Abstract
Exposure of Brown Norway rats to mercuric chloride induces systemic autoimmunity, involving T- and B-lymphocyte activation, (auto-)antibody production and multiorgan inflammation. Several divalent metal ions, such as Mg2+ and Mn2+, can activate binding of integrins to their ligands, thus causing lymphocyte adhesion. To test the hypothesis that Hg2+ acts in a similar way, we studied the effect of HgCl2 on integrin-mediated T-cell adhesion. HgCl2 induced cell–cell aggregation of human T lymphoblasts. Exposure of a human T-cell clone to HgCl2 for 1 hr enhanced, in a dose-dependent way, cell binding to fibronectin (FN) and to intercellular adhesion molecules (ICAM) -1, -2 and -3. Furthermore, HgCl2 induced strong binding of Jurkat T cells to FN. These effects of HgCl2 were of similar magnitude as the effects of phorbol 12-myristate 13-acetate (PMA) or MnCl2. Studies using blocking antibodies indicated the involvement of CD11a in binding to ICAMs, and of CD49d, CD49e, and CD29 in binding to FN. Adhesion to FN induced by HgCl2 or by PMA, but not by MnCl2, was dependent on temperature and on extracellular Ca2+ or Mg2+. Addition of cytochalasin B enhanced synergistically the FN adhesion induced by MnCl2, whereas the effects of PMA and HgCl2 were not modified. These results indicate that Hg2+ is a potent activator of T-cell adhesion, mediated by several integrins and ligands. In contrast to the effect of MnCl2, HgCl2-induced cell adhesion probably involves an intracellular pathway. Activation of integrins by HgCl2 may play an important role in activation and migration of leucocytes involved in HgCl2-induced immune dysregulation in vivo.
Introduction
A number of drugs and environmental pollutants can induce immune dysregulation in susceptible individuals, which may for example lead to autoimmune diseases involving the kidney as a target organ.1 Using mercuric chloride as a model compound, immune dysregulation induced by chemicals has been frequently studied in rodents. Exposure of Brown Norway (BN) rats to HgCl2 induces a systemic lupus-like autoimmune syndrome, characterized by activation and proliferation of T and B lymphocytes, high production of (auto-) antibodies, infiltration of leucocytes in many organs, glomerulonephritis and proteinuria.2,3 T-cell depletion experiments revealed an essential role for T lymphocytes in disease induction.4 Furthermore, high production of interleukin-4 (IL-4) and immunoglobulin E (IgE) in HgCl2-exposed BN rats indicate a strong and preferential activation of T helper type 2 (Th2) cells.5,6 The mechanism of T-cell activation by exposure to HgCl2 has not been identified in detail. HgCl2 may alter the structure, processing, presentation and/or recognition of (auto-)antigens, thus leading to T-cell autoreactivity.7 In addition, non-antigen-specific T-lymphocyte activation by HgCl2 is likely to be involved.8,9
We have previously shown that in vivo exposure of BN rats to HgCl2 enhances T-lymphocyte expression of various cell adhesion and activation molecules, such as lymphocyte function-associated antigen-1 (LFA-1; CD11a/CD18), intercellular adhesion molecule-1 (ICAM-1; CD54), integrin α4 (CD49d) and OX40 (CD134), at an early stage of disease.10 Furthermore, it has been reported by Molina et al. that in vivo treatment with a blocking monoclonal antibody (mAb) directed against integrin α4 inhibits a major part of autoimmune manifestations induced by HgCl2 in BN rats.11 These data indicate a role for cell adhesion molecules in the immune dysregulation induced by HgCl2. In the present study, we examined whether HgCl2 may have a direct effect on T-cell adhesion mediated by integrins.
Integrins are heterodimeric cell membrane receptors involved in cell-to-cell and cell-to-matrix adhesion. Integrin-mediated adhesive capacity is determined by the levels of expression of the receptors and their ligands.12 Furthermore, the receptors require activation for ligand binding.13 Ligand binding results in cell adhesion, as well as in signal transduction via the integrin receptor,14 and, in several cases, via the ligand.15 Thus, these processes play an essential role in numerous functions of lymphocytes.
Activation of integrins can take place either as a result of extracellular events, or via an intracellular pathway. Certain integrin-binding mAbs can stimulate ligand binding, probably by stabilizing an active conformation of the receptor.16–18 Furthermore, several divalent metal ions that bind to extracellular domains of integrins, such as Mg2+ and Mn2+, increase integrin affinity for its ligand,19,20 which can also be demonstrated using isolated integrins and ligands in a cell-free system, indicating its independence on cell function.21 In contrast, integrin activation can also result from a process named inside-out signalling. This can be achieved by ligation of cell surface molecules, such as CD3 and CD2,22,23 as well as by agents that induce intracellular signalling events, such as phorbol esters, which activate protein kinase C, and ionomycin, which elevates the intracellular calcium level.24 Evidence has been presented that integrin activation by the phorbol ester phorbol 12-myristate 13-acetate (PMA) can take place independent of changes in integrin affinity, but involves modification of the association of integrins with cytoskeletal proteins, and their mobility in the plasma membrane.20,25–27
Induction of lymphocyte adhesion by HgCl2, thus modifying the physiological regulation of this process, could be of direct importance for the immune dysregulatory capacity of this compound. In the present study, we investigated the effect of exposure of T lymphocytes to HgCl2 on their binding to several ligands of integrins: fibronectin (FN), ICAM-1, ICAM-2 and ICAM-3. The effects of HgCl2 were compared to adhesion induced by MnCl2 and by PMA. The results show that HgCl2 induces integrin-mediated T-cell adhesion, which involves several integrins and ligands and is probably mediated via an intracellular pathway.
Materials and methods
Cells and cell culture
Human peripheral blood obtained from healthy donors was used for isolation of peripheral blood mononuclear cells (PBMC) by density gradient centrifugation, using Ficoll-Paque (Pharmacia Biotech, Uppsala, Sweden). T lymphoblasts were generated by culturing PBMC for 5–7 days in the presence of soluble OKT3 (mAb anti-CD3) and of a cytokine mixture, that was obtained from activated PBMC, as described,28 using RPMI culture medium (RPMI-1640 supplemented with 10% heat-inactivated fetal calf serum, 100 IU/ml penicillin, 100 µg/ml streptomycin, and 2 mm glutamin). The human Th0 cell clone ACC6 (kindly provided by Dr M. L. Kapsenberg, Amsterdam, the Netherlands), was stimulated every 14 days by allogeneic feeder cells and phytohaemagglutinin, as described,29 and cultured in Iscove's modified Dulbecco's medium (Gibco BRL Life Technologies, Paisley, UK), supplemented with 10% heat-inactivated normal human serum, 25 U/ml recombinant IL-2 (Eurocetus, Amsterdam, the Netherlands), 100 IU/ml penicillin, 100 µg/ml streptomycin and 2 mm glutamin. ACC6 T cells were used for experiments when they were in a resting phase, at day 10 or 11 after stimulation. The T-cell leukaemia line Jurkat [obtained from the American Type Tissue Collection (ATCC), Rockville, MD] was cultured in RPMI culture medium.
Monoclonal antibodies
Mouse mAb were used, directed against CD3 (OKT3, IgG2a; supernatant from hybridoma obtained from the ATCC), CD11a (F8.8,30 IgG1; kindly provided by Dr A. Bloem, Utrecht, the Netherlands, and LFA 1/2,14 IgG1; donation from Dr R. A. W. van Lier, Amsterdam, The Netherlands), CD29 (4B4,31 IgG1; Coulter Immunology, Hialeah, FL), CD49d (HP2/1,11,32 IgG1; a gift from Dr F. Sánchez-Madrid, Madrid, Spain), CD49e (SAM-1, IgG2b; provided by Dr C. G. Figdor, Nijmegen, the Netherlands) and CD54 (84H10,33 IgG1; from Dr S. Shaw, Bethesda, MD). Monoclonal mouse IgG1 (Zymed Laboratories, San Francisco, CA) was applied as a non-binding control mAb. All antibody preparations used were free of NaN3.
Homotypic aggregation assay
Cells were cultured in flat-bottom 96-well plates (2 × 105/100 µl/well) at 37°, using adhesion buffer [Hanks' balanced salt solution (HBSS) without Ca2+ and Mg2+ (Gibco BRL), supplemented with d-glucose (2 mg/ml), bovine serum albumin (BSA; 10 mg/ml), MgCl2 (1 mm) and CaCl2 (1 mm)], in the presence or absence of HgCl2 (Sigma, St Louis, MO). Monoclonal antibodies were preincubated with the cells during 30 min at 4°. Cell aggregation was examined by light microscopy after 3–4 hr of culture.
Production of chimeric proteins
Four different chimeric proteins containing the extracellular domains of either ICAM-1, ICAM-2, ICAM-3, or CD8, linked to the hinge domain of human IgG1, were expressed in COS cells. The appropriate expression plasmids for ICAM-1-IgG and CD8-IgG were kindly provided by Dr B. Seed (Boston, MA) and Dr A. Aruffo (Seattle, WA), and have been described previously.34,35 The cDNA sequences encoding the two or five amino-terminal immunoglobulin-like domains of ICAM-2,36 or ICAM-3,37 respectively, were amplified by polymerase chain reaction (PCR) using synthetic oligonucleotides and cDNA from U937 cells. Primer sequences were as follows: ICAM-2-sense, 5′ GCCCGGTCGACGCCGCCACCATGTCCTCTTTCGGTTACAGGACCCTG (position 43–90); and antisense, 5′ GACTATGATGGGATCCTGGCTGTCCGACACAG-GCTCATAGATCTC (position 702–746);36 ICAM-3-sense, 5′ CAGGTCGACGTAGCCATCGCCACCATGGTACCATCC (position 2–22); and antisense, 5′ CACGAAGACGGGGA-GATCTTGGGAGCTCCCAGCCTC (position 1439–1474).37 Specificity of amplification products was verified by restriction site analysis. Subsequently, they were cloned into the CD8-IgG1-CDM8 vector,35 followed by transfection into COS7m6 cells, as described.37 Seven days after transfection, the supernatants were harvested and stored at −80° until use. Immunoprecipitation and sodium dodecyl sulphate–polyacrylamide gel electrophoresis revealed production of recombinant proteins of the expected molecular weights; concentrations of chimeric proteins in culture supernatants were determined by enzyme-linked immunosorbent assay.
Cell adhesion assays
For coating of chimeric proteins, flat-bottom 96-wells plates were precoated using goat anti-human Fcγ (10 µg/ml in 50 mm Tris, pH 9·5; Jackson Immunoresearch Laboratories, West Grove, PA) for 1 hr at 37°. After blocking (4% BSA in PBS; 1 hr at 37°) and washing, diluted COS cell-supernatants containing chimeric proteins (0·2 µg/ml in PBS) were incubated for 1 hr at 37°. Human plasma fibronectin (10 µg/ml in PBS), kindly provided by Dr J. van Mourik (Amsterdam, the Netherlands), was coated for 2 hr at 37°, followed by a blocking step (4% BSA in PBS; 1 hr at 37°). All plates were washed with adhesion buffer before use.
Jurkat T cells or ACC6 T cells were washed in adhesion buffer, and added to the coated wells at 0·8 × 105/well and 1·25 × 105/well, respectively, followed by centrifugation for 5 min at 20 g and incubation for 1 hr at 37°, unless otherwise indicated. Subsequently, the plates were gently washed, using warm adhesion buffer, five to seven times, and stored without buffer at −80° until measurement.
Incubation of the cells was performed in the presence or absence of the following stimuli: HgCl2 (2·5 µm), MnCl2 (500 µm; Sigma) and PMA (50 ng/ml; Sigma); they were applied at the indicated concentrations, unless otherwise specified. In some experiments, soluble purified OKT3 was used (5 µg/ml). All mAb were preincubated with the cells for 30 min at 4°. For testing the dependence on extracellular cations, cells were washed and incubated in Ca2+- and Mg2+-free adhesion buffer containing BSA which had been previously extensively dialysed against Ca2+- and Mg2+-free HBSS. Some experiments were performed using cytochalasin B (5 µg/ml; Sigma), which was preincubated with the cells for 30 min at 37°; subsequently, it was present during the assay. Cytochalasin B was dissolved in dimethyl sulphoxide, which was added to the control cultures at equivalent concentration (≤ 0·05% v/v).
Quantification of cell adhesion
Cells which were bound to the wells were quantified by a fluorometric assay, using the DNA-binding fluorochromes Hoechst 33258 and Syto 13 (both from Molecular Probes, Leiden, the Netherlands) for Jurkat T cells and ACC6 T cells, respectively. A cell lysis procedure was performed which was previously described by Rago et al.38 Briefly, the plates were thawed at room temperature, and, after addition of 100 µl distilled water per well, were incubated at 37° for 1 hr. Subsequently, the plates were again frozen at −80°. After thawing, 100 µl TNE buffer (10 mm Tris, 2 m NaCl, 1 mm ethylenediaminetetraacetic acid, pH 7·4) containing Hoechst 33258 (32 µm) or Syto 13 (10 µm) was added, and fluorescence intensity was measured by a fluorometer (Perkin Elmer LS50), using appropriate wavelengths for excitation and emission (345/460 nm for Hoechst 33258 and 488/509 nm for Syto 13). The number of cells bound to each well was calculated by interpolation in a calibration line. Binding to the ligand and to the control-coating was assessed in parallel for each stimulus: ICAM-1, ICAM-2 and ICAM-3 were compared with CD8, and FN was compared with BSA. The percentage specific binding was calculated from triplicate wells using the following formula: 100 × [(number of cells bound to the ligand−number of cells bound to control coating)/number of input cells]. Results were summarized as the mean percentage specific binding to the ligand ±standard deviation.
Results
Integrin-mediated cell adhesion induced by HgCl2
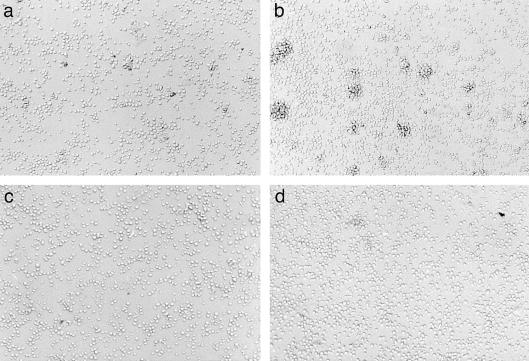
In order to examine whether exposure to HgCl2 modifies cell adhesion, its effect on lymphocyte aggregation was examined. Addition of 5 µm HgCl2 to T lymphoblasts clearly increased the number and size of cell aggregates, as compared to the control culture (Fig. 1a,b). Cell aggregation in control cultures and in HgCl2-stimulated cultures was completely inhibited when the cells were preincubated with mAb against CD11a and CD54, indicating the involvement of LFA-1 and ICAM-1 in this process (Fig. 1c,d). Exposure of the Th0 clone ACC6 to HgCl2 also induced cell aggregation (not shown). In contrast, the human leukaemic T-cell line Jurkat did not aggregate upon exposure to HgCl2, to PMA, or to MnCl2.
Figure 1.
Lymphocyte aggregation induced by HgCl2. T lymphoblasts, generated from human PBMC as explained in the Materials and Methods, were incubated in control medium (a, c), or in medium containing 5 µm HgCl2 (b, d). Cells were preincubated in the presence (c, d) or absence (a, b) of LFA1/2 (anti-CD11a) and 84H10 (anti-CD54). Photographs were taken after 4 hr of incubation.
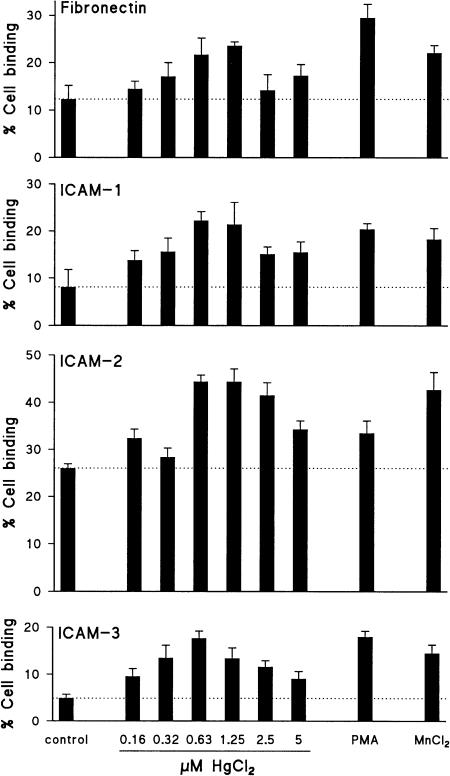
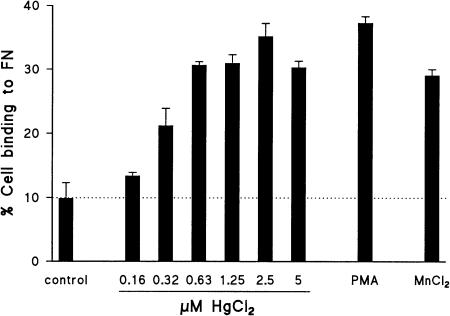
Cell adhesion induced by HgCl2 was further examined in quantitative ligand binding assays, in which the number of adhered cells was measured using DNA-binding fluorochromes. This method has the convenience and accuracy of a fluorometric assay, and excludes any possible interference of the detection method in the biological process, since the detecting agent is not present during the adhesion assay. As illustrated in Fig. 2, HgCl2 enhanced the binding of ACC6 T cells to FN, ICAM-1, ICAM-2 and ICAM-3 dose-dependently, with a maximal effect at 0·6–1·25 µm. Cell binding to ICAM-1, ICAM-2 and ICAM-3 could be completely prevented by blocking antibodies directed against CD11a, irrespective of the stimulus used (not shown). Exposure of Jurkat T cells to HgCl2 induced strong cell binding to FN, to a similar level as that induced by PMA or by MnCl2, as shown in Fig. 3. Fibronectin adhesion of Jurkat T cells exposed to HgCl2 was maximal at a concentration of 2·5 µm; this concentration did not induce any cytotoxicity as assessed by trypan blue exclusion and by microscopic evaluation of cell morphology. Notably, the effect of HgCl2 was much stronger than the effect of MnCl2, when compared at equimolar concentrations (2·5–5 µm; not shown). Analysis of the kinetics of HgCl2-induced FN adhesion revealed maximal binding after 1 hr of stimulation, followed by a gradual decrease (not shown). In agreement with other investigators,39 as well as with the lack of inducible cellular aggregation in Jurkat T cells, these cells did not bind to ICAM-2, neither under control conditions, nor after stimulation with HgCl2, MnCl2, or PMA.
Figure 2.
HgCl2 induces binding of the Th0 clone ACC6 to FN, ICAM-1, ICAM-2 and ICAM-3. Adhesion of ACC6 T cells was assessed without stimulation (control) or in the presence of HgCl2 (concentrations as indicated), PMA (50 ng/ml) or MnCl2 (500 µm). One of two similar experiments is shown.
Figure 3.
HgCl2 induces adhesion of Jurkat T cells to FN. Jurkat T-cell adhesion was analysed without stimulation (control) or in the presence of HgCl2 (concentrations as indicated), PMA (50 ng/ml) or MnCl2 (500 µm). Data are representative for four experiments.
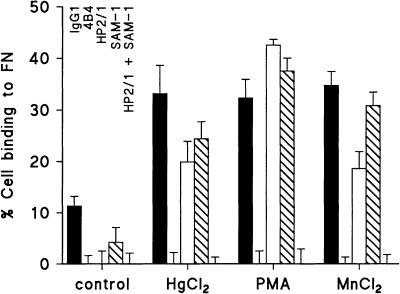
Inhibition studies using blocking mAb were performed to examine which integrins are involved in HgCl2-induced binding of Jurkat T cells to FN (Fig. 4). A mAb directed against the integrin β1 subunit (CD29) completely blocked FN adhesion under control conditions, as well as after stimulation with HgCl2, MnCl2, or PMA. Monoclonal antibodies directed against integrin α4 (CD49d) or α5 (CD49e) subunits, when applied alone, did partially and variably inhibit FN adhesion. However, when they were applied in combination, FN adhesion was completely blocked, indicating that both integrin α4β1 (VLA-4) and α5β1 (VLA-5) were able to mediate cell adhesion to FN after stimulation with HgCl2, MnCl2, or PMA. Flow cytometric analysis revealed membrane expression of CD29, CD49d and CD49e on Jurkat T cells at a high level (not shown). Expression was not modified after incubation for 1 hr with HgCl2, PMA, or MnCl2, indicating that induction of cell adhesion by these stimuli is not caused by an increase in receptor number.
Figure 4.
HgCl2-induced FN adhesion of Jurkat T cells is mediated via VLA-4 and VLA-5. Jurkat T cells were preincubated with control IgG1, 4B4 (anti-CD29), HP2/1 (anti-CD49d), SAM-1 (anti-CD49e), or HP2/1 plus SAM-1. Subsequently, FN adhesion was assessed in the presence or absence of HgCl2 (2·5 µm), PMA (50 ng/ml), or MnCl2 (500 µm), as indicated. In separate experiments, optimal blocking concentrations of the mAb were determined, in which SAM-1 was titrated in the presence of HP2/1, and vice versa. Data represent three similar experiments.
HgCl2 and MnCl2 induce cell adhesion via different pathways
Induction of Jurkat T-cell adhesion to FN by HgCl2, MnCl2, or PMA, was compared with respect to its dependence on extracellular cations and on a physiological temperature, in order to study the pathways used in the adhesion process.
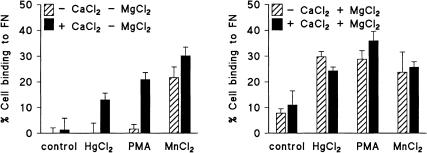
When the adhesion assay was performed in a buffer without Ca2+ or Mg2+, Jurkat T-cell adhesion to FN was completely blocked, unless MnCl2 was present (Fig. 5a). Addition of 1 mm CaCl2 (Fig. 5a) or 1 mm MgCl2 (Fig. 5b) restored the effects of HgCl2 and PMA but did not significantly modify the effect of MnCl2. The presence of MgCl2 (1 mm) also enhanced adhesion of untreated cells (Fig. 5); when MgCl2 was applied at higher concentrations (3 mm), adhesion of unstimulated cells was further increased, and the stimulatory effects of HgCl2, PMA and MnCl2 were undetectable (not shown). These experiments indicate that Mn2+, but not Hg2+, bypasses the requirement of extracellular Ca2+ or Mg2+ for integrin-mediated cell adhesion.
Figure 5.
HgCl2-induced adhesion of Jurkat T cells to FN is dependent on the presence of extracellular Ca2+ or Mg2+. The experiment was performed as indicated in the Materials and Methods; CaCl2 or MgCl2 were present or absent as indicated, at a concentration of 1 mm. Data shown in (a) and (b) were obtained in a parallel experiment; they are representative for at least three experiments. Data obtained in the absence of CaCl2 were confirmed by experiments in which the cells were preincubated with 1 mm EGTA, followed by extensive washing, which procedure yielded similar results (not shown).
Cell adhesion induced by HgCl2 or by PMA was completely prevented when the assay was performed at 4° instead of at 37° whereas the effect of MnCl2 was unaffected by incubation at low temperature (similar data were obtained in three independent experiments; not shown). Apparently, an active cellular process is required for adhesion induced by HgCl2 or by PMA but, as expected, not for MnCl2-induced adhesion.
Involvement of the actin cytoskeleton in cell adhesion
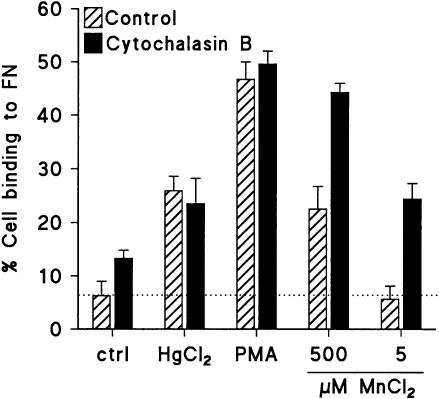
The compound cytochalasin B, which disrupts the intracellular actin cytoskeleton, was used to investigate the role of the cytoskeleton in FN adhesion of Jurkat T cells, induced by different stimuli. Pre-incubation with cytochalasin B induced a dose-dependent increase of adhesion of Jurkat T cells to FN (not shown). Furthermore, treatment with cytochalasin B had a synergistic effect when combined with MnCl2, also when MnCl2 was applied at a dose which was in itself not effective in induction of cell adhesion (Fig. 6). However, no effect of cytochalasin B was detected when it was applied in combination with PMA or HgCl2 (Fig. 6).
Figure 6.
Modification of FN adhesion of Jurkat T cells by cytochalasin B. Cells were preincubated with cytochalasin B (5 µg/ml), followed by stimulation of the cells as indicated. Data represent two similar experiments.
Discussion
The present study identifies the immune dysregulatory compound HgCl2 as a potent activator of integrin-mediated T-cell adhesion. This effect of HgCl2 is not specific for a single integrin or ligand: HgCl2 activates cell binding via LFA-1 to ICAM-1, ICAM-2 and ICAM-3, as well as cell binding via VLA-4 and VLA-5 to FN. Furthermore, adhesion-promoting effects of HgCl2 could be demonstrated in T lymphoblasts derived from normal human PBMC, in a human T-cell clone and in Jurkat T cells, indicating that its effect is not a unique feature of a particular cell line.
Obviously, activation of integrin-mediated ligand binding by a metal ion is not surprising.19–21 However, comparison of the induction of Jurkat T-cell adhesion by Hg2+ with the effect of Mn2+ revealed important differences: the effect of HgCl2, but not the effect of MnCl2, was dependent on the presence of extracellular Ca2+ or Mg2+, and on a physiological temperature. MnCl2 supports binding of ligands to a variety of integrins. High-affinity binding sites for Mn2+ have been mapped in the extracellular domain of several integrins,21,40–42 and on some of these sites, Ca2+ and Mg2+ competed for Mn2+ binding.21,41,42 Mn2+ is able to induce ligand binding to isolated integrin molecules or integrin domains.21,40,43 This metal ion stabilizes a high-affinity state of the integrin that resembles the ligand-bound conformation,18 as detected by mAb which bind to specific epitopes in this conformation.19,44 In contrast to adhesion induced by Mn2+, induction of integrin-mediated ligand binding by PMA requires active cell metabolism and the presence of Ca2+ or Mg2+, and does in general not induce a high-affinity state.18,20,22,25 Since the requirements for induction of lymphocyte adhesion by HgCl2 resemble those of PMA but not those of MnCl2, we propose that HgCl2 acts via an intracellular signalling pathway. Observations from our own group and from others have clearly shown that HgCl2 is able to induce cell signalling events in T lymphocytes. Exposure to HgCl2 can change the redox balance towards an oxidative state by decreasing the intracellular glutathione level, which can lead to signalling events such as intracellular calcium mobilization and production of oxygen radicals.8,45 Furthermore, HgCl2 can induce tyrosine phosphorylation in T lymphocytes and activation of the tyrosine kinases p56lck and p60c-src46,47 Hg2+ has an extreme high affinity for protein thiol groups, and can induce crosslinking of cystein residues on proteins.48 Crosslinking of T-cell membrane proteins by HgCl2 was proposed to trigger tyrosine phosphorylation;46 however, these studies were performed using 10- to 1000-fold higher concentrations of HgCl2 than required to support T-cell adhesion. We tested the involvement of tyrosine kinases in HgCl2-induced T-cell adhesion by using the tyrosine kinase-specific inhibitor herbimycin A. This compound inhibited the induction of cell adhesion by all three stimuli used in our study (not shown) and therefore did not provide further clues about the mechanistic difference in cell adhesion induced by the stimuli examined.
Integrin activation induced by inside-out signalling has been frequently shown to require an intact cytoskeleton.20,22 However, depending on the cell type, disruption of the actin cytoskeleton by cytochalasins can enhance LFA-1-mediated binding to ICAM-1,27,49 probably by induction of integrin clustering on the membrane.49,50 Kucik et al. have shown that exposure to PMA strongly increases the lateral movement of LFA-1 in the cell membrane, which may very well play a major role in increasing the cellular capacity to bind ligand.27 We demonstrate here that cytochalasin B increases integrin β1-mediated adhesion of Jurkat T cells, thus indicating that cytoskeletal anchoring regulates integrin β1-mediated adhesion in this cell line. Furthermore, the effect of this compound was synergistically increased by MnCl2, but not by HgCl2 or PMA. This finding supports the hypothesis that MnCl2 increases the affinity of integrins for their ligands, thereby synergizing with the action of cytochalasin B, whereas PMA and HgCl2 mainly act by modification of integrin association with cytoplasmic proteins, resulting in a similar functional effect as that induced by cytochalasin B.27
A recent publication describes that HgCl2 can induce expression of an activation-dependent epitope of integrin β1, recognized by the mAb HUTS21, on rat lymphocytes.51 Expression of the HUTS21 epitope is correlated to integrin adhesive function, and can be induced by various stimuli, including MnCl2 and a phorbol ester.52 These results are in line with the functional effects of HgCl2 on integrin-mediated adhesion described in the present study. HUTS21 expression was also demonstrated in BN rats exposed to HgCl2,51 but the kinetics of the response suggest that lymphocyte activation secondary to treatment with HgCl2 is at least partially responsible for the effects observed. A functional relevance of expression of activated integrins in HgCl2-induced autoimmunity is given by an in vivo experiment in which a treatment with the HUTS21 mAb prevented renal inflammation and reduced autoantibody production induced by HgCl2.51
Our study provides clear evidence that HgCl2 is able to activate T lymphocytes in the absence of antigen presentation. Whether integrin-mediated ligand binding directly induced by HgCl2 plays a role in its immune dysregulatory abilities in vivo will obviously depend on the concentration of HgCl2 in the microenvironment of lymphocytes. The peak concentrations of mercury in the spleens of mice exposed to HgCl2 at a dose which induces autoimmunity were reported to vary between 646 and 955 ng per gram wet tissue,53,54 which roughly corresponds to 3·2 and 4·8 µm HgCl2. These data suggest that integrin activation by HgCl2 can take place in vivo, in the induction phase of the disease.
Integrin-mediated ligand binding is of great importance for many aspects of the immune system, including costimulation of T lymphocytes during antigen presentation, B-cell activation and antibody production, extravasation and migration of leucocytes, cell-mediated cytotoxicity and regulation of cell survival. Interactions mediated via integrin α4 and β1 are required for the full development of HgCl2-induced disease in vivo.11,51 Therefore, we postulate that the mechanism described in the present study plays an important role in the induction and effector phases of the HgCl2-induced autoimmune syndrome.
Acknowledgments
The authors thank Drs A. Aruffo, A. Bloem, C. G. Figdor, M. L. Kapsenberg, R. A. W. van Lier, J. van Mourik, F. Sánchez-Madrid, B. Seed, and S. Shaw for kindly providing reagents used in this study and Dr G. Koopman for helpful discussion. This study was financially supported by the European Community, BIOTECH programme no. BIO-CT92-0316.
Abbreviations
- FN
fibronectin
- ICAM
intercellular adhesion molecule
- LFA
lymphocyte function-associated antigen
- mAb
monoclonal antibody
- VLA
very late antigen
References
- 1.Fillastre JP, Druet P, Méry JP. Proteinuric nephropathies associated with drugs and substances of abuse. In: Cameron JS, Glassock RJ, editors. The Nephrotic Syndrome. 16. New York, Basel: Marcel Dekker; 1988. p. 697. [Google Scholar]
- 2.Sapin C, Druet E, Druet P. Induction of anti-glomerular basement membrane antibodies in the Brown-Norway rat by mercuric chloride. Clin Exp Immunol. 1977;28:173. [PMC free article] [PubMed] [Google Scholar]
- 3.Aten J, Bosman CB, Rozing J, Stijnen T, Hoedemaeker PJ, Weening JJ. Mercuric chloride-induced autoimmunity in the Brown Norway rat. Cellular kinetics and major histocompatibility complex antigen expression. Am J Pathol. 1988;133:127. [PMC free article] [PubMed] [Google Scholar]
- 4.Pelletier L, Pasquier R, Vial MC, Mandet C, Moutier R, Salomon JC, Druet P. Mercury-induced autoimmune glomerulonephritis: requirement for T cells. Nephrol Dial Transplant. 1987;1:211. [PubMed] [Google Scholar]
- 5.Prouvost-Danon A, Abadie A, Sapin C, Bazin H, Druet P. Induction of IgE synthesis and potentiation of anti-ovalbumin IgE antibody response by HgCl2 in the rat. J Immunol. 1981;126:699. [PubMed] [Google Scholar]
- 6.Gillespie KM, Saoudi A, Kuhn J, Whittle CJ, Druet P, Bellon B, Mathieson PW. Th1/Th2 cytokine gene expression after mercuric chloride in susceptible and resistant rat strains. Eur J Immunol. 1996;26:2388. doi: 10.1002/eji.1830261018. [DOI] [PubMed] [Google Scholar]
- 7.Griem P, Gleichmann E. Metal ion induced autoimmunity. Curr Opin Immunol. 1995;7:831. doi: 10.1016/0952-7915(95)80056-5. [DOI] [PubMed] [Google Scholar]
- 8.Aten J, Claessen N, Chand MA, Weening JJ. The intracellular redox status modifies susceptibility to mercuric chloride-induced T cell activation. Kidney Int. 1995;48:1364. (Abstract). [Google Scholar]
- 9.Prigent P, Saoudi A, Pannetier C, Graber P, Bonnefoy JY, Druet P, Hirsch F. Mercuric chloride, a chemical responsible for T helper cell (Th) 2-mediated autoimmunity in Brown Norway rats, directly triggers T cells to produce interleukin-4. J Clin Invest. 1995;96:1484. doi: 10.1172/JCI118185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roos A, Claessen N, Weening JJ, Aten J. Enhanced T lymphocyte expression of LFA-1, ICAM-1, and the TNF receptor family member OX40 in HgCl2-induced systemic autoimmunity. Scand J Immunol. 1996;43:507. doi: 10.1046/j.1365-3083.1996.d01-66.x. [DOI] [PubMed] [Google Scholar]
- 11.Molina A, Sánchez-Madrid F, Bricio T, Martín A, Barat A, Alvarez V, Mampaso F. Prevention of mercuric chloride-induced nephritis in the Brown Norway rat by treatment with antibodies against the α4 integrin. J Immunol. 1994;153:2313. [PubMed] [Google Scholar]
- 12.Palecek SP, Loftus JC, Ginsberg MH, Lauffenburger DA, Horwitz AF. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature. 1997;385:537. doi: 10.1038/385537a0. [DOI] [PubMed] [Google Scholar]
- 13.Lub M, Van Kooyk Y, Figdor CG. Ins and outs of LFA-1. Immunol Today. 1995;16:479. doi: 10.1016/0167-5699(95)80031-x. [DOI] [PubMed] [Google Scholar]
- 14.Van Noesel C, Miedema F, Brouwer M, De Rie MA, Aarden LA, Van Lier RAW. Regulatory properties of LFA-1 alpha and beta chains in human T-lymphocyte activation. Nature. 1988;333:850. doi: 10.1038/333850a0. [DOI] [PubMed] [Google Scholar]
- 15.Arroyo AG, Campanero MR, Sánchez-Mateos P, Zapata JM, Ursa MA, Angel del Pozo M, Sánchez-Madrid F. Induction of tyrosine phosphorylation during ICAM-3 and LFA-1-mediated intercellular adhesion, and its regulation by the CD45 tyrosine phosphatase. J Cell Biol. 1994;126:1277. doi: 10.1083/jcb.126.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faull RJ, Kovach NL, Harlan JM, Ginsberg MH. Affinity modulation of integrin α5β1: regulation of the functional response by soluble fibronectin. J Cell Biol. 1993;121:155. doi: 10.1083/jcb.121.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arroyo AG, García-Pardo A, Sánchez-Madrid F. A high affinity conformational state on VLA integrin heterodimers induced by an anti-beta 1 chain monoclonal antibody. J Biol Chem. 1993;268:9863. [PubMed] [Google Scholar]
- 18.Bazzoni G, Hemler ME. Are changes in integrin affinity and conformation overemphasized? Trends Biochem Sciences. 1998;23:30. doi: 10.1016/s0968-0004(97)01141-9. 10.1016/s0968-0004(97)01141-9. [DOI] [PubMed] [Google Scholar]
- 19.Dransfield I, Cabañas C, Craig A, Hogg N. Divalent cation regulation of the function of the leucocyte integrin LFA-1. J Cell Biol. 1992;116:219. doi: 10.1083/jcb.116.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stewart MP, Cabañas C, Hogg N. T cell adhesion to intercellular adhesion molecule-1 (ICAM-1) is controlled by cell spreading and the activation of integrin LFA-1. J Immunol. 1996;156:1810. [PubMed] [Google Scholar]
- 21.Smith JW, Cheresh DA. Labeling of integrin αvβ3 with 58Co (III). Evidence of metal ion coordination sphere involvement in ligand binding. J Biol Chem. 1991;266:11429. [PubMed] [Google Scholar]
- 22.Dustin ML, Springer TA. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 1989;341:619. doi: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- 23.Van Kooyk Y, Van de Wiel-Van Kemenade P, Weder P, Kuijpers TW, Figdor CG. Enhancement of LFA-1-mediated cell adhesion by triggering through CD2 or CD3 on T lymphocytes. Nature. 1989;342:811. doi: 10.1038/342811a0. [DOI] [PubMed] [Google Scholar]
- 24.Van Kooyk Y, Weder P, Heije K, De Waal Malefijt R, Figdor CG. Role of intracellular Ca2+ levels in the regulation of CD11a/CD18 mediated cell adhesion. Cell Adh Commun. 1993;1:21. doi: 10.3109/15419069309095679. [DOI] [PubMed] [Google Scholar]
- 25.Danilov YN, Juliano RL. Phorbol ester modulation of integrin-mediated cell adhesion: a postreceptor event. J Cell Biol. 1989;108:1925. doi: 10.1083/jcb.108.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faull RJ, Kovach NL, Harlan JM, Ginsberg MH. Stimulation of integrin-mediated adhesion of T lymphocytes and monocytes: two mechanisms with divergent biological consequences. J Exp Med. 1994;179:1307. doi: 10.1084/jem.179.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kucik DF, Dustin ML, Miller JM, Brown EJ. Adhesion-activating phorbol ester increases the mobility of leukocyte integrin LFA-1 in cultured lymphocytes. J Clin Invest. 1996;97:2139. doi: 10.1172/JCI118651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koopman G, De Graaff M, Huysmans ACLM, Meijer CJLM, Pals ST. Induction of homotypic T cell adhesion by triggering of leukocyte function-associated antigen-1α (CD11a): differential effects on resting and activated T cells. Eur J Immunol. 1992;22:1851. doi: 10.1002/eji.1830220726. [DOI] [PubMed] [Google Scholar]
- 29.Wierenga EA, Snoek M, De Groot C, Chrétien I, Bos JD, Jansen HM, Kapsenberg ML. Evidence for compartmentalization of functional subsets of CD4+ T lymphocytes in atopic patients. J Immunol. 1990;144:4651. [PubMed] [Google Scholar]
- 30.Ahsmann EJM, Lokhorst HM, . Dekker AW, Bloem AC. Lymphocyte function-associated antigen-1 expression on plasma cells correlates with tumor growth in multiple myeloma. Blood. 1992;79:2068. [PubMed] [Google Scholar]
- 31.Takada Y, Puzon W. Identification of a regulatory region of integrin beta 1 subunit using activating and inhibiting antibodies. J Biol Chem. 1993;268:17597. [PubMed] [Google Scholar]
- 32.Sánchez-Madrid F, Landázuri MO, Morago G, Cebrián M, Acevedo A, Bernabeu C. VLA-3: a novel polypeptide association within the VLA molecular complex: cell distribution and biochemical characterization. Eur J Immunol. 1986;16:1343. doi: 10.1002/eji.1830161106. [DOI] [PubMed] [Google Scholar]
- 33.Makgoba MM, Sanders ME, Ginther Luce GE, Dustin ML, Springer TA, Clark EA, Mannoni P, Shaw S. ICAM-1 a ligand for LFA-1-dependent adhesion of B,T and myeloid cells. Nature. 1988;331:86. doi: 10.1038/331086a0. [DOI] [PubMed] [Google Scholar]
- 34.Damle NK, Klussman K, Aruffo A. Intercellular adhesion molecule-2, a second counter-receptor for CD11a/CD18 (leukocyte function-associated antigen-1), provides a costimulatory signal for T-cell receptor-initiated activation of human T cells. J Immunol. 1992;148:665. [PubMed] [Google Scholar]
- 35.Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61:1303. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- 36.Staunton DE, Dustin ML, Springer TA. Functional cloning of ICAM-2, a cell adhesion ligand for LFA-1 homologous to ICAM-1. Nature. 1989;339:61. doi: 10.1038/339061a0. [DOI] [PubMed] [Google Scholar]
- 37.Fawcett J, Holness CLL, Needham LA, Turley H, Gatter KC, Mason DY, Simmons DL. Molecular cloning of ICAM-3, a third ligand for LFA-1, constitutively expressed on resting leukocytes. Nature. 1992;360:481. doi: 10.1038/360481a0. [DOI] [PubMed] [Google Scholar]
- 38.Rago R, Mitchen J, Wilding G. DNA fluorometric assay in 96-well tissue culture plates using Hoechst 33258 after cell lysis by freezing in distilled water. Anal Biochem. 1990;191:31. doi: 10.1016/0003-2697(90)90382-j. [DOI] [PubMed] [Google Scholar]
- 39.Van Kooyk Y, van de Wiel-van Kemenade E, Weder P, Huijbens RJF, Figdor CG. Lymphocyte function-associated antigen 1 dominates very late antigen 4 in binding of activated T cells to endothelium. J Exp Med. 1993;177:185. doi: 10.1084/jem.177.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mould AP, Akiyama SK, Humphries MJ. Regulation of integrin α5β1–fibronectin interactions by divalent cations. Evidence for distinct classes of binding sites for Mn2+, Mg2+, and Ca2+ J Biol Chem. 1995;270:26270. doi: 10.1074/jbc.270.44.26270. [DOI] [PubMed] [Google Scholar]
- 41.Michishita M, Videm V, Arnaout MA. A novel divalent cation-binding site in the A domain of the β2 integrin CR3 (CD11b/CD18) is essential for ligand binding. Cell. 1993;72:857. doi: 10.1016/0092-8674(93)90575-b. [DOI] [PubMed] [Google Scholar]
- 42.Griggs DW, Schmidt CM, Carron CP. Characteristics of cation binding to the I domain of LFA-1 and MAC-1. The LFA-1 I domain contains a Ca2+-binding site. J Biol Chem. 1998;273:22113. doi: 10.1074/jbc.273.34.22113. [DOI] [PubMed] [Google Scholar]
- 43.Smith JW, Piotrowicz RS, Mathis D. A mechanism for divalent cation regulation of β3-integrins. J Biol Chem. 1994;269:960. [PubMed] [Google Scholar]
- 44.Bazzoni G, Shih DT, Buck CA, Hemler ME. Monoclonal antibody 9EG7 defines a novel β1 integrin epitope induced by soluble ligand and manganese, but inhibited by calcium. J Biol Chem. 1995;270:25570. doi: 10.1074/jbc.270.43.25570. [DOI] [PubMed] [Google Scholar]
- 45.Roos A, Claessen N, Schilder-Tol EJM, Chand MA, Weening JJ, Aten J. Thiol levels in CD134-defined subsets of rat T lymphocytes: possible implications for HgCl2-induced immune dysregulation. Biochem Biophys Res Commun. 1997;240:452. doi: 10.1006/bbrc.1997.7679. 10.1006/bbrc.1997.7679. [DOI] [PubMed] [Google Scholar]
- 46.Nakashima I, Pu M, Nishizaki A, et al. Redox mechanism as alternative to ligand binding for receptor activation delivering disregulated cellular signals. J Immunol. 1994;152:1064. [PubMed] [Google Scholar]
- 47.Pu M, Akhand AA, Kato M, et al. Evidence of a novel redox-linked activation mechanism for the Src kinase which is independent of tyrosine 527-mediated regulation. Oncogene. 1996;13:2615. [PubMed] [Google Scholar]
- 48.Utschig LM, Bryson JW, O'halloran TV. Mercury-199 NMR of the metal receptor site in MerR and its protein-DNA complex. Science. 1995;268:380. doi: 10.1126/science.7716541. [DOI] [PubMed] [Google Scholar]
- 49.Lub M, Van Kooyk Y, Van Vliet SJ, Figdor CG. Dual role of the actin cytoskeleton in regulating cell adhesion mediated by the integrin lymphocyte function-associated molecule-1. Mol Biol Cell. 1997;8:341. doi: 10.1091/mbc.8.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stewart MP, McDowall A, Hogg N. LFA-1-mediated adhesion is regulated by cytoskeletal restraint and by a Ca2+-dependent protease, calpain. J Cell Biol. 1998;140:699. doi: 10.1083/jcb.140.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Escudero E, Martín A, Nieto M, et al. Functional relevance of activated β1 integrins in mercury-induced nephritis. J Am Soc Nephrol. 2000;11:1075. doi: 10.1681/ASN.V1161075. [DOI] [PubMed] [Google Scholar]
- 52.Luque A, Gómez M, Puzon W, Takada Y, Sánchez-Madrid F, Cabañas C. Activated conformations of very late activation integrins detected by a group of antibodies (HUTS) specific for a novel regulatory region (355–425) of the common β1 chain. J Biol Chem. 1996;271:11067. doi: 10.1074/jbc.271.19.11067. [DOI] [PubMed] [Google Scholar]
- 53.Hultman P, Eneström S. Dose–response studies in murine mercury-induced autoimmunity and immune-complex disease. Toxicol Appl Pharmacol. 1992;113:199. doi: 10.1016/0041-008x(92)90115-9. [DOI] [PubMed] [Google Scholar]
- 54.Griem P, Scholz E, Turfeld M, Zander D, Wiesner U, Dunemann L, Gleichmann E. Strain differences in tissue concentrations of mercury in inbred mice treated with mercuric chloride. Toxicol Appl Pharmacol. 1997;144:163. doi: 10.1006/taap.1997.8124. 10.1006/taap.1997.8124. [DOI] [PubMed] [Google Scholar]