Abstract
Oligodeoxynucleotides containing CpG motifs (CpG-ODN) are potent in vitro B-cell activators and they have been successfully used to increase in vivo antibody responses to T-dependent peptide and protein antigens. In contrast, the use of CpG-ODN to enhance in vivo antibody responses to various T-independent type 2 (TI-2) antigens has recently generated contradictory results. In this study, we compared the CpG-ODN stimulatory effect on antibody responses of adult and young BALB/c mice to trinitrophenylaminoethyl-carboxymethyl (TNP) -Ficoll and to polysaccharides (PS) from several distinct serotypes of Streptococcus pneumoniae (SPn). CpG-ODN co-administration significantly enhanced antigen-specific immunoglobulin M (IgM), IgG, IgG1 and IgG2a titres to TNP-Ficoll. The depletion of CD4+ cells by monclonal antibodies (GK1.5) identified their essential role in CpG-ODN-mediated enhancement of antibody responses. In contrast to TNP-Ficoll, CpG-ODN failed to enhance IgM and IgG responses to any of the 18 SPnPS serotypes tested. Providing T-cell epitopes by the conjugation of SPnPS to the carrier protein tetanus toxoid again allowed CpG-ODN to mediate enhancement of IgG, IgG2a and IgG3 responses to most SPnPS serotypes. Thus, antigen-presenting cell/T-cell interaction appears to largely mediate the in vivo influence of CpG-ODN on antibody responses to TI-2 antigens. In early life, additional factors limit CpG-ODN modulation of antibody responses to TI-2 antigens.
Introduction
Thymus-independent type-2 (TI-2) antigens were originally described as evoking primarily immunoglobulin M (IgM) responses, with little or no IgG antibody formation, and were further defined as capable of inducing antibody responses in T-cell-deficient (nu/nu) mice, but not in mice bearing the xid mutation or in young mice.1,2 Among these TI-2 antigens, distinct polysaccharides (PS) constituting the cell wall of encapsulated bacteria, dextran, or synthetic PS, such as Ficoll, share common features such as large molecular weight, ordered display of multiple identical epitopes and poor biodegradability.3 Although T cells are not directly primed by TI-2 antigens, a certain regulatory role of T cells and T-cell- or macrophage-derived factors on B-cell responses has been described for most TI-2 antigens, including pneumococcal PS and trinitrophenylaminoethyl-carboxymethyl (TNP) -Ficoll.3 Recently, mimicking antigen-presenting cell (APC) and T-cell help by co-administration of recombinant interleukin-12 (IL-12) and anti-CD40 monoclonal antibodies (mAb) resulted in a significant increase in antibody titres to pneumococcal PS.4,5 Furthermore, although Ficoll was initially considered as a model TI-2 antigen, APC and T cells were later shown to control antibody responses to Ficoll.6,7 Thus, TI-2 antigens represent a rather heterogeneous group of different molecules where the nature of the B-cell activation signal(s) is extremely critical for the determination of both the qualitative and quantitative profiles of immunoglobulin isotype production, probably in response to various cytokines.3
Recently, non-methylated CpG-motifs present in bacterial DNA or contained in short synthetic oligodeoxynucleotides (ODN) were shown to induce strong B-cell activation in vitro.8 Although the exact mechanisms responsible for this B-cell-stimulatory effect are not yet known, they are likely to involve the induction of immediate early genes such as erg-1, c-fos and c-myc.9 CpG-ODN sequences have also been demonstrated to strongly activate APC and natural killer (NK) cells, to promote the secretion of cytokines such as IL-6, IL-10, IL-12, IL-18, tumour necrosis factor-α, type I interferons, and interferon-γ and to induce the expression of major histocompatibility complex and various co-stimulatory molecules.10 This activation has been shown to result in a strong increase of antibody and T-cell responses to a variety of protein antigens, as reviewed by Wagner.10 In contrast, controversial results were recently reported from experiments using CpG-ODN to increase antibody responses to TI-2 antigens. In one study CpG-ODN failed to enhance or even suppressed Pseudomonas aeruginosa-PS-specific antibody responses,11 whereas another study reported that CpG-ODN increases TNP-Ficoll specific IgG but not IgM responses.12 Most recently, CpG-ODN were reported to enhance Hemophilus influenzae b (HIB) vaccine responses, but only when HIB-PS were conjugated to a carrier protein.13 CpG-ODN administration was also shown to enhance antibody responses against two pneumococcal PS conjugated to a diphtheria toxin protein carrier,14 but responses to plain, unconjugated pneumococcal PS were not evaluated. To understand better the features of CpG-ODN modulation of antibody responses to TI-2 antigens, we assessed the capacity of CpG-ODN to enhance in vivo antibody responses to TNP-Ficoll and to a panel of distinct Streptococcus pneumoniae (Spn) PS serotypes, which were either administered as plain PS or as protein-conjugated vaccines. Given the potential importance of an enhancement of early-life antibody responses to bacterial PS, and the capacity of CpG-ODN to enhance early-life murine antibody responses to peptide and protein vaccines,15,16 we also assessed the influence of CpG-ODN on antibody responses elicited by PS immunization in early life.
Materials and methods
Mice
Specific pathogen-free adult BALB/c inbred mice were purchased from BRL (Füllinsdorf, Switzerland) and kept under specific pathogen-free conditions. Breeding cages were checked daily for new births. Pups were kept with mothers until weaning at the age of 4 weeks. The evaluation of early-life responses was performed in 2-week-old mice, the earliest age allowing preimmunization bleeding. Mice were bled before and at several time-points after immunization at the tail vein except for mice at 2 weeks of age, which were bled retro-orbitally. Antigen formulations were injected subcutaneously (s.c.) in groups of four to eight mice, unless otherwise indicated in the figure legends. Aliquots of serum from individual mice were assessed either individually or pooled (by group and time-point) for the presence of antigen-specific antibodies.
Antigens, adjuvants and immunization procedures
Pneumovax-23 [Merck, Sharp and Dohuse (MSD), West Point, PA] is a polyvalent pneumococcal vaccine containing 23 different purified cell wall PS from SPn. In our experiments, it was used at a dose of 25 µg (20 µl) of SPnPS per mouse.5 Pn11-TT, a polyvalent SPn glycoconjugate vaccine containing 11 different purified cell wall PS from SPn individually conjugated to tetanus toxoid (TT), was kindly provided by Pasteur Mérieux Connaught (Marcy l'Étoile, France). It was used at one-fifth of the human dose (0·1 µg to 0·5 µg of each SPnPS per dose, and 1 µg for serotype 26/6B). Immunizations were performed s.c. at the neck. TNP45-Ficoll and TNP8-OVA were obtained from Biosearch Technologies (Novato, CA). Both antigens were diluted in saline and each mouse was injected s.c. with a dose of 50 µg. In experiments with CD4 depletions, a dose of 25 µg TNP-Ficoll was used. Where specified, the antigens were mixed with aluminium hydroxide (AlOH, Chiron, Italy) (0·25 mg for young mice, 1 mg for adult mice) immediately prior to immunization. ODN [CpG-ODN 1826, TCCATGACGTTCCTGACGTT, or control (ctr)-ODN 1982, TCCAGGACTTCTCTCAGGTT] were used at a dose of 3 µg in young mice and 50 µg in adult mice, as described previously.16 ODN with a nuclease-resistant phosphorothioate backbone were provided by the Coley Pharmaceutical Group (Wellesley, MA). Endotoxin levels in all ODN were < 0·075 EU/ml tested by Limulus assay (Whittaker Bioproducts, Walkersville, MD). The Na+ salts of the ODN were ethanol-precipitated and then resuspended in 10 mm Tris (pH 7·0), 1 mm ethylenediaminetetraacetic acid (EDTA) for storage at −20° prior to dilution into phosphate-buffered saline (PBS) for injection. Where indicated, CD4+ cells were depleted in mice by weekly intraperitoneal (i.p.) injections of 0·3 mg anti-CD4 mAb (GK1.5), starting 1 week before immunization with antigen. The extent of CD4+ cell depletion was monitored on peripheral blood samples by flow cytometry and was generally > 95%.
Quantification of antigen-specific antibodies
Sera from mice immunized with pneumococcal vaccines, either individual or pooled by experimental groups and time-points, were preadsorbed with Pneumococcal C-polysaccharide (CPS) (Serum Institute Kopenhagen, Denmark, 2 µg/ml), for 1 hr at room temperature. Sera were then tested by enzyme-linked immunosorbent assay (ELISA) on Nunc Maxisorb plates coated with one of several different pneumococcal serotypes (American Type Culture Collection, Manassas, VA), according to the World Health Organisation Consensus Protocol for determination of specific pneumococcal antibodies, modified for detection of mouse-specific immunoglobulin by use of the relevant isotype-specific (IgM, IgG, IgG1, or IgG2a) peroxidase-conjugated goat or rabbit anti-mouse antibodies (Zymed, San Francisco, CA).17
For the detection of TNP-specific antibodies, Nunc Maxisorb plates were coated overnight at 4° with TNP-bovine serum albumin (BSA; Biosearch Technologies, Novato, CA) before saturation with 1% BSA (Sigma, St Louis, MI) in PBS-0·05% Tween-20, for 1 hr at room temperature. Sera were diluted in saturation buffer and serial twofold dilutions (starting at 1/100) were incubated for 2 hr at room temperature. After washing, the relevant isotype-specific peroxidase-conjugated goat or rabbit anti-mouse antibody (Zymed) was added for 2 hr at 37° prior to washing, incubation with substrate and reading. For both tests antibody levels were expressed as ELISA titres by reference to serial dilution of a previously out-titrated serum pool from immunized adult mice. Antibody titres below the cut-off of the assay were given an arbitrary titre of one-half of the cut-off value in order to allow calculation of geometric mean antibody titres.
Determination of in vitro proliferative responses
Single spleen cell suspensions were prepared from spleens and cultured at 37° in a 5% CO2 humidified incubator, in RPMI-1640 supplemented with 10% (v/v) heat inactivated fetal calf serum (FCS), 1·5 mm l-glutamine, 50 µm 2-mercaptoethanol, 100 U/ml penicillin and 100 µg/ml streptomycin. Cells were seeded on to 96-well plates and treated with media or ODN (at the indicated concentrations) at a density of 105 cells/200 µl/well. This procedure was previously shown to induce proliferation of only B cells.8 At the end of the incubation period, cells were pulsed with 1 µCi of [3H]thymidine before harvesting and scintillation counting, as described previously.8,17 Standard deviations of the triplicate wells were < 5%.
Determination of IgG/IgM titres after in vitro proliferative responses
For the detection of IgM and IgG in culture supernatants, spleen cells were seeded into 96-well plates and treated with media or ODN (at the indicated concentrations) at a density of 106 cells/200 µl/well. After incubation for 48 hr cell supernatants were harvested and immunoglobulin titres determined by ELISA. Nunc Maxisorb plates were coated overnight at 4° with goat anti-mouse IgG Fc (5 µg/ml) or goat anti-mouse IgM (2 µg/ml) in PBS (Cappel/ICN, Costa Mesa, CA), before saturation with 1% BSA (Sigma) in PBS-0·05% Tween-20, for 1 hr at room temperature. Sera were diluted in saturation buffer and twofold serial dilutions of supernatant (starting at 1/2) were incubated overnight at 4°. After washing, peroxidase-conjugated goat anti-mouse IgG or goat anti-mouse IgM (Zymed) were added for 2 hr at 37° prior to washing, followed by incubation with substrate and measurement of optical density (OD). Results of the assays were expressed as ELISA titres by reference to serial dilution of a previously out-titrated serum pool from naïve mice.
Statistical analysis
Significance analysis between results obtained from various groups of mice was performed by using the Mann–Whitney U-test. Differences with probability values > 0·05 were considered insignificant.
Results
CpG-ODN enhance in vivo antibody responses to TNP-Ficoll
Adult BALB/c mice (> 8-week-old) were first immunized with 50 µg of AlOH-adjuvanted TNP-Ficoll together with either CpG- or ctr-ODN. CpG-ODN administration significantly enhanced peak TNP-specific IgM titres (measured 10 days post-immunization) compared to ctr-ODN (Table 1). CpG-ODN only slightly enhanced total IgG (not shown) and IgG1 titres, but they markedly increased IgG2a antibodies (Table 1). When immunization was performed in 2-week-old mice, CpG-ODN administration significantly enhanced IgG1 but not IgG2a responses to TNP-Ficoll (Table 1). In contrast, when TNP was conjugated to the ovalbumin protein carrier (TNP-OVA), a strong CpG-mediated enhancement of both IgG1 and IgG2a titres was observed regardless of age at immunization (Table 1).
Table 1.
Anti-TNP antibody ELISA titres (log10) at 10 days (IgM) or at 28 days (IgG1 and IgG2a) postimmunization with TNP-Ficoll or TNP-OVA. Means and SD of each group
| Mice | Antigen | ODN | IgM | IgG1 | IgG2a |
|---|---|---|---|---|---|
| Ad (n = 4) | TNP-Ficoll | CpG- | 4·7 ± 0·1* | 5·9 ± 0·2 | 5·5 ± 0·1* |
| ctr- | 4·5 ± 0·1* | 5·4 ± 0·3 | 3·5 ± 0·1* | ||
| 2w (n = 8) | TNP-Ficoll | CpG- | 4·5 ± 0·1 | 5·1 ± 0·2* | 2·8 ± 0·5 |
| ctr- | 4·4 ± 0·1 | 4·2 ± 0·3* | 2·6 ± 0·3 | ||
| Ad (n = 4) | TNP-OVA | CpG- | ND | 5·6 ± 0·2¶ | 5·7 ± 0·1* |
| ctr- | ND | 4·8 ± 0·5¶ | 3·1 ± 0·5* | ||
| 1w (n = 8) | TNP-OVA | CpG- | ND | 5·4 ± 0·1* | 4·0 ± 0·5¶ |
| ctr- | ND | 4·0 ± 0·1* | 2·7 ± 0·4¶ |
and
= P < 0·02; ND, not done; Ad, adults; 2w, 2-week-old; 1w, 1-week-old.
CpG-ODN fail to enhance antibody responses to plain pneumococcal polysaccharides
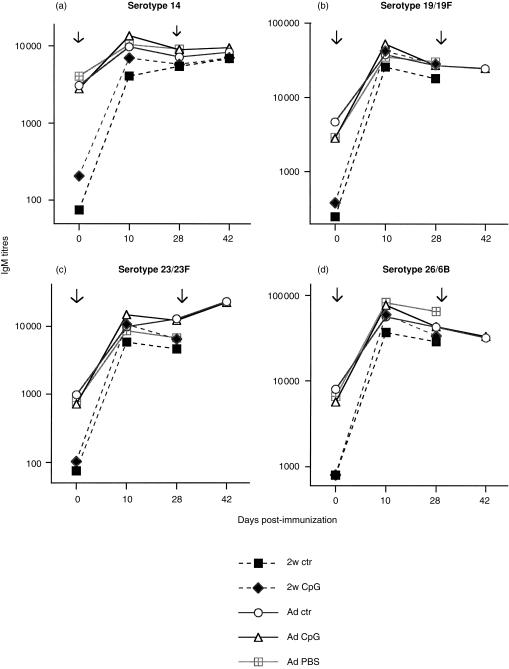
When immunization was performed with 25 µg of a mixture of SPnPS from 23 distinct serotypes (Pn-23), SPnPS IgM responses were detected against serotypes 14, 19/19F, 23/23F and 26/6B (Fig. 1a–d) and against most other serotypes contained in the vaccine (data not shown). As expected, IgM responses peaked 10 days after priming, with no further increase after booster immunization. In contrast to responses to TNP-Ficoll, CpG-ODN had no significant influence on the magnitude or the kinetics of IgM responses against any of 18/23 tested SPnPS (Fig. 1a–d and data not shown). CpG-ODN also failed to enhance IgG, IgG2a and IgG3 responses to plain SPnPS. IgG to all tested serotypes remained at very low titres (< 1000 ELISA units), regardless of the administration of CpG- or ctr-ODN (data not shown). Modification of the experimental conditions, including the change of the immunization route (i.p. versus s.c.) or addition of AlOH, failed to result in a CpG-ODN-mediated enhancement of IgM/IgG responses to SPnPS. CpG-ODN also failed to enhance IgM (Fig. 1a–d) or IgG (not shown) responses when immunization was performed at 2 weeks of age.
Figure 1.
Lack of influence of CpG-ODN on IgM responses to S. pneumoniae polysaccharides (SPnPS). BALB/c mice were immunized with 25 µg of SPnPS (Pn-23) together with 50 µg (adults: Ad) or 3 µg (2 weeks-old: 2w) of CpG-ODN, ctr-ODN or PBS (controls), and boosted 4 weeks later with the same formulation. IgM against SPn serotypes 14 (a), 19/19F (b), 23/23F (c) and 26/6B (d) was measured by ELISA in sera obtained before (day 0) and at several time-points after immunization. Titres are expressed as ELISA units obtained from serum pools of each experimental group containing five to eight mice. Arrows indicate the time of immunization.
Pneumococcal PS do not interfere with CpG-ODN B-cell activation in vitro
In view of the lack of enhancement of IgM/IgG responses to SPnPS, we asked whether SPnPS could interfere with B-cell activation. Indeed, complex PS taken up by APC are known to be retained for extended periods in the endosomal compartment18,19 in which CpG-ODN must undergo acidification or other processing steps in order to be able to bind to cytoplasmic CpG-binding proteins and hence deliver their activation signals to the cell nucleus.10,20 Splenocytes from naïve mice were thus incubated in vitro with a mixture of SPnPS and increasing concentrations of CpG- or ctr-ODN, under conditions previously described as leading to B-cell proliferation and polyclonal IgM secretion.8,21
As previously observed,16 B-cell proliferative responses to CpG-ODN stimulation were dose- and age-dependent (Table 2). In spite of lower proliferative responses of cells from 1-week-old mice compared to adult mice, CpG-ODN significantly increased polyclonal IgM production from cells of all age groups (Table 2). Increasing concentrations of SPnPS (0·1–10 µg/ml) remained without inhibitory influence on CpG-ODN mediated B-cell proliferation and IgM production (Table 2). Thus, the B-cell stimulatory activity mediated by CpG-ODN was not inhibited, at least not in vitro, by the presence of high concentrations of SPnPS.
Table 2.
In vitro responses of spleen cells from naïve mice of different ages to CpG-ODN
| B cell proliferation* (stimulation index) | IgM production† (end-point titres) | |||||
|---|---|---|---|---|---|---|
| 1 week | 4 week | adults | 1 week | 4 weeks | adults | |
| Cells alone | 1× | 1× | 1× | 1373 | 3069 | 7178 |
| CpG-ODN | ||||||
| 3 µg/ml | 20× (11–29) | 69× (38–86) | 127× (89–165) | 48 600 | 16 100 | 22 950 |
| 0·3 µg/ml | 11× (4–13) | 45× (20–54) | 61× (20–104) | 29 550 | 10 189 | 17 633 |
| 0·03 µg/ml | 1× (0–2) | 1× (0–1) | 1× (0–1) | 1516 | 3610 | 6874 |
| 3 µg/ml + Pn-23‡ | ND | 38× | 164× | ND | 12 441 | ND |
| 0·3 µg/ml + Pn-23‡ | ND | 41× | 105× | ND | 8362 | ND |
| 0 µg/ml + Pn-23‡ | ND | 1× | 1× | ND | 1075 | ND |
| ctr-ODN | ||||||
| 3 µg/ml | 3× (1–6) | 3× (2–4) | 3× (2–3) | 3266 | 4695 | 7034 |
| 0·3 µg/ml | 2× (1–5) | 1× (0–2) | 1× (0–2) | 1286 | 3303 | 4434 |
| 0·03 µg/ml | 1× (0–3) | 1× (0–2) | 1× (0–1) | 1222 | 2933 | 4466 |
Mean SI from three experiments (adults) to five experiments (1-week- and 4-week-old mice) and the range (in brackets);
Production of non-specific IgM by splenocytes in vitro. Results of a representative experiment;
Pn-23 was added to in vitro cultures in concentrations of 0·1 µg/ml to 10 µg/ml (indicated is 10 µg/ml); different experiment to
; ND, not done.
Conjugation of SPnPS to a protein carrier allows modulation of IgG responses by CpG-ODN, in a serotype- and age-dependent manner
To investigate the role of T-cell epitopes in the CpG-ODN-mediated enhancement of IgG responses to SPnPS, mice were next immunized with a glycoconjugate vaccine containing 11 distinct SPnPS individually conjugated to the TT carrier protein (Pn11-TT). As expected, CpG-ODN strongly enhanced total IgG, IgG1 and IgG2a responses to TT, the protein moiety of the vaccine (Table 3).
Table 3.
Anti-TT antibody ELISA titres (log10) at 28 days postimmunization with Pn11-TT. Means and SD of each group
| Mice | n | ODN | IgG | IgG1 | IgG2a |
|---|---|---|---|---|---|
| Ad | (5) | CpG- | 5·3 ± 0·1* | 5·3 ± 0·1¶ | 4·3 ± 0·2* |
| Ad | (5) | ctr- | 4·5 ± 0·0* | 4·6 ± 0·1¶ | 2·4 ± 0·2* |
| 2w | (10) | CpG- | 4·5 ± 0·2¶ | 4·5 ± 0·2* | 2·8 ± 0·4¶ |
| 2w | (8) | ctr- | 4·1 ± 0·2¶ | 4·2 ± 0·2* | 2·0 ± 0·0¶ |
and
=P < 0·02; Ad, adults; 2w, 2 weeks-old.
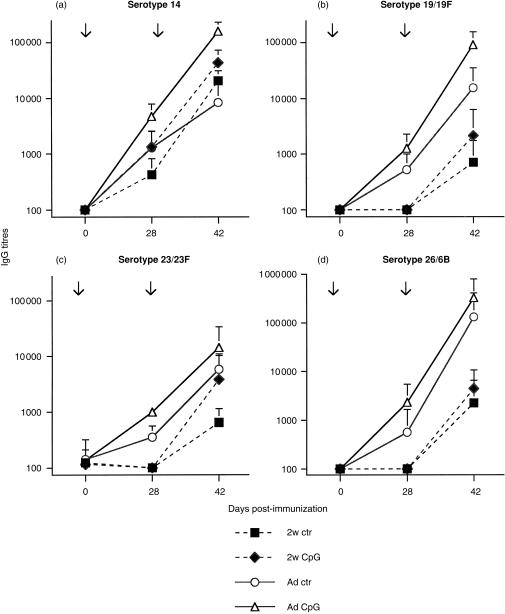
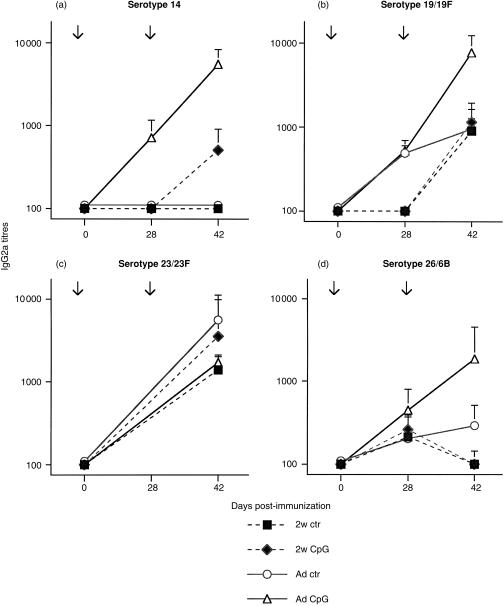
In contrast, CpG-ODN remained without any influence on SPnPS-specific IgM responses induced by Pn11-TT (data not shown). CpG-ODN co-administration significantly enhanced IgG responses for four major serotypes (Fig. 2a–d), however, the magnitude of this CpG-ODN-mediated enhancement was modest and serotype-dependent. A significant enhancement of both primary and secondary IgG responses in adult mice was observed for serotypes 14, 19/19F, 23/23F and 56/18C (similar to 23/23F, results not shown), whereas responses to serotype 26/6B were not increased (Fig. 2d). The influence of CpG-ODN on total IgG responses was confirmed by antibody subclass determination, showing an increase of IgG2a (Fig. 3a–d) and of IgG3 antibody responses, which mirrored the IgG2a responses (data not shown). When Pn11-TT immunization was performed in 2-week-old mice, co-administration of CpG-ODN failed to significantly enhance IgG responses to any serotype of SPnPS (Figs 2 and 3), in spite of their enhancement of TT-specific antibodies (Table 3). Thus, addition of CD4 T-cell epitopes to SPnPS allowed CpG-ODN to mediate an enhancement of IgG and IgG2a antibody responses, although only to a modest degree and in a serotype- and age-dependent manner.
Figure 2.
CpG-ODN modulation of SPnPS-specific IgG responses to a SPn glycoconjugate vaccine. BALB/c mice were immunized with the glycoconjugate Pn11-TT vaccine together with 50 µg (adults: Ad) or 3 µg (2 weeks-old: 2w) of CpG-ODN or control-ODN, and boosted 4 weeks later with the same formulation. Total IgG against SPn serotypes 14 (a), 19/19F (b), 23/23F (c) and 26/6B (d) was measured by ELISA in individual sera obtained on day 28 (primary response) and on day 42 (secondary response). Expressed are mean titres and SD in ELISA units of five to ten mice per group. Serotype 14, P < 0·02 adults day 28 and day 42; serotype 19/19F, P < 0·02 adults day 28; serotype 23/23F, P < 0·02 adults day 28 and day 42. Arrows indicate the time of immunization.
Figure 3.
CpG-ODN modulation of SPnPS-specific IgG2a responses to a SPn glycoconjugate vaccine. BALB/c mice were immunized with the glycoconjugate Pn11-TT vaccine together with 50 µg (adults: Ad) or 3 µg (2 weeks-old: 2w) of CpG-ODN or control-ODN, and boosted 4 weeks later with the same formulation. IgG2a against SPn serotypes 14 (a), 19/19F (b), 23/23F (c) and 26/6B (d) was measured by ELISA in individual sera obtained on day 28 (primary response) and on day 42 (secondary response). Expressed are mean titres and SD in ELISA units of five to ten mice per group. Serotype 14, P < 0·02 adults day 28 and day 42; serotype 19/19F, P < 0·02 day 42; serotype 26/6B, not different due to large variations on day 42. Arrows indicate the time of immunization.
Role for CD4+ cells in CpG modulation of antibody responses to TI-2 antigens
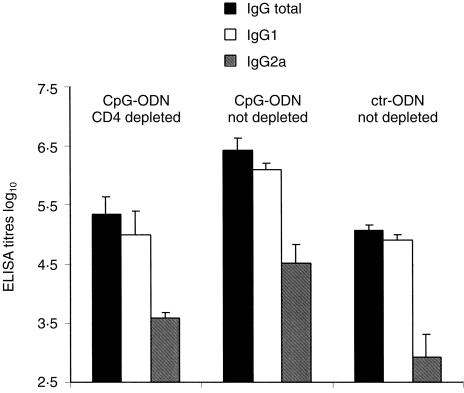
The observation of a CpG modulation of antibody responses to TNP-Ficoll and to TT-conjugated SPn but not to unconjugated PS suggested an essential participation for APC/T-cell interaction in the CpG modulation of antibody responses to TI-2 antigens. This was directly assessed in adult mice depleted of CD4+ cells prior to TNP-Ficoll and CpG-ODN administration. CD4-depletion reduced anti-TNP total IgG measured 3 weeks after immunization in CpG-ODN adjuvanted mice compared to non-depleted mice, such that antibody titres were now similar to those of non-depleted mice immunized with ctr-ODN formulations (Fig. 4). Similar results were obtained for IgG1 titres (Fig. 4). CD4 depletion also reduced IgG2a titres following TNP-Ficoll immunization with CpG-ODN, although IgG2a antibodies remained slightly higher than in mice injected with antigen and ctr-ODN (Fig. 4). Thus, although the depletion of CD4+ cells did not abrogate IgG, IgG1 and IgG2a responses to alum-adsorbed TNP-Ficoll, the CpG-ODN-mediated enhancement of antibody responses was completely (IgG and IgG1) or largely (83%, IgG2a) dependent on CD4+ cells.
Figure 4.
Depletion of CD4+ cells abolishes the CpG-ODN-mediated enhancement of IgG responses to TNP-Ficoll. CD4-depleted or non-depleted adult BALB/c mice were immunized with TNP-Ficoll together with either CpG- or ctr-ODN. Three weeks later mice were bled and anti-TNP total IgG, IgG1 and IgG2a titres were measured by ELISA in individual sera. Expressed are mean titres and SD in ELISA units of four or five mice per group. P < 0·04 group CpG-ODN CD4-depleted versus group CpG-ODN not depleted; P < 0·03 group CpG-ODN not depleted versus group ctr-ODN not depleted.
Discussion
Our observations indicate that the in vivo enhancement of antibody responses to TI-2 antigens by CpG-ODN is dependent on the nature of the PS antigen. B-cell responses to TNP-Ficoll were enhanced by CpG-ODN-mediated immunostimulatory signals, whereas responses to plain SPnPS were not. Our results suggest that these differences might reflect the degree of the participation of other immune cells likely to be modulated by CpG-ODN, such as APC and T cells.
Our observation that CpG-ODN enhance both adult and early-life in vivo antibody responses to TNP-Ficoll is in accordance with the recent description of their capacity to enhance the in vitro generation of TNP-Ficoll-specific antibody-forming cells by adult and neonatal splenocytes.21 In vivo, CpG-ODN are shown here to essentially enhance adult IgG2a responses to TNP-Ficoll, similar to their influence on IgG2a responses to protein vaccines22,23 and model protein antigens.24–27 This ‘T helper type 1 driving’ immunomodulatory property of CpG-ODN has been largely associated with their capacity to enhance IL-12 production by APC,28 which suggests that TNP-Ficoll responses may be influenced by APC activation. In contrast, CpG-ODN failed to increase IgG2a responses in mice primed at 2 weeks of age, despite significant enhancement of TNP-specific IgG1 antibodies and in spite of their previously reported influence on early-life IgG2a responses to protein antigens.15,16 Immunization with the T-dependent TNP-OVA formulation confirmed the capacity of CpG-ODN to mediate induction of TNP-specific IgG2a antibodies in early life.
Next, we examined whether CpG-ODN could directly activate Ficoll-specific B cells or if their stimulatory effect was mediated by other immune cells, such as APC or T cells. Responses to TNP-Ficoll have previously been shown to require macrophages, both in vitro6 and in vivo.7,29 The contribution of T cells to anti-Ficoll B-cell responses was also demonstrated by infusing T cells into nude mice.30 In addition, activated T cells were shown by histology to be co-localized with TNP-specific antibody-secreting cells.7 To investigate the role of the APC/T-cell interaction in the CpG-mediated influence on TNP-Ficoll responses, CD4+ cell-depleted mice were immunized with TNP-Ficoll together with CpG- or ctr-ODN. In contrast, although CD4 depletion did not suppress antibody responses to alum-adsorbed TNP-Ficoll, it almost completely abolished the CpG-ODN-mediated enhancement of antibody responses. Thus, the influence of CpG-ODN on antibody responses to TNP-Ficoll seems largely dependent on the presence of CD4+ cells. Not only T cells but also certain dendritic cell subpopulations can express CD431 and could therefore represent targets for the depleting GK1.5 antibody. We thus conclude that the influence of CpG-ODN on TNP-Ficoll responses is mediated by APC–T-cell interactions, which are abrogated by CD4+ cell depletion.
In contrast to Ficoll PS, purified pneumococcal PS induced almost exclusively IgM responses. Although the weak or absent IgG responses to Pn-23 immunization observed in adult BALB/c mice do not correlate to the situation in human adults, where IgG responses can be generated by the same Pn-23 vaccine, they are in accordance with results obtained in human infants as well as in CBA/J mice.32 Unexpectedly, CpG-ODN co-administration failed to enhance either IgM or IgG responses to unconjugated SPnPS. This failure could have been due to interference of SPnPS on CpG-ODN mediated B-cell activation, but this phenomenon was not observed even when high concentrations of SPnPS were added during in vitro incubation of splenocytes with CpG-ODN. Alternatively, CpG-ODN and SPnPS could have drained to distinct sites after s.c. injection, given the preferential localization of PS antigens in the spleen rather than in lymph nodes.33,34 However, the use of an i.p. immunization route, resulting in preferential draining towards the spleen, also failed to result in a CpG-mediated enhancement of antibody responses to SPnPS. Apart from being different in structure there are also differences in size between TNP-Ficoll and the various SPnPS. Thus, in order to evaluate the influence of CpG-ODN supplementation under optimal conditions for both TI-2 antigens, we used antigen doses formerly reported to induce optimal B-cell responses. Comparison of similar doses (i.e. 25–50 µg) is indeed prevented by inhibition of antibody responses to high doses of SPnPS35,36 Interestingly, mixing the pneumococcal vaccine/CpG-ODN combination with AlOH prior to its administration did not significantly alter the immunogenicity of the preparation. This was in contrast to preparations of TNP-Ficoll, TNP-OVA and several other T-dependent antigens,15,16 where the supplementation with AlOH was required in order effectively transmit the CpG-ODN-activating effect. These results indicate a fundamental difference in the CpG-ODN-mediated enhancement of T-dependent and TI-2 (SPnPS) immune responses. We hypothesize that the activation of innate immune responses by CpG-DNA evolved primarily as a defence against intracellular pathogens, which commonly lack TI-2 antigens, and that the failure to promote TI-2 responses more effectively may be a mechanism to avoid inducing responses to DNA or other self molecules with repeating epitopes.
In contrast to the lack of influence of CpG-ODN on plain SPnPS responses, it was effective on responses to certain conjugated SPnPS serotypes. To most serotypes, this CpG-ODN-mediated enhancement of IgG responses was of a modest magnitude, but evidenced by the particular enhancement of IgG2a and IgG3 responses. These results are in accordance with a recent report showing that CpG-ODN enhanced antibody responses to the HIB conjugate vaccine, but not to unconjugated HIB-PS.13 It is interesting to note that the magnitude of the CpG-ODN-mediated enhancement of IgG2a and IgG3 titres was dependent on the pneumococcal serotype. Serotype 14-specific antibody responses were most susceptible to CpG-ODN activation, followed by serotype 23/23F and 56/18C (not shown). In contrast, we observed no enhancement of antibody responses to 26/6B by CpG-ODN administration. Thus, even when additional T-cell epitopes were provided, the capacity of CpG-ODN to enhance adult antibody responses to TI-2 antigens remained dependent on the nature of the SPnPS. This might suggest a distinct modulatory role of APC and/or T cells on B-cell responses to various SPn serotypes.
Unexpectedly, the CpG-mediated enhancement of SPnPS-specific antibody responses to the Pn11-TT glycoconjugate vaccine in adult mice was not observed when in mice immunized at 2 weeks of age. Although we cannot formally exclude that immature APC or T cells are the limiting factors for early-life antibody responses to SPnPS, as described previously for other polysaccharides (Ficoll and Dextran),37,38 neonatal APC and T cells have been shown to be fully receptive to CpG-ODN, both in previous experiments15,16 and in this report (TNP-OVA, Table 1). Hence, we hypothesize that a certain immaturity of early-life B cells limits the CpG-ODN modulation of early-life antibody responses to PS.37,39
Therefore, it appears that contradictory reports recently issued on the influence of CpG-ODN on antibody responses to PS antigens may be largely explained by the various nature of the PS antigens used and the relative role for APC/T-cell interaction in the modulation of PS-specific B-cell responses. Whether or not CpG-ODN would be able to enhance human adult or early-life responses to plain or conjugated PS can thus not be readily predicted by preclinical studies.
Acknowledgments
We thank Paolo Quirighetti, Gianna Cadau and Christine Brighouse for excellent assistance with animal care and technical and secretarial support. This research was supported by the Swiss National Research Foundation, the WHO Global Programme for Vaccines and Immunization, the Fondation pour la Recherche Médicale and Coley Pharmaceutical Group, Inc.
Abbreviations
- CpG-ODN
oligodeoxynucleotides containing CpG motifs
- ctr-ODN
oligodeoxynucleotides containing no CpG motifs
- Pn-23
Pneumovax® vaccine containing purified pneumococcal polysaccharides
- Pn11-TT
tetanus toxin conjugate pneumococcal polysaccharide vaccine
- PS
polysaccharides
- TI-2
thymus-independent type 2 antigens
- TNP-OVA
trinitrophenyl hapten conjugated to ovalbumin
- TNP-Ficoll
trinitrophenylaminoethyl-carboxymethyl-Ficoll
- TT
tetanus toxoid
- SPnPS
Streptococcus pneumoniae serotype-specific polysaccharides
References
- 1.Mosier DE, Mond JJ, Goldings EA. The ontogeny of thymic independent antibody responses in vitro in normal mice and mice with an X-linked B cell defect. J Immunol. 1977;119:1874–8. [PubMed] [Google Scholar]
- 2.Subbarao B, Mosier DE, Ahmed A, Mond JJ, Scher I, Paul WE. Role of a nonimmunoglobulin cell surface determinant in the activation of B lymphocytes by thymus-independent antigens. J Exp Med. 1979;149:495–506. doi: 10.1084/jem.149.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mond JJ, Lees A, Snapper CM. T cell-independent antigens type 2. Annu Rev Immunol. 1995;13:655–92. doi: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- 4.Buchanan RM, Arulanandam BP, Metzger DW. IL-12 enhances antibody responses to T-independent polysaccharide vaccines in the absence of T and NK cells. J Immunol. 1998;161:5525–33. [PubMed] [Google Scholar]
- 5.Dullforce P, Sutton DC, Heath AW. Enhancement of T cell-independent immune responses in vivo by CD40 antibodies. Nat Med. 1998;4:88–91. doi: 10.1038/nm0198-088. [DOI] [PubMed] [Google Scholar]
- 6.Boswell HS, Sharrow SO, Singer A. Role of accessory cells in B cell activation. I. Macrophage presentation of TNP-Ficoll: evidence for macrophage–B cell interaction. J Immunol. 1980;124:989–96. [PubMed] [Google Scholar]
- 7.Van den Eertwegh AJ, Noelle RJ, Roy M, Shepherd DM, Aruffo A, Ledbetter JA, Boersma WJ, Claassen E. In vivo CD40–gp39 interactions are essential for thymus-dependent humoral immunity. I. In vivo expression of CD40 ligand, cytokines, and antibody production delineates sites of cognate T–B cell interactions. J Exp Med. 1993;178:1555–65. doi: 10.1084/jem.178.5.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krieg AM, Yi AK, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, Koretzky GA, Klinman DM. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546–9. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 9.Han SS, Chung ST, Robertson DA, Chelvarajan RL, Bondada S. CpG oligodeoxynucleotides rescue BKS-2 immature B cell lymphoma from anti-IgM-mediated growth inhibition by up-regulation of egr-1. Int Immunol. 1999;11:871–9. doi: 10.1093/intimm/11.6.871. 10.1093/intimm/11.6.871. [DOI] [PubMed] [Google Scholar]
- 10.Wagner H. Bacterial CpG DNA activates immune cells to signal infectious danger. Adv Immunol. 1999;73:329–68. doi: 10.1016/s0065-2776(08)60790-7. [DOI] [PubMed] [Google Scholar]
- 11.Threadgill DS, McCormick LL, McCool TL, Greenspan NS, Schreiber JR. Mitogenic synthetic polynucleotides suppress the antibody response to a bacterial polysaccharide. Vaccine. 1998;16:76–82. doi: 10.1016/s0264-410x(97)00151-5. 10.1016/s0264-410x(97)00151-5. [DOI] [PubMed] [Google Scholar]
- 12.Tygrett L, Wiechert S, Li X, Takahashi K, Krieg A, Waldschmidt T. Capacity of CpG ODN to enhance TNP-Ficoll responses in mice. FASEB J. 1999;13:A990. [Google Scholar]
- 13.von Hunolstein C, Teloni R, Mariotti S, Recchia S, Orefici G, Nisini R. Synthetic oligodeoxynucleotide containing CpG motifs induces an anti-polysaccharide type 1-like immune response after immunization of mice with Haemophilus influenzae type b conjugate vaccine. Int Immunol. 2000;12:295–303. doi: 10.1093/intimm/12.3.295. 10.1093/intimm/12.3.295. [DOI] [PubMed] [Google Scholar]
- 14.Chu RS, McCool T, Greenspan NS, Schreiber JR, Harding CV. CpG oligodeoxynucleotides act as adjuvants for pneumococcal polysaccharide-protein conjugate vaccines and enhance antipolysaccharide immunoglobulin G2a (IgG2a) and IgG3 antibodies. Infect Immun. 2000;68:1450–6. doi: 10.1128/iai.68.3.1450-1456.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brazolot Millan CL, Weeratna R, Krieg AM, Siegrist CA, Davis HL. CpG DNA can induce strong Th1 humoral and cell-mediated immune responses against hepatitis B surface antigen in young mice. Proc Natl Acad Sci U S A. 1998;95:15553–8. doi: 10.1073/pnas.95.26.15553. 10.1073/pnas.95.26.15553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kovarik J, Bozzotti P, Love-Homan L, Pihlgren M, Davis HL, Lambert PH, Krieg AM, Siegrist CA. CpG oligodeoxynucleotides can circumvent the Th2 polarization of neonatal responses to vaccines but may fail to fully redirect Th2 responses established by neonatal priming. J Immunol. 1999;162:1611–17. [PubMed] [Google Scholar]
- 17.Barrios C, Brawand P, Berney M, Brandt C, Lambert PH, Siegrist CA. Neonatal and early life immune responses to various forms of vaccine antigens qualitatively differ from adult responses: predominance of a Th2-biased pattern which persists after adult boosting. Eur J Immunol. 1996;26:1489–96. doi: 10.1002/eji.1830260713. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez-Fernandez M, Carrasco-Marin E, Alvarez-Dominguez C, Outschoorn IM, Leyva-Cobian F. Inhibitory effects of thymus-independent type 2 antigens on MHC class II-restricted antigen presentation: comparative analysis of carbohydrate structures and the antigen presenting cell. Cell Immunol. 1997;176:1–13. doi: 10.1006/cimm.1996.1078. 10.1006/cimm.1996.1078. [DOI] [PubMed] [Google Scholar]
- 19.Leyva-Cobian F, Outschoorn IM, Carrasco-Marin E, Alvarez-Dominguez C. The consequences of the intracellular retention of pathogen-derived T-cell-independent antigens on protein presentation to T cells. Clin Immunol Immunopathol. 1997;85:1–15. doi: 10.1006/clin.1997.4426. 10.1006/clin.1997.4426. [DOI] [PubMed] [Google Scholar]
- 20.Yi AK, Tuetken R, Redford T, Waldschmidt M, Kirsch J, Krieg AM. CpG motifs in bacterial DNA activate leukocytes through the pH-dependent generation of reactive oxygen species. J Immunol. 1998;160:4755–61. [PubMed] [Google Scholar]
- 21.Chelvarajan RL, Raithatha R, Venkataraman C, Kaul R, Han SS, Robertson DA, Bondada S. CpG oligodeoxynucleotides overcome the unresponsiveness of neonatal B cells to stimulation with the thymus-independent stimuli anti-IgM and TNP-Ficoll. Eur J Immunol. 1999;29:2808–18. doi: 10.1002/(SICI)1521-4141(199909)29:09<2808::AID-IMMU2808>3.0.CO;2-E. 10.1002/(sici)1521-4141(199909)29:09<2808::aid-immu2808>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 22.Liu HM, Newbrough SE, Bhatia SK, Dahle CE, Krieg AM, Weiner GJ. Immunostimulatory CpG oligodeoxynucleotides enhance the immune response to vaccine strategies involving granulocyte-macrophage colony-stimulating factor. Blood. 1998;92:3730–6. [PubMed] [Google Scholar]
- 23.Davis HL, Weeratna R, Waldschmidt TJ, Tygrett L, Schorr J, Krieg AM, Weeranta R. CpG DNA is a potent enhancer of specific immunity in mice immunized with recombinant hepatitis B surface antigen. J Immunol. 1998;160:870–6. [PubMed] [Google Scholar]
- 24.Roman M, Martin-Orozco E, Goodman JS, et al. Immunostimulatory DNA sequences function as T helper-1-promoting adjuvants. Nat Med. 1997;3:849–54. doi: 10.1038/nm0897-849. [DOI] [PubMed] [Google Scholar]
- 25.Lipford GB, Bauer M, Blank C, Reiter R, Wagner H, Heeg K. CpG-containing synthetic oligonucleotides promote B and cytotoxic T cell responses to protein antigen: a new class of vaccine adjuvants. Eur J Immunol. 1997;27:2340–4. doi: 10.1002/eji.1830270931. [DOI] [PubMed] [Google Scholar]
- 26.Weiner GJ, Liu HM, Wooldridge JE, Dahle CE, Krieg AM. Immunostimulatory oligodeoxynucleotides containing the CpG motif are effective as immune adjuvants in tumor antigen immunization. Proc Natl Acad Sci USA. 1997;94:10833–7. doi: 10.1073/pnas.94.20.10833. 10.1073/pnas.94.20.10833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chu RS, Targoni OS, Krieg AM, Lehmann PV, Harding CV. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J Exp Med. 1997;186:1623–31. doi: 10.1084/jem.186.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krieg AM, Love-Homan L, Yi AK, Harty JT. CpG DNA induces sustained IL-12 expression in vivo and resistance to Listeria monocytogenes challenge. J Immunol. 1998;161:2428–34. [PubMed] [Google Scholar]
- 29.Kraal G, Ter Hart H, Meelhuizen C, Venneker G, Claassen E. Marginal zone macrophages and their role in the immune response against T-independent type 2 antigens: modulation of the cells with specific antibody. Eur J Immunol. 1989;19:675–80. doi: 10.1002/eji.1830190416. [DOI] [PubMed] [Google Scholar]
- 30.Mongini PK, Stein KE, Paul WE. T cell regulation of IgG subclass antibody production in response to T-independent antigens. J Exp Med. 1981;153:1–12. doi: 10.1084/jem.153.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vremec D, Pooley J, Hochrein H, Wu L, Shortman K. CD4 and CD8 expression by dendritic cell subtypes in mouse thymus and spleen. J Immunol. 2000;164:2978–86. doi: 10.4049/jimmunol.164.6.2978. [DOI] [PubMed] [Google Scholar]
- 32.Aaberge IS, North RJ, Groeng EC, Lovik M. Antibody response to pneumococcal polysaccharide vaccine in young, adult and old mice. Scand J Immunol. 1993;38:17–30. doi: 10.1111/j.1365-3083.1993.tb01689.x. [DOI] [PubMed] [Google Scholar]
- 33.Goud SN, Muthusamy N, Subbarao B. Differential responses of B cells from the spleen and lymph node to TNP-Ficoll. J Immunol. 1988;140:2925–30. [PubMed] [Google Scholar]
- 34.Amlot PL, Grennan D, Humphrey JH. Splenic dependence of the antibody response to thymus-independent (TI-2) antigens. Eur J Immunol. 1985;15:508–12. doi: 10.1002/eji.1830150516. [DOI] [PubMed] [Google Scholar]
- 35.Baker PJ, Stashak PW, Amsbaugh DF, Prescott B. Characterization of the antibody response to type 3 pneumococcal polysaccharide at the cellular level. I. Dose–response studies and the effect of prior immunization on the magnitude of the antibody response. Immunology. 1971;20:469–80. [PMC free article] [PubMed] [Google Scholar]
- 36.Siskind GW, Howard JG. Studies on the induction of immunological unresponsiveness to pneumococcal polysaccharide in mice. J Exp Med. 1966;124:417–29. doi: 10.1084/jem.124.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chelvarajan RL, Gilbert NL, Bondada S. Neonatal murine B lymphocytes respond to polysaccharide antigens in the presence of IL-1 and IL-6. J Immunol. 1998;161:3315–24. [PubMed] [Google Scholar]
- 38.Halista SM, Johnson-Robbins LA, El-Mohandes AE, Lees A, Mond JJ, Katona IM. Characterization of early activation events in cord blood B cells after stimulation with T cell-independent activators. Pediatr Res. 1998;43:496–503. doi: 10.1203/00006450-199804000-00010. [DOI] [PubMed] [Google Scholar]
- 39.Snapper CM, Rosas FR, Moorman MA, Mond JJ. Restoration of T cell-independent type 2 induction of Ig secretion by neonatal B cells in vitro. J Immunol. 1997;158:2731–5. [PubMed] [Google Scholar]