Abstract
Major histocompatibility complex (MHC) class I-specific inhibitory receptors are expressed not only on natural killer (NK) cells but also on some subsets of T cells. We here show Ly49 expression on γ/δ T cells in the thymus and liver of β2-microglobulin-deficient (β2m−/−) and C57BL/6 (β2m+/+) mice. Ly49C/I or Ly49A receptor was expressed on NK1.1+γ/δ T cells but not on NK1.1−γ/δ T cells. The numbers of NK1.1+γ/δ T cells were significantly smaller in β2m+/+ mice than in β2m−/− mice with the same H-2b genetic background. Among NK1.1+γ/δ T cells, the proportions of Ly49C/I+ cells but not of Ly49A+ cells, were decreased in β2m+/+ mice, suggesting that cognate interaction between Ly49C/I and H-2Kb is involved in the reduction of the number of Ly49C/I+ γ/δ T cells in β2m+/+ mice. The frequency of Ly49C/I+ cells in NK1.1+γ/δ T cells was lower in both lethally irradiated β2m+/+ mice transplanted with bone marrow (BM) from β2m−/− mice and lethally irradiated β2m−/− mice transplanted with BM from β2m+/+ mice than those in adult thymectomized BM-transplanted chimera mice. These results suggest that reduction of Ly49C/I+ NK1.1+γ/δ T cells in β2m+/+ mice is at least partly due to the down-modulation by MHC class I molecules on BM-derived haematopoietic cells or radioresistant cells in the thymus.
Introduction
The major histocompatibility complex (MHC) class I-specific receptors currently identified have been assigned to two distinct groups that belong to the immunoglobulin and C-type lectin superfamilies.1,2 In mice, C-type lectin inhibitory receptor Ly49 family and CD94/NKG2 homologue are identified. Most mouse natural killer (NK) cells simultaneously express two or more Ly49 receptors, and MHC class I haplotype influences the frequency and the level of expression of these receptors on NK cells expressing self-MHC-specific receptors.3 There is accumulating evidence concerning the mechanisms underlying the Ly49 receptor repertoire formation of NK cells, and it is most likely that host MHC class I molecules specifically interact with Ly49 receptors, resulting in down-regulation of Ly49 expression during a phase of NK-cell differentiation.3–8 Ly49 receptors are also expressed on certain subsets of α/β T cells such as NK1.1+α/β T cells.9–12 The study of TAP1-deficient mice which express CD1 [the positively selecting ligand for the canonical T-cell receptor (TCR) on NK1.1+α/β T cells] but not MHC class I (the ligand for Ly49 inhibitory receptors) demonstrated that loss of Ly49 expression is not due to the selection against NK1.1+α/β T cells expressing self-MHC-reactive Ly49 receptors, but rather the simple down-regulation of receptor expression which may be caused in a lineage-specific fashion.9 Thus, it is conceivable that both NK and NK1.1+α/β T cells adapted to the MHC environment for their maturation and function by simple down-regulation of self-reactive MHC inhibitory receptor expression.
A majority of human CD8+γ/δ T cells, particularly the Vγ9/Vδ2 subsets in the peripheral blood and gut, express CD94, killer cell inhibitory receptor (KIR) or both.13–15 The KIR expressed on human γ/δ T cells can impair antigen-specific cytotoxic T lymphocyte functions, anti-tumour responses and cytokine production.14–16 Thus, the inhibitory receptors on γ/δ T cells also serve to raise the threshold of activation, which may act as a safeguard against autoreactivity or terminate a primary response.
In this study, we compared the expression of Ly49 receptors on γ/δ T cells in the thymus and liver between C57BL/6 [β2-microglobulin (β2m)+/+] and β2m-deficient (β2m−/−) mice with H-2b genetic background. Ly49 receptors were expressed only on the NK1.1+ fraction of γ/δ T cells, and a reduced frequency of NK1.1+γ/δ T cells was correlated with the decrease of NK1.1+γ/δ T cells, with Ly49C/I+ receptor recognizing H-2Kb in β2m+/+ mice. Analysis of lethally irradiated bone marrow (BM)-transplanted chimera mice revealed that expression of β2m-associated molecules either on radioresistant host cells or BM-derived cells in the thymus is essential for the reduction of the frequency of Ly49C/I+ NK1.1+γ/δ T-cell fraction. These results suggest that expression of MHC class I molecules in the thymus plays a key role in shaping the repertoire formation of Ly49-positive cells in NK1.1+γ/δ T cells.
Materials and methods
Mice
All mice used in this study were bred in the Nagoya University School of Medicine (Nagoya, Japan) animal barrier facility under specific pathogen-free conditions. B6-Ly5.1 mice (H-2b, Ly5.1) were kindly provided by Dr Kenji Kishihara (Department of Immunology, Medical Institute of Bioregulation, Kyushu University, Fukuoka, Japan). Mice genetically deficient for β2m gene expression bred to the C57BL/6 (H-2b, Ly5.2) background were obtained from Taconic (Germantown, NY). Age- and sex-matched C57BL/6 mice obtained from Japan SLC (Hamamatsu, Japan) were used as controls.
Antibodies and reagents
Phycoerythrin (PE)-conjugated anti-NK1.1 monoclonal antibody [mAb; PK136, mouse immunoglobulin G2a (IgG2a)]; fluorescein isothiocyanate (FITC)-conjugated anti-TCR γ/δ mAb (UC7-13D5, hamster IgG), anti-Ly49C/I mAb (5E6, mouse IgG2a), anti-Ly49A mAb (A1, mouse IgG2a) and anti-CD3 mAb (145-2C11, hamster IgG); biotin-conjugated anti-CD4 mAb (RM4-5, rat IgG2a), anti-CD8α mAb (53-6.7, rat IgG2a) and anti-TCR γ/δ; purified anti-Ly5.1 mAb (A20, mouse IgG2a) and anti-NK1.1 mAb were purchased from PharMingen (San Diego, CA). Red613™-conjugated streptavidin and Cy5-conjugated anti-mouse IgG (H + L) were obtained from Life Technologies (Gaithersburg, MD) and Amersham Pharmacia Biotech (Uppsala, Sweden), respectively.
Cell preparation
Mononuclear cells (MNCs) from the thymus were obtained by standard methods. Hepatic MNCs were prepared as previously described.17 Briefly, the liver was removed in Hanks' balanced salt solution (HBSS) and gently minced on a fine stainless steel mesh. After washing with HBSS, the pellet was resuspended in 45% Percoll (Amersham Pharmacia Biotech, Uppsala, Sweden) solution and gently layered on 66% Percoll solution, then centrifuged at 800 g for 20 min at room temperature. The interface was harvested and washed with HBSS. T-cell-depleted BM cells were prepared by treatment of the BM cells with anti-Thy-1.2 mAb plus complement (LOW-TOX® Guinea-Pig Complement, Cederlane Lab., Ontario, Canada).
Flow cytometry
γ/δ T cells were stained with PE-, FITC-, or biotin-conjugated mAbs. To block FcR-mediated binding of the mAb, 2.4G2 (anti-FcγR mAb, rat IgG2b) was added. All incubation steps were performed at 4° for 30 min. To detect biotin-conjugated mAb, cells were stained with Red613™-conjugated streptavidin after incubation with primary mAb. For four-colour fluorescence-activated cell sorter analysis, cells were stained with purified anti-NK1.1 mAb (PK136) followed by Cy5 anti-mouse IgG. After vigorous washing, cells were stained with PE-anti γ/δ TCR mAb, biotin-anti-CD4 mAb, biotin-anti-CD8 mAb or biotin-anti-CD3 mAb and FITC-anti-Ly49C/I mAb, or FITC-anti-Ly49A mAb followed by streptavidin-RED613™. The stained cells were analysed with a FACSCalibur (Becton Dickinson, San Jose, CA). Small lymphocytes were gated by forward and side scattering.
Generation of bone marrow chimera mice
Six- to eight-week-old B6-Ly5.1 (β2m+/+) and β2m−/− adult mice were either thymectomized (ATX) or sham-thymectomized (sham-ATX). Seven days after the surgical procedure, these mice were subjected to total body irradiation with a dose of 800 rads and reciprocally reconstituted by an intravenous injection of 1 × 107 Thy1.2+ T-cell-depleted bone marrow (BM) cells from B6-Ly5.1 or β2m−/− mice. Sixteen to 20 weeks after BM transplantation, each group of mice was subjected to analysis. The proportions of donor-derived cells were checked by the positive expression of Ly5.1 for B6-Ly5.1 BM-transplanted β2m−/− mice (designated β2m+/+→β2m−/−) or the negative expression of Ly5.1 for β2m−/− BM-transplanted B6-Ly5.1 mice (β2m−/−→β2m+/+). Mice in which donor-derived cells were proven to account for 95% or more were used for analysis.
Statistics
The data were analysed by Student's t-test, and a P-value of less than 0·05 was taken as significant.
Results
Increased expression of Ly49C/I receptors on NK1.1+γ/δ T cells in β2m-deficient mice
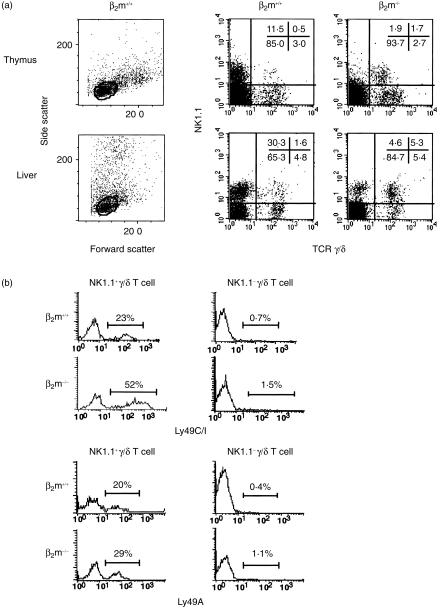
We previously reported that a significant fraction of γ/δ T cells has NK1.1 molecules.18 To examine the difference in NK1.1+γ/δ T-cell development between β2m+/+ and β2m−/− mice, we compared the proportion and absolute numbers in the thymus or liver between β2m+/+ and β2m−/− naïve mice. As shown in Fig. 1(a) and Table 1, the numbers of NK1.1+ cells in γ/δ T cells were significantly smaller in each organ of β2m+/+ mice than those in β2m−/− mice.
Figure 1.
(a) Flow cytometric analysis of NK1.1 expression on thymocytes and liver mononuclear cells (MNCs) from naive C57BL/6 (β2m+/+) and β2m-deficient (β2m−/−) mice. The gates used to distinguish small lymphocytes in the thymus and liver of β2m+/+ mice were shown (left panels). Analysis gates were set on CD4− CD8−γ/δ TCR+ cells for thymocytes and CD3+ cells for liver MNCs. Data were representative of nine independent experiments and shown as typical profiles. (b) Expression of Ly49 receptors on NK1.1+γ/δ T cells in the thymus of β2m+/+ and β2m−/− mice. For four-colour FACS analysis, thymocytes from naive β2m+/+ or β2m−/− mice were stained with purified-anti NK1.1 mAb (PK136) followed by Cy5-anti-mouse IgG. After vigorous washing, cells were stained with PE-anti-γ/δ TCR mAb (GL3), biotin-anti-CD4 mAb (RM4–5), biotin-anti-CD8 mAb (53–6·7) and FITC-anti-Ly49C/I mAb (5E6), or FITC-anti-Ly49A mAb (A1) followed by streptavidin-RED613™. Analysis gates were set on CD4− CD8−, NK1.1+ and γ/δ TCR+, and expression of each Ly49 receptor was shown as single histogram. Data were representative of three independent experiments and shown as typical profiles.
Table 1.
Proportion and absolute number of NK1.1+γ/δ T cells in the thymus and the liver of β2m-deficient mice
| Thymus | Liver | |||
|---|---|---|---|---|
| NK1.1+γ/δ T cells in CD4− CD8−γ/δ T cells (%) | Total NK1.1+ γ/δ Τ cells ( × 104) | NK1.1+γ/δ T cells in γ/δ T cells (%) | Total NK1.1+ γ/δ T cells ( × 104) | |
| β2m−/− | 38·1 ± 2·8* | 2·9 ± 0·7* | 43·9 ± 11·5* | 5·6 ± 1·9* |
| β2m+/+ | 15·0 ± 3·4 | 0·7 ± 0·2 | 23·2 ± 4·8 | 1·4 ± 0·9 |
Values represent mean±SD of nine individual determinations.
P < 0·05 by Student's t-test compared with the values for β2m+/+ mice.
MHC class I-specific inhibitory receptors such as Ly49 have been reported to play a role in shaping the repertoire of murine NK cells and NK1.1+α/β T cells.3–9 Therefore, we next examined the expression of these receptors on NK1.1+γ/δ T cells in the thymus of β2m−/− and β2m+/+ mice. As shown in Fig. 1(b) and Table 2, the proportion of NK1.1+γ/δ T cells expressing Ly49C/I, specific for H-2b,k,d,s, was significantly lower in β2m+/+ mice than in β2m−/− mice with the same H-2b background (22·9 ± 7·6% versus 52·4 ± 7·7%, respectively, P < 0·01). On the other hand, the proportion of NK1.1+γ/δ T cells expressing Ly49A, which recognizes H-2Dd,k, did not differ in β2m+/+ mice from β2m−/− mice (20·0 ± 4·4% versus 27·8 ± 3·3%, respectively). NK1.1−γ/δ T cells expressed neither receptor to any significant degree. This predisposition of Ly49 receptor expression was also observed on the liver MNCs (Table 2).
Table 2.
Proportions of Ly49 receptor-positive subsets in the liver NK1.1+γ/δ T cells of bone marrow-transplanted chimera mice
| Percentage of Ly49C/I positive cells | Percentage of Ly49A positive cells | |
|---|---|---|
| β2m+/+ | 22·5 ± 7·3*** | 20·7 ± 4·3 |
| β2m−/− | 50·5 ± 6·5 | 22·5 ± 3·5 |
| β2m+/+→β2m−/− (sham-ATX)* | 29·2 ± 6·2*** | 24·8 ± 5·3 |
| β2m+/+→β2m−/− (ATX) * | 60·6 ± 9·7 | 21·7 ± 8·3 |
| β2m−/−→β2m+/+ (sham-ATX)* | 34·2 ± 6·2** | 19·0 ± 1·4 |
| β2m−/−→β2m+/+ (ATX) * | 55·4 ± 4·6 | 20·9 ± 3·4 |
Values represent average percentages±SD of three to five individual determinations.
Liver mononuclear cells were obtained from each chimera after 16–20 weeks after in vivo transfer.
P < 0·05 by Student's t-test compared with the values for β2m−/− mice.
P < 0·01 by Student's t-test compared with the values for β2m−/− mice.
Both BM-derived cells and radioresistant thymic cells determine the frequency of Ly49C/I+ NK1.1+γ/δ T cells
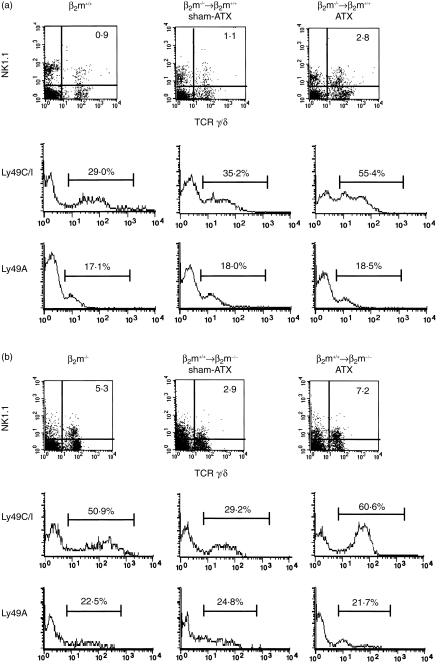
Although a significant fraction of γ/δ T cells can develop outside the thymus, most γ/δ T cells develop under the influence of the thymus. To examine the role of the thymus in determining the frequency of Ly49C/I+ NK1.1+γ/δ T cells, BM-transplanted chimera mice were generated in either euthymic (sham-ATX) or athymic (ATX) conditions using β2m−/− and B6-Ly5.1 mice (β2m+/+) with H-2b background. First, we prepared BM-transplanted chimeras by reconstituting lethally irradiated adult B6-Ly5.1 mice with T-cell-depleted BM cells obtained from syngeneic β2m−/− mice (Ly5.2), in which β2m-associated molecules including classical MHC class I are expressed only on radioresistant host cells (β2m−/−→β2m+/+). Sixteen to 20 weeks after BM transplantation, mice in which the proportion of donor-derived cells was proven to be 95% or more were used for analysis. Total numbers of NK1.1+ γ/δ T cells were increased in the liver of β2m−/−→β2m+/+ ATX chimeras compared with β2m−/−→β2m+/+ sham-ATX chimeras as control mice (3·8 × 104 ± 1·7 × 104 versus 0·7 × 104 ± 0·5 × 104 cells/liver, respectively, P < 0·05). As shown in Fig. 2(a) and Table 2, the proportion of Ly49C/I+ NK1.1+γ/δ T cells in the liver of β2m−/−→β2m+/+ ATX chimeras was markedly higher than that in β2m−/−→β2m+/+ sham-ATX chimeras (P < 0·05). The frequency and expression levels of Ly49A on NK1.1+γ/δ T cells were not changed with these manipulations.
Figure 2.
Expression of Ly49 receptors on NK1.1+ γ/δ T cells in the liver of BM-transplanted chimera mice. BM chimera mice were generated by transplanting T-cell-depleted BM from β2m−/− mice into lethally irradiated 8-week-old B6-Ly5.1 mice [β2m−/−→β2m+/+ (a)] or BM from B6-Ly5.1 into β2m−/− mice [β2m+/+→β2m−/− (b)] either under euthymic (sham-ATX) or athymic (ATX) conditions. Sixteen to 20 weeks after BM transplantation, the proportions of host-derived cells were checked by the negative expression of Ly5.1 for β2m−/−→β2m+/+ chimeras and by the positive expression for β2m+/+→β2m−/− chimeras. For four-colour FACS analysis, liver MNCs from naive β2m+/+ or β2m−/− mice were stained with purified-anti Ly5.1 mAb (A20) followed by Cy5-anti-mouse IgG. After vigorous washing, cells were stained with PE-anti-NK1.1 mAb (PK136), biotin-anti-γ/δ TCR mAb (GL3) and FITC-anti-Ly49C/I mAb (5E6), or FITC-anti Ly49A mAb (A1) followed by streptavidin-RED613™. Chimera mice in which the percentage of donor-derived cells was proven to be 95% or more were used for analysis. Expressions of NK1.1 and γ/δ TCR are displayed by two-dimensional dot plots, and the expressions of Ly49C/I and Ly49A on NK1.1+ and γ/δ TCR+ cells are shown as single histograms. Representative data from three to five mice in each group are shown as typical profiles.
We next prepared BM-transplanted chimeras by reconstituting lethally irradiated adult β2m−/− mice with T-cell-depleted BM obtained from B6-Ly5.1 mice (β2m+/+→β2m−/−), in which β2m-associated molecules are expressed only on the BM-derived donor cells, under either euthymic or athymic conditions. As shown in Fig. 2(b) and Table 2, in β2m+/+→β2m−/− ATX chimeras, a remarkable increase was seen in the number of NK1.1+γ/δ T cells accompanied with the marked expansion of Ly49C/I-expressing cells. On the other hand, in β2m+/+→β2m−/− sham-ATX chimeras, a reduced proportion of Ly49C/I-expressing NK1.1+γ/δ T cells was observed. The frequency and expression levels of Ly49A on NK1.1+γ/δ T cells were not different between β2m+/+→β2m−/− sham ATX and ATX chimeras.
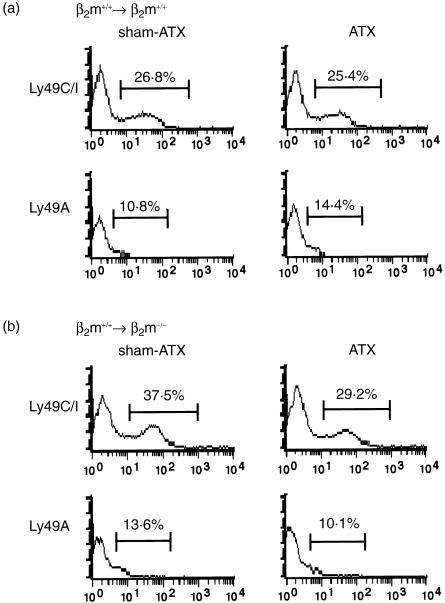
In contrast to NK1.1+γ/δ T cells, there were no differences in the proportion of Ly49C/I+ NK cells in lethally irradiated β2m−/− or β2m+/+ mice reconstituted with B6-Ly5.1 BM cells between ATX and sham-ATX mice, although the intensity of Ly49C/I was lower than that in β2m−/− mice (Fig. 3). These results clearly indicate that the radioresistant host cells and/or BM-derived donor cells in the thymus are required for the reduction of the proportion of NK1.1+γ/δ T cells that express Ly49C/I receptors.
Figure 3.
Expression of Ly49 receptors on CD3− NK1.1+cells in BMT chimera mice. Liver MNCs were stained with biotin-anti-CD3 mAb (145–2C11), PE-anti-NK1.1 mAb (PK136) and FITC-anti-Ly49C/I mAb (5E6) or FITC-anti-Ly49A (A1) followed by streptavidin-RED613™. Ly49C/I and Ly49A expressions on CD3−NK1.1+ liver MNCs from lethally irradiated B6-Ly5.1 (β2m+/+) (a) and β2m-deficient (β2m−/−) (b) mice reconstituted with B6-Ly5.1 bone marrow either under euthymic (sham-ATX) or athymic (ATX) conditions are displayed as single histograms. Representative data from three mice in each group are shown as typical profiles.
Discussion
The present study revealed that Ly49C/I or Ly49A inhibitory receptor was expressed on the NK1.1+ fraction of γ/δ T cells. The decreased frequency of NK1.1+γ/δ T cells was correlated with the reduction of the NK1.1+γ/δ T cells with Ly49C/I receptor recognizing H-2Kb in β2m+/+ mice as compared with those in β2m−/− mice with the same H-2b background. Analysis of BM-transplanted chimera mice revealed that expression of β2m-associated molecules either on radioresistant host cells or BM-derived cells in thymus is required for the reduction of the frequency of Ly49C/I+ population in NK1.1+γ/δ T cells.
Several mechanisms could account for this modulation of the frequency of NK1.1+γ/δ T cells which express self-MHC reactive Ly49 inhibitory receptors. One obvious possible mechanism for regulation of Ly49 expression is that host MHC class I molecules specifically interact with Ly49 molecules, resulting in down-regulation of the Ly49 receptor expression. This hypothesis corresponds to the receptor calibration model for NK cells, which relies on flexibility in the expression of Ly49 molecules during a phase in NK-cell differentiation.19,20 Positive and negative selections of α/β T cells are tightly regulated in the thymus by their antigen-specific receptors for MHC class I or II molecules with antigens.21–26 The analysis of γ/δ TCR transgenic mice provided evidence for positive and negative selections of γ/δ T cells specific for the nonclassical MHC class I molecule TL,27–29 raising a possibility that clonal selections of γ/δ T cells specific for β2m-associated molecules in the thymus may cause the difference in repertoire in γ/δ T cells between β2m+/+ and β2m−/− mice. If γ/δ T cells specialized to recognize self-β2m-associated molecules are the major part of the Ly49C/I+ NK1.1+γ/δ T-cell population, the deletion of this population in β2m+/+ mice would result in the decrease in the Ly49C/I+ population on NK1.1+γ/δ T-cell development. Compulsory expression of Ly49 receptor by transgene is reported to impair NK1.1+α/β T-cell development,9 suggesting that signalling via Ly49 receptor inhibits the positive selection against α/β T cells expressing self-MHC reactive Ly49 receptor during their development and maturation. We have previously reported that a significant fraction of NK1.1+γ/δ T cells was positively selected in the presence of MHC class II molecules in thymus.18 Therefore, it is alternatively possible that signalling via Ly49 inhibitory receptor may impair the positive selection of the Ly49C/I+ NK1.1+γ/δ T cells by MHC class II molecules, resulting in the reduction of this population in the thymus. However, in contrast to α/β T cells, given the paucity of information about the selection molecules and physiological ligands, the requirement of these interactions is still a matter of debate with regard to γ/δ T cells. The involvement of Ly49 inhibitory receptor in the thymic selection of immature thymocytes awaits further analysis of positive and negative selection of γ/δ T cells in thymus.
The modulations of the frequencies of Ly49-defined NK-cell subsets has been shown to be induced by relevant MHC class I molecules in a thymus-independent manner.30 Consistent with this finding on NK cells, we observed down-regulation of Ly49C/I expression on NK1.1+γ/δ T cells in both β2m−/−→β2m+/+ and β2m+/+→β2m−/− chimera mice irrespective of the presence or absence of thymus. These results suggest that expression of MHC class I molecules either on radioresistant host cells or BM-derived cells outside thymus down-modulate Ly49C/I expression on NK1.1+γ/δ T cells. However, in sharp contrast to NK cells, the presence of thymus greatly influences the number of Ly49C/I+ NK1.1+γ/δ T cells. At present, it is unknown why the MHC class I environment outside thymus does not affect the Ly49 repertoire in NK1.1+γ/δ T cells. Since large numbers of Ly49C/I+ NK1.1+γ/δ T cells were present in athymic BM-transplanted chimera mice, Ly49C/I+ NK1.1+γ/δ T cells may be able to develop outside thymus and escape from intrathymic selection during their development. We speculate that this escape from intrathymic selection may partly explain the increase in the number of NK1.1+γ/δ T cells in ATX chimeras. Although the positively selecting ligand for extrathymic NK1.1+γ/δ T cells is not clear, it is probable that this subsisted population is selected properly by its ligand beyond the inhibitory signalling via Ly49 receptors in the periphery. On the other hand, in the thymic environment, Ly49 inhibitory receptor signalling might be strong enough to inhibit the TCR signal for positive selection of γ/δ T cells. We speculate that this selective balance of positive and negative signalling might be required for maturation in the thymus and periphery. The MHC class I environment outside the thymus may induce only slight down-regulation of the Ly49 receptor expression on NK1.1+γ/δ T cells.
A number of γ/δ T cells have been shown to be specific for β2m-associated molecules such as classical MHC class I, TL, Qa and CD1 molecules.27,31–38 It can be speculated that γ/δ T cells recognizing self-β2m-associated molecules preferentially express Ly49 inhibitory receptors. The inhibitory signal by Ly49 receptors may inhibit the signaling from γ/δ TCR and thereby restrain the cytotoxic activity of the self-reactive γ/δ T cells against normal cells expressing both MHC class I and their ligands on its surface. Thus, inhibitory receptors on γ/δ T cells serve to raise the threshold of activation, which may act as a safeguard against autoreactivity.
In conclusion, our results suggest that reduction of Ly49C/I+ NK1.1+γ/δ T cells in β2m+/+ mice is at least partly due to the down-modulation by MHC class I molecules on BM-derived haematopoietic cells or radioresistant cells in the thymus.
Acknowledgments
We wish to thank Dr Kenji Kishihara for providing B6-Ly5.1 mice, and Mrs K. Itano for technical assistance. This work was supported by grants from the Mochida Foundation, Suzuken Memorial Foundation, the Tokyo Biochemical Research Foundation, the Yasuda Medical Research Foundation, the Yakult Bioscience Foundation, the Ministry of Education, Science, Sports and Culture of Japan, the Center of Excellence Foundation, and JSPS-RFTF97L00703.
Abbreviations
- ATX
adult thymectomized
- BM
bone marrow
- β2m
β2 microglobulin
- KIR
killer cell inhibitory receptor
- MNC
mononuclear cell
References
- 1.Lanier LL. NK cell receptors. Annu Rev Immunol. 1998;16:359–93. doi: 10.1146/annurev.immunol.16.1.359. [DOI] [PubMed] [Google Scholar]
- 2.Long EO. Regulation of immune responses through inhibitory receptors. Annu Rev Immunol. 1999;17:875–904. doi: 10.1146/annurev.immunol.17.1.875. [DOI] [PubMed] [Google Scholar]
- 3.Held W, Roland J, Raulet DH. Allelic exclusion of Ly49-family genes encoding class I MHC-specific receptors on NK cells. Nature. 1995;376:355–8. doi: 10.1038/376355a0. [DOI] [PubMed] [Google Scholar]
- 4.Salcedo M, Diehl AD, Olsson-Alheim MY, Sundback J, Van Kaer L, Karre K, Ljunggren HG. Altered expression of Ly49 inhibitory receptors on natural killer cells from MHC class I-deficient mice. J Immunol. 1997;158:3174–80. [PubMed] [Google Scholar]
- 5.Ryan JC, Seaman WE. Divergent functions of lectin-like receptors on NK cells. Immunol Rev. 1997;155:79–89. doi: 10.1111/j.1600-065x.1997.tb00941.x. [DOI] [PubMed] [Google Scholar]
- 6.Dorfman JR, Raulet DH. Acquisition of Ly49 receptor expression by developing natural killer cells. J Exp Med. 1998;187:609–18. doi: 10.1084/jem.187.4.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olsson-Alheim MY, Salcedo M, Ljunggren HG, Karre K, Sentman CL. NK cell receptor calibration: effects of MHC class I induction on killing by Ly49Ahigh and Ly49Alow NK cells. J Immunol. 1997;159:3189–94. [PubMed] [Google Scholar]
- 8.Renard V, Cambiaggi A, Vely F, Blery M, Olcese L, Olivero S, Bouchet M, Vivier E. Transduction of cytotoxic signals in natural killer cells: a general model of fine tuning between activatory and inhibitory pathways in lymphocytes. Immunol Rev. 1997;155:205–21. doi: 10.1111/j.1600-065x.1997.tb00953.x. [DOI] [PubMed] [Google Scholar]
- 9.Robson MacDonald H, Lees RK, Held W. Developmentally regulated extinction of Ly-49 receptor expression permits maturation and selection of NK1.1+ T cells. J Exp Med. 1998;187:2109–14. doi: 10.1084/jem.187.12.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khoo NK, Fahlen L, Sentman CL. Modulation of Ly49A receptors on mature cells to changes in major histocompatibility complex class I molecules. Immunology. 1998;95:126–31. doi: 10.1046/j.1365-2567.1998.00577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ortaldo JR, Winkler-Pickett R, Mason AT, Mason LH. The Ly-49 family: regulation of cytotoxicity and cytokine production in murine CD3+ cells. J Immunol. 1998;160:1158–65. [PubMed] [Google Scholar]
- 12.MacDonald HR. NK.1.1+ T cell receptor-alpha/beta+ cells: new clues to their origin, specificity, and function. J Exp Med. 1995;182:633–8. doi: 10.1084/jem.182.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carena I, Shamshiev A, Donda A, Colonna M, Libero GD. Major histocompatibility complex class I molecules modulate activation threshold and early signaling of T cell antigen receptor-gamma/delta stimulated by nonpeptidic ligands. J Exp Med. 1997;186:1769–74. doi: 10.1084/jem.186.10.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fisch P, Meuer E, Pende D, et al. Control of B cell lymphoma recognition via natural killer inhibitory receptors implies a role for human Vgamma9/Vdelta2 T cells in tumor immunity. Eur J Immunol. 1997;27:3368–79. doi: 10.1002/eji.1830271236. [DOI] [PubMed] [Google Scholar]
- 15.Poccia F, Cipriani B, Vendetti S, et al. CD94/NKG2 inhibitory receptor complex modulates both anti-viral and anti-tumoral responses of polyclonal phosphoantigen-reactive V gamma 9V delta 2 T lymphocytes. J Immunol. 1997;159:6009–17. [PubMed] [Google Scholar]
- 16.Bakker AB, Phillips JH, Figdor CG, Lanier LL. Killer cell inhibitory receptors for MHC class I molecules regulate lysis of melanoma cells mediated by NK cells, gamma delta T cells, and antigen-specific CTL. J Immunol. 1998;160:5239–45. [PubMed] [Google Scholar]
- 17.Ishigami M, Nishimura H, Naiki Y, et al. The roles of intrahepatic Valpha14 (+) NK1.1 (+) T cells for liver injury induced by Salmonella infection in mice. Hepatology. 1999;29:1799–808. doi: 10.1002/hep.510290605. [DOI] [PubMed] [Google Scholar]
- 18.Nishimura H, Washizu J, Naiki Y, Hara T, Fukui Y, Sasazuki T, Yoshikai Y. MHC class II-dependent NK1.1+ gammadelta T cells are induced in mice by Salmonella infection. J Immunol. 1999;162:1573–81. [PubMed] [Google Scholar]
- 19.Olsson MY, Karre K, Sentman CL. Altered phenotype and function of natural killer cells expressing the major histocompatibility complex receptor Ly-49 in mice transgenic for its ligand. Proc Natl Acad Sci USA. 1995;92:1649–53. doi: 10.1073/pnas.92.5.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raulet DH, Held W, Correa I, Dorfman JR, Wu MF, Corral L. Specificity, tolerance and developmental regulation of natural killer cells defined by expression of class I-specific Ly49 receptors. Immunol Rev. 1997;155:41–52. doi: 10.1111/j.1600-065x.1997.tb00938.x. [DOI] [PubMed] [Google Scholar]
- 21.Teh HS, Kisielow P, Scott B, Kishi H, Uematsu Y, Bluthmann H, von Boehmer H. Thymic major histocompatibility complex antigens and the alpha beta T-cell receptor determine the CD4/CD8 phenotype of T cells. Nature. 1988;335:229–33. doi: 10.1038/335229a0. [DOI] [PubMed] [Google Scholar]
- 22.Kruisbeek AM, Mond JJ, Fowlkes BJ, Carmen JA, Bridges S, Longo DL. Absence of the Lyt-2–,L3T4+ lineage of T cells in mice treated neonatally with anti-I-A correlates with absence of intrathymic I-A-bearing antigen-presenting cell function. J Exp Med. 1985;161:1029–47. doi: 10.1084/jem.161.5.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scott B, Bluthmann H, Teh HS, von Boehmer H. The generation of mature T cells requires interaction of the alpha beta T-cell receptor with major histocompatibility antigens. Nature. 1989;338:591–3. doi: 10.1038/338591a0. [DOI] [PubMed] [Google Scholar]
- 24.Bill J, Palmer E. Positive selection of CD4+ T cells mediated by MHC class II-bearing stromal cell in the thymic cortex. Nature. 1989;341:649–51. doi: 10.1038/341649a0. [DOI] [PubMed] [Google Scholar]
- 25.Benoist C, Mathis D. Positive selection of the T cell repertoire: where and when does it occur? Cell. 1989;58:1027–33. doi: 10.1016/0092-8674(89)90501-1. [DOI] [PubMed] [Google Scholar]
- 26.Berg LJ. Generation of the T cell repertoire. Curr Opin Immunol. 1989;2:87–92. doi: 10.1016/0952-7915(89)90102-7. [DOI] [PubMed] [Google Scholar]
- 27.Ito K, Van Kaer L, Bonneville M, Hsu S, Murphy DB, Tonegawa S. Recognition of the product of a novel MHC TL region gene (27b) by a mouse gamma delta T cell receptor. Cell. 1990;62:549–61. doi: 10.1016/0092-8674(90)90019-b. [DOI] [PubMed] [Google Scholar]
- 28.Pereira P, Zijlstra M, McMaster J, Loring JM, Jaenisch R, Tonegawa S. Blockade of transgenic gamma delta T cell development in beta 2-microglobulin deficient mice. Embo J. 1992;11:25–31. doi: 10.1002/j.1460-2075.1992.tb05023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wells FB, Gahm SJ, Hedrick SM, Bluestone JA, Dent A, Matis LA. Requirement for positive selection of gamma delta receptor-bearing T cells. Science. 1991;253:903–5. doi: 10.1126/science.1831565. [DOI] [PubMed] [Google Scholar]
- 30.Sykes M, Harty MW, Karlhofer FM, Pearson DA, Szot G, Yokoyama W. Hematopoietic cells and radioresistant host elements influence natural killer cell differentiation. J Exp Med. 1993;178:223–9. doi: 10.1084/jem.178.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ciccone E, Viale O, Pende D, et al. Specificity of human T lymphocytes expressing a gamma/delta T cell antigen receptor. Recognition of a polymorphic determinant of HLA class I molecules by a gamma/delta clone. Eur J Immunol. 1989;19:1267–71. doi: 10.1002/eji.1830190718. [DOI] [PubMed] [Google Scholar]
- 32.Bluestone JA, Cron RQ, Cotterman M, Houlden BA, Matis LA. Structure and specificity of T cell receptor gamma/delta on major histocompatibility complex antigen-specific CD3+, CD4−, CD8− T lymphocytes. J Exp Med. 1988;168:1899–916. doi: 10.1084/jem.168.5.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vandekerckhove BA, Datema G, Koning F, Goulmy E, Persijn GG, Van Rood JJ, Claas FH, De Vries JE. Analysis of the donor-specific cytotoxic T lymphocyte repertoire in a patient with a long term surviving allograft. Frequency, specificity, and phenotype of donor-reactive T cell receptor (TCR) -alpha beta+ and TCR-gamma delta+ clones. J Immunol. 1990;144:1288–94. [PubMed] [Google Scholar]
- 34.Spits H, Paliard X, Engelhard VH, de Vries JE. Cytotoxic activity and lymphokine production of T cell receptor (TCR) -alpha beta+ and TCR-gamma delta+ cytotoxic T lymphocyte (CTL) clones recognizing HLA-A2 and HLA-A2 mutants. Recognition of TCR-gamma delta+ CTL clones is affected by mutations at positions 152 and 156. J Immunol. 1990;144:4156–62. [PubMed] [Google Scholar]
- 35.Bonneville M, Ito K, Krecko EG, et al. Recognition of a self major histocompatibility complex TL region product by gamma delta T-cell receptors. Proc Natl Acad Sci USA. 1989;86:5928–32. doi: 10.1073/pnas.86.15.5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Faure F, Jitsukawa S, Miossec C, Hercend T. CD1c as a target recognition structure for human T lymphocytes: analysis with peripheral blood gamma/delta cells. Eur J Immunol. 1990;20:703–6. doi: 10.1002/eji.1830200336. [DOI] [PubMed] [Google Scholar]
- 37.Van Kaer L, Wu M, Ichikawa Y, Ito K, Bonneville M, Ostrand-Rosenberg S, Murphy DB, Tonegawa S. Recognition of MHC TL gene products by gamma delta T cells. Immunol Rev. 1991;120:89–115. doi: 10.1111/j.1600-065x.1991.tb00589.x. [DOI] [PubMed] [Google Scholar]
- 38.Porcelli S, Brenner MB, Greenstein JL, Balk SP, Terhorst C, Bleicher PA. Recognition of cluster of differentiation 1 antigens by human CD4– CD8–cytolytic T lymphocytes. Nature. 1989;341:447–50. doi: 10.1038/341447a0. [DOI] [PubMed] [Google Scholar]