Abstract
The ability of neutrophils to degrade cartilage proteoglycan suggests that the neutrophils that accumulate in the joints of rheumatoid arthritis patients are mediators of tissue damage. The regulatory mechanisms which are relevant to the proteoglycan-degrading activity of neutrophils are poorly understood. Since phosphatidylinositol 3-kinase (PI3-K), protein kinase C (PKC), the extracellular signal-regulated protein kinase (ERK)1/ERK2 and cyclic adenosine monophosphate (cAMP) have been reported to regulate neutrophil respiratory burst and/or degranulation, a role for these signalling molecules in regulating proteoglycan degradation was investigated. Preincubation of human neutrophils with GF109203X (an inhibitor of PKC), PD98059 (an inhibitor of MEK, the upstream regulator of ERK1/ERK2) or with forskolin or dibutyryl cAMP, failed to suppress proteoglycan degradation of opsonized bovine cartilage. In contrast, preincubation of neutrophils with wortmannin or LY294002, specific inhibitors of PI3-K, inhibited proteoglycan degradation. Incubation of neutrophils with cartilage resulted in the activation of PI3-K in neutrophils, consistent with a role for PI3-K in proteoglycan degradation. Activation of PI3-K and proteoglycan degradation was enhanced by tumour necrosis factor-α. Degradation caused by neutrophils from the synovial fluid of rheumatoid arthritis patients was also inhibited by wortmannin. These data demonstrate that the proteoglycan degradative activity of neutrophils required PI3-K but not PKC or the ERK1/ERK2/ERK5 cascades and was insensitive to increases in intracellular cAMP concentrations.
Introduction
Large numbers of neutrophils accumulate in the synovial fluid and joints of rheumatoid arthritis patients during the early stages and exacerbations of the disease.1 The demonstration that neutrophils degrade cartilage proteoglycan in vitro2–7 suggests that neutrophils are important mediators of cartilage destruction. The tissue-damaging action of neutrophils is enhanced by the pro-inflammatory cytokines, tumour necrosis factor-α (TNF-α), interferon-γ and granulocyte–macrophage colony-stimulating factor,3–5 which are present in the synovial fluid of rheumatoid arthritis patients.8 In general, the ability of these cytokines to prime neutrophils for enhanced degradative activity is associated with enhanced adherence of neutrophils to cartilage.3–5 The cartilage-degrading activity of neutrophils is enhanced by opsonizing cartilage with heat-aggregated immunoglobulin G3–5 but can be greatly reduced by preventing neutrophils from adhering to cartilage,4 by the addition of anti-CD11a or anti-CD11b antibody to the incubation medium,4 or by the use of non-opsonized cartilage.3–5 These observations demonstrate that β2-integrin and Fcγ receptors are involved in triggering proteoglycan degradation by neutrophils.
The intracellular signalling mechanisms that regulate the cartilage-degrading behaviour of neutrophils have not been defined. Since the ability of neutrophils to degrade proteoglycan is dependent to a large degree on contact between neutrophils and cartilage, and the engagement of β2-integrin and Fcγ receptors,4 intracellular signalling pathways activated through the interaction of neutrophils with cartilage and the engagement of β2-integrin and Fcγ receptors are likely to play prominent roles in regulating the proteoglycan degradative actions of the neutrophils. Stimulation of neutrophils through either the engagement of integrin molecules or surface receptors triggers the activation of intracellular signalling molecules such as protein kinase C (PKC), tyrosine kinases, the extracellular signal-regulated protein kinase (ERK), p38 Mitogen-activated protein kinase (MAP) kinase and phosphatidylinositol-3-kinase (PI3-K).1,9–14 Inhibitor studies have implicated some of these kinases in the regulation of neutrophil migration, respiratory burst and/or degranulation stimulated by soluble ligands.15–21 However, the ability of some of the agents to inhibit soluble ligand-stimulated neutrophil responses is poorly correlated with their ability to inhibit neutrophil functions in a complex setting, such as the killing of micro-organisms.15,21
The object of this study was to identify some of the signalling pathways that control the neutrophil-mediated degradation of cartilage proteoglycan. Our data show that incubation of neutrophils with cartilage resulted in the adherence of neutrophils to cartilage and the release of cartilage proteoglycan. Preincubation of neutrophils with inhibitors of PI3-K, but not with inhibitors of PKC or the ERK cascade, blocked proteoglycan degradation. The neutrophil-mediated proteoglycan degradation was also insensitive to increases in intracellular cyclic AMP levels. Neutrophil adherence to cartilage was associated with activation of PI3-K, a response that was enhanced by TNF-α. These results demonstrate that neutrophil-mediated proteoglycan degradation required PI3-K.
Materials and methods
Materials
Anti-p85α (sc 423) antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Human immunoglobulin G was from the Commonwealth Serum Laboratories (Melbourne, Australia). γ-32P-ATP (specific activity 4000 Ci/mmol) was obtained from Geneworks (Adelaide, Australia). Sodium 51Cr-chromate and sodium 35S-sulphate (specific activity 25–40 Ci/mg sulphur) were obtained from Amersham Pharmacia Biotec (UK). Phosphatidylinositol was purchased from Sigma Chem. Co. (St Louis, MO). Wortmannin, GF109203X and LY294002 were obtained from Biomol Research Labs (Plymouth Meeting, PA). PD98059 was purchased from New England Biolabs (Beverly, MA). Silica gel 60 thin-layer chromatography plates were purchased from E. Merck (Darmstadt, Germany). The inhibitors were dissolved in dimethylsulphoxide (0.1% v/v final) which did not affect neutrophil responses. All media used were certified to be lipopolysaccharide-free.
Preparation and culture of cartilage explants
Bovine articular cartilage was obtained from the South Australian Meat Corporation abattoir and was prepared and cultured essentially as described previously.4 Cartilage pieces (approximately 3 g) were cultured in Dulbecco's modified Eagle's medium (DMEM, Cell Image, Adelaide, Australia) (100 ml) supplemented with HEPES, antibiotics, and 20% newborn calf serum (NBCS) (Trace Scientific, Melbourne, Australia). To label the proteoglycan in the extracellular matrix, cartilage was incubated with Na2[35S]-SO4 (590 µCi) overnight.4
Heat-aggregated immunoglobulin G
Heat-aggregated immunoglobulin G was prepared by heating human immunoglobulin G at 63° for 1 hr at 20 mg/ml in phosphate-buffered saline.
Opsonization of cartilage
Cartilage was incubated with heat-aggregated immunoglobulin G at 5 mg/100 mg cartilage in DMEM containing 20% NBCS for 1 hr at 37°. Cartilage was washed three times in Hanks' balanced salt solution (HBSS) without phenol red to remove unbound opsonin and then distributed into tubes as described below.
TNF-α
TNF-α was donated by Dr G. R. Adolf (Ernst Boehringer Institut, Vienna, Austria). The cytokine was a recombinant protein produced in Escherichia coli, with a specific activity of 6 × 107 units/mg (see Boehringer Mannheim catalogue for definition) and contained < 0.125 endotoxin units/ml (Limulus lysate assay). Fresh dilutions of TNF-α in HBSS were made for each experiment.
Synovial fluid
Joint fluids were obtained from the knees of two juvenile rheumatoid arthritis patients by aspiration. Patient 1, with systemic onset arthritis, had received right total hip replacement and intra-articular triamcinolone hexacetonide in the left knee 7 months before aspiration. Patient 2 had oligoarticular onset arthritis, was not responsive to non-steroidal anti-inflammatory agents and had mild uveitis. Both patients were due to receive intra-articular triamcinolone hexacetonide in both knees. At the time of aspiration, patient 1 was being treated with methotrexate, ibuprofen and prednisolone while patient 2 was treated with piroxicam. To isolate neutrophils from synovial fluid, cells in the fluid were first pelleted (1000 g for 5 min) and resuspended in Medium 199 (Cell Image, Adelaide, Australia) before being subjected to purification as described below.
Preparation of neutrophils
Human neutrophils were isolated from the peripheral blood of healthy volunteers or from the synovial fluid from the joints of juvenile rheumatoid arthritis patients by the rapid single-step method of Ferrante and Thong.22 The preparation of neutrophils was of > 98% purity and > 99% viability as judged by morphological examination of cytospin preparations and the ability of viable cells to exclude trypan blue.
Incubation of neutrophils with cartilage
Neutrophils (4 × 106/2 ml HBSS/tube) were preincubated with GF109203X (10 min), PD98059 (45 min), wortmannin or LY294002 (15 min) or with solvent (dimethylsulphoxide, 0.1% v/v) before being added to cartilage. Wortmannin- and LY294002-pretreated cells were washed (three times in HBSS) after the preincubation period. Approximately 100 mg of opsonized cartilage/tube was used. Within each experiment, neutrophils were derived from the same donor or synovial fluid. Cartilage and neutrophils were incubated together in DMEM containing 20% NBCS, in the presence or absence of TNF-α (1000 U/ml), for up to 2.5 hr at 37° in a humidified atmosphere of 5% CO2 in air.
Neutrophil-mediated cartilage damage
Proteoglycan degradation was measured by determining the release of 35S-labelled proteoglycan into the culture medium. Samples were fractionated on Sephadex G-25 columns as described previously.4 Results were expressed as a percentage of the total radioactivity present in the cartilage.
Adherence of neutrophils to cartilage
Adherence was measured essentially as described previously.4 Briefly, neutrophils (6 × 106) prelabelled with sodium 51Cr-chromate (50 µCi/ml, 1 hr) were incubated with unlabelled cartilage for up to 2.5 hr at 37°. To ensure an adequate interaction between neutrophils and cartilage, the tubes were gently inverted (twice) every 30 min. At the times indicated in the Results section, tubes were removed from the incubator and unbound neutrophils were removed by washing (three times in 2 ml HBSS). Adherent cells and aliquots of cells that had not been incubated with cartilage were lysed in 2 ml of 0.5 m NaOH for 48 hr at room temperature. The radioactivity in the NaOH extracts was determined in a gamma-counter (LKB Wallac 1282 Compu-gamma) and the number of adherent neutrophils was determined.
Superoxide production
Superoxide production was measured by monitoring the chemiluminescence resulting from the oxidation of lucigenin (9,9′,-bis-N-methyl-acridinium nitrate). Briefly, neutrophils (1 × 106 cells, 100 µl) were preincubated with wortmannin (10–150 nm, 300 µl) before being challenged with fMLP (100 nm, 100 µl). When TNF-α was used, cells were further preincubated with the cytokine (1000 U/ml, 100 µl) for 20 min. The cells were placed in a water-jacketed (37°) luminometer chamber (Model 1250 or 1251 with MultiUse software, Bio-Orbit Oy, Turku, Finland), lucigenin (250 mm, 500 µl) was added and the resulting chemiluminescence (mV) was recorded over time immediately after the addition of the lucigenin. Results are expressed as the maximum rate of superoxide production achieved (in mV) during a 10-min period.
Degranulation
Degranulation was determined by assaying the release of β-glucuronidase from the azurophilic granules. Briefly, cells (1 × 106/ml) were preincubated with wortmannin followed by a 10-min preincubation with 2 mg/ml cytochalasin B. Then, fMLP (50 nm) was added and incubation was continued for 30 min. After centrifugation, aliquots of the supernatants were collected. To estimate the amount of released β-glucuronidase, excess methylumbelliferyl glucuronide was added and the amount of methylumbelliferone formed was determined in a fluorometer. The amount of fluorescent product that was formed was proportional to the amount of β-glucuronidase in the sample and results are expressed in fluorescence units.
Preparation of neutrophil lysates
After incubation of unlabelled neutrophils (3 × 107) with cartilage pieces as described above for adherence, the contents of the tubes were pelleted by centrifugation (800 g for 5 min at 4°). Neutrophils were lysed in buffer A [20 mm HEPES, pH 7.4, 0.5% (v/v) nonidet P-40, 100 mm NaCl, 1 mm ethylenediaminetetraacetic acid (EDTA), 2 mm Na3VO4, 2 mm dithiothreitol, 1 mm phenylmethylsulphonyl fluoride (PMSF), and 10 µg/ml each of leupeptin, aprotinin, pepstatin A and benzamidine] for 2 hr at 4°. After centrifugation (16 000 g for 5 min), the supernatants were collected for kinase assay. Incubation of cartilage pieces per se in buffer A for 2 hr did not result in the release of any protein into the buffer, indicating that all the soluble proteins in the lysate came from neutrophils.
Phosphatidylinositol 3-kinase immunoprecipitation and kinase assay
The lysates were precleared with Protein A–Sepharose (15 µl, 20 min, 4°) and 3 µg of anti-p85α antibody was added. After 2 hr (at 4°), PI3-K was precipitated by the addition of 15 µl Protein A–Sepharose. The immunoprecipitated PI3-K was washed once with buffer A and once with buffer B (10 mm Tris–HCl, pH 7.6, 100 mm NaCl, 1 mm EDTA and 100 µm Na2VO4) before being resuspended in 50 µl of buffer B. After this, 10 µl of MgCl2 (100 mm) and 10 µl of phosphatidylinositol (2 µg/ml) that had been sonicated in Tris–HCl (10 mm, pH 7.6) containing 1 mm EDTA, were added and the reaction was started by the addition of 15 µCi [32P]ATP. The tubes were incubated at room temperature for 10 min with constant mixing. The reaction was terminated by the addition of 40 µl of 8 m HCl and 320 µl of CHCl3: CH3OH (1 : 1). After vigorous mixing, and centrifugation, the upper phase was removed and the lower phase was washed (three times) with 500 µl CH3OH:H2O (1 : 1). The lipid samples were air dried and phosphorylated products were separated by thin-layer chromatography on Silica gel 60 plates, developed with CHCl3: CH3OH:H2O: 30% NH4OH (90 : 70 : 14.6 : 5.4, v/v).23 The PI332P spots were located, based on the relative fractionation (RF) value,23 and the radioactivity in the spots was determined using an Instant Imager (Packard Instrument, Canberra-Packard, Victoria, Australia).
Statistical analysis
Results were analysed by analysis of variance (anova) and followed where appropriate by Student's t-test or Tukey's test for multiple comparisons. Differences were considered significant when P < 0.05.
Results
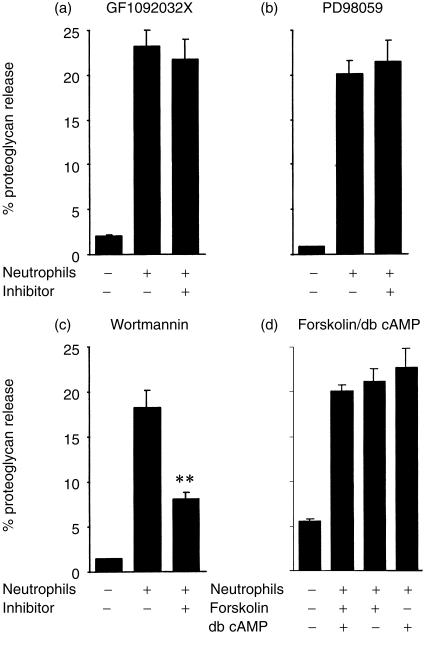
As reported previously,4 incubation of human blood neutrophils with opsonized cartilage resulted in the degradation of cartilage proteoglycan (Fig. 1). To investigate the roles of PKC, the ERK1 and ERK2 cascades and PI3-K, neutrophils were preincubated with GF109203X, PD98059 and wortmannin, relatively specific inhibitors of PKC, MAP kinase/ERK kinase (MEK) and PI3-K, respectively, at concentrations that have been reported to maximally inhibit cellular responses. While GF109203X (Fig. 1a) and PD98059 (Fig. 1b) lacked effects on neutrophil-mediated proteoglycan degradation, the ability of the neutrophils to degrade cartilage was partially inhibited by wortmannin (Fig. 1c). Neutrophil responses have generally been reported to be inhibited by increases in intracellular cyclic adenosine monophosphate (cAMP) levels.24 The data in Fig. 1(d) demonstrate that proteoglycan degradation was not affected by the addition of either dibutyryl cAMP or forskolin in the presence of isobutylmethylxanthine. In contrast to their inability to inhibit proteoglycan degradation, GF109203X, PD98059, dibutyryl cAMP and forskolin all inhibited fMLP-stimulated respiratory burst (refs 15, 19, 21, 24, and data not shown).
Figure 1.
Effects of GF109203X, PD98059, wortmannin and dibutyryl cAMP or forskolin on neutrophil-mediated proteoglycan degradation. Neutrophils (4 × 106) were preincubated in the absence or presence of 0·5 µm GF109203X (a), 50 µm PD98059 (b), 150 nm wortmannin (c) and 0·5 mm dibutyryl cAMP or 1 µm forskolin in the presence of 1 mm isobutylmethylxanthine (d) for 15, 45, 30 and 30 min, respectively, before being incubated with 35S-sulphur prelabelled bovine articular cartilage. Proteoglycan degradation was assessed as described in the Materials and Methods. Results, as means ±SEM of at least three experiments conducted in triplicates and using cells from different donors, are expressed as percentage of 35S-labelled proteoglycan released during a 2·5-h incubation period. Significance of difference between the presence and absence of an inhibitor/agent: **P < 0·001.
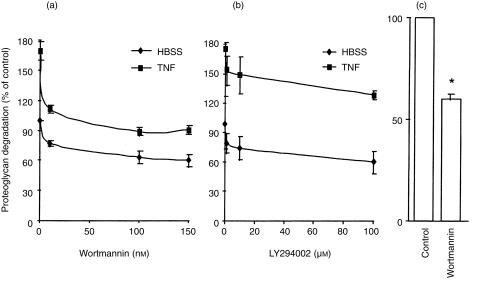
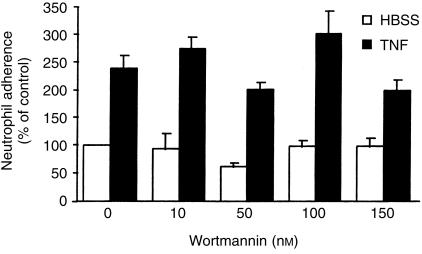
To further characterize the effects of wortmannin on neutrophil-mediated proteoglycan degradation, cells were pretreated with various concentrations of wortmannin and the effects were compared with those of another specific PI3-K inhibitor, LY294002. Both inhibitors partially suppressed the ability of the neutrophils to degrade cartilage either in the presence or absence of TNF-α (Fig. 2a,b). The ability of neutrophils to degrade proteoglycan was enhanced by TNF-α, consistent with the cytokine's ability to enhance the pro-inflammatory activity of neutrophils.25 The IC50 for wortmannin and LY294002 were approximately 10 nm and 1 µm, respectively, and these values are in close agreement with the published IC50 of approximately 5 nm and 1 µm, respectively, for wortmannin and LY294002.26 The viability of the neutrophils over this period of time was not affected by the PI3-K inhibitors as determined by trypan blue exclusion test (data not shown). Since the neutrophils were thoroughly washed after being preincubated with either wortmannin or LY294002, the above effects were independent of any effects of wortmannin or LY294002 on the cartilage per se.
Figure 2.
Suppression of neutrophil-mediated proteoglycan degradation by inhibitors of PI3-K. Neutrophils (4 × 106) were preincubated with various concentrations of wortmannin (a) or LY294002 (b), or with wortmannin (150 nm) (c) for 15 min before being added to labelled bovine articular cartilage in the presence (a and b) or absence (a–c) of TNF-α. Proteoglycan degradation was assessed as described in the Materials and Methods. Results, as means ±SEM of three experiments performed in triplicates and using cells from different donors (a and b) or means ±SEM of six replicate incubations using cells from the synovial fluid of two juvenile rheumatoid arthritis patients (c), are expressed as percentage of 35S-labelled proteoglycan released in the absence of inhibitor and TNF-α over 150 min. Significance of difference between the presence and absence of inhibitor (anova) (a and b): 0·05 < P < 0·001; between the presence and absence of wortmannin (c):* P < 0·001.
Neutrophils from inflamed joints are constantly being bathed in a cocktail of pro-inflammatory cytokines/mediators that promote cartilage destruction. Consequently, it was important to determine whether wortmannin was able to suppress proteoglycan degradation caused by these prestimulated neutrophils. Although synovial fluid from 10 juvenile rheumatoid arthritis patients was obtained, an inability to isolate sufficient numbers of neutrophils from the fluid of eight patients meant that the effects of wortmannin could only be examined on cells from two patients. Interestingly, wortmannin also suppressed proteoglycan degradation caused by the neutrophils from both patients, either in the absence (Fig. 2c) or presence of TNF-α (data not shown). Peripheral blood neutrophils from these patients were not examined since our previous studies using these cells have failed to find any differences in their responsiveness to stimulation compared with cells obtained from the blood of healthy donors.7
The above data suggest that neutrophil-mediated proteoglycan degradation is regulated by PI3-K. To confirm this, it was necessary to demonstrate activation of PI3-K under the conditions in which proteoglycan degradation was observed. PI3-K phosphorylates inositol-containing phospholipids on the D3 position of the inositol ring, resulting in the formation of phosphatidylinositol (PI) 3P, PI3,4,P2 and PI3,4,5P3.26 There are multiple forms of PI3-K that are divided into three classes.26 Leucocytes have been reported to express all four known Class I PI3-K isoforms α, β, γ and δ which are heterodimers of a regulatory subunit and a catalytic subunit (ref. 27 and references therein). Class Ia kinases, coupled to tyrosine kinases, have three catalytic isoforms (p110α, p110β and p110δ) and seven adaptor proteins derived from three separate genes (p85α, p85β and p55γ).26 Class Ib PI3-K, coupled to heterotrimeric G protein-coupled receptors, is represented by PI3-Kγ and is composed of p110γ catalytic subunit and a 101 000 MW regulatory subunit. Genetic studies with PI3-Kγ null mice have demonstrated that this lipid kinase is essential for fMLP receptor-mediated accumulation of PI 3,4,5 P.27
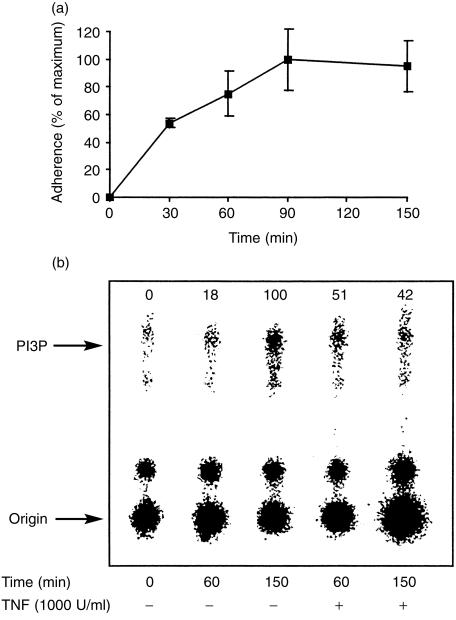
An anti-p85α subunit antibody that reacts with all three currently known Class Ia regulatory subunits was used to immunoprecipitate PI3-K. Since all the neutrophils in each tube were lysed at the end of the incubation period and equal amounts of protein were subjected to immunoprecipitation, the data represent an increase in the specific activity of the kinase. Incubation of neutrophils with cartilage resulted in a time-dependent adherence of the neutrophils to cartilage (Fig. 3a) and an increase in the activity of PI3-K as determined by the formation of PI332P (Fig. 3b). At 60 min, the activity of PI3-K was further enhanced by TNF-α although kinase activity was reduced by TNF-α at 150 min. Although a number of neutrophil ligands have now been demonstrated to cause an up-regulation of the activity of PI3-K,12,18,20 an effect of TNF-α on PI3-K activity in neutrophils has not yet been reported. Analyses of the kinetics of adherence and kinase activity indicate that a change in the number of adherent neutrophils preceded a change in the activity of PI3-K, consistent with a causal relationship between adherence and kinase activity.
Figure 3.
Time-dependent increases in the adherence of neutrophils to cartilage and activity of PI3-K. Neutrophils (3 × 107 for kinase activity or 6 × 106 51Cr-chromate-labelled for adherence) were incubated with unlabelled cartilage for up to 150 min. The tubes were gently inverted every 30 min to ensure adequate interaction between neutrophils and cartilage. At the end of the incubation period, the pieces of cartilage were washed and the number of adherent neutrophils (a) and PI3-K activity (b) were determined as described in the Materials and Methods. Results are means ±SEM of two experiments (a) performed in triplicates, or representative of three experiments (b) using cells from different donors. The numbers above (b) represent kinase activities, expressed as percentage of the maximum level (150 min in the absence of TNF-α), after deduction of basal kinase activity at 0 min.
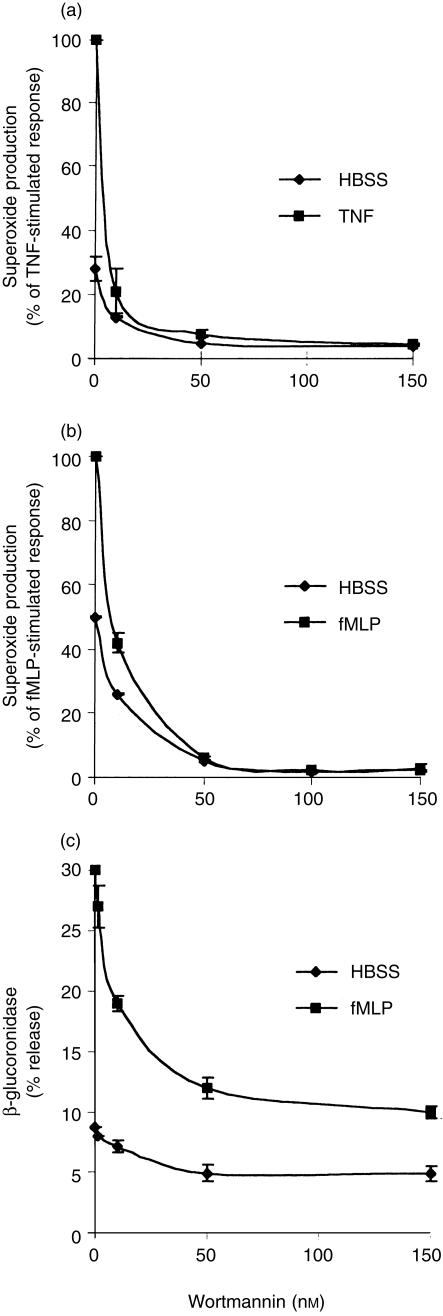
Oxygen-derived reactive species and proteolytic enzymes such as elastase have been implicated as mediators of proteoglycan degradation,2 and wortmannin has previously been demonstrated to inhibit neutrophil superoxide production21 and degranulation.28 In order to gain a better understanding of the relative importance of oxygen radicals and secretory products towards proteoglycan degradation, the effects of wortmannin on neutrophil respiratory burst and degranulation were investigated. As shown in Fig. 4(a,b), wortmannin inhibited superoxide production with an IC50 of approximately 10 nm. In contrast to the partial suppression of proteoglycan degradation, suppression of superoxide production was near complete. Furthermore, the stimulatory effects of fMLP and TNF-α were abolished. Wortmannin inhibited basal and fMLP-stimulated release of β-glucuronidase, a marker of the azurophilic granules, with an IC50 of approximately 10 nm (Fig. 4c). Similar to the suppression of proteoglycan degradation, degranulation was only partially suppressed by wortmannin and the suppressive effect was greater in fMLP-stimulated cells than in non-stimulated cells (Fig. 4c).
Figure 4.
Suppression of superoxide production and release of β-glucuronidase by wortmannin. Cells were pretreated with wortmannin for 15 min before being stimulated with fMLP (100 nm) or TNF-α (1000 U/ml). The production of superoxide and release of β-glucuronidase were assessed as described in the Materials and Methods. Results, means ±SEM of three determinations, are representative of three (c) or four (a, b) experiments, each using cells from a different donor.
Our previous studies have demonstrated that adherence of neutrophils to cartilage is required for proteoglycan degradation4 and it is possible that the inhibitors could exert their effects by inhibiting adherence. However, the data in Fig. 5 demonstrate that the ability of neutrophils to adhere to cartilage was not significantly affected by wortmannin. These data suggest that step(s) downstream of adherence, such as degranulation, required PI3-K.
Figure 5.
Neutrophil adherence to cartilage was not inhibited by wortmannin. Neutrophils (6 × 106), prelabelled with sodium 51Cr-chromate, were preincubated with wortmannin (150 nm) for 30 min before being incubated with unlabelled bovine articular cartilage for 150 min. Cells were washed before being added to cartilage. At the end of the incubation period, the cartilage was washed (three times in 2 ml HBSS) to remove non-adherent neutrophils and the number of adherent neutrophils was determined as described in the Materials and Methods. Results, means ±SEM of three experiments conducted in triplicates and using cells from different donors, are expressed as percentage of the number of adherent neutrophils in the HBSS control group in the absence of inhibitor. Significance of difference between the presence and absence of inhibitor (anova): P > 0·05.
Discussion
Neutrophil-derived agents such as elastase and oxygen-derived reactive species have been implicated as mediators of proteoglycan degradation.2,29 Under our experimental conditions, the trigger for the production and release of these tissue-injuring agents is likely to be the direct contact between neutrophils and cartilage which facilitates the engagement of the β2-integrin molecules and Fcγ receptors on the neutrophils. Consistent with this, we have previously demonstrated that separation of neutrophils from cartilage with a semi-permeable barrier almost totally abolished the proteoglycan degrading activity of the neutrophils.4 Our studies have also demonstrated that the addition of anti-CD11a or anti-CD11b antisera to the incubation medium greatly reduced the degree of proteoglycan degradation,4 and non-opsonized cartilage was poorly degraded by the neutrophils.4 Indeed, engagement of β2-integrins on neutrophils has been demonstrated to be sufficient or necessary for the stimulation of neutrophil degranulation and the generation of oxygen-derived reactive species.30,31 This is likely to be due to the activation of signalling molecules such as PKC, tyrosine kinases, ERK and PI3-K when integrin molecules are appropriately engaged.9,10 Similarly, engagement of Fcγ receptors also results in the activation of these signalling molecules.9–14 Inhibitor studies have implicated some of these kinases in the regulation of neutrophil degranulation, respiratory burst and migration.15–21,27 However, it is currently not known whether these kinases play any roles in neutrophil-mediated inflammation of joint tissues in rheumatoid arthritis.
With the exception of TNF, soluble neutrophil agonists such as fMLP, platelet-activating factor and interleukin-8 stimulate a very similar range of intracellular signalling molecules in the neutrophils. However, current evidence indicates that the relative importance of each of these signalling molecules in regulating a neutrophil function depends on the nature of the challenge or the ligand being used.11,15,16,20,32 For example, while both phorbol 12-myristate 13-acetate (PMA) and C5a increased degranulation-dependent expression of CD11b/CD18 on neutrophils and the adherence of neutrophils to endothelial cells or to plastic, pharmacological inhibition of PKC only suppressed the actions of PMA but not those of C5a,32 despite the C5a receptor being coupled to PI 4,5-bisphosphate phospholipase C through a heterotrimeric G-protein.33 While lipopolysaccharide, TNF-α, fMLP and PMA all stimulate p38 MAP kinase activity in neutrophils and enhance neutrophil adherence, only lipopolysaccharide- and TNF-α-stimulated adherence was inhibited by SB203580, a specific inhibitor of the p38.11 PMA- and fMLP-stimulated adherence was not affected.11 Consequently, extrapolation of results from one setting to another may not be valid, especially in the comparison between the biochemical regulation of neutrophil inflammatory activity under complex conditions when a number of receptors are engaged and less complex conditions when responses such as the respiratory burst and degranulation are triggered by a single receptor, as demonstrated in this and other studies.15,21
The present study demonstrates that the cartilage-degrading activity of blood- and synovial fluid-derived neutrophils is regulated, at least partially, by PI3-K. In contrast, PKC, the ERK1/ERK2 cascade and protein kinase A do not play any roles in regulating this activity. Since PD98059 cannot distinguish between MEK1, MEK2 and MEK5,34 our data also exclude the ERK5 cascade as being involved in regulating proteoglycan degradation. Although both oxygen radicals and proteolytic enzymes are required for proteoglycan degradation, our data demonstrate that inhibition of proteoglycan degradation by wortmannin is better correlated with inhibition of degranulation than with inhibition of superoxide production. Thus, despite an IC50 of approximately 10 nm for all three parameters, inhibition of superoxide production by wortmannin was near complete while inhibition of proteoglycan degradation and degranulation was only partial. These data imply that tissue damage, albeit reduced, can occur in the presence of very small quantities of oxygen radicals. Similarly, studies into the regulation of antibacterial and antifungal activities of neutrophils by various intracellular signalling molecules have found poor correlation in the ability of inhibitors to suppress superoxide production and microbicidal activity.15,21 Thus, while inhibitors of ERK, p38 and PI3-K inhibited superoxide production by 20–100%, maximum inhibition of neutrophil-mediated killing of Staphlyococcus aureus and Candida albicans were well below these figures,15,21 despite the established role of oxygen radicals in the killing of these micro-organisms.35 The possibility that the cartilage could produce oxygen radicals to compensate for the reduced production of superoxide by the neutrophils is excluded by the observation that the inhibitory effect of wortmannin was not altered when the experiments were repeated with cartilage that had been killed by repeated freezing and thawing (unpublished data).
Stimulation of neutrophils with fMLP, IL8, crystals or via the Fcγ receptors enhances the activity of PI3-K.12,18,20 Although a small number of studies have now reported that TNF-α can stimulate the activity of PI3-K,36,37 this effect has not yet been reported in neutrophils. Our data that TNF-α enhanced the activity of PI3-K in neutrophils adhering to cartilage are therefore the first demonstration in neutrophils that TNF-α can increase the activity of PI3-K, albeit in a transient manner. The ability of wortmannin to abolish TNF-α-stimulated superoxide production suggests that PI3-K is a major mediator of the actions of TNF-α on the respiratory burst. On the other hand, TNF-α-stimulated proteoglycan degradation is only partially dependent on PI3-K.
In summary, the present study demonstrates that the cartilage proteoglycan degrading activity of neutrophils is mediated, at least in part, by PI3-K and is insensitive to cAMP and inhibitors of PKC and MEK1/MEK2/MEK5. Intra-articular suppression of PI3-K may be a means to reduce neutrophil-mediated joint tissue damage in rheumatoid arthritis patients.
Acknowledgments
The authors thank Ms Gina Parashakis for technical assistance and the Channel 7 Children's Research Foundation and the National Health and Medical Research Council of Australia for supporting this work.
Abbreviations
- DMEM
Dulbecco's modified Eagle's medium
- ERK
the extracellular signal-regulated protein kinase
- HBSS
Hanks' balanced salt solution
- MEK
MAP kinase/ERK kinase, PI3-K, phosphatidylinositol-3-kinase
- PKC
protein kinase C
- TNF
tumour necrosis factor α
References
- 1.Brown KA. The polymorphonuclear cell in rheumatoid arthritis. Br J Rheumatol. 1988;27:150–5. doi: 10.1093/rheumatology/27.2.150. [DOI] [PubMed] [Google Scholar]
- 2.Kowanko IC, Bates EJ, Ferrante A. Mechanisms of neutrophil-mediated cartilage damage in vitro: the role of lysosomal enzymes, hydrogen peroxide and hypochlorous acid. Immunol Cell Biol. 1989;67:321–9. doi: 10.1038/icb.1989.47. [DOI] [PubMed] [Google Scholar]
- 3.Kowanko IC, Ferrante A. Interferon gamma increases human neutrophil-mediated cartilage proteoglycan degradation. Clin Exp Rheumatol. 1992;10:123–9. [PubMed] [Google Scholar]
- 4.Kowanko IC, Ferrante A. Adhesion and TNF priming in neutrophil-mediated cartilage damage. Clin Immunol Immunopathol. 1996;79:36–42. doi: 10.1006/clin.1996.0048. 10.1006/clin.1996.0048. [DOI] [PubMed] [Google Scholar]
- 5.Kowanko IC, Ferrante A. Granulocyte-macrophage colony-stimulating factor augments neutrophil-mediated cartilage degradation and neutrophil adherence. Arthritis Rheum. 1991;34:1452–60. doi: 10.1002/art.1780341117. [DOI] [PubMed] [Google Scholar]
- 6.Kowanko IC, Bates EJ, Ferrante A. Platelet-activating factor inhibits proteoglycan synthesis and enhances neutrophil-mediated proteoglycan degradation in cartilage explants. Arthritis Rheum. 1992;35:918–25. doi: 10.1002/art.1780350811. [DOI] [PubMed] [Google Scholar]
- 7.Kowanko IC, Ferrante A, Clemente G, Youssef PP, Smith M. Tumour necrosis factor priming of peripheral blood neutrophils from rheumatoid arthritis patients. J Clin Immunol. 1996;16:216–21. doi: 10.1007/BF01541227. [DOI] [PubMed] [Google Scholar]
- 8.Feldman M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996;14:397–440. doi: 10.1146/annurev.immunol.14.1.397. [DOI] [PubMed] [Google Scholar]
- 9.Kolanus W, Seed B. Integrins and inside out signal transduction: converging signals from PKC and PIP3. Curr Opin Cell Biol. 1977;9:725–31. doi: 10.1016/s0955-0674(97)80127-5. [DOI] [PubMed] [Google Scholar]
- 10.Schlaepfer DD, Hunter T. Integrin signalling and tyrosine phosphorylation: just the FAKs? Trends Cell Biol. 1998;8:151–7. doi: 10.1016/s0962-8924(97)01172-0. 10.1016/s0962-8924(97)01172-0. [DOI] [PubMed] [Google Scholar]
- 11.Detmers PA, Zhou D, Polizzi E, et al. Role of stress-activated mitogen-activated protein kinase (p38) in β2 integrin-dependent neutrophil adhesion and the adhesion-dependent oxidative burst. J Immunol. 1998;161:1921–9. [PubMed] [Google Scholar]
- 12.Tilton B, Andjelkovic M, Didichenko SA, Hemmings BA, Thelen M. G-protein-coupled receptors and Fcγ-receptor mediate activation of Akt/protein kinase B in human phagocytes. J Biol Chem. 1997;272:28096–101. doi: 10.1074/jbc.272.44.28096. [DOI] [PubMed] [Google Scholar]
- 13.McLeish KR, Klein JB, Coxon PY, Head KZ, Ward RA. Bacterial phagocytosis activates extracellular signal-regulated kinase and p38 mitogen-activated protein kinase cascade in human neutrophils. J Leukoc Biol. 1998;64:835–44. [PubMed] [Google Scholar]
- 14.Liang L, Huang CK. Activation of multiple protein kinases induced by cross-linking of Fc gamma RII in human neutrophils. J Leukoc Biol. 1995;57:326–31. doi: 10.1002/jlb.57.2.326. [DOI] [PubMed] [Google Scholar]
- 15.Hii CST, Stacey K, Moghaddami N, Murray AW, Ferrante A. Role of the extracellular signal-regulated protein kinase cascade in human neutrophil killing of S. aureus and C. albicans and in migration. Infect Immun. 1999;67:1297–302. doi: 10.1128/iai.67.3.1297-1302.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zu YL, Qi J, Gilchrist A. p38 mitogen-activated protein kinase activation is required for human neutrophil function triggered by TNFα or fMLP. J Immunol. 1998;160:1982–9. [PubMed] [Google Scholar]
- 17.Merritt JE, Sullivan JA, Tse J, Wilkinson SE, Nixon JS. Different sensitivities of neutrophil responses to a selective protein kinase C inhibitor Ro 31–8425: redundancy in signal transduction. Cell Signal. 1997;9:53–7. doi: 10.1016/s0898-6568(96)00097-6. 10.1016/s0898-6568(96)00097-6. [DOI] [PubMed] [Google Scholar]
- 18.Jackson JK, Lauener R, Duronio V, Burt HM. The involvement of phosphatidylinositol 3-kinase in crystal-induced human neutrophil activation. J Rheumatol. 1997;24:341–8. [PubMed] [Google Scholar]
- 19.Rane MJ, Carrithers SL, Arthur JM, Klein JB, McLeish KR. Formyl peptide receptors are coupled to multiple mitogen-activated protein kinase cascades by distinct signal transduction pathways: role in activation of reduced nicotinamide adenine dinucleotide oxidase. J Immunol. 1997;159:5070–8. [PubMed] [Google Scholar]
- 20.Knall. C, Worthen GS, Johnson GL. Interleukin 8-stimulated phosphatidylinositol-3-kinase activity regulates the migration of human neutrophils independent of extracellular signal-regulated kinase and p38 mitogen activated protein kinase. Proc Natl Acad Sci USA. 1997;94:3052–7. doi: 10.1073/pnas.94.7.3052. 10.1073/pnas.94.7.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schnyder B, Meunier PC, Car BD. Inhibition of kinases impairs neutrophil activation and killing of Staphylococcus aureus. Biochem J. 1998;331:489–95. doi: 10.1042/bj3310489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrante A, Thong YH. Separation of mononuclear and polymorphonuclear leukocytes from human blood by the one-step Hypaque-Ficoll method is dependent on blood column height. J Immunol Methods. 1982;48:81–5. doi: 10.1016/0022-1759(82)90212-5. [DOI] [PubMed] [Google Scholar]
- 23.Crawley JB, Williams LM, Mander T, Brennan FM, Foxwell BMJ. Interleukin-10 stimulation of phosphatidylinositol-3-kinase and p70, S6 kinase is required for the proliferation but not the anti-inflammatory effects of the cytokine. J Biol Chem. 1996;271:16357–62. doi: 10.1074/jbc.271.27.16357. [DOI] [PubMed] [Google Scholar]
- 24.Bessler H, Gilgal R, Djaldetti M, Zahavi I. Effect of pentoxifylline on the phagocytic activity, cAMP levels, and superoxide anion production by monocytes and polymorphonuclear cells. J Leukoc Biol. 1986;40:747–54. doi: 10.1002/jlb.40.6.747. [DOI] [PubMed] [Google Scholar]
- 25.Klebanoff SJ, Vadas MA, Harlan JM, et al. Stimulation of neutrophils by tumour necrosis factor. J Immunol. 1986;136:4220–5. [PubMed] [Google Scholar]
- 26.Vanhaesebroeck B, Alessi DR. The PI3K-PDK1 connection: more than just a road to PKB. Biochem J. 2000;346:561–76. 10.1042/0264-6021:3460561. [PMC free article] [PubMed] [Google Scholar]
- 27.Hirsch E, Katanaev VL, Garlanda C, et al. Central role for G protein-coupled phosphoinositide 3-kinase γ in flammation. Science. 2000;287:1049–53. doi: 10.1126/science.287.5455.1049. 10.1126/science.287.5455.1049. [DOI] [PubMed] [Google Scholar]
- 28.Capodici C, Hanft S, Feoktistov M, Pillinger MH. Phosphatidylinositol 3-kinase mediates chemoattractant-stimulated, CD11b/CD18-dependent cell-cell adhesion of human neutrophils: evidence for an ERK-independent pathway. J Immunol. 1998;160:1901–9. [PubMed] [Google Scholar]
- 29.Velvart M, Fehr K. Degradation in vivo of articular cartilage in rheumatoid arthritis and juvenile chronic arthritis by cathepsin-G and elastase from polymorphonuclear leukocytes. Rheumatol Int. 1987;7:195–202. doi: 10.1007/BF00541377. [DOI] [PubMed] [Google Scholar]
- 30.Nathan C, Srimal S, Farber C, et al. Cytokine-induced respiratory burst of human neutrophils: dependence on extracellular matrix and CD11/CD18. J Cell Biol. 1991;109:1341–9. doi: 10.1083/jcb.109.3.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schleiffenbaum B, Moser R, Patarroyo M, Fehr J. The cell surface glycoprotein Mac-1 (CD11b/CD18) mediates neutrophil adhesion and modulates degranulation independently of its quantitative cell surface expression. J Immunol. 1989;142:3537–45. [PubMed] [Google Scholar]
- 32.Sullivan JA, Merritt JE, Budd JM, Booth RF, Hallam TJ. Effect of a selective protein kinase C inhibitor, Ro 31–8425, on MAC-1 expression and adhesion of human neutrophils. Eur J Immunol. 1994;24:621–6. doi: 10.1002/eji.1830240320. [DOI] [PubMed] [Google Scholar]
- 33.Rollins TE, Siciliano S, Kobayashi S, Cianciarulo DN, Bonilla-Argudo V, Collier K. Purification of the active C5a receptor from human polymorphonuclear leukocytes as a receptor-Gi complex. Proc Natl Acad Sci USA. 1991;88:971–5. doi: 10.1073/pnas.88.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kamakura S, Moriguchi T, Nishida E. Activation of the protein kinase ERK5/BMK1 by receptor tryosine kinases. J Biol Chem. 1999;274:26563–71. doi: 10.1074/jbc.274.37.26563. [DOI] [PubMed] [Google Scholar]
- 35.Klebanoff SJ. Phagocytic cells: products of oxygen metabolism. In: Gallin JI, Goldstein IM, Snyderman R, editors. InflammationBasic Principles and Clinical Correlates. New York: Raven Press; 1988. p. 391. [Google Scholar]
- 36.Hanna AN, Chan EYW, Xu J, Stone JC, Brindley DN. A novel pathway for tumour necrosis factor-α and ceramide signalling involving sequential activation of tyrosine kinase, p21ras and phosphatidylinositol 3-kinase. J Biol Chem. 1999;274:12722–9. doi: 10.1074/jbc.274.18.12722. [DOI] [PubMed] [Google Scholar]
- 37.Ozes ON, Mayo LD, Gustin JA, Pfeffer LM, Donner DB. NF-κB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–5. doi: 10.1038/43466. 10.1038/43466. [DOI] [PubMed] [Google Scholar]