Abstract
The androgen receptor (AR) is activated by both ligand-dependent and -independent mechanisms. Current therapies for prostate cancer target the ligand-binding domain in the C terminus of the AR. However, ligand-independent activation of the AR occurs by the N-terminal domain (NTD), making the NTD a potential novel target for the treatment of hormone refractory prostate cancer. A possible therapeutic approach is to overexpress an AR NTD peptide to create decoy molecules that competitively bind the interacting proteins required for activation of the endogenous full-length AR. We provide evidence that in vivo expression of AR NTD decoys decreased tumor incidence and inhibited the growth of prostate cancer tumors. This growth inhibition was characterized by a 10-fold decrease in serum levels of prostate-specific antigen (PSA) (46.7 ng/ml ± 19.9 vs. 432.4 ng/ml ± 201.3; P = 0.0299) and a 4-fold decrease in tumor volume (92.2 mm3 ± 43.4 vs. 331.4 mm3 ± 85.5; P = 0.011). AR NTD decoy molecules also delayed hormonal progression, as determined by time to rising PSA levels after castration of the host. The tumors treated with AR NTD decoys contained more apoptotic cells and fewer proliferating cells, whereas no effect was seen on the viability of cells that did not depend on the AR. This work provides further evidence of the importance of the NTD of the AR in the progression of prostate cancer and presents a target for the development of antagonists of the AR in the clinical management of this disease.
Keywords: N-terminal domain, steroid receptor, ligand, independent activation, androgen independent, homone refractory
The androgen receptor (AR) has distinct functional domains that include a C-terminal ligand-binding domain (LBD), a DNA-binding domain (DBD), and an N-terminal domain (NTD) containing one or more transcriptional activation domains (1, 2). Binding of androgen (ligand) to the LBD of the AR results in its activation and allows the receptor to initiate transcription after binding androgen response elements (ARE) in the promoter and enhancer regions of its target genes. The AR is also activated in the absence of androgen by cAMP-dependent PKA, IL-6, Her-2/neu, and other growth factors (3–8). Ligand-independent transformation of the AR results in increased nuclear AR protein, increased AR/ARE complex formation, and transactivation by the AR NTD (4–7). Thus, in the absence of androgens, the AR can be activated by alternative pathways to circumvent the need for ligand.
The role of AR in hormone-refractory disease is supported by the observation that the genes increased by androgens in androgen-dependent prostate cancer also become elevated in androgen-independent (hormone refractory) prostate cancer (9). One example is the gene for prostate-specific antigen (PSA), which is transcriptionally regulated by the AR. Rising serum levels of PSA indicate biochemical failure of hormone ablation therapy and the emergence of hormone refractory disease. In vivo, reexpression of PSA occurs at the level of transcription in castrated hosts bearing the lymph node-derived carcinoma of the prostate (LNCaP) human prostate cancer xenograft or the LNCaP hollow-fiber models (10, 11). These findings suggest the AR is activated in the absence of androgens by alternative signal transduction pathways in androgen-independent disease. This hypothesis is consistent with the observation that nuclear AR protein is present in hormone-refractory prostate cancer (12). Other studies demonstrated that the AR gene is amplified in 20–30% of androgen-independent tumors (13, 14) and that the AR is necessary for the proliferation of androgen-independent prostate cancer cells (15). An additional study showed that varying the timing and sequence of use of antiandrogens may prolong the time to androgen independence (16). Therefore, activation of the AR is strongly implicated in the underlying molecular mechanism of hormone refractory disease. Antiandrogens currently used in the clinic target the LBD; however, activation of the AR through its NTD is also a potential therapeutic target. Copies of the AR NTD, residues 1–558 (AR1–558), would theoretically compete with the unknown activating protein(s), thereby preventing activation of the full-length endogenous AR (17). Decoy AR1–558 molecules lack the DBD, hinge region, and LBD, as well as any known nuclear localization sequences. We provide proof of concept that AR NTD decoy molecules inhibit activation of the endogenous full-length AR. In addition, we provide in vivo evidence implicating the AR NTD in the mechanism of prostate cancer growth and hormonal progression.
Results
Decoy Molecules Block Expression of PSA.
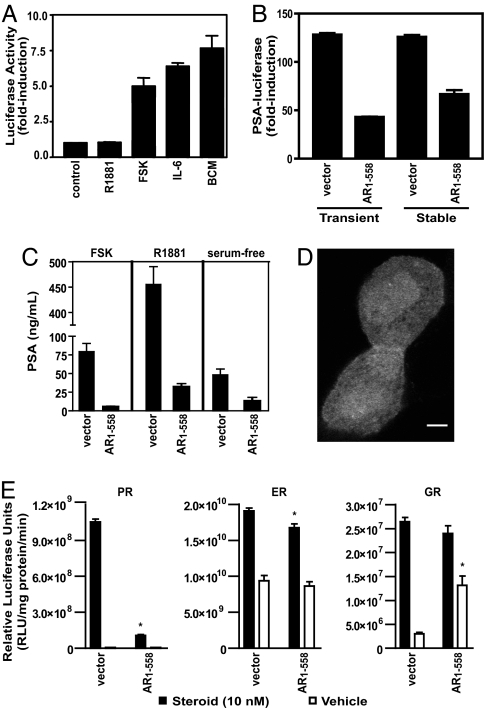
The AR is activated by its NTD in response to bone-derived factors and compounds that stimulate the PKA and IL-6 pathways (5–7, 18). To confirm this finding, LNCaP human prostate cancer cells were transfected with a construct encoding amino acids 1–558 of the AR NTD (AR1–558) fused to the DBD of Gal4. Upon activation of PKA or treatment with IL-6 or bone-conditioned medium, AR1–558 transactivation was induced (Fig. 1A). Androgen (R1881) does not bind to the AR NTD and did not mediate transactivation of this construct.
Fig. 1.
Inhibition of AR activation by decoy molecules. (A) Transactivation assay of AR1–558 in LNCaP cells cotransfected with p5xGal4UAS-TATA-luciferase and Gal4DBD-AR1–558 before incubation with R1881 (10 nM), FSK (50 μM), IL-6 (50 ng/ml), or 50% conditioned media from primary cultures of human osteoblast-like cells (bone-conditioned medium). The results are presented as fold induction of luciferase activity in treated cells vs. control cells. (B) Decoy AR1–558 blocked ligand-independent activation of the AR by FSK. Cells transiently or stably transfected with vector or decoy AR1–558 were transiently transfected with the PSA(−630/+12)-luciferase reporter. All cells were induced with FSK for 24 h. (C) Under serum-free conditions, induction of endogenous PSA secretion was inhibited in LNCaP cells stably expressing decoy AR1–558 in response to FSK, R1881, and serum-free conditions. Cells stably transfected with empty vector are shown in lanes 1, 3, and 5. Cells that stably expressed the decoy AR1–558 protein are shown in lanes 2, 4, and 6. Levels of PSA protein secreted into the media were measured after 72 h of treatment. Error bars represent the mean ± SE of three separate experiments performed in triplicate. (D) Confocal fluorescent micrograph of GFP-AR1–558 expression in transduced LNCaP cells maintained in vitro for 3 days after addition of lentiviral particles. (Scale bar, 5 μm.) (E) Transactivation assays to monitor activity of the PR, ER, and GR in the presence of decoy AR1–558. Three independent experiments were performed in triplicate for each receptor, and representative results are shown. Error bars represent mean ± SE of triplicate wells. ∗, P < 0.05 between vector and decoy AR1–558.
We previously hypothesized that expression of AR NTD decoy molecules may block such ligand-independent activation of the AR (17). The effect of these decoy molecules (AR1–558) on transactivation of the full-length endogenous AR was measured by using the PSA(−630/+12)-luciferase reporter in LNCaP prostate cancer cells. This region of the PSA promoter contains several well characterized androgen response elements (19, 20) and, through a mechanism that depends on functionally active AR, is induced by forskolin (FSK) in LNCaP cells devoid of serum and androgens (5). Treatment of transiently transfected LNCaP cells with FSK to stimulate PKA activity increased PSA-luciferase expression by >125-fold (Fig. 1B). This induction of PSA-luciferase by FSK was inhibited by 66% in the presence of peptides of the AR NTD (AR1–558). Inhibition of PSA-luciferase was also observed in LNCaP cells stably expressing the AR1–558 decoy. Western blot analysis confirmed expression of decoy AR1–558 in both transiently and stably transfected cells [supporting information (SI) Fig. 6A and B]. Decoy AR1–558 also inhibited expression of the endogenous PSA gene in response to FSK and androgen, as measured by secretion of PSA protein by LNCaP cells (Fig. 1C). Together, these data indicate that decoy AR1–558 blocked both ligand-dependent and -independent activation of the AR, as well as induction of PSA gene expression. Confocal fluorescence microscopy revealed diffuse expression of GFP-AR1–558 decoys in both the cytoplasm and nuclei of LNCaP cells transduced in vitro (Fig. 1D). Thus, it is not clear whether the inhibitory activity of decoy AR1–558 occurs in the cytosol or nucleus, but these decoys do not appear to localize to a particular cellular organelle.
To determine whether decoy AR1–558 specifically inhibited AR, or whether it caused general inhibition of steroid receptors, we tested its ability to alter the transcriptional activity of the progesterone receptor (PR), estrogen receptor (ER), and glucocorticoid receptor (GR). Luciferase reporters containing response elements for the PR, ER, and GR were induced only by their respective ligands upon transient expression of PRβ, ERα, or GRα in LNCaP cells (Fig. 1E). Coexpression of decoy AR1–558 led to a 90% inhibition (P < 0.001) of PR activity and a small but significant (P < 0.03) decrease in ER transactivation after treatment of cells with estradiol. In contrast, the presence of decoy AR1–558 did not significantly inhibit transcriptional activity of GR after treatment with dexamethasone. Interestingly, expression of AR1–558 decoys increased GR activity in the absence of ligand by >3-fold (P < 0.01).
Decoy AR1–558 Inhibited Incidence, Growth, and Hormonal Progression of Prostate Cancer.
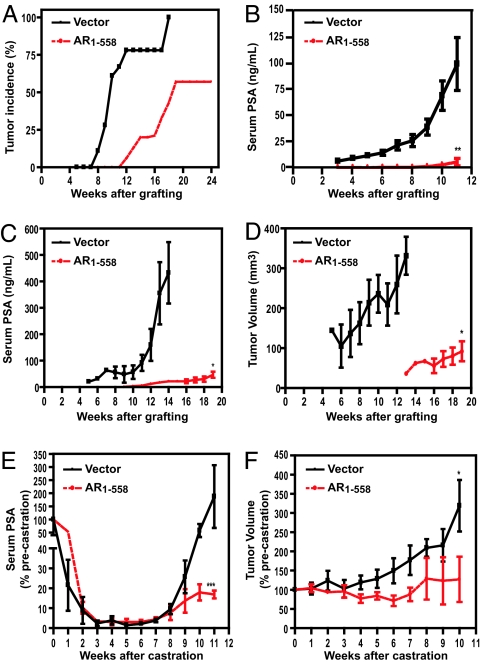
We used the LNCaP prostate cancer xenograft model to determine whether expression of decoy AR1–558 would be sufficient to prevent or delay the growth of prostate cancer in vivo (10). LNCaP cells stably expressing decoy AR1–558 or control vector were inoculated into intact male SCID mice. Tumor incidence and serum PSA were monitored for 24 weeks in intact hosts. Over this period, both the development of tumors and serum PSA levels were significantly decreased in hosts bearing decoy AR 1–558 as compared with hosts bearing tumors with vector alone. Tumor incidence remained at zero in hosts bearing tumors with decoy AR 1–558 during the first 11 weeks when using the criteria of a tumor as ≥40 mm3 (15) (Fig. 2A). Twenty-four weeks after implantation, a tumor take rate of only 58% was observed in mice bearing tumors expressing decoy AR 1–558, compared with 100% tumor take in mice bearing tumors containing vector alone. A comparable incidence rate was observed in parental LNCaP cells 3 months after inoculation (21). These results were consistent with the extremely low levels of serum PSA measured in this group of animals (Fig. 2B).
Fig. 2.
Tumor incidence, growth, and serum PSA in SCID mice bearing s.c. tumors of cells stably transfected with vector or decoy AR1–558. (A and B) Tumor incidence (A) and corresponding serum PSA (B) in intact mice bearing tumors stably transfected with vector (n = 18) or decoy AR1–558 (n = 16). (C and D) Serum PSA levels (C) and corresponding tumor volume (D) over a period of 19 weeks in intact SCID mice bearing allografts with vector or decoy AR1–558. (E and F) Progression to androgen independence after castration was determined by levels of serum PSA (E) and corresponding tumor volume (F) over a period of 11 weeks after castration. ∗, P ≤ 0.05; ∗∗, P ≤ 0.01; ∗∗∗, P ≤ 0.001.
Tumors bearing decoy AR 1–558 or vector were immediately passaged into another cohort of animals to form allografts, such that tumor growth and serum PSA could be compared in hosts bearing tumors of the same age and size (5 × 5 × 3 mm3). These studies revealed a decrease in serum PSA in intact animals bearing allografts expressing decoy AR 1–558 as compared with vector (Fig. 2C). Serum PSA was detectable 5 weeks after implantation of LNCaP cells expressing the vector, as compared with 9 weeks in mice bearing allografts expressing decoy AR 1–558. Serum PSA rapidly increased in mice bearing allografts expressing vector as compared with decoy AR 1–558 and upon completion of the experiment was ≈10 times greater than in mice bearing tumors with decoy AR 1–558 (432.4 ng/ml ± 201.3 vs. 46.7 ng/ml ± 19.9, P = 0.0299). Serum PSA levels corresponded to tumor volume in these two cohorts of animals, with control tumors being ≈4 times larger than those expressing decoy AR1–558 at the experimental endpoint (331.4 mm3 ± 82.5 vs. 92.2 mm3 ± 43.4, P = 0.011) (Fig. 2D). Thus, consistent with the observed decrease in PSA expression in vitro, expression of decoy AR 1–558 decreased androgen-dependent growth of tumors relative to controls.
The time to androgen independence in castrated animals was also compared in mice bearing tumors expressing vector or decoy AR 1–558. These experiments revealed that castration caused a >90% decrease in serum PSA in all animals by 4 weeks after castration (Fig. 2E). This drop in serum PSA was comparable to levels observed previously with the parental cell line (10) as well as clinically (22). After reaching PSA nadir, serum PSA started to rapidly rise in animals bearing tumors with vector alone, comparable to the parental cell line (10). Mice bearing tumors expressing decoy AR 1–558 also showed a >90% drop in serum PSA by 4 weeks after castration. In contrast to control tumors, serum PSA remained <20% of the precastrate level throughout the duration of the experiment. Decoy AR 1–558 protein was still expressed in tumors after 38 weeks (SI Fig. 6C), and PSA protein levels were also detected in harvested tumors bearing decoy AR 1–558 (SI Fig. 6D). Tumor volume was also measured in these animals (Fig. 2F). Similar to the parental cell line (10), tumor volume continued to increase in castrated mice bearing vector despite the reduction in circulating androgen. In contrast, a marked decrease in tumor volume was observed for up to 7 weeks after castration in mice bearing tumors expressing decoy AR 1–558. Upon completion of the experiment, tumors expressing decoy AR 1–558 had not increased significantly in volume from when the hosts were castrated, and they were one-third the volume of tumors bearing the vector. These data indicate that decoy AR 1–558 delays progression to the androgen-independent stage of tumor growth.
Decoy AR1–558 Inhibits Proliferation and Increases Apoptosis.
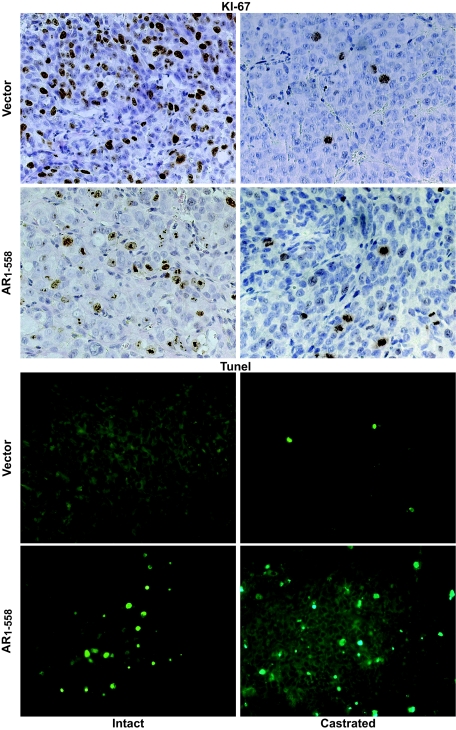
H&E staining revealed altered morphology in tumors bearing decoy AR 1–558, especially in castrated animals (SI Fig. 7). Staining of tumors for the proliferation marker Ki-67 (brown) revealed high levels of proliferation in control tumors in the presence of circulating androgen (Fig. 3). The number of proliferating cells in control tumors was substantially reduced with castration. In contrast, tumors expressing the AR 1–558 decoy contained fewer Ki-67-positive cells, suggesting reduced proliferation even in the presence of androgens. Castration further decreased Ki-67 staining in tumors bearing AR 1–558 decoy to levels comparable to those observed in castrated mice carrying tumors with vector alone. This observation implies that in the presence of androgen, AR 1–558 decoy slowed the proliferation of prostate cancer cells. Because tumor growth is the net of proliferation and cell death, we also measured apoptotic cell death by TUNEL staining. Control tumors grown in intact mice showed no positive staining for TUNEL, and castration induced only low levels of cell death (Fig. 3). Decoy AR 1–558-expressing tumors showed a marked increase in TUNEL staining in the presence of androgen and even more so with castration, suggesting that the reduced growth and poor tumor take of tumors expressing decoy AR 1–558 may be due to a combination of increased cell death and reduced proliferation in these cells. Together, these data indicate that the decoy AR 1–558 inhibits the growth of prostate cancer in vivo in both the presence and absence of androgens.
Fig. 3.
Decoy AR1–558 decreased proliferation and increased apoptosis in prostate cancer tumors. Prostate cancer tumors stably expressing vector or decoy AR1–558 were harvested, sectioned, and stained for Ki67 or TUNEL. Left displays sections of tumors from intact hosts, whereas Right displays sections from castrated hosts with androgen-independent tumors.
Lentivirus Delivery of Decoy AR1–558 Inhibits the Growth of Established Tumors.
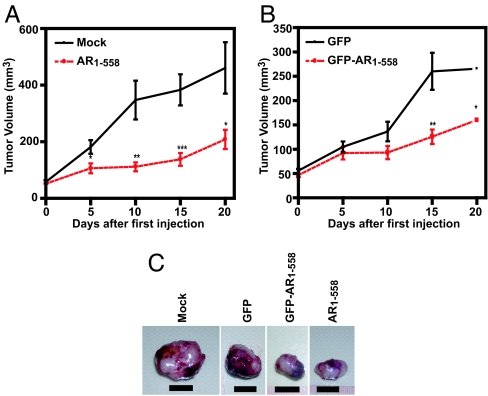
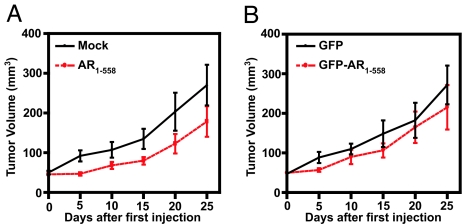
Activation of the AR by alternative signal transduction pathways leads to androgen-independent increases in expression of PSA (23). In vivo, stably expressed decoy AR 1–558 inhibited both tumor growth and PSA secretion in intact and castrated animals (Fig. 2), indicating that these decoy molecules may have therapeutic value. However, these tumors were initiated with the prostate cancer cells already expressing decoy AR 1–558. Therefore, we next tested the application of decoy AR 1–558 to established tumors to more closely reproduce the clinical scenario. For this experiment, decoy AR 1–558 was delivered to established xenografts using lentivirus technology. GFP, AR1–558, and GFP-AR1–558 plasmids were generated, and their sequences, direction, and protein expression were confirmed by sequencing and Western blot analysis (SI Fig. 8A and B). Expression was also confirmed by fluorescence microscopy in unfixed sections of tumors harvested 5 days after intratumoral injection of decoy GFP-AR1–558 (SI Fig. 8C). LNCaP s.c. xenografts were grown to 50 mm3 before the random assignment of animals to each of four lentivirus treatment groups. Within 5 days of the first injection, decoy AR1–558 had significantly (P = 0.017) inhibited tumor growth relative to tumors that were mock-injected (Fig. 4A). Tumors transduced with decoy AR1–558 were less than half the size of mock injected tumors (55% smaller, P = 0.031) by the end of the study. Tumors transduced with decoy GFP-AR1–558 were also significantly decreased in volume relative to tumors treated with GFP alone (40% smaller, P = 0.013) (Fig. 4B). Representative tumors harvested from each treatment are shown in Fig. 4 C. Decoy AR1–558, GFP-AR1–558, and GFP proteins were still expressed in tumors harvested at the end of the experiment (SI Fig. 9A). PSA protein was present at markedly reduced levels in tumors transduced with decoy AR1–558 relative to mock-injected tumors (SI Fig. 9B). These data confirm that decoy AR1–558 is effective in blocking the growth of established prostate cancer tumors.
Fig. 4.
Tumor volume was decreased by lentivirus delivery of decoy AR1–558. (A and B) The volume of s.c. LNCaP tumors was monitored after mock injection (media, n = 9) or inoculation with viral particles for decoy AR1–558 (n = 11) (A) or GFP (n = 14) or decoy GFP-AR1–558 (n = 14) (B). Tumors were 50 mm3 at the first inoculation and were subsequently injected every 5 days to a total of four injections. Error bars represent the means ± SEM. Student's t test: ∗, P ≤ 0.05; ∗∗, P ≤ 0.01; ∗∗∗, P ≤ 0.001. (C) Representative tumors harvested 20 days after the first injection. (Scale bars, 10 mm.)
Parallel lentivirus injection studies were performed in s.c. xenografts of PC3 human prostate cancer cells that do not express endogenous AR (24). No significant differences in tumor volume were observed among the different treatments by the end of the experiment (Fig. 5A and B). Relative to mock treatment, there were nonsignificant decreases in the GFP, GFP-AR1–558, and AR1–558 groups. Decoy GFP-AR1–558 and GFP proteins were still expressed in tumors harvested upon completion of the experiment (SI Fig. 9C). Transduction with viral particles carrying the vector for GFP had a small but significant effect on overall body weight of hosts bearing the highly vascularized LNCaP tumors, but this finding may be due to GFP, because AR1–558 alone caused no significant differences (SI Fig. 10A). No difference in body weight was detected in hosts bearing PC3 xenografts (SI Fig. 10B). These results suggest that decoy AR1–558 did not cause nonspecific toxicity to tissues that did not depend on AR activity. Overall, these data provide evidence that decoy AR1–558 molecules can inhibit the growth of prostate cancer tumors that express endogenous AR.
Fig. 5.
Lentivirus delivery of decoy AR1–558 did not affect the growth of tumors that do not express full-length AR. (A and B) Volume of s.c. PC3 tumors mock injected (media, n = 7) or inoculated with viral particles for decoy AR1–558 (n = 8) (A) or GFP (n = 9) or decoy GFP-AR1–558 (n = 9) (B). Tumors were 50 mm3 at the first inoculation and were subsequently injected every 5 days to a total of four injections. Error bars represent means ± SEM.
Discussion
The only effective systemic treatment for patients with advanced prostate cancer is androgen ablation therapy. This therapy initially causes tumor regression in most patients; however, these malignancies invariably progress to an androgen-independent stage, to which the patient will succumb within 2 years. The AR has been implicated in maintaining the growth of prostate cancer cells in an androgen-depleted state (15). Activation of the AR in prostate cells maintained in vitro in serum- and androgen-free conditions can occur in response to stimulation of protein kinase pathways and the IL-6 pathway that converge upon the AR NTD (5–7). The importance of the AR NTD is strongly supported by our demonstration here that decoy AR1–558 inhibited tumor incidence, growth, and hormonal progression of prostate cancer tumors that expressed endogenous AR. These effects appeared to be due to the decreased proliferation and increased apoptosis of prostate cancer cells. In contrast, these decoys exhibited no effect on tumors whose growth did not depend on functionally active AR, and lentiviral delivery of decoy AR1–558 caused no apparent adverse effects on the host.
Our experiments showed that decoy AR1–558 inhibited androgen-dependent and -independent expression of the AR-regulated gene PSA. Decoy AR1–558 also caused a large decrease in the activity of a PR-dependent luciferase reporter. In contrast, the presence of decoy AR1–558 caused only a small decrease in ER activity and no inhibition of GR activity. Despite the large effect on PR transactivation, treatment of mice carrying LNCaP or PC3 tumors with decoy AR1–558 caused no obvious side effects. Additionally, levels of progesterone are low in males, and thus the significance of inhibition of PR activity in elderly men remains to be investigated.
Decoy AR1–558 contains the entire AR NTD but lacks the DBD. The AR NTD has three regions that are highly conserved among the steroid receptor family: amino acids 1–30, 224–258, and 500–541. In the presence of ligand, amino acids 1–30, containing the sequence 23FxxLF27, are important for optimal orientation and association of the NTD with the LBD between AR dimers. Deletion of this motif does not alter dimerization affinity (25). The second area of conservation, containing residues 224–258, is within the AF1 transactivation domain (amino acids 142–485) and uniquely provides the main determinant for transcriptional activation of AR (2, 26). The last area of conservation is amino acids 500–541, adjacent to the DBD. This region has a negative influence on AR binding to the androgen response elements within the first intron of the prostatic binding protein gene (27). Future experiments should determine whether smaller regions of the AR NTD would be as effective at inhibiting the growth of prostate cancer cells. If so, it may be possible to find an active region of the AR NTD that would retain its inhibitory effect on AR transactivation while not significantly affecting other steroid receptors.
The AR NTD contains numerous phosphorylation sites and interacts with multiple proteins (23). For example, TAB2 was recently shown to interact with the AR NTD at residues 179–188 in response to IL-1β and induce a switch whereby antiandrogens were able to activate the AR (28). Steroid receptor coactivator-1 was also shown to bind the AR NTD and increase ligand-independent activation of the AR downstream of IL-6 (7). It is possible that decoy AR1–558 competitively binds one or more upstream kinases or coactivators to decrease AR transcriptional activity. Decoy AR1–558 may also alter the dissociation of the various chaperone proteins, such as Hsp70 and -90, that are bound to the ligand-free receptor. Given that decoy AR1–558 did not greatly inhibit ER or GR activity, it is likely that these decoys are binding to proteins specifically required for activation of AR and PR. A careful analysis of the specific coregulators bound by AR and PR under these conditions may aid in the elucidation of any functional interactions with decoy AR1–558. Alternatively, AR and PR may require unique posttranslational modification(s) to achieve full transactivation potential, and decoy AR1–558 may inhibit these events. Identifying the subcellular location of these interactions may allow for refinement of this treatment approach by targeting AR NTD decoys to specific regions of the cell. It is also possible that decoy AR1–558 alters nuclear translocation of full-length AR, and future experiments should determine why AR activity was decreased, while no inhibition of the closely related GR was observed.
Decoy AR1–558 was effective in slowing the growth of prostate cancer both in the presence (intact) and absence (castrated) of androgens, implying that decoy AR1–558 competes away a protein or proteins essential for transcriptional activity of the endogenous AR. AR1–558 decoys did not completely block the full-length AR in these experiments, because PSA protein was still detectable, but these studies also showed that levels of the decoy AR1–558 were not enormously elevated over levels of full-length endogenous AR. It is therefore conceivable that higher concentrations of decoy AR1–558 relative to full-length endogenous AR may result in an even greater response. Lentivirus delivery of decoy AR1–558 also led to expression in PC3 cells, yet decoy AR1–558 had no significant effect on tumor volume or on the overall body weight of the hosts. This finding suggests that decoy AR1–558 competes away a protein(s) that is not essential for the growth of cells that do not depend on AR activity. Identification of the proteins specifically interacting with decoy AR1–558 may help clarify the mechanism by which these decoys are able to inhibit the growth of prostate cancer tumors. Once identified, these interactions could be specifically targeted by smaller peptide decoys or by small molecules. Our findings here strongly implicate the AR NTD as a previously unexplored therapeutic target for the treatment of most prostate cancers that express AR and support the need to further enhance and exploit this therapeutic approach.
Materials and Methods
Animals and Cell Culture.
Male NOD-SCID mice were obtained from the Animal Research Center of the British Columbia Cancer Agency. All procedures were performed in compliance with regulations on the humane use and care of laboratory animals under an appropriate animal license issued by the University of British Columbia (Vancouver, BC, Canada). LNCaP human prostate cancer cells were provided by L. W. K. Chung (Emory University School of Medicine, Atlanta, GA), whereas PC3 cells were from American Type Culture Collection (Rockville, MD). Synthetic androgen R1881 was purchased from Perkin–Elmer (Wellesley, MA). All chemicals were obtained from Sigma (St. Louis, MO), unless stated otherwise.
Plasmids.
The human AR cDNA was a gift from A. O. Brinkmann (Erasmus University, Rotterdam, The Netherlands). The PSA(−630/+12)-luciferase, Gal4DBD, p5× Gal4UAS-TATA-luciferase, and AR1–558Gal4DBD plasmids have been described (5, 6). AR1–558 was made by excising the insert from the AR1–558Gal4DBD plasmid and inserting it into the BamHI site of pcDNA3.1HisA plasmid (Invitrogen, Carlsbad, CA). Lentivirus vectors for AR1–558, GFP, and GFP-AR1–558 were prepared by PCR by using pGFP-AR1–558 plasmid as a template and inserted into pLenti6/V5-D-TOPO plasmid (Invitrogen). The orientation and sequence were confirmed by DNA sequence analysis. Protein expression was confirmed by Western blot analysis. The ERE-E1b-Luc and PRE-E1b-Luc reporters and pCR3.1-hERα were gifts from C. L. Smith, and pCR3.1-hPRβ was from N. L. Weigel (Baylor College of Medicine, Houston, TX). The full-length GRα expression plasmid was purchased from GeneCopoeia (Germantown, MD), whereas the pGR-Luc reporter construct was purchased from Panomics (Fremont, CA).
Transient Transfections and Luciferase Assay.
LNCaP cells (3 × 105 per well) were transiently transfected according to published methods (5). After 24 h, the medium was replaced with serum-free RPMI medium 1640 with R1881, progesterone, estradiol, dexamethasone, FSK, IL-6 (50 ng/ml; R&D Systems, Minneapolis, MN), or conditioned media from primary cultures of osteoblasts (18). Luciferase activities were measured by using the Dual Luciferase Assay System (Promega, Madison, WI) with the aid of a multiplate luminometer (EG&G Berthold, Wildbad, Germany) and normalized to protein concentration. All transfection experiments were performed in three separate experiments by using triplicate wells.
Stable Transfections.
Cells stably expressing decoy AR1–558 were created by excising AR1–558 from the AR1–558Gal4 plasmid and cloning into the BamHI site of the mammalian episomal expression vector Prep9 (Invitrogen). The orientation and sequence were confirmed by DNA sequence analysis. The G418-resistant LNCaP clones of decoy AR1–558 were screened for high protein expression by Western blot analysis and assayed for inhibition of FSK induction of the transiently transfected PSA-luciferase reporter plasmid in cells maintained in vitro. Upon confirmation that decoy AR1–558 was expressed and that it blocked ligand-independent activation of the AR by FSK, the clones expressing this peptide were inoculated s.c. into animals to create xenografts.
Lentivirus Particle Preparation, Transduction, and Titration.
The lentivirus particles were prepared by using the ViraPower expression system (Invitrogen). For in vitro transduction, LNCaP cells (105 per well) were plated in six-well plates. After 24 h, virus stocks were added at various dilutions to medium containing 6 μg/ml polybrene. After 18 h, culture medium was replaced with fresh medium, and the cells were harvested 72 h after infection for protein expression. Lentiviral concentrations were determined by counting the number of GFP- and GFP-AR1–558-positive cells by either UV microscopy or flow cytometry (FACScalibur; Becton Dickinson, Franklin Lakes, NJ) and by p24 viral protein concentration as assayed by ELISA (PerkinElmer).
Xenografts and Castration.
Male NOD-SCID mice, 6–8 weeks old, were inoculated s.c. with LNCaP cells stably transfected with empty vector (4 × 106) or with decoy AR1–558 (8 × 106). The cells were suspended in 75 μl of RPMI medium 1640 (5% FBS) with 75 μl of Matrigel (Becton Dickinson Labware) and injected in the flank region under methoxyfluorane anesthesia. Established xenografts were harvested, and 5 × 5 × 3-mm pieces were immediately passaged in vivo to create allografts of equal size for cell lines carrying either the vector or AR1–558. Castration was performed under anesthesia by making a small incision in the scrotum to remove each testicle after ligation of the cord. For lentivirus studies, LNCaP cells (106 per ml) or PC3 cells (5 × 106 per ml) were dispersed in medium with 50% Matrigel and injected s.c. in the right and left flanks of 6-week-old male NOD-SCID mice. When tumors averaged ≈40–50 mm3 in size, the animals were randomly divided into four groups (Mock-media-control, GFP, GFP-AR1–558, and AR1–558) per cell line. Treatment consisted of injections every 5 days with 1–2 × 107 particles for GFP-AR1–558 and AR1–558 and 1 × 108 particles for GFP to a total of four inoculations. Tumors were measured weekly, and their volumes were calculated by the formula length × width × height × 0.5236. At 5 (LNCaP) or 10 (PC3) days after the last inoculation, mice were killed, and the tumors were excised and prepared for immunohistochemistry and Western blot analyses.
In Vitro PSA Secretion.
Stably transfected LNCaP cells (25,000) were plated in 12-well dishes in RPMI medium 1640/5% FBS/100 μg/ml G418. After 24 h, the media were replaced with serum-free RPMI medium 1640 for 48 h before treating with vehicle, R1881, or FSK. The media were removed after 72 h and assayed for PSA protein using the PSA ELISA kit (Medicorp, Montreal, QC, Canada) and a Versamax microplate reader (Molecular Devices, Sunnyvale, CA).
Serum PSA.
Blood samples were obtained from mice weekly before and after castration. Serum PSA levels were determined by an enzymatic immunoassay kit with a lower limit of sensitivity of 0.2 μg/liter according to the manufacturer's protocol (Abbott IMX, Montreal, QC, Canada).
Immunohistochemistry.
Tissue sections (5 μm) were blocked in immunohistochemistry solution (Immunovision Technologies, Brisbane, CA) and immunostained with anti-Ki67 (Dako, Carpenteria, CA). Detection steps used the ABC kit (Vector Laboratories, Burlingame, CA). Peroxidase activity was localized with 3,3-diaminobenzidine. The sections were counterstained with H&E.
Fluorescent Microscopy.
For the TUNEL assay, tumor sections (5 μm) were stained by using the ApopTag Fluorescein in situ apoptosis detection kit (TdT Tunel assay; Chemicon International, Hampshire, U.K.). After fluorescein staining, the slides were counterstained with mounting medium containing DAPI, mounted on coverslips, and examined by using a Zeiss Axioplan-2 Fluorescence Microscope (Zeiss, Toronto, ON, Canada). Confocal micrographs were obtained at the BioImaging Facility at the University of British Columbia by using a Zeiss LSM 510 Meta laser-scanning microscope.
Supplementary Material
Acknowledgments
We are grateful to Country Meadows Senior Men's Golf Charity for financial support to purchase essential equipment. This research was supported by grants from the U.S. Army Medical Research and Materiel Command Prostate Cancer Research Program (W81XWH-04-1-0292) and Health Canada (to M.D.S.).
Abbreviations
- AR
androgen receptor
- ER
estrogen receptor
- GR
glucocorticoid receptor
- NTD
N-terminal domain
- PR
progesterone receptor
- PSA
prostate-specific antigen
- LBD
ligand-binding domain
- DBD
DNA-binding domain
- FSK
forskolin
- LNCaP
lymph node-derived carcinoma of the prostate
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0606718104/DC1.
References
- 1.Jenster G, van der Korput HA, van Vroonhoven C, van der Kwast TH, Trapman J, Brinkmann AO. Mol Endocrinol. 1991;5:1396–1404. doi: 10.1210/mend-5-10-1396. [DOI] [PubMed] [Google Scholar]
- 2.Jenster G, van der Korput HA, Trapman J, Brinkmann AO. J Biol Chem. 1995;270:7341–7346. doi: 10.1074/jbc.270.13.7341. [DOI] [PubMed] [Google Scholar]
- 3.Culig Z, Hobisch A, Cronauer MV, Radmayr C, Trapman J, Hittmair A, Bartsch G, Klocker H. Cancer Res. 1994;54:5474–5478. [PubMed] [Google Scholar]
- 4.Nazareth LV, Weigel NL. J Biol Chem. 1996;271:19900–19907. doi: 10.1074/jbc.271.33.19900. [DOI] [PubMed] [Google Scholar]
- 5.Sadar MD. J Biol Chem. 1999;274:7777–7783. doi: 10.1074/jbc.274.12.7777. [DOI] [PubMed] [Google Scholar]
- 6.Ueda T, Bruchovsky N, Sadar MD. J Biol Chem. 2002;277:7076–7085. doi: 10.1074/jbc.M108255200. [DOI] [PubMed] [Google Scholar]
- 7.Ueda T, Mawji NR, Bruchovsky N, Sadar MD. J Biol Chem. 2002;277:38087–38094. doi: 10.1074/jbc.M203313200. [DOI] [PubMed] [Google Scholar]
- 8.Craft N, Shostak Y, Carey M, Sawyers CL. Nat Med. 1999;5:280–285. doi: 10.1038/6495. [DOI] [PubMed] [Google Scholar]
- 9.Gregory CW, Hamil KG, Kim D, Hall SH, Pretlow TG, Mohler JL, French FS. Cancer Res. 1998;58:5718–5724. [PubMed] [Google Scholar]
- 10.Sato N, Gleave ME, Bruchovsky N, Rennie PS, Goldenberg SL, Lange PH, Sullivan LD. J Steroid Biochem Mol Biol. 1996;58:139–146. doi: 10.1016/0960-0760(96)00018-0. [DOI] [PubMed] [Google Scholar]
- 11.Sadar MD, Akopian VA, Beraldi E. Mol Cancer Ther. 2002;1:629–637. [PubMed] [Google Scholar]
- 12.van der Kwast TH, Schalken J, Ruizeveld de Winter JA, van Vroonhoven CC, Mulder E, Boersma W, Trapman J. Int J Cancer. 1991;48:189–193. doi: 10.1002/ijc.2910480206. [DOI] [PubMed] [Google Scholar]
- 13.Moul JW, Srivastava S, McLeod DG. Semin Urol. 1995;13:157–163. [PubMed] [Google Scholar]
- 14.Visakorpi T, Hyytinen E, Koivisto P, Tanner M, Keinanen R, Palmberg C, Palotie A, Tammela T, Isola J, Kallioniemi OP. Nat Genet. 1995;9:401–406. doi: 10.1038/ng0495-401. [DOI] [PubMed] [Google Scholar]
- 15.Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, Rosenfeld MG, Sawyers CL. Nat Med. 2004;10:33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- B16.Bruchovsky N, Goldenberg SL, Mawji NR, Sadar M. Proceedings of the Seventh International Congress of Andrology; Englewood, NJ: Medimond; 2001. pp. 609–623. [Google Scholar]
- 17.Sadar MD, Hussain M, Bruchovsky N. Endocr Relat Cancer. 1999;6:487–502. doi: 10.1677/erc.0.0060487. [DOI] [PubMed] [Google Scholar]
- 18.Blaszczyk N, Masri BA, Mawji NR, Ueda T, McAlinden G, Duncan CP, Bruchovsky N, Schweikert HU, Schnabel D, Jones EC, Sadar MD. Clin Cancer Res. 2004;10:1860–1869. doi: 10.1158/1078-0432.ccr-0974-3. [DOI] [PubMed] [Google Scholar]
- 19.Riegman PH, Vlietstra RJ, van der Korput JA, Brinkmann AO, Trapman J. Mol Endocrinol. 1991;5:1921–1930. doi: 10.1210/mend-5-12-1921. [DOI] [PubMed] [Google Scholar]
- 20.Cleutjens KB, van Eekelen CC, van der Korput HA, Brinkmann AO, Trapman J. J Biol Chem. 1996;271:6379–6388. doi: 10.1074/jbc.271.11.6379. [DOI] [PubMed] [Google Scholar]
- 21.Sato N, Gleave ME, Bruchovsky N, Rennie PS, Beraldi E, Sullivan LD. Cancer Res. 1997;57:1584–1589. [PubMed] [Google Scholar]
- 22.Arai Y, Yoshiki T, Yoshida O. J Urol. 1990;144:1415–1419. doi: 10.1016/s0022-5347(17)39757-4. [DOI] [PubMed] [Google Scholar]
- 23.Wang G, Sadar MD. J Cell Biochem. 2006;98:36–53. doi: 10.1002/jcb.20802. [DOI] [PubMed] [Google Scholar]
- 24.Kaighn ME, Lechner JF, Narayan KS, Jones LW. Natl Cancer Inst Monogr. 1978:17–21. [PubMed] [Google Scholar]
- 25.Schaufele F, Carbonell X, Guerbadot M, Borngraeber S, Chapman MS, Ma AA, Miner JN, Diamond MI. Proc Natl Acad Sci USA. 2005;102:9802–9807. doi: 10.1073/pnas.0408819102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simental JA, Sar M, Lane MV, French FS, Wilson EM. J Biol Chem. 1991;266:510–518. [PubMed] [Google Scholar]
- 27.Liu GZ, Wang H, Wang Z. J Biol Chem. 2003;278:14956–14960. doi: 10.1074/jbc.M212229200. [DOI] [PubMed] [Google Scholar]
- 28.Zhu P, Baek SH, Bourk EM, Ohgi KA, Garcia-Bassets I, Sanjo H, Akira S, Kotol PF, Glass CK, Rosenfeld MG, Rose DW. Cell. 2006;124:615–629. doi: 10.1016/j.cell.2005.12.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.