Abstract
Chronic stress is known to induce immunological disorders. In the present study we examined the consequences of chronic restraint stress on the immune response to tetanus toxin in mice. We investigated the repartition of subsets of lymphoid cells in blood and spleen, the functional ability of lymphocytes to proliferate and to produce cytokines, and antibody titres against tetanus toxin following stress. We report discordance of the stimulation index of lymphocytes in the restraint group: the proliferating rate severely decreased following stimulation with a relevant antigen, whereas it increased with mitogen. Thus, we report a decrease in cytokine production with relevant antigen (interferon-γ and interleukin-10), without a T helper type 1 and 2 secretion imbalance. Moreover, we observed an alteration in the humoral response, including a delay in isotype maturation and an immunoglobulin G1/G2a imbalance.
Introduction
Acute and chronic stress can both have short and long-term consequences, either protective or damaging, as described more than 64 years ago by Selye.1 According to Dhabhar and McEwen, stress is a constellation of events, beginning with a stimulus (stressor) that precipitates a brain reaction (stress perception) that subsequently activates physiological systems in the body (stress response).2 Allostasis – the ability to achieve stability through change – is critical to survival, and the price of physiological accommodation to stress is considered as allostatic load.3,4 Numerous studies have shown that stress, through the activation of the sympathetic nervous system and the hypothalamic–pituitary–adrenal (HPA) axis can be immunosuppressive and hence detrimental to health. The communication circuits within and between the immune system and the nervous system are complex, including shared ligands and their receptors. Moreover, interactions between the immune and nervous systems are bidirectional.5 The immune system, in addition to its well-known functions, could be considered as a diffuse sense organ scattered through the body, which communicates with the central nervous system. The sharing of ligands (hormones, neurotransmitters and cytokines) and their receptors constitutes a biochemical information network between each of these systems.
However, the interactions between the immune system and the HPA axis are not always deleterious.3,6 The immune system itself responds to pathogens or other antigens with its own form of allostasis. At the same time, other allostatic systems, such as the HPA axis and the autonomous nervous system, interfere with the immune system. It seems relevant to distinguish between acute and chronic effects of stress. For example, acute stress enhances the trafficking of lymphocytes and macrophages to the site of acute challenge through HPA axis stimulation;7 the effects of stress are probably beneficial. By contrast, repeated stress induces a decrease or a disruption of cellular immunity,6,8,9 a decrease of the different subsets of lymphoid cells in secondary lymphoid organs that correlates with a decrease of antibody levels,10 and/or a disruption of cytokine secretion.11
Several studies have reported an alteration of the antibody response and lymphocyte proliferation to hepatitis B or influenza vaccine in the context of stress.12–16 In order to understand the mechanisms involved in the alteration of the immune response, we investigated the effects of a chronic restraint (RST) stress on the response to tetanus toxin (TeNT) vaccine in mice. We assessed the repartition of lymphoid cell subsets in blood and spleen, the functional ability of lymphocytes to proliferate and to produce cytokines and the antibody response to TeNT.
This study highlights the fact that chronic stress may have important deleterious effects on both cellular and humoral vaccine-induced responses. We report here a discordance of the stimulation index of lymphocytes in the restraint group: the proliferating rate severely decreased following stimulation with relevant antigen, whereas it increased with mitogen.
Materials and methods
Mice, restraint protocol and immunization
Six- to eight-week-old male BALB/c mice were purchased from CERJ (Mayenne, France). All experiments were approved by the institutional animal experimentation ethical committee. The experiments were performed twice. Upon arrival, mice were kept in quarantine for a week under standardized housing conditions (five animals per cage with water and food ad libitum) with inversion of the light cycle (light 19.00–07.00 hr). Three groups of 10 mice (RST groups) were stressed according to the following protocol: mice were restrained daily (5 days out of 7) in well-ventilated, horizontal 50-ml conical polypropylene tubes for 6 hr (09.00–15.00 hr). Three groups of 10 mice were used as control. Mice were intraperitoneally (i.p.) immunized on day 0 and boosted on days 7 and 15, at 11.00 hr with 0·5 ml of 1/20 of the human dose of TeNT vaccine (40 Lf/ml, Tétavax, Pasteur vaccins, Marnes-la-Coquette, France) diluted in sterile phosphate-buffered saline (PBS; Sigma, St. Louis, MO). Two groups of 10 mice (one RST and one control) were killed serially on days 5, 12 and 19. Mice were decapitated and blood was collected on EDTA at 09.30 hr in order to avoid fluctuations in the plasma corticosterone levels resulting from differences in circadian rhythm. Plasma were collected and stored at −80°. Plasma corticosterone levels were measured in duplicate with a radioimmunoassay kit ([125I]Corticosterone RIA kit; ICN Pharmaceuticals, Costa Mesa, CA).
Analysis of lymphocyte subsets from spleen and peripheral blood
Spleens were removed immediately after killing and placed in a sterile plastic Petri dish containing 3 ml PBS. Isolation of spleen cells was achieved using a potter homogenizer, transferred into a 50-ml polypropylene tube, and filtered through a 70-µm cell strainer (Becton Dickinson, Franklin Lakes, NJ). Potters were washed with Hanks' balanced salt solution (Sigma) and the final volume of each cell suspension was adjusted to 5 ml. Cells were then washed once in Hanks' solution. Red blood cells were removed by mixing the pellet with lysis NH4Cl buffer (5 min at room temperature, 5 ml per spleen). Cells were washed and adjusted to 106 cells/ml in PBS containing 0·1% sodium azide and 3% bovine serum albumin (BSA; Sigma).
After red cell lysis in NH4Cl buffer (15 min at 25°), peripheral blood leucocytes (PBL) were washed twice with PBS and then suspended at 106 cell/ml in 0·1% sodium azide/3% BSA in PBS. Two-hundred-microlitre samples of cell suspensions were labelled for 30 min at 4° in 96-well plates (V-bottom, Nunc, Roskilde, Denmark) with the following antibodies: fluorescein isothiocyanate (FITC) -labelled anti-CD45, phycoerythrin (PE) -labelled anti-CD8α, cyanine5-phycoerythrin (Cy5) -labelled anti-CD3 complex, Cy5-labelled anti-CD4, cy5-labelled anti-CD45R/B220, PE-labelled anti-CD45RB, PE-labelled anti-B7.2 and PE-labelled anti-Iad (Pharmingen, San Diego, CA). Cells were washed twice with 0·1% sodium azide/3% BSA in PBS and fixed with 1% formaldehyde (Sigma) in the washing buffer. Three-colour flow cytometry analysis was performed on a FACS Vantage™ (Becton Dickinson) using the CellQuest™ software.
Spleen cell proliferation assay and cytokine quantification
Spleen cells were isolated as described above and adjusted to 2 × 106 cells/ml in RPMI-1640 (Sigma) containing 5% fetal calf serum (FCS; Gibco, Merelbeke, Belgium), 100 U/ml penicillin, 0·1 mg/ml streptomycin (Sigma), 2 mm l-glutamine (Sigma), referred to as complete medium. Two-hundred-microlitre samples of these cell suspensions were added in triplicate to the wells of a sterile 96-well plate (Costar-Corning, Cambridge, MA). Each sample was tested in the presence or in the absence of activating agents [concanavalin A (Con A) at 5 µg/ml (Sigma) or TeNT at 10 µg/ml (a gift from Dr Marche, Inserm U 238, Grenoble, France)]. Plates were incubated at 37° in a 5% CO2 atmosphere for 5 days. On day 5, cells were pulsed with [3H]thymidine (ICN Pharmaceuticals) for 18 hr and then harvested. Incorporation of [3H]thymidine was measured in a β-scintillation counter (microbeta, EG & G Wallac, Turku, Finland).
Interferon-γ (IFN-γ) and interleukin-10 (IL-10) secretion by splenocytes in the presence of TeNT or Con A was measured in culture supernatants on day 5. Cytokine levels were determined using enzyme-linked immunosorbent assay (ELISA) kits (Quantikine system, R & D, Oxon, UK).
Detection of anti-TeNT-specific antibody production by ELISA, and antibody-forming cells (AFCs) by ELISPOT assay
Plasma antibody titres were determined by ELISA.17 Briefly, 96-well plates (Maxisorp immunoplates, Nunc) were coated with TeNT at 5 µg/ml. Serial twofold dilutions of plasma were incubated for 2 hr at 37°. Biotinylated goat antibody anti-mouse immunoglobulin M (IgM), IgG1, or IgG2a (Southern Biotechnology Associates, Birmingham, AL) was then added. End-point titres were expressed as the log2 of the reciprocal of the last dilution of sample which gave an optical density (OD) value ≥2 × OD of a pool of control plasma.
Total immunoglobulin-secreting cells and antigen-specific AFCs were assessed with an ELISPOT assay.18 Ninety-six-well nitrocellulose plates (MAHA millipore, Bedford, MA) were coated overnight at room temperature with goat IgG anti-mouse IgG (Sigma) or IgM (Bethyl, Montgomery, TX) at 5 µg/ml (100 µl per well) for total IgG or IgM AFCs, respectively, or with TeNT at 5 µg/ml for anti-TeNT-specific AFCs. After washing (four times with PBS), uncoated sites were blocked with 1% BSA in PBS (200 µl per well) for 1 hr at 37°. Serial twofold dilutions of cell suspensions at 2 × 106 cells/ml in complete medium were added in duplicate (100 µl per well) and incubated for 4 hr at 37° under 5% CO2. Plates were washed three times with PBS and three times with 0·1% Tween-20–PBS. Then, 100 µl of biotinylated goat IgG anti-mouse IgG or IgM (1/20 000, Southern Biotechnology Associates) in PBS−0·1% Tween-20 was added and incubated overnight at 4°. After washing, 100 µl of 1/1000 dilution of alkaline phosphatase-conjugated streptavidin (1 mg/ml, Jackson Laboratories, West Grove, PA) were added and incubated for 30 min at 37°. Plates were then washed four times with PBS and developed by addition of 5-bromo-4-chloro-3-indolyl phosphate (Sigma). Plates were incubated for 10 min at room temperature and washed with water. Wells were photographed and AFCs in each well were counted.
Statistical analysis
Analysis of variance was performed using the Staview™ software. Differences among groups of animals were calculated by the two-tailed analysis using Fisher's Protected Least Significance Difference test.
Results
Assessment of stress
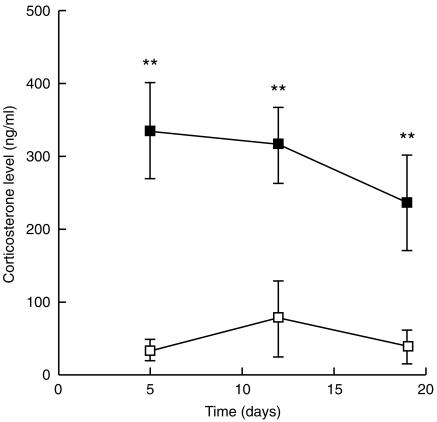
The stress was assessed by plasma corticosterone levels on the day of killing. The levels were three- to fourfold higher in the RST than in the control group with high statistical significance (P < 0·001), and decreased over time (Fig. 1).
Figure 1.
Corticosterone level: blood samples from control mice (□) and RST mice (▪) were taken at 09.30 hr on the day of killing to avoid fluctuations of plasma corticosterone levels resulting from circadian rhythms. Corticosterone levels were measured by radioimmunoassay; results are expressed as ng/ml plasma. Data are shown as mean ± SD for each group of mice. **P < 0·001.
Redistribution of lymphocytes subsets in spleen and peripheral blood
We addressed the question of the redistribution of relative percentages of leucocyte subsets in blood and spleen in mice under stressful conditions. Table 1 shows that spleen subsets were distributed similarly in both the RST and the control group, but peripheral cell subsets clearly differed, with a decrease of the relative percentages of B and CD4+ T cells in the RST group (P < 0·05).
Table 1.
Cell subsets in the spleen and peripheral blood lymphocytes
| Time (days) | Group | B cells | T cells | T CD4+ | T CD8+ |
|---|---|---|---|---|---|
| Spleen | |||||
| 5 | control | 56·0±5·6 | 38·5±2·0 | 30·5±2·3 | 11·5±2·3 |
| restraint | 46·7±3·6 | 37·8±3·0 | 29·9±2·6 | 13·0±1·0 | |
| 12 | control | 53·1±2·38 | 36·2±1·6 | 30·0±2·1 | 11·1±0·8 |
| restraint | 55·0±2·5 | 34·3±2·7 | 28·9±1·4 | 10·3±1·1 | |
| 19 | control | 43·0±8·4 | 37·7±3·6 | 29·5±3·6 | 8·8±3·2 |
| restraint | 48·6±6·8 | 38·5±3·6 | 30·3±2·2 | 10·1±2·4 | |
| Peripheral blood lymphocytes | |||||
| 5 | control | 34·1±6·5 | 49·5±4·2 | 42·4±3·1 | 11·5±1·5 |
| restraint | 21·8±9·0 | 59·2±6·4 | 47·8±4·8 | 15·3±1·9 | |
| 12 | control | 42·8±8·2 | 60·6±6·1 | 49·3±4·3 | 14·5±1·4 |
| restraint | 38·2±2·7 | 62·3±2·9 | 49·4±4·1 | 16·5±2·2 | |
| 19 | control | 40·2±6·1* | 57·2±6·8* | 43·6±4·3* | 14·0±1·7 |
| restraint | 25·8±11·7 | 46·9±7·8 | 33·7±5·3 | 14·9±2·1 | |
Cells were labelled with FITC-, PE- and Cy5-conjugated antibodies and further analysed by flow cytometry. Results are expressed for each group of mice as mean percentages of cells expressing relevant markers±SD.
P < 0·05.
The modulation of cell surface marker expression has been reported to be related to maturation or cell activation.19,20 Therefore, we investigated the expression of several cell surface markers on splenocytes and PBLs (Table 2). CD45RB expression increases as T cells progress from naive to memory cells,21 B7.2 expression on antigen-presenting cells (APC) plays an important role in T–B-cell costimulatory interactions,22 and major histocompatibility complex (MHC) class II expression on APCs plays a role in restriction of the T-cell recognition. In the spleen, on day 5, B7.2 expression tended to increase whereas this modification disappeared on days 12 and 19, but we cannot rule out a lower labelling efficiency. We observed no significant modification of CD45RB expression on CD4+ T cells.
Table 2.
Cell surface marker expression in the spleen and in peripheral blood lymphocytes
| B Cells | |||||
|---|---|---|---|---|---|
| Time (days) | Group | CD4 cells CD45RB | MHC II | B 7.2 | |
| Spleen | |||||
| 5 | control | 91·8 ± 3·7 | 64·2 ± 11·5 | 3·1 ± 1·1* | |
| restraint | 87·7 ± 2·1 | 80·5 ± 6·9 | 6·3 ± 1·6 | ||
| 12 | control | 96·4 ± 0·6 | 91·4 ± 2·9 | 16·5 ± 4·6 | |
| restraint | 96·9 ± 0·8 | 90·6 ± 2·5 | 13·7 ± 2·2 | ||
| 19 | control | 86·1 ± 3·0 | 77·9 ± 3·4 | 8·0 ± 1·8 | |
| restraint | 88·1 ± 1·8 | 82·3 ± 10·2 | 10·6 ± 3·4 | ||
| Peripheral blood lymphocytes | |||||
| 5 | control | 98·4 ± 0·8 | 65·8 ± 18·0 | 20·9 ± 16·6 | |
| restraint | 97·6 ± 1·5 | 60·1 ± 12·7 | 19·6 ± 14·7 | ||
| 12 | control | 89·3 ± 1·6 | 74·8 ± 4,2* | 12·3 ± 6·8* | |
| restraint | 89·4 ± 1·5 | 51·5 ± 10·4 | 5·7 ± 3·9 | ||
| 19 | control | 95·4 ± 1·7* | 80·2 ± 6·1* | nd | |
| restraint | 91·9 ± 2·1 | 65·5 ± 15·4 | nd | ||
Cells were labelled with FITC-, PE- and Cy5-conjugated antibodies and further analysed by flow cytometry. Results are expressed for each group of mice as mean percentages of cells expressing relevant markers ± SD.
P < 0·05; nd, not done.
By contrast, PBLs exhibited severe modulation of cell surface markers in RST mice: the relative percentage of B cells expressing MHC class II molecules (P < 0·05) and of B7.2 (P < 0·05) was lower. Indeed, the relative percentage of T CD4+ cells expressing the CD45RB memory marker was significantly lower on day 19 (P < 0·05).
Spleen cell activation upon antigen and mitogen challenge: proliferation and cytokine expression
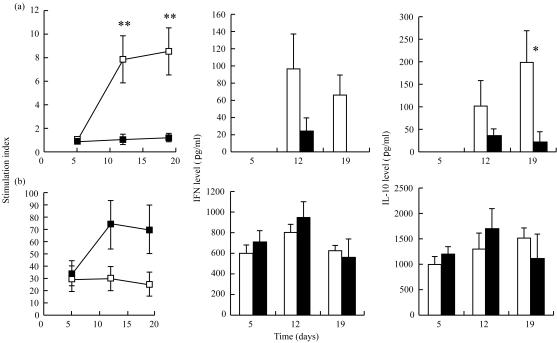
We next asked if T-cell responses could be modified by stress. Spleen cell proliferation was measured on days 5, 12 and 19. Our preliminary studies had shown that optimal proliferation rates with TeNT were obtained after 5 days of culture. Using this protocol, we observed striking differences in the proliferating capacity with TeNT as compared to Con A (Fig. 2). On days 12 and 19, after the first and second boost, the stimulation index (SI) with TeNT increased significantly in the control group but not in the RST group (P < 0·001). By contrast, a trend towards a higher SI in the RST group was observed after mitogen stimulation.
Figure 2.
Stimulation index and cytokine production levels in splenocyte cultures. Splenocytes from each mouse were culture for 5 days. Incorporation of [3H]thymidine was measured in a β-scintillation counter. The stimulation indices of control mice (□) and RT mice (▪) were calculated as a ratio of counts per minute (c.p.m.) in stimulated wells/c.p.m. in control wells. IFN-γ and IL-10 quantification of control mice (open bars) and of RST mice (filled bars) were performed on supernatants using ELISA. After culture with TeNT, IFN-γ and IL-10 were undetectable on day 5 in both groups, and IFN-γ was undetectable on day 19 in the RST group. Results with either TeNT (a) or Con A (b) are expressed as mean ± SE. *P < 0·05; **P < 0·001.
We further investigated the ability of lymphocytes to produce cytokines in culture with TeNT or mitogen. When cultured with TeNT, splenocytes from RST mice displayed a significantly reduced production of cytokines from both T helper type 1 (Th1; IFN-γ) and Th2 (IL-10) subtypes compared to the splenocytes of control mice (Fig. 2). After mitogenic stimulation, cytokine production was similar in both RST and control groups.
Kinetics of the antibody production
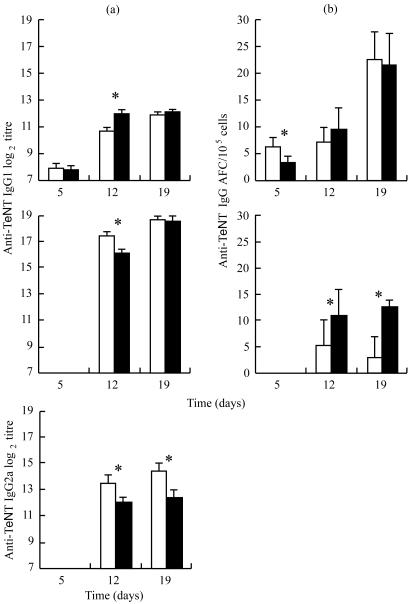
To investigate the effects of stress on the humoral response, anti-TeNT antibody titres were assessed on days 5, 12 and 19. No differences between RST and control mice were found 5 days after the first immunization (Fig. 3). On day 12, anti-TeNT IgM titres increased (P < 0·05) in RST mice, whereas anti-TeNT IgG1 and IgG2a decreased compared to the control group (P < 0·05). In contrast, 19 days after the first immunization and two booster injections, the titre of anti-TeNT IgM was similar in both groups, but a significant decrease was observed in anti-TeNT IgG. These differences between the RST and control groups were significant only with anti-TeNT IgG2a isotype (P < 0·05).
Figure 3.
Anti-TeNT antibody titre in the plasma and anti-TeNT antibody-forming cells in the spleen. (a) Titres of anti-TeNT IgM, IgG1 and IgG2a in plasma were evaluated by ELISA. Results are expressed as mean of log2 titre ± SE of control mice (open bars) and of RST mice (filled bars). (b) Anti-TeNT-specific AFCs IgM and IgG were quantified by ELISPOT. Results are expressed as mean ± SE of control mice (open bars) and of RST mice (filled bars). Anti-TeNT IgG and anti-TeNT IgG FACs were undetectable on day 5. *P < 0·05.
We evaluated the number of anti-TeNT and total IgM and IgG AFCs (Fig. 3). A difference in the number of specific IgM AFCs was observed on day 5 (P < 0·05), while total IgM and IgG AFCs were not affected by stress (data not shown). On days 12 and 19, anti-TeNT IgM AFC numbers were similar, whereas anti-TeNT IgG AFCs were increased at days 12 and 19 in RST group (P < 0·05).
Discussion
Our results provide new insight into the modulation of the immune response in a context of chronic restraint stress.
First, we confirmed the effects of chronic restraint stress on lymphocyte tissue distribution, in agreement with a previous study.10 In the spleen, the percentages of different subsets were not affected, whereas in blood we observed a significant decrease in B and CD4+ T cell numbers on day 19. Several studies have reported that stress induces a modulation of leucocyte trafficking.23,24 Cell trafficking is crucial to surveillance as well as to the effector function. Glucocorticoids, a major stress mediator, interact with leucocyte trafficking. In the delayed type hypersensitivity model, it was shown that glucocorticoids induce a redeployment of leucocytes throughout the body that enhances cell-mediated immunity in acute stress, while they are immunosuppressive in chronic stress.2,6 However, in our study we observed in parallel a decline in B and T CD4+ lymphocyte numbers and a decrease in corticosterone levels.
Moreover, we observed an important down regulation of surface molecules on peripheral B cells, strategic for efficient T-cell activation (MHC II and B7.2). These modifications may compromise the full development of B-cell functions independently of the effects of stress on leucocyte redeployment. The question remains whether circulating B cells could further fully develop in lymphoid organs.
More important is the information on lymphocyte function. We have shown that lymphocytes from RST mice tend to be more susceptible to mitogen activation. Thus, mediators of stress (e.g. glucocorticoids or sympathetic neuromediators) seem to enhance directly or indirectly the proliferation capacity of splenocytes. However, in culture with the relevant antigen, splenocytes of RST mice had a lower SI than splenocytes of control mice. Similar results have been reported in a model of inescapable footshock stress.8 Splenocytes of rats immunized with cholera toxin did not respond to the relevant antigen but proliferated in the presence of lectin (Con A and phytohaemagglutinin). We referred to this situation as a restraint stress-induced discordance of SI. These results are compatible with two main hypotheses: either mediators of stress interact directly with specific proliferating T cells after presentation of TeNT-derived peptides by APC, or APCs, in a context of stress mediators, present peptides loaded on MHC without stimulating T cells. In both cases, anti-TeNT T cells would be induced to become anergic or to undergo apoptosis, explaining the SI differences between RST and control mice splenocytes. It is noteworthy that these hypotheses are not mutually exclusive, and that mediators of stress could interact with both T cells and APCs.
On the one hand, we could suppose that T cells are sensitive to mediators of stress. Glucocorticoids that are produced within the thymic environment have been shown to induce thymocyte apoptosis.25 Furthermore, steroid-induced apoptosis of thymocytes has been suggested as a potential mechanism for the removal of non-selected thymocytes, and thymus-derived glucocorticoids set the thresholds for thymocyte selection by inhibiting T-cell-receptor-mediated thymocyte activation.26 Thus, we can draw the hypothesis that corticosterone released by the HPA axis during stress could interfere with T lymphocytes after engagement of T-cell receptors. Anti-TeNT T-cell clones could have been stimulated in a context of mediators of stress, including glucocorticoids. In these conditions, the physiological stimulation of anti-TeNT clones would lead to a stress-induced abortion of these clones after a rapid expansion (as for corticosteroid-induced cell death in thymus). Moreover, other mediators of stress could induce deletion of lymphocytes. A recent report sheds new light on the promotion of apoptosis of splenocytes in a model of restraint stress.27 This study suggests that stress-induced changes in CD95 (Fas/Apo-1) expression and lymphocyte number could be blocked by specific opioid receptor antagonists, indicating a pivotal role of endogenous opioids in this process.
On the other hand, the dendritic cell system of APCs, is the initiator and the modulator of the immune response.28–30 The efficiency of presentation by dendritic cells depends on their maturation status, which is driven by complex environmental interactions. Previous studies have shown that mediators of stress, such as glucocorticoids, modulate the efficiency of presentation by DCs. Glucocorticoids can either induce tolerance31 or modulate the capacity to stimulate T cells.32,33 In our model, we can hypothesize that an alteration of microenvironment involving stress mediators could modulate the APCs' ability to stimulate T cells. In this case, APCs that present TeNT-derived peptides without costimulation signals or with a low rate of peptides loaded on MHC class II could drive anergy of TeNT-specific T cells, or deletion of these clones.
We observed other disruptions in the maturation of the humoral response. First, anti-TeNT antibody titres showed a delay in isotype maturation in RST mice (higher titres of IgM and lower titres of IgG1 and IgG2a on day 12). The maturation of B cells into plasma cells was correlated with their migration from spleen germinal centres to the bone marrow. A delay in the maturation of B cells could therefore account for the observed increase in numbers of anti-TeNT IgG-secreting cells in the spleen of RST mice. Second, we observed an IgG1/IgG2a imbalance in RST mice. Although no single cytokine appears to be required for the generation of IgG1/IgG2a responses in vivo, IFN-γ has been shown to enhance IgG2a production, while IL-4 stimulates IgG1 responses.34,35 Thus, the IgG1/IgG2a imbalance is proposed to reflect a shift toward Th2.10 However, our results show that Th1 and Th2 cytokine production did not shift with time from Th1 toward Th2. In the absence of Th lymphocytes, because of their stress-induced functional depletion, cytokines (either Th1 or Th2) would not be produced in the microenvironment of the germinal centres of RST mice. In our model of stress, we could hypothesize that the IgG1/IgG2a imbalance reflects an absence of cytokine signalling rather than a positive shift toward Th2 secretion, in opposition to other current hypotheses.36
This study is of particular interest since it suggests that chronic stress could be detrimental to the T-cell vaccine response. The clinical impact remains to be evaluated. The identification of stress mediators and the deciphering of the molecular mechanisms involved in these alterations warrant further investigations.
Acknowledgments
This study was supported by grants from DGA (PEA 980816). We thank Drs O. Attree, X. Bigard and A. Quesnel-Hellmann for their helpful discussions and comments.
Abbreviations
- Ab
antibody
- APC
antigen-presenting cell
- Cy5
cyanine5-phycoerythrin
- Con A
concanavalin A
- DC
dendritic cell
- FCS
fetal calf serum
- HPA
hypothalamic–pituitary–adrenal
- IFN
interferon
- OD
optical density
- PBL
peripheral blood leucocytes
- PE
phycoerythrin
- RST
restraint
- SI
stimulation index
- TeNT
tetanus toxin
REFERENCES
- 1.Selye H. Syndrome produced by diverse nocuous agents. Nature. 1936;138:32. doi: 10.1176/jnp.10.2.230a. [DOI] [PubMed] [Google Scholar]
- 2.Dhabhar FS, Mcewen BS. Enhancing versus suppressive effects of stress hormones on skin immune function. Proc Natl Acad Sci USA. 1999;96:1059–64. doi: 10.1073/pnas.96.3.1059. 10.1073/pnas.96.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–9. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 4.Sapolsky RM. McEwen-induced modulation of endocrine history: a partial review. Stress. 1997;2:1–12. doi: 10.3109/10253899709014733. [DOI] [PubMed] [Google Scholar]
- 5.Maier SF, Watkins LR. Cytokines for psychologists: Implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychol Rev. 1998;105:83–107. doi: 10.1037/0033-295x.105.1.83. [DOI] [PubMed] [Google Scholar]
- 6.Dhabhar FS, McEwen BS. Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: a potential role for leukocyte trafficking. Brain Behav Immun. 1997;11:286–306. doi: 10.1006/brbi.1997.0508. 10.1006/brbi.1997.0508. [DOI] [PubMed] [Google Scholar]
- 7.Dhabhar FS, Ewen BSM. Stress-induced enhancement of antigen-specific cell-mediated immunity. J Immunol. 1996;156:2608–15. [PubMed] [Google Scholar]
- 8.Kusnecov AW, Rabin BS. Unescapable footshock exposure differentially alters antigen- and mitogen-stimulated spleen cell proliferation in rats. J Neuroimmunol. 1993;44:33–42. doi: 10.1016/0165-5728(93)90265-z. [DOI] [PubMed] [Google Scholar]
- 9.Stefanski V, Engler H. Effects of acute and chronic social stress on blood cellular immunity in rats. Physiol Behav. 1998;64:733–41. doi: 10.1016/s0031-9384(98)00127-9. 10.1016/s0031-9384(98)00127-9. [DOI] [PubMed] [Google Scholar]
- 10.Fukui Y, Sudo N, Yu X-N, Nukina H, Sogawa H, Kubo C. The restraint stress-induced reduction in lymphocyte cell number in lymphoid organs correlates with the suppression of in vivo antibody production. J Neuroimmunol. 1997;79:211–5. doi: 10.1016/s0165-5728(97)00126-4. 10.1016/s0165-5728(97)00126-4. [DOI] [PubMed] [Google Scholar]
- 11.Zhang D, Kishihara K, Wang B, Mizobe K, Kubo C, Nomoto K. Restraint stress-induced immunosuppression by inhibiting leukocyte migration and Th1 cytokine expression during intraperitoneal infection of Listeria monocytogenes. J Neuroimmunol. 1998;92:139–51. doi: 10.1016/s0165-5728(98)00197-0. 10.1016/s0165-5728(98)00197-0. [DOI] [PubMed] [Google Scholar]
- 12.Glaser R, Kiecolt-Glaser JK, Malarkey WB, Sheridan JF. The influence of psychological stress on the immune response. Ann N Y Acad Sci. 1998;840:649–55. doi: 10.1111/j.1749-6632.1998.tb09603.x. [DOI] [PubMed] [Google Scholar]
- 13.Glaser R, Kiecolt-Glaser JK, Bonneau RH, Malarkey W, Kennedy S, Hughes J. Stress-induced modulation of the immune response to recombinant hepatitis B vaccine. Psychosom Med. 1992;54:22–9. doi: 10.1097/00006842-199201000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Jabaaij L, Hattum JV, Vingerhoets JJ, Oostveen FG, Duivenvoorden HJ, Baillieux RE. Modulation of immune response to rDNA hepatitis B vaccination by psychological stress. J Psychosom Res. 1996;41:129–37. doi: 10.1016/0022-3999(96)00123-7. 10.1016/0022-3999(96)00123-7. [DOI] [PubMed] [Google Scholar]
- 15.Kiecolt-Glaser JK, Glaser R, Gravenstein S, Malarkey WB, Sheridan J. Chronic stress alters the immune response to influenza virus vaccine in older adults. Proc Natl Acad Sci USA. 1996;93:3043–7. doi: 10.1073/pnas.93.7.3043. 10.1073/pnas.93.7.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vedhara K, Cox NKM, Wilcok GKW, Perks P, Hunt M, Anderson S, Lightman SL, Shanks NM. Chronic stress in elderly carers of dementia patients and antibody responses to influenza vaccination. Lancet. 1999;353:627–31. doi: 10.1016/S0140-6736(98)06098-X. 10.1016/s0140-6736(98)06098-x. [DOI] [PubMed] [Google Scholar]
- 17.Xu-Amano J, Kiyono H, Jackson RJ, et al. Helper T cell subsets for immunoglobulin A responses: Oral immunization with tetanus toxoid and cholera toxin as adjuvant selectively induces Th2 cells in mucosa associated tissues. J Exp Med. 1993;178:1309–20. doi: 10.1084/jem.178.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jackson RJ, Fujihashi K, Xu-Amano J, Kiyono H, Elson CO, Mcghee JR. Optimizing oral vaccines: induction of systemic and mucosal B-cell and antibody responses to tetanus toxoid by use of cholera toxin as an adjuvant. Infect Immun. 1993;61:4272–9. doi: 10.1128/iai.61.10.4272-4279.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lydyard P, Grossi C. Development of the immune system. In: Roitt I, Brostoff J, Male D, editors. Immunology. London: C. V. Mosby; 1998. pp. 155–69. [Google Scholar]
- 20.Liu Y-J, Arpin C. Germinal center development. Immunol Rev. 1997;156:111–26. doi: 10.1111/j.1600-065x.1997.tb00963.x. [DOI] [PubMed] [Google Scholar]
- 21.Dianzani U, Luqman M, Rojo J, Yagi J, Baron JL, Woods A, Janeway Ca, Jr, Bottomly K. Molecular associations on the T cell surface correlate with immunologic memory. Eur J Immunol. 1990;20:2249–57. doi: 10.1002/eji.1830201014. [DOI] [PubMed] [Google Scholar]
- 22.Bluestone JA. New perspectives of CD28-B7-mediated T cell costimulation. Immunity. 1995;2:555–9. doi: 10.1016/1074-7613(95)90000-4. [DOI] [PubMed] [Google Scholar]
- 23.Dhabhar FS, Miller AH, Mcewen B, Spencer RL. Effects of stress on immune cell distribution. Dynamics and hormonal mechanisms. J Immunol. 1995;154:5511–27. [PubMed] [Google Scholar]
- 24.Dhabhar FS, Miller AH, Ewen BSM, Spencer RL. Stress-induced changes in blood leukocyte distribution. Role of adrenal steroid hormones. J Immunol. 1996;157:1638–44. [PubMed] [Google Scholar]
- 25.Wagner Dh, Jr, Hagman J, Linsley PS, Hodson W, Freed JH, Newell MK. Rescue of thymocytes from glucocorticoid-induced cell death mediated by CD28/CTLA-4 costimulatory interactions with B7-1/B7-2. J Exp Med. 1996;184:1631–8. doi: 10.1084/jem.184.5.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vacchio MS, Ashwell JD. Thymus-derived glucocorticoids regulate antigen-specific positive selection. J Exp Med. 1997;185:2033–8. doi: 10.1084/jem.185.11.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yin D, Tuhthill D, Mufson RA, Shi Y. Chronic restraint stress promotes lymphocyte apoptosis by modulating CD95 expression. J Exp Med. 2000;191:1423–8. doi: 10.1084/jem.191.8.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–96. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 29.Hart DNJ. Dendritic cells: Unique leukocyte populations which control the primary immune response. Blood. 1997;90:3245–87. [PubMed] [Google Scholar]
- 30.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 31.Jong ED, Vieira PL, Kalinski P, Kapsenberg ML. Corticosteroids inhibit the production of inflammatory mediators in immature monocyte-derived DC and induce the development of tolerogenic DC3. J Leukocyte Biol. 1999;66:201–4. doi: 10.1002/jlb.66.2.201. [DOI] [PubMed] [Google Scholar]
- 32.Piemonti L, Monti P, Allavena P, Leone BE, Caputo A, Carlo VD. Glucocorticoids increase the endocytic activity of human dendritic cells. Int Immunol. 1999;11:1519–26. doi: 10.1093/intimm/11.9.1519. 10.1093/intimm/11.9.1519. [DOI] [PubMed] [Google Scholar]
- 33.Moser M, Smedt TD, Sornasse T, et al. Glucocorticoids down-regulate dendritic cell function in vitro and in vivo. Eur J Immunol. 1995;25:2818–24. doi: 10.1002/eji.1830251016. [DOI] [PubMed] [Google Scholar]
- 34.Coffman RL, Carty J. A T cell activity that enhances polyclonal IgE production and its inhibition by interferon-gamma. J Immunol. 1986;136:949–54. [PubMed] [Google Scholar]
- 35.Snapper CM, Paul WE. Interferon-gamma and B-cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987;236:944–77. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- 36.Rook GAW, Zumia A. Gulf War syndrome: is it due to a systemic shift in cytokine balance towards a Th2 profile? Lancet. 1997;349:1831–3. doi: 10.1016/S0140-6736(97)01164-1. 10.1016/s0140-6736(97)01164-1. [DOI] [PubMed] [Google Scholar]