechnol. 1999;17:974–978. doi: 10.1038/13664. [DOI] [PubMed] [Google Scholar]
- 10.Lam KS, Salmon SE, Hersh EM, Hruby V, Kazmierski WM, Knapp RJ. Nature. 1991;354:82–84. doi: 10.1038/354082a0. [DOI] [PubMed] [Google Scholar]
- 11.Noda H, Kohara Y, Okano K, Kambara H. Anal Chem. 2003;75:3250–3255. doi: 10.1021/ac020674n. [DOI] [PubMed] [Google Scholar]
- 12.Rettig JR, Folch A. Anal Chem. 2005;77:5628–5634. doi: 10.1021/ac0505977. [DOI] [PubMed] [Google Scholar]
- 13.Yamamura S, Kishi H, Tokimitsu Y, Kondo S, Honda R, Rao SR, Omori M, Tamiya E, Muraguchi A. Anal Chem. 2005;77:8050–8056. doi: 10.1021/ac0515632. [DOI] [PubMed] [Google Scholar]
- 14.Yang MS, Li CW, Yang J. Anal Chem. 2002;74:3991–4001. doi: 10.1021/ac025536c. [DOI] [PubMed] [Google Scholar]
- 15.Wheeler AR, Throndset WR, Whelan RJ, Leach AM, Zare RN, Liao YH, Farrell K, Manger ID, Daridon A. Anal Chem. 2003;75:3581–3586. doi: 10.1021/ac0340758. [DOI] [PubMed] [Google Scholar]
- 16.Di Carlo D, Aghdam N, Lee LP. Anal Chem. 2006;78:4925–4930. doi: 10.1021/ac060541s. [DOI] [PubMed] [Google Scholar]
- 17.Auerswald J, Widmer D, de Rooij NF, Sigrist A, Staubli T, Stockli T, Knapp HF. Electrophoresis. 2005;26:3697–3705. doi: 10.1002/elps.200500401. [DOI] [PubMed] [Google Scholar]
- 18.Auerswald J, Knapp HF. Microelectron Eng. 2003;67–68:879–886. [Google Scholar]
- 19.Toriello NM, Douglas ES, Mathies RA. Anal Chem. 2005;77:6935–6941. doi: 10.1021/ac051032d. [DOI] [PubMed] [Google Scholar]
- 20.Tam JM, Biran I, Walta DR. Appl Phys Lett. 2004;84:4289–4291. [Google Scholar]
- 21.Lilliehorn T, Nilsson M, Simu U, Johansson S, Almqvist M, Nilsson J, Laurell T. Sensor Actuat B. 2005;106:851–858. [Google Scholar]
- 22.Maxwell RB, Gerhardt AL, Toner M, Gray ML, Schmidt MA. J Microelectromech Syst. 2003;12:630–640. [Google Scholar]
- 23.Yin Z, Prosperetti A. J Micromech Microeng. 2005;15:643–651. [Google Scholar]
- 24.Ho CT, Lin RZ, Chang HY, Liu CH. Lab Chip. 2005;5:1248–1258. doi: 10.1039/b507575k. [DOI] [PubMed] [Google Scholar]
- 25.White FM. Fluid Mechanics. New York: McGraw–Hill; 1994. p. 333. [Google Scholar]
- 26.Jeon NL, Dertinger SKW, Chiu DT, Choi IS, Stroock AD, Whitesides GM. Langmuir. 2000;16:8311–8316. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.