Abstract
The contribution of early splenic B-cell populations to the colonization of the ileal Peyer's patch was investigated following the surgical removal of the spleen in a series of 56-day-old fetal sheep. The fetuses were killed at 140 days of gestation and the ileal Peyer's patch, the distal jejunal lymph node which drains the Peyer's patch, and a peripheral lymph node, the superficial cervical lymph node, were examined. Enzyme and immunohistochemical evaluation concluded that the distribution of B cells, T cells and stromal cells in the ileal Peyer's patch was similar in splenectomized and normal fetal sheep. Thus, the presence of the fetal spleen was not essential for the colonization of the ileal Peyer's patch and other early sites of B-cell accumulation would appear capable of generating the necessary precursor populations. Investigation of B-cell populations in lymph nodes used a combination of terminal deoxynucleotidyl-transferase-mediated deoxyuridine-triphosphate nick-end-labelling (TUNEL) histochemistry and immunofluorescence to determine the average number of apoptotic B cells in the primary follicles of the outer cortex of splenectomized and normal lambs. A significantly increased number of apoptotic B cells was present in the distal jejunal lymph node but not in the superficial cervical lymph node of splenectomized lambs. This finding suggests that splenectomy affected prenatal B-cell development in fetal sheep and raises questions as to the regulation of B-cell lymphopoiesis in a species using a post-rearrangement organ of diversification.
Introduction
In sheep, the ileal Peyer's patch is responsible for producing the vast majority of B cells and for the generation of the preimmune antibody repertoire.1 Investigation of the relationship between these early B-cell populations and the ileal Peyer's patch has shown that the precursor population destined to colonize the Peyer's patch follicles exists in fetal sheep from at least 63 days of gestation2 (gestation in sheep is 150 days). At the time of existence of this precursor population, the spleen contains the major accumulation of B cells in the fetal sheep.3 Studies in the chicken have differed in their assessment of the significance of the accumulation of B cells in the early spleen. Reynaud et al.4 suggested that the early fetal spleen was a ‘dead-end’ for B cells, having no influence on the subsequent colonization of the bursa of Fabricius. These investigators found that B-cell precursors seeded various organs but only expanded in the bursa of Fabricius. The lack of both cell proliferation and selection for functional immunoglobulin gene sequences was interpreted to indicate that the embryonic chicken spleen was not a site supporting further differentiation of B-cell progenitors. However, others have speculated that B cells originating in the spleen emigrate to the bursal follicles where they expand and undergo gene diversification.5 In sheep, early splenic B cells are engaged in proliferation,3 which may suggest an on-going role in B-cell development.
The contribution of splenic B cells to the colonization of the ileal Peyer's patch and subsequent B-cell formation can be addressed through the early splenectomy of fetal sheep. If splenic B cells are solely responsible for the colonization of the ileal Peyer's patch, then splenectomy at 56 days of gestation, as with anti-immunoglobulin M (IgM) treatment at this stage,2,6 should result in the failure of development of lymphoid follicles in the Peyer's patch. Conversely, if splenic B cells make no contribution to the colonization of the ileal Peyer's patch, that is they are a ‘dead-end’ population, then splenectomy should not affect the ileal Peyer's patch or the B-cell populations that emigrate from the patch and populate other lymphoid tissues.7,8 In the present study, a surgical procedure for the splenectomy of fetal sheep at an early stage of gestation (56 days) was developed and the effect of splenectomy on B-cell development in prenatal lambs was investigated. The results indicate that splenectomy does not prevent the development of the ileal Peyer's patch but the increased levels of apoptosis observed in primary follicles in draining lymph nodes suggest that processes occurring in the early fetal spleen may influence B-cell development.
Materials and methods
Animals and tissue collection
Fetal sheep were obtained from timed matings of Australian merino ewes. At the time of splenectomy, the fetal lambs were 56 days of gestation and both the fetal sheep and the ewes were killed and tissues were collected from the fetuses at 140 days of gestation. Tissues were collected from 30 splenectomized fetal sheep and from 33 normal fetal sheep. The tissues were collected from the ileal Peyer's patch at the attachment of the ileocaecal fold to the ileum, and from the distal jejunal and superficial cervical lymph nodes. To facilitate orientation during sectioning, the transversely resected ileum was cut along the mesenteric border and the tissue was placed with the mucosa down onto a piece of the animal's own liver. Similarly, the lymph nodes were cut transversely through the hilus into two approximately equal halves. The tissues were embedded in Tissue-Tek OTC compound (Miles Inc., Elkhart, IN) and frozen in isopentane (Rectapur, Prolabo, Paris, France) or chlorodifluoromethane (Isceon, ISC Chemicals Ltd, Bristol, UK), chilled in liquid nitrogen, wrapped in aluminium foil, and stored at −70°.
Surgical removal of the spleen from fetal sheep at 56 days of gestation
Anaesthesia of ewes was induced with intravenous thiopentone and maintained by halothane inhalation. The uterus was delivered through a paramedian abdominal incision. The myometrium was incised using electrocautery and the fetal membranes were exposed. The rear end of the 56-day (approximately 20 g) fetus was propelled into a bubble formed by the protruding membranes. Following decompression of the amniotic cavity by removal of fluid into a syringe, the membranes overlying the fetus were incised and its rear end was drawn out onto the surface of the uterus with the left side uppermost. An incision of 7–8 mm was placed immediately caudal to the twelfth rib. Under an operating microscope, gentle traction was applied to draw the rumen out of the abdomen. The spleen, which is closely applied to the rumen, was avulsed and the rumen was replaced in the abdomen. The fetal abdominal wall was repaired with 6/0 atraumatic sutures and the fetus, together with the reserved amniotic fluid, was replaced in the uterus.
The previous splenectomy was observed to be complete at post-mortem examination of each fetus. Survival rates after splenectomy performed at 56 days gestation are of the order of 80%. In another study of the postnatal immunological reactivity of lambs splenectomized at 56 days gestation, the animals were observed to have developed normally (P. McCullagh, unpublished data).
Enzyme and immunohistochemistry
The detection of reactivity for the enzyme 5′-nucleotidase has been described previously.9
An avidin–biotin–peroxidase immunohistochemical technique was used to stain frozen tissue sections, using a protocol that has been described previously.10 The primary antibodies used in this procedure were mouse monoclonal antibodies directed against sheep CD5 (SBU-T1; Centre for Animal Biotechnology, Parkville, Victoria, Australia)11 and sheep CD21 (Du2-87-6).12
TUNEL histochemistry
For the demonstration of apoptotic cells, a cell-death detection kit (Boehringer Mannheim, Mannheim, Germany) that specifically labels cells with extensive DNA fragmentation by template-independent enzymatic addition of fluorescein-conjugated nucleotides to DNA-strand breaks (terminal deoxynucleotidyl-transferase-mediated deoxyuridine-triphosphate nick-end-labelling (TUNEL)) was used according to the manufacturer's recommendations. This procedure was combined with an indirect immunofluorescence technique for the detection of IgM-positive cells. Briefly, frozen sections were allowed to dry for at least 2 hr and then fixed in 4% paraformaldehyde in Dulbecco's phosphate-buffered saline (PBS) for 20 min. Following a 30-min wash in PBS, the sections were permeabilized with 0·1% Triton-X-100/0·1% sodium citrate for 2 min at 4° and rinsed twice with PBS. The sections were then incubated with the TUNEL reaction mixture for 60 min at 37°. Following washing in PBS, the sections were incubated with a rabbit polyclonal antibody directed against sheep IgM µ-chain (Cappel Research Products, Durham, NC) for 1 hr. Following washing in PBS, the sections were incubated for 30 min with a 7-Amino-4-methylcoumarin-3-acetic acid (AMCA)-conjugated goat anti-rabbit immunoglobulin (Vector Laboratories, Burlingame, CA) diluted in 1% bovine serum albumin/Tris-buffered saline. Following a further washing in PBS, the sections were mounted with polyvinyl alcohol pH 7·5 and examined with a Leitz Aristoplan microscope equipped for fluorescence. All incubations were performed in a humid chamber. Negative control sections were incubated in a TUNEL reaction mixture lacking the transferase enzyme.
Evaluation of B-cell development
Tissues from the ileal Peyer's patch, distal jejunal lymph node and superficial cervical lymph node from all animals included in this study were cut and stained for 5′-nucleotidase reactivity. The stained sections were examined for the presence of strong reactivity associated with B-cell follicles. The pattern of 5′-nucleotidase reactivity in the ileal Peyer's patch and lymph nodes is altered in B-cell-depleted sheep.6
As previous studies have shown that a group size of 5–10 animals is sufficient to detect differences in leucocyte populations in lymphoid tissues,10,13 the further analysis of the distribution and phenotype of leucocyte populations was performed on randomly selected groups of splenectomized and normal lambs. The limited use of tissues collected from splenectomized and control lambs in the present study was undertaken so that tissues were available for a number of on-going studies of early fetal splenectomy and to reduce the overall number of animals killed in this series of investigations.
Tissue sections from the ileal Peyer's patch of splenectomized and normal lambs were stained to detect the presence of B cells (CD21+ cells) and T cells (CD5+ cells) and the distribution of leucocyte populations was compared between the two groups. To compare the size of lymphoid follicle, the area occupied by Peyer's patch and lymph node follicles was determined in sections stained for CD21, using a video-image analysis system (Optilab/Pro 2·5, Graftek, Mirmande, France). Images were captured from a CCD-72 video camera (Dage-MTI, Inc., Michigan City, IN) mounted on a Leitz Orthoplan microscope using a Neotech Image Grabber (Neotech Limited, Hampshire, UK). The ileum and lymph nodes had both been cut transversely to standardize the orientation of the tissue sections. Every follicular accumulation of CD21+ cells in one tissue section from the Peyer's patch and lymph nodes of an animal was traced interactively and the area in pixel units was determined. The total follicle area, mean follicle area and number of follicles in a tissue section were calculated. A single tissue section was used because previous studies have indicated that variation in B-cell populations between sections from the same tissue block does not make a major contribution to the total variance.13
The extent of cell death in IgM+ cells in the follicles of the distal jejunal and superficial cervical lymph nodes was determined by counting the number of TUNEL-positive cells in lymph node follicles from randomly selected groups of lambs. All follicles in one tissue section from each lymph node of each animal were counted. Follicles were readily distinguished as large accumulations of IgM+ cells. TUNEL-positive cells lying adjacent to, but separated from, the confluent IgM+ cell accumulations were not counted. The average number of apoptotic B cells in a follicle was calculated for each lymph node from an animal.
Statistical analysis
As some of the data sets were not normally distributed, a Wilcoxon/Kruskal–Wallis Test (Rank Sums) as executed by JMP 3.2.2 (SAS Institute Inc., 1997) was performed to evaluate the differences between the groups of splenectomized and normal lambs. The level of significance chosen was P = 0·05.
Results
Development of ileal Peyer's patch following splenectomy early in gestation
The pattern of enzyme reactivity for 5′-nucleotidase and the immunohistochemical distribution of CD21 showed no obvious differences between the lymphoid follicles in the ileal Peyer's patch of normal and splenectomized lambs (Fig. 1). The appearance of the interfollicular T-cell areas, as assessed by the immunohistochemical distribution of CD5, also showed no obvious differences between the ileal Peyer's patches of the two groups of lambs (not shown).
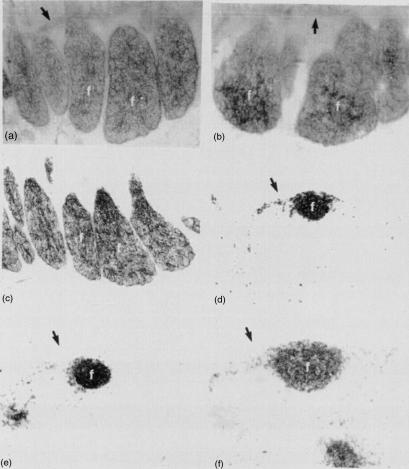
Figure 1.
Fetal lambs at 140 days of gestation. Splenectomies had been performed at 56 days of gestation. (a) Ileal Peyer's patch of a splenectomized fetal lamb showing intense enzyme reactivity in the B-cell follicles (f); (→) muscularis mucosa; 5′-nucleotidase. (b) Ileal Peyer's patch of a normal fetal lamb at 140 days of gestation showing intense enzyme reactivity in the B-cell follicles (f); (→) muscularis mucosa; 5′-nucleotidase. (c) Ileal Peyer's patch of a splenectomized fetal lamb showing CD21+ cells. Note strong staining of cells in the B-cell follicles (f). (d) Distal jejunal lymph node of a splenectomized fetal lamb showing CD21+ cells. Note strong staining of cells in the B-cell follicles (f); (→) capsule. (e) Superficial cervical lymph node of a splenectomized fetal lamb showing CD21+ cells. Note strong staining of cells in the B-cell follicles (f); (→) capsule. (f) Distal jejunal lymph node of a normal fetal lamb showing CD21+ cells. Note strong staining of cells in the B-cell follicles (f); (→) capsule. Magnification in (a)–(f) ×95.
Computer-assisted morphometric analysis was used to determine the total area and mean area of follicles and number of follicles in the ileal Peyer's patch and in the distal jejunal and superficial cervical lymph nodes for the splenectomized and age-matched control lambs (Table 1). For these populations of follicles, there were no significant differences in the total or mean follicular area or in the number of follicles between the groups (P > 0·05).
Table 1.
The total and average follicular area (in pixel unit2) and the number of follicles in a tissue section were determined for the ileal Peyer's patch and the distal jejunal and superficial cervical lymph nodes from normal and splenectomized fetal lambs at 140 days of gestation using a video-image analysis system
| Normal lambs | Splenectomized lambs | |||||||
|---|---|---|---|---|---|---|---|---|
| Total follicle area | Average follicle area | Number of follicles | Total follicle area | Average follicle area | Number of follicles | |||
| Ileal Peyer's patch | ||||||||
| Median | 7227·1 | 267·7 | 30 | 4872·2 | 171·9 | 32 | ||
| Mean | 9284·9 | 213·8 | 36·2 | 8656·2 | 212·4 | 35·5 | ||
| SD | 8631·4 | 115·7 | 25·5 | 8249·0 | 169·7 | 22·4 | ||
| n | 5 | 5 | 5 | 8 | 8 | 8 | ||
| Distal jejunal lymph node | ||||||||
| Median | 731·5 | 128·9 | 5 | 1396·4 | 173·9 | 7 | ||
| Mean | 1479·6 | 136·0 | 9·6 | 3257·7 | 229·0 | 11·6 | ||
| SD | 1647·0 | 70·0 | 9·5 | 5198·6 | 177·5 | 12·7 | ||
| n | 7 | 7 | 7 | 10 | 10 | 10 | ||
| Superficial cervical lymph node | ||||||||
| Median | 1099·2 | 106·1 | 9 | 1372·4 | 135·1 | 9 | ||
| Mean | 1125·9 | 97·7 | 10·9 | 1456·2 | 125·5 | 12·4 | ||
| SD | 720·1 | 26·9 | 6·2 | 776·1 | 42·7 | 7·7 | ||
| n | 7 | 7 | 7 | 9 | 9 | 9 | ||
The median, mean and standard deviation (SD) for these groups of tissues were calculated; n = number of lambs.
Apoptotic B cells in primary follicles of lymph nodes
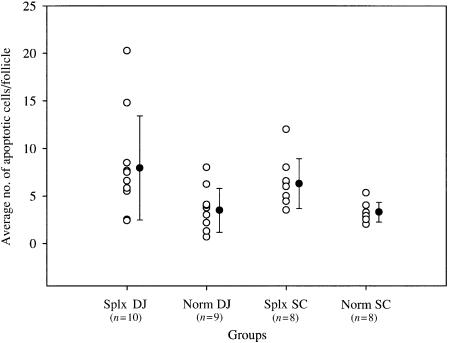
The level of apoptosis in B cells in the primary follicles of the distal jejunal and superficial cervical lymph nodes in splenectomized and normal fetal sheep was investigated using a combination of TUNEL histochemistry and immunofluorescence for IgM. There were few apoptotic B cells present in the lymph nodes of normal fetuses but the level was higher in the splenectomized fetuses (Fig. 2). The number of apoptotic B cells in the primary follicles of the distal jejunal lymph node and the superficial cervical lymph node of splenectomized and normal fetal lambs at 140 days of gestation was counted (Fig. 3). The average number of apoptotic B cells in the follicles of the distal jejunal lymph node was significantly higher in the splenectomized lambs compared with the average number in the normal lambs (P < 0·05). In contrast with this result, the average number of apoptotic B cells in the follicles of the superficial cervical lymph node was not significantly higher in the splenectomized than in the normal lambs (Fig. 3).
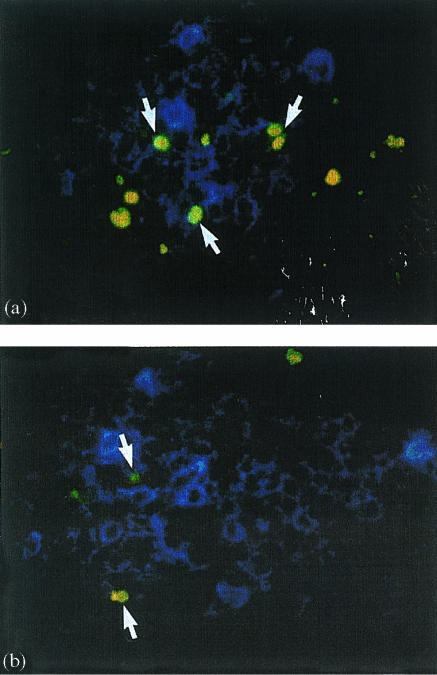
Figure 2.
(a) Distal jejunal lymph node. Splenectomized fetal lamb at 140 days of gestation. Splenectomy was performed at 56 days of gestation. Double immunofluorescence showing many TUNEL+ cells (yellow-green; arrows) in an accumulation of IgM+ cells (blue) in the outer cortex of the lymph node. (b) Distal jejunal lymph node. Normal fetal lamb at 140 days of gestation. Double immunofluorescence showing some TUNEL+ cells (yellow-green; arrows) in an accumulation of IgM+ cells (blue) in the outer cortex of the lymph node. Magnification ×450.
Figure 3.
The average number of apoptotic cells (○) in B-cell follicles was determined in the outer cortex of the distal jejunal lymph node (DJ) and superficial cervical lymph node (SC) of splenectomized (Splx) and normal, age-matched control (Norm) lambs. The group means (± SD) are shown as •. The mean of the SplxDJ group was significantly larger (P < 0·05) than the mean of age-matched control group (NormDJ).
Discussion
The present study shows that B-cell populations in the early fetal spleen are not essential for the colonization and development of the ileal Peyer's patch. Previous studies have used treatment with anti-IgM antibody at 63 days of gestation to prevent the colonization of the ileal Peyer's patch.2,6 The injection of antibody resulted in an absence of B cells from ileal Peyer's patch follicles at 140 days of gestation and a markedly reduced presence of B cells in secondary lymphoid tissues, which hindered the development of primary follicles. Prior to the commencement of lymphopoiesis in the ileal Peyer's patch at around 100 days of gestation,14,15 B cells are present in a number of sites, including the spleen, lymph nodes and circulation.16 The surgical removal of the early spleen shows that sites other than the spleen are able to produce B cells capable of colonizing Peyer's patch follicles.
While the ileal Peyer's patches were similar in normal and splenectomized fetal sheep, the increased levels of apoptosis detected in the primary follicles of the distal jejunal lymph node of splenectomized lambs suggest that B-cell development was affected by splenectomy. In the chicken, Reynaud et al.4 considered the fetal spleen a ‘dead-end’ for B cells while others5 have speculated that B cells from the spleen seed the follicles of the bursa of Fabricius. The present findings support the suggestion that proliferating B cells, which are prominent in the sheep spleen between 55 and 77 days of gestation, have an on-going role in B-cell development.3 A recent study in cattle17 found that diversification of the immunoglobulin light chain gene was occurring in the early fetal spleen, prompting speculation that the spleen may provide a partially diversified B-cell stock from which B cells emigrate to colonize the developing follicles of the ileal Peyer's patch.17 Interestingly, the study in cattle found little diversity outside the spleen, which suggests that splenectomy and the removal of a large source of diversified B cells may skew the antibody repertoire of the colonizing population. The demonstration of increased cell death in lymph nodes draining the ileal Peyer's patch but not in other peripheral lymph nodes following splenectomy provides support for this notion and draws attention to the regulation and control of B-cell lymphopoiesis during ontogeny.
Studies in transgenic mice have shown that several distinct checkpoints exist for the deletion of self-reactive B cells from the preimmune repertoire.18 In mice, self-reactive immature B cells are eliminated through processes of developmental arrest and apoptosis.19 Similarly, apoptosis is associated with the extensive B-cell death that occurs in the ileal Peyer's patch and has been proposed as an important mechanism in the selection of non-self-reactive B cells.20,21 In mice, self-reactive B cells that escape the bone marrow have been shown to localize in the white pulp of the spleen and the paracortex of lymph nodes. A process of follicular exclusion has been described whereby competition among B cells of different specificities prevents the migration of self-reactive B cells into primary follicles trapping them in the outer T-cell zones, which leads to their death by apoptosis in 1–3 days.22,23 Contact with stromal cells is important for the survival of B cells24,25 and the transmission of survival signals from stromal cells to recirculating B cells within primary follicles of secondary lymphoid tissues is implied in the studies of follicular exclusion.22 However, stromal cells are also known to deliver negative signals to B cells inducing apoptosis.25 B cells emigrating from the ileal Peyer's patch travel via lymphatic vessels first to the mesenteric lymph nodes including the distal jejunal lymph node before entering the general circulation.26 In comparison with the levels in peripheral lymph nodes, the high level of apoptosis in B cells in the primary follicles of the distal jejunal lymph node of splenectomized fetuses suggests that negative signals are being delivered to the newly emigrated B cells. This finding raises the possibility that the primary follicles in lymph nodes receiving B cells released from the Peyer's patch follicles represent an additional checkpoint rather than a safe haven.
The present study has shown that the creation of splenectomized fetal lambs is a useful model for the investigation of B-cell ontogeny in sheep. In addition to allowing the assessment of the contribution of B cells emanating from splenic microenvironments to the colonization of the ileal Peyer's patch, the present study has identified areas for further investigation that are relevant to the induction of B-cell tolerance in a species using a post-rearrangement organ of diversification.
Acknowledgments
The authors acknowledge the skilful technical assistance of Karen King, Bernie Barancewicz, Laila Aune, Elfy Bodhal and Ingjerd Andersen. This study was supported in part by grants from the Norwegian Research Council.
References
- 1.Griebel PJ, Hein WR. Expanding the role of Peyer's patches in B-cell ontogeny. Immunol Today. 1996;17:30–9. doi: 10.1016/0167-5699(96)80566-4. 10.1016/0167-5699(96)80605-0. [DOI] [PubMed] [Google Scholar]
- 2.Press CMcL, Reynolds JD, McClure SJ, Simpson-Morgan MW, Landsverk T. Fetal lambs are depleted of IgM+ cells following a single injection of an anti-IgM antibody early in gestation. Immunology. 1996;88:28–34. doi: 10.1046/j.1365-2567.1996.d01-641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Press CMcL, Hein WR, Landsverk T. Ontogeny of leucocyte populations in the spleen of fetal lambs with emphasis on the early prominence of B cells. Immunology. 1993;80:598–604. [PMC free article] [PubMed] [Google Scholar]
- 4.Reynaud C-A, Imhof BA, Anquez V, Weill J-C. Emergence of committed B lymphoid progenitors in the developing chicken embryo. EMBO J. 1992;11:4349–58. doi: 10.1002/j.1460-2075.1992.tb05534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCormack WT, Thompson CB. Somatic diversification of the chicken immunoglobulin light-chain gene. Adv Immunol. 1990;48:41–67. doi: 10.1016/s0065-2776(08)60751-8. [DOI] [PubMed] [Google Scholar]
- 6.Press CMcL, Reynolds JD, McClure SJ, Landsverk T. Development of accessory cells in B-cell compartments is retarded in B-cell-depleted fetal sheep. Dev Immunol. 1998;6:223–31. doi: 10.1155/1998/27025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reynolds JD, Kennedy L, Peppard J, Pabst R. Ileal Peyer's patch emigrants are predominantly B cells and travel to all lymphoid tissues in sheep. Eur J Immunol. 1991;21:283–9. doi: 10.1002/eji.1830210207. [DOI] [PubMed] [Google Scholar]
- 8.Pabst R, Reynolds JD. Evidence of extensive lymphocyte death in sheep Peyer's patches. II. The number and fate of newly-formed lymphocytes that emigrate from Peyer's patches. J Immunol. 1986;136:2011–17. [PubMed] [Google Scholar]
- 9.Halleraker M, Landsverk T, Nicander L. Organization of ruminant Peyer's patches as seen with enzyme histochemical markers of stromal and accessory cells. Vet Immunol Immunopathol. 1990;26:93–104. doi: 10.1016/0165-2427(90)90135-f. [DOI] [PubMed] [Google Scholar]
- 10.Gunnes G, Jörundsson E, Press CMcL, Skjerve E, Ulvund M, Landsverk T. Increased γδ T-cell populations in draining lymph nodes of lambs during the elicitation phase of dinitrochlorobenzene-induced contact hypersensitivity. Dev Comp Immunol. 1999;23:665–75. doi: 10.1016/s0145-305x(99)00045-2. 10.1016/s0145-305x(99)00045-2. [DOI] [PubMed] [Google Scholar]
- 11.Beya MF, Miyasaka M, Dudler L, Ezaki T, Trnka Z. Studies on the differentiation of T lymphocytes in sheep. II. Two monoclonal antibodies that recognize all ovine T lymphocytes. Immunology. 1987;57:115–21. [PMC free article] [PubMed] [Google Scholar]
- 12.Hein WR, Dudler L, Marston WL, Landsverk T, Young AJ, Avila D. Ubiquitination and dimerization of complement receptor type 2 on sheep B cells. J Immunol. 1998;161:458–66. [PubMed] [Google Scholar]
- 13.Gunnes G, Press CMcL, Tverdal A, Landsverk T. Compartments within the lymph node cortex of calves and adult cattle differ in the distribution of leukocyte populations: an immunohistochemical study using computer-assisted morphometric analysis. Dev Comp Immunol. 1998;22:111–23. doi: 10.1016/s0145-305x(97)00038-4. 10.1016/s0145-305x(97)00038-4. [DOI] [PubMed] [Google Scholar]
- 14.Press CMcL, Halleraker M, Landsverk T. Ontogeny of leukocyte populations in the ileal Peyer's patch of sheep. Dev Comp Immunol. 1992;16:229–41. doi: 10.1016/0145-305x(92)90022-5. [DOI] [PubMed] [Google Scholar]
- 15.Reynolds JD, Morris B. The evolution and involution of Peyer's patches in fetal and postnatal sheep. Eur J Immunol. 1983;13:627–35. doi: 10.1002/eji.1830130805. [DOI] [PubMed] [Google Scholar]
- 16.Miyasaka M, Morris B. The ontogeny of the lymphoid system and immune responsiveness in sheep. Prog Vet Microbiol Immunol. 1988;4:21–55. [PubMed] [Google Scholar]
- 17.Lucier MR, Thompson RE, Waire J, Lin AW, Osborne BA, Goldsby RA. Multiple sites of Vλ diversification in cattle. J Immunol. 1998;161:5438–44. [PubMed] [Google Scholar]
- 18.Goodnow CC. Balancing immunity, autoimmunity and self-tolerance. Ann N Y Acad Sci. 1997;815:55–66. doi: 10.1111/j.1749-6632.1997.tb52044.x. [DOI] [PubMed] [Google Scholar]
- 19.Hartley SB, Cooke MP, Fulcher DA, Harris AW, Cory S, Basten A, Goodnow CC. Elimination of self-reactive B lymphocytes proceeds in two stages: arrested development and cell death. Cell. 1993;72:325–35. doi: 10.1016/0092-8674(93)90111-3. [DOI] [PubMed] [Google Scholar]
- 20.Motyka B, Reynolds JD. Apoptosis is associated with the extensive B cell death in the sheep ileal Peyer's patch and the chicken bursa of Fabricius: a possible role in B cell selection. Eur J Immunol. 1991;21:1951–8. doi: 10.1002/eji.1830210825. [DOI] [PubMed] [Google Scholar]
- 21.Reynolds JD. Evidence of extensive lymphocyte death in sheep Peyer's patches. I. A comparison of lymphocyte production and export. J Immunol. 1986;136:2005–10. [PubMed] [Google Scholar]
- 22.Cyster JG, Hartley SB, Goodnow CC. Competition for follicular niches excludes self-reactive cells from the recirculating B-cell repertoire. Nature. 1994;371:389–95. doi: 10.1038/371389a0. [DOI] [PubMed] [Google Scholar]
- 23.Cyster JG, Goodnow CC. Antigen-induced exclusion from follicles and anergy are separate and complementary processes that influence peripheral B cell fate. Immunity. 1995;3:691–701. doi: 10.1016/1074-7613(95)90059-4. [DOI] [PubMed] [Google Scholar]
- 24.Illera VA, Perandones CE, Stunz LL, Mower DA, Ashman RF. Apoptosis in splenic B lymphocytes: regulation by protein kinase C and IL-4. J Immunol. 1993;151:2965–73. [PubMed] [Google Scholar]
- 25.Borghesi LA, Smithson G, Kincade PW. Stromal cell modulation of negative regulatory signals that influence apoptosis and proliferation of B lineage lymphocytes. J Immunol. 1997;159:4171–9. [PubMed] [Google Scholar]
- 26.Lowden S, Heath T. Lymphatic drainage from the distal small intestine in sheep. J Anat. 1993;183:13–20. [PMC free article] [PubMed] [Google Scholar]